Abstract
Binding kinetic studies with the adenosine analogues [3H]CPA (0.250–50 nM) and [3H]CGS21680 (0.1–100 nM) were performed in renal tissue from control (NL) and thyroidectomised (HTX) rats. We propose that the low renal adenosine content reported in hypothyroid rats may induce changes in the density and/or affinity of adenosine receptor, distributed in the cortex (C), outer medulla (OM), and inner medulla (IM) of the kidney.
[3H]CPA and [3H]CGS21680 binding saturation isotherms were fitted by nonlinear regression analysis and evaluated by Furchgott's method. These results revealed high (KH) and low (KL) affinity (KD) sites for both compounds. As expected, a heterogeneous pattern was observed for Bmax and KD values.
Bound [3H]CPA and [3H]CGS21680 were displaced by increasing concentrations of nonlabelled DPCPX and NECA, respectively, indicating the presence of A1 and A2A adenosine receptors distributed in the renal segments studied.
The relative intrinsic efficacy (ɛ) for [3H]CPA and [3H]CGS21680 showed extreme values (far from 1.0), 0.5 in IM NL and 2.70 in IM HTX for [3H]CGS21680.
Our results indicate that A2A adenosine receptor is predominant in IM from HTX, but A1 receptors are expressed preferentially in C in NL.
We conclude that the changes observed in number, affinity, and ɛ for the A2A receptor in IM from HTX might be responsible from alterations in medullary function, that is, incapacity for urine concentration as observed in the hypothyroid kidney.
Keywords: Adenosine A1 receptors, adenosine A2A receptors, adenosine agonist CPA, adenosine agonist CGS21680, hypothyroidism and renal adenosine
Introduction
Adenosine receptors participate in the regulation of renal haemodynamics, renin secretion and transport processes in the kidney (Spielman & Thompson, 1982; Mccoy et al., 1993). The concentration of the nucleoside responsible for the activation of adenosine receptors depends on the activity of adenosine-metabolising enzymes (Spielman & Arend, 1991), and becomes crucial in certain conditions such as acute renal failure (Osswald et al., 1977) and hypothyroidism (Franco et al., 1996), in which adenosine contributes to the alterations of renal function. In this regard, hypothyroidism induces profound changes in cellular metabolism, in particular that of ATP and adenosine (Capasso & DeSanto, 1987). An increase in 5′-nucleotidase activity and a decrease in adenosine kinase activity have been reported in brain tissue of hypothyroid rats (Mazurkiewics & Saggerson, 1989). Moreover, 5′-nucleotidase and adenosine deaminase activities in brown and white adipocytes have been shown to be decreased (Jamal & Saggerson, 1987), associated with a lower adenosine content (Ohisalo et al., 1987). In addition, an enhanced sensitivity to the nucleoside is observed in those cells (Saggerson, 1986). Thus, the alteration of adenosine-metabolising enzymes results in changes in the overall adenosine concentration, which could induce changes in the response of extracellular receptors (Saggerson, 1986; Ohisalo et al., 1987).
Previous studies from our laboratory have demonstrated a low adenosine content in the kidney of hypothyroid rats, which induces the preferential activation of A1 receptors, which in turn leads to the renal vasoconstriction observed in this model; these findings suggest an increased sensitivity to this nucleoside (Franco et al., 1996). Furthermore, in this condition, adenosine receptor blockers restore renal function to near normal levels, and exogenous adenosine induces paradoxical renal vasodilatation (Franco et al., 1996).
It is possible that the low adenosine content in the hypothyroid kidney (Franco et al., 1996) induces changes in the number and/or sensitivity of adenosine receptors. The purpose of this study was to investigate whether the functional renal response observed in hypothyroidism is associated with changes in adenosine receptor binding. The selective A1 and A2A adenosine receptor agonists [3H]CPA and [3H]CGS21680 were used to perform binding studies in kidney slices.
Methods
Induction of hypothyroidism
Male Wistar rats weighing 300–350 g were subjected to surgical thyroidectomy with parathyroid reimplantation as previously described (Franco et al., 1996; Martínez et al., 1997). Briefly, the trachea was exposed under ether anaesthesia, and under a stereoscopic microscope (model M5, Wild, Heerbrugg, Switzerland), the parathyroid glands were visualised, dissected from the thyroid gland, and reimplanted into the surrounding neck muscles. The thyroid gland was then carefully dissected, to avoid injury to the laryngeal nerves, and completely excised. The effectiveness of this procedure was assessed by determining the serum calcium concentration in 10 control and 10 thyroidectomised rats, chosen by a randomised method using standard techniques (Ca2+ of 10.2±3 in control vs 10.3±0.2 mM in HTX; phosphorous of 6.5±0.3 in control vs 6.3±0.5 mM in HTX; T4 of 6.4±0.77 in control vs 1.18±0.19 μg dl−1 in HTX, (P<0.05). Since sham-operated rats had similar values of thyroxin, calcium, and phosphorous, NL rats were chosen as controls (data not shown). Rats were killed 15 days after surgery, and the kidneys were collected.
Radioligand binding assays
The binding assays were performed in slices from the cortex (C), outer medulla (OM), and inner medulla (IM) of normal and HTX hypothyroid rat kidneys, 15 days after surgery.
The rats were anaesthetised with sodium pentobarbital (35 mg kg−1). The kidneys were rapidly removed and transferred to ice-cold buffer (4°C) of the following composition in mM: 300 mannitol, 50 Tris-HCl and 2.5 glucose, pH 7.4. The kidneys were dissected in a Petri dish placed on ice. Slices from C, OM, and IM, were prepared with a kidney slicer. Slices of approximately 1 mm thickness were weighed and suspended in buffer solution (W V−1), 100 mg of fresh tissue ml−1. The final protein content was 100–200 mg protein ml−1. Before the start of the binding assays, slices were divided into 100 mg samples, warmed to 37°C, and allowed to equilibrate for 20 min in aerated buffer solution in a shaker bath. Adenosine deaminase (2 mU ml−1) was added, and the tissue was incubated for 120 min before starting the binding studies; the enzyme diffusion in this preparation cannot be estimated.
Binding assays were performed as described elsewhere (Blanco et al., 1992), with the adenosine analogues [3H]CGS21680 [-2-carboxyethyl(phenethylamino)]-5′-N-ethylcarboxamido adenosine at a concentration range of 0.5–100 mM, and [3H]CPA (N6-cyclopentyladenosine) at a concentration range of 0.250–50 nM to determine total binding. Reversible binding assays and selectivity were assessed with nontritiated compounds, the A1-adenosine receptor antagonist DPCPX (8-cyclopenthyl-1,3-dipropylxanthine) and the A2-adenosine receptor agonist NECA (5′-N-ethylcarboxamido adenosine), used at concentrations of 1–100 nM. Adenosine receptors have a higher affinity for this adenosine analogue than for adenosine. Nonspecific binding was determined by incubating the labelled compound with a 500-fold excess amount of nontritiated compound. This value was subtracted from the total binding to obtain specific binding, and it was less than 30% in all the experiments. Treatment with adenosine deaminase increased specific binding by 40% in C and OM, and by 60% in IM, indicating that a great extent of specific binding was masked by endogenous adenosine.
The reaction was started with 10 μCi ml−1 of the radioactive adenosine analogue and with different amounts of the nonlabelled compound added to the samples, in order to obtain suitable concentrations. After 120 min incubation, the reaction achieved equilibrium conditions and was stopped with 2 ml ice-cold buffer (4°C) containing in mM: 300 mannitol, 80 Na2SO4, 10 Tris, and 0.01 dipyridamole, pH 7.4. Free and bound radioligands were separated by rapid filtration through Millipore filters (0.65 μm pore size, DAWP-025) moistened with stop solution, and kept under suction. The filters were washed twice with 1 ml of ice-cold stop solution and oven-dried overnight at 65°C. The filters were then dissolved in 5 ml scintillation spectrophotometer solution (1209-Rackbeta, Wallac-Pharmacia). All radiolabelled compound binding values were corrected for nonspecific and filter bindings. Binding results were expressed in fmol mg prot.−1
Protein determination
Protein content in kidney slices was determined according to the bicinchoninic acid (BCA) method (Smith et al., 1985) using a BCA protein assay kit (Pierce Rockford III, U.S.A.), with bovine plasma gamma globulin as standard. A standard curve was constructed for concentrations from 0 to 1.5 mg ml−1, and the absorbance of the samples was measured at 562 nm.
Materials
[3H]CGS21680, ([3H]2-[carboxyethyl(phenethylamino)]-5′-N-ethyl carboxamide adenosine (45 Ci mmol−1; 1665 GBq mmol−1), [3H]CPA ([3H]N6-cyclopentyladenosine) and Aquasol-2-NEF were from New England Nuclear (Life Sci. Boston U.S.A.). A1-adenosine receptor antagonist DPCPX (8-cyclopentyl-1,-3-dipropylxanthine), the A2A-adenosine receptor agonist CGS-21680-HCl(2-p-carboxyethyl)phenethylamino-5′-N-ethylcarboxamido-adenosine hydrochloride and A2-receptor agonist NECA (5′-N-ethylcarboxamidoadenosine) were purchased from RBI-Sigma (Nattick, MA, U.S.A.). All other products were purchased from Sigma (St Louis, MO, U.S.A.).
Data analysis
Binding experiments with different preparations were performed a minimum of three times, and each data point represents assays in triplicate. Data from saturation and displacement curves were analysed by a nonlinear regression method using the computer program Enzfitter (Elsevier-Biosoft, U.K., 1999). Since the data did not fit well to single binding site kinetics in any of the experiments when examined by the F-test, fitting of the saturation isotherms obtained was then carried out based on kinetics for two independent binding sites using the following equation:
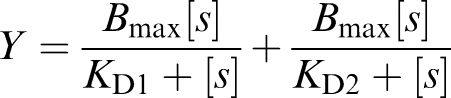 |
where [s] represents the ligand concentration. After successive iterations with a χ2 test, the best curve fit was obtained and the data were plotted and expressed as the mean±standard error of the mean (s.e.m.). Radioligand binding relative intrinsic efficacies (ɛ) were calculated by Furchgott's validation method according to Morey et al. (1998). Comparisons among groups were performed by two-way analysis of variance (ANOVA) for multiple comparisons followed by Newmann–Keuls test (Sigma Stat, Jandel Scientific, U.S.A.). Differences among the curves obtained were established by analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons (Sigma Stat, Jandel Scientific U.S.A.). Differences were considered statistically significant when P<0.05.
Results
Binding saturation experiments with [3H]CPA and [3H]CGS 21680
Saturation curves with [3H]CPA were performed in each renal section of C, OM, and IM in the concentration range of 0.250 to 50 nM (data not shown). Table 1 shows the binding constants (KD and Bmax) for [3H]CPA in each kidney area for both the NL and HTX group. In each renal section of both groups, the high (KH) and low (KL) affinity binding sites and their respective capacities Bmax were examined. The order of affinity for KH values in each renal section was different in NL (OM>IM>C, P<0.05) but similar in HTX (NS). The KL values were found to be different in NL (IM>C>OM, P<0.05) and in HTX (C>OM>IM, P<0.05). The high-affinity state of the adenosine receptor in the NL group showed no differences in the number of sites (Bmax) between C and OM sections, which was greater than in IM (P<0.05). Differing in the low-affinity state, the Bmax pattern was as follows: C>IM>OM (P<0.05). In the HTX group Bmax values were different among all sections in both the high-affinity state IM>OM>C (P<0.05) and the low-affinity state IM>OM>C, (P<0.05). In the high-affinity state, C was significantly lower in HTX than NL (P<0.05), while no changes were observed in OM and IM when comparing NL vs HTX rats (Table 1). When comparisons were performed among groups, Bmax values in the low-affinity state were NL C>HTX C; NL OM<HTX OM; NL IM<HTX IM (P<0.05) (Table 1).
Table 1.
[3H]CPA-binding renal cortex, outer medulla, and inner medulla from normal and hypothyroid rats
| Normal rats | Hypothyroid rats | |||
| Kidney section | KD (nM) | Bmax (fmol mg protein−1) | KD (nM) | Bmax (fmol mg protein−1) |
| Cortex | KH=1.74±0.25 | 257±21 | KH=1.91±0.92 | 205±13* |
| KL=126.94±5.80 | 5936±128 | KL=21.55±3.01* | 559±24* | |
| Outer medulla | KH=0.82±0.07 | 291±73 | KH=1.47±0.16* | 346±19 |
| KL=8810±646 | 180±83 | KL=384.7±68* | 6445±82* | |
| Inner medulla | KH=0.96±0.05 | 384±13 | KH=1.87±0.38* | 426±41 |
| KL=41.23±1.53 | 2802±29 | KL=32391±91* | 8511±269* | |
P<0.05 normal vs hypothroid rats.
Mean binding parameters KD and Bmax (n=3±s.e.m) of [3H]2-chloro-N6-cyclopentyl-adenosine ([3H]CPA) in renal slices of the cortex, outer medulla, and inner medulla, derived from saturation experiments. Experiments were carried out in triplicate. The specific binding of [3H]CPA corresponds to about 40–60% for the three renal sections studied. KH denotes the high-affinity state of receptor; KL denotes the low-affinity state of receptor.
Saturation curves with [3H]-CGS21680 were determined in the concentration range of 0.1–100 nM (data not shown). Table 2 shows the binding constants (KD and Bmax) for [3H]CGS21680 in each kidney section for both NL and HTX groups. In both groups, a high (KH) and low (KL) affinity binding sites and their respective capacities Bmax were examined in each section. KH values in C and OM from NL did not differ between them, but they were significantly greater than in IM (C=OM>IM, P<0.05). In contrast, KH values in HTX differed in the three sections and showed the following potency order: IM>OM>C (P<0.05). KL values were as follows: IM>OM>C (P<0.05) for NL, and C>IM>OM (P<0.05) for HTX. Differences were observed (P<0.05) when KH and KL values were compared in the two groups, with the exception of the C from HTX rats.
Table 2.
[3H]CGS 21680-binding renal cortex, outer medulla, and inner medulla from normal and hypothyroid rats
| Kidney | Normal rats | Hypothyroid rats | ||
| KD (nM) | Bmax (fmol mg protein−1) | KD (nM) | Bmax (fmol mg protein−1) | |
| Cortex | KH=0.07±0.01 | 97±9 | KH=19.41±0.81* | 898±96* |
| KL=111.73±1.30 | 620±118 | KL=20.68±2.13* | 269±23* | |
| Outer medulla | KH=0.04±0.02 | 96±9 | KH=5.92±0.31* | 1288±123* |
| KL=37.09±1.60 | 360±11 | KL=40821±8313* | 7270±93* | |
| Inner medulla | KH=1.00±0.29 | 0.50±0.04 | KH=0.18±0.04* | 188±30* |
| KL=1.99±0.27 | 781±39 | KL=64.10±26* | 862±173* | |
P<0.05 normal vs hypothroid rats.
Mean binding parameters KD and Bmax (n=3±s.e.m) of [3H]2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamidoadenosine ([3H]CGS 21680) in renal slices of the cortex, outer medulla, and inner medulla, derived from saturation experiments. Experiments were carried out in triplicate. The specific binding of [3H]CGS 21680 corresponds to about 40–60% for the three renal sections studied. KH denotes the high-affinity state of receptor; KL denotes the low-affinity state of receptor.
The high-affinity state of the receptor in the NL group had a significantly higher capacity value in C and OM compared to IM (P<0.05); no difference was seen between C and OM (NS). For KL values (OM<C=IM P>0.05), capacity was lower in OM than in C and IM. When comparing KL capacities in NL vs HTX, the values for IM were similar (NS); these values were significantly lower for C and higher for OM in HTX (P<0.05).
Binding competition experiments with [3H]CPA and [3H]CGS 21680
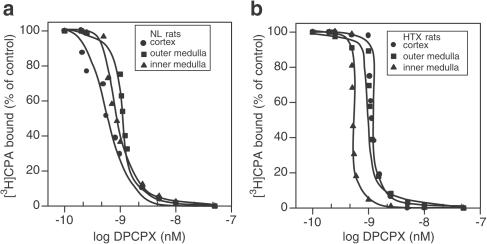
To study the competitive antagonism of A1-adenosine receptors, displacement curves of 1 nM [3H]CPA were performed in the presence of different concentrations of the A1-adenosine antagonist DPCPX. Concentrations ranging from 0.5 to 50 nM of the antagonist inhibited 1 nM [3H]CPA binding in a concentration-dependent pattern in both NL (Figure 1a) and HTX (Figure 1b) for the three renal sections C, OM, and IM. [3H]CPA-binding Ki values for all the renal sections in NL and HTX in the presence of DPCPX are shown in Table 3 . The order of potency for DPCPX-mediated inhibition of [3H]CPA binding, when comparing Ki values, was C>IM>OM in NL, and IM>C=OM in HTX. In examining this parameter, a significant shift of the displacement curve to the right was observed only in renal cortex from HTX (P<0.05).
Figure 1.
[3H]CPA (1 nM) displaced by DPCPX over a concentration range from 0.5 to 50 nM in C, OM, and IM of normal (a) and hypothyroid (b) kidneys. Slices were incubated with the radiolabelled compound for 120 min and exposed to different concentrations of nonlabelled DPCPX. The experiments were performed at 25°C. The rest of the procedure was as described in the Methods section. Results are expressed as the percentage of the control binding. Only specific binding was plotted. Results are the means of five experiments.
Table 3.
Ki values from [3H]CPA-binding displacement in the presence of DPCPX and NECA in renal sections from normal and hypothyroid rats
| A1 ([3H]CPA vs DPCPX) Ki (nM) | A2A ([3H]CPA vs NECA) Ki (nM) | |
| Cortex | ||
| Normal rats | 0.12±0.04 | 0.78±0.21* |
| Hypothyroid rats | 0.82±0.10 | 0.71±0.19 |
| Outer medulla | ||
| Normal rats | 0.74±0.17 | 0.10±0.04* |
| Hypothyroid rats | 0.84±0.12 | ND |
| Inner medulla | ||
| Normal rats | 0.20±0.05 | 0.50±0.05* |
| Hypothyroid rats | 0.22±0.05 | 0.90±0.20* |
Data are means±s.e.m.
P<0.05 DPCPX vs NECA; ND=not detectable.
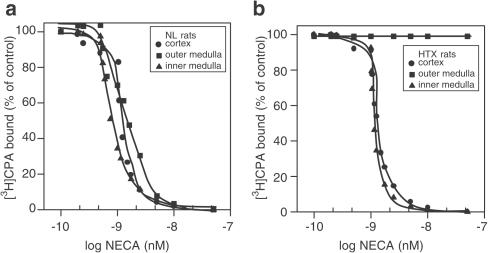
Experiments to test competitive agonist binding were carried out with the nonspecific A2-adenosine receptor agonist NECA in NL (Figure 2a) and HTX (Figure 2b) rats. Bound [3H]CPA was displaced by 0.5–20 nM NECA in a concentration-dependent manner in NL and HTX renal sections, with the exception of OM from HTX, in which no reversal of [3H]CPA-specific binding was induced by NECA. Displacement with the nonspecific A2-adenosine agonist was as follows, OM>IM>C in NL, and C=IM (OM, not detectable) in HTX. When comparing NL vs HTX, displacement curves for both C and IM were significantly shifted to the right in the latter (P<0.05).
Figure 2.
[3H]CPA (1 nM) displaced by NECA over a concentration range of 0.5–50 nM in C, OM, and IM of normal (a) and hypothyroid (b) kidneys. Slices were incubated with the radiolabelled compound for 120 min and exposed to different concentrations of the non-labelled NECA. The experiments were performed at 25°C. The rest of the procedure was as described in the Methods section. Results are expressed as the percentage of the control binding. Only specific binding was plotted. Results are the means of five experiments.
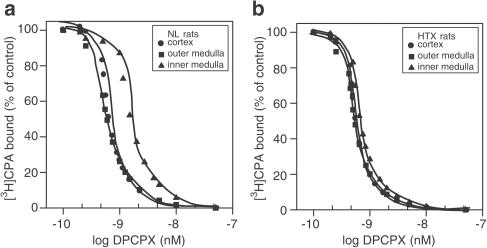
As a highly specific A2A-adenosine antagonist receptor was still not available at the time, we tested for competitive antagonism with the displacement of 1 nM [3H]CGS21680 in the presence of DPCPX, an A1 antagonist for which A2-adenosine receptors have some affinity (Lee & Redington, 1986; Cunha et al., 1996; Müller, 1997). The results in Figure 3a and b indicate a complete inhibition of specific [3H]CGS21680 binding with 0.5–50 nM DPCPX in a concentration-dependent manner in both the NL and HTX group, respectively, and in the C, OM, and IM. The Ki values indicate that the order of potency was similar between groups (Table 4 ).
Figure 3.
[3H]CGS21680 (1 nM) displaced by DPCPX over a concentration range of 0.5–50 nM in C, OM, and IM of normal (a) and hypothyroid (b) kidneys. Slices were incubated with the radiolabelled compound for 120 min and exposed to different concentrations of the nonlabelled DPCPX. The experiments were performed at 25°C. The rest of the procedure was as described in the Methods section. Results are expressed as the percentage of the control binding. Only specific binding was plotted. Results are the means of five experiments.
Table 4.
Ki values from [3H]CGS-21680 binding displacement in the presence of DPCPX and NECA in renal sections from normal and hypothyroid rats
| A1 ([3H]CGS vs DPCPX) Ki (nM) | A2A ([3H]CGS vs NECA) Ki (nM) | |
| Cortex | ||
| Normal rats | 0.14±0.03 | 0.26±0.04* |
| Hypothyroid rats | 0.16±0.01 | 0.28±0.04* |
| Outer medulla | ||
| Normal rats | 0.17±0.04 | 0.14±0.06 |
| Hypothyroid rats | 0.16±0.04 | 0.22±0.05 |
| Inner medulla | ||
| Normal rats | 0.12±0.01 | 0.23±0.05* |
| Hypothyroid rats | 0.17±0.03 | 0.24±0.01* |
Data are means±s.e.m.
P<0.05 DPCPX vs NECA.
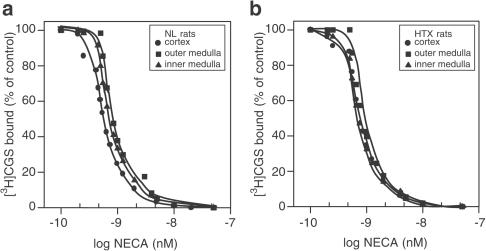
Experiments to determine competitive agonist displacement with [3H]CGS21680-specific binding were performed with 0.5–20 nM NECA. The results in Figure 4a and b indicate a complete displacement in this concentration range. The Ki values shown in Table 4 were significantly higher in OM (0.14±0.06 nM, P<0.05) from NL than in IM and C for the same group. Thus, the order of potency for the displacement of specifically bound [3H]CGS21680 in the presence of NECA was as follows, OM>IM=C in NL and, C=OM=IM in HTX.
Figure 4.
[3H]CGS21680 (1 nM) displaced by NECA over a concentration range of 0.5–50 nM in C, OM, and IM of normal (a) and hypothyroid (b) kidneys. Slices were incubated with the radiolabelled compound for 120 min and exposed to different concentrations of the nonlabelled DPCPX. The experiments were performed at 25°C. The rest of the procedure was as described in the Methods section. Results are expressed as the percentage of the control binding. Only specific binding was plotted. Results are the means of five experiments.
Since the data from the binding saturation experiments were fitted with a two-site model, the information provided by Ki values was limited because in some experiments the differences were not significant. Therefore, we calculated the KL/KH ratio for [3H]CPA (Table 5 ) and for [3H]CGS21680 (Table 6 ), in the presence of DPCPX and NECA, to determine whether these differences were in affinity or intrinsic efficacy. In the case of [3H]CPA binding experiments, KL/KH ratio values ranged from 2.85 to 4.15. These values corresponded to OM from HTX in the presence of DPCPX and IM in the presence of NECA. With regard to their respective ɛ CPA values (Table 5), the range was from nondetectable in OM of HTX to 1.36 in IM of HTX. For [3H]CGS21680-binding experiments, KL/KH ratios were in the range of 1.50–4.71 (Table 6), both values corresponding to the IM from HTX and NL rats, respectively. The ɛ values for CGS21680 were in the range of 0.50–2.70, both extreme values corresponding to the IM from the NL and HTX groups.
Table 5.
KL/KH ratios and intrinsic efficacies (ɛ ) from [3H]CPA binding curves in the presence of DPCPX and NECA in renal sections from normal and hypothyroid rats
| [3H]CPA (KL/KH) DPCPX | ([3H]CPA (KL/KH) NECA | ɛCPA | |
| Cortex | |||
| Normal rats | 3.12 | 3.90 | 1.25 |
| Hypothyroid rats | 3.01 | 3.44 | 1.14 |
| Outer medulla | |||
| Normal rats | 3.24 | 3.82 | 1.17 |
| Hypothyroid rats | 2.85 | — | — |
| Inner medulla | |||
| Normal rats | 3.12 | 3.58 | 1.14 |
| Hypothyroid rats | 3.05 | 4.15 | 1.36 |
ɛ the intrinsic efficacy of an agonist as defined by Morey et al. (1998).
Table 6.
KL/KH ratios and intrinsic efficacies (ɛ) from [3H]CGS-21680 binding curves in the presence of DPCPX and NECA in renal sections from normal and hypothyroid rats
| [3H]CGS (KL/KH) DPCPX | ([3H]CGS (KL/KH) NECA | ɛCGS | |
| Cortex | |||
| Normal rats | 3.20 | 3.40 | 1.06 |
| Hypothyroid rats | 3.00 | 3.29 | 1.09 |
| Outer medulla | |||
| Normal rats | 2.46 | 2.80 | 1.14 |
| Hypothyroid rats | 2.00 | 2.70 | 1.35 |
| Inner medulla | |||
| Normal rats | 4.71 | 2.40 | 0.50 |
| Hypothyroid rats | 1.50 | 4.00 | 2.70 |
ɛ is the intrinsic efficacy of an agonist as defined by Morey et al. (1998).
Discussion
Changes in the affinity and number of sites of A1- and A2A-adenosine receptors were demonstrated in kidneys from thyroidectomised rats when they were pharmacologically evaluated with the specific radioligands [3H]CPA and [3H]CGS21680. Binding studies conducted in the three sections of NL and HTX kidneys, the C, OM, and IM, demonstrated a predominance of A2A adenosine receptors over A1 adenosine receptors in the hypothyroid kidney, for the higher affinity state of the receptor (KH).
The renal cortex is characterised by a high blood flow and cortical nephrons whose principal structures consist of glomeruli and proximal convoluted tubules. In this study, [3H]CPA binding indicated a specificity and saturability for A1 receptors as observed by other authors in isolated glomeruli (Toya et al., 1993). Our results, however, demonstrated the presence of two binding sites in renal cortex from both NL and HTX rats. The KH and Bmax values for NL rats are similar to those determined by Toya et al. (1993), whereas KL and Bmax values are similar to those reported by Blanco et al. (1992) for A3 adenosine receptors in membranes from the proximal tubule. In the HTX renal cortex, no differences appear to exist in the high specificity state of the A1 adenosine receptor; however, significantly lower values were observed in the low specificity state for KL and Bmax. These findings suggest a reduction of the lower state of this receptor in HTX rats. Transitional states in adenosine receptors coupled to G proteins may be the explanation for the changes in binding kinetics (Blanco et al., 1992; Sho et al., 1999). Similar states have been described in synaptosomes from hypothyroid rats even in the presence of GTPγS, a nonhydrolysable analogue of GTP (Fideu et al., 1994), and in thyroid cells stimulated by TSH in the presence of pertussis toxin (Matias et al., 1993). Thus, the changes detected in [3H]CPA binding in renal cortex from HTX rats involve a decrease in low-affinity state and number of the A1 adenosine receptor, probably dependent on transitional-inactivated adenosine receptors coupled to G-proteins. Other mechanisms, such as downregulation, receptor endocytosis and low recycling of the receptors, could also explain, at least in part, the findings mentioned above (Cunha et al., 1995); however, binding experiments do not allow us to determine the involvement of these mechanisms.
From the [3H]CGS21680 binding studies, significant differences were found between groups indicating an increase in KH and Bmax in the HTX cortex, which suggests that A2A adenosine receptors expand in number with a decreased affinity. When examining the values obtained with [3H]CPA binding, we found that differences arise in the high-affinity state of the receptor (KH). Since [3H]CGS21680 is a more specific analogue for A2A adenosine receptors (Levens et al., 1991), this explains the similar values of KH and KL values in [3H]CGS21680 binding vs KL [3H]CPA binding in renal cortex from HTX rat. This strongly suggests that the A1/A2A adenosine receptor ratio is increased in C from HTX rats. In addition, the high-affinity state and number of sites revealed by [3H]CGS21680 binding indicates that the A2A adenosine receptor is a predominant receptor over A1 in renal cortex from HTX rats, and may regulate glomerular haemodynamics (Franco et al., 1996; Smith et al., 2000) and adenosine transport in the proximal tubule in the hypothyroid rat (Martínez et al., 1997).
The OM collecting ducts and loop of Henle of the juxtaglomerulary nephrons are located mainly in the outer strip of the medulla. The fine regulation of blood flow and solute reabsorption normally occur in these structures (Harrison-Bernard & Navar, 1996), and the medullary segments regulate the concentration of urine. When [3H]CPA binding was measured in this section, two affinity sites were determined in both the NL and HTX group; the high-affinity state (KH) of the A1 receptor was higher in NL than in HTX rats, whereas the number of A1 receptor sites predominated in the low-affinity state of the OM and IM from the HTX rat. In hypothyroidism, it is recognised that there is an inability to achieve either maximal concentration or dilution of urine (Holmes & DiScala 1970; Emmanouel et al., 1974; Michael et al., 1976). A2 receptors have been proposed to have a role in the regulation of blood flow in the vasa recta and transport mechanisms in the medullary segments of the nephron (Miyamoto et al., 1988; Silldorff et al., 1996). Thus, the changes in A1 and A2A adenosine receptors in the medullary segments may increase blood flow in these vessels, which results in a washout of interstitial solutes, decreasing medullary tonicity, thereby producing a concentration impairment of the tubular fluid, and a decrease of sodium and water reabsorption (Reineck & Parma, 1982; Miyamoto et al., 1988; Silldorff et al., 1996). Thus, this mechanism may induce an inability to concentrate urine and a decrease in tubular reabsorption, as observed in hypothyroidism.
Although DPCPX and NECA inhibited [3H]CPA binding in OM from NL rats, the A2 nonspecific adenosine receptor agonist NECA did not inhibit specific CPA binding in spite of a Ki of 0.10±0.04 nM in the OM from normal rats. CPA bound to A1 receptors was not displaced by NECA in our binding experiments in the OM from HTX rats. The presence of A2A was demonstrated in this tissue section by [3H]CGS21680 binding in both groups. Since no increased displacement with GTPγS (guanosine 5′-O-3-(thio)triphosphate) was observed in similar preparations (Fideu et al., 1994; Sho et al., 1999), another possible explanation for this unexpected result may be that the receptor–agonist complex is internalised to a greater extent than in the other renal sections, as has been observed in a smooth muscle cell line (Saura et al., 1998) and rat brain (Ruiz et al., 1996). This can also be explained by a low rate of transition of the coupled adenosine receptors to G proteins (Blanco et al., 1992; Sho et al., 1999), resulting in an increased transitional state of the receptor to reach its active state at the studied incubation time in our experiments, as already mentioned.
CPA and CGS21680 saturation binding experiments indicated a predominance of the KH for A1 receptors in NL and A2A in HTX in the OM. The Ki values appeared contradictory since CPA displacement by NECA showed a lower Ki for NL than CGS21680 displacement by DPCPX, in HTX. One possible explanation for the discrepancy in Ki values in CPA and CGS binding is that although both compounds are considered highly selective agonists for A1 and A2A, respectively (Müller & Scior, 1993), amino-acid substitution in either transmembrane or extracellular domains of A1 adenosine receptors results in changes of affinity for agonists and antagonists, such as in the case for [3H]CPA and [3H]CGS21680 binding (Rivkees et al., 1999; Olah & Stiles, 2000). Marked changes in the lipid composition of cell membranes is observed in hypothyroidism (Brasitus & Dudeja, 1988) and may influence drug–receptor interactions. Therefore, the molecular properties of adenosine receptor ligands can also modify binding to receptors in the HTX status. DPCPX exhibits a lipophilic domain that can better interact with receptor transmembrane loops in NL rats, and NECA molecular groups at the 6-amino or 2-position or at the sugar moiety at the 5′-position participate in the binding to adenosine receptors and are essential in eliciting specific responses (Müller & Scior, 1993; Olah & Stiles, 2000). It has been reported that in some conditions such as acute renal failure, the expression of A1 adenosine receptors is increased in the renal cortex while reduced in the IM, which helps to explain the abnormalities in renal function (Smith et al., 2000). There is no clear explanation for these alterations in receptor expression, but mechanisms such as up and downregulation and G protein coupling may be involved (Lee et al., 2002). We could speculate that in hypothyroidism, thyroid hormone imbalance may induce changes in adenosine receptor expression in the kidney.
It should be mentioned that ɛ values confirmed a higher A2A receptor affinity state in HTX and that ɛ values for CPA and CGS21680 binding experiments obtained in our study using the validated Furchgott's method are low, from 0.5 to 2.7, when compared with the results of other authors, for example, 5.9 to 6.6 in cardiovascular tissues (Morey et al., 1998). However, ɛ values for adenosine and purine analogues have not been reported for the kidney.
In summary, this study demonstrated that hypothyroidism in the rat induces a modification of the binding characteristics of A1 and A2A adenosine receptors, relative to the adenosine analogues [3H]CPA and [3H]CGS21680. This indicates a relative predominance of the high-affinity state A1 adenosine receptor in the C, and a relative predominance of A2A adenosine receptors in the three sections of the hypothyroid kidney when compared to [3H]CPA binding receptors. The induced imbalance in A1/A2 receptor ratio may contribute to the alterations in renal function observed in this condition.
Acknowledgments
This work was supported by grants from the National Council of Science and Technology (CONACyT-Mexico) No. 359P-M9506 to Martha Franco and No. 25887-M to Flavio Martínez. We thank Dr Albert Leyva for reading the manuscript.
Abbreviations
- A1
adenosine A1 receptor
- A2A
adenosine A2A receptors
- Bmax
density receptor capacity
- C
renal cortex
- [3H]CGS21680
2-[(carboxyethyl)phenethylamino]-5′-N-ethylcarboxamido adenosine, adenosine A2 agonist
- [3H]CPA
cyclopentyl adenosine, adenosine A1 analogue
- DPCPX
8-cyclopenthy-1,3-dipropylxanthine, A1 receptor antagonist
- ɛ
intrinsic efficacy
- GTPγS
guanosine 5′-O-3-(thio)triphosphate
- HTX
thyroidectomised rats
- IM
renal inner medulla
- KD
equilibrium dissociation constant
- KH
high-affinity binding sites constant
- KL
low-affinity binding sites constant
- NECA
5′ethylcarboxamido adenosine, A2/A1 agonist
- NL
control
- OM
renal outer medulla
- TSH
thyroid-stimulating hormone
- T4
thyroxine
References
- BLANCO J., CANELA E.I., MALLOL L., FRANCO R. Characterization of adenosine receptors in brush border membranes from pig kidney. Br. J. Pharmacol. 1992;107:671–678. doi: 10.1111/j.1476-5381.1992.tb14505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRASITUS T.A., DUDEJA P.K. Effect of hypothyroidism on the lipid composition and fluidity of rat colonic apical plasma membrane. Biochim. Biophys. Acta. 1988;939:189–196. doi: 10.1016/0005-2736(88)90062-4. [DOI] [PubMed] [Google Scholar]
- CAPASSO G., DeSanto N.G. Thyroid hormones and renal transport: cellular and biochemical aspects. Kidney Int. 1987;32:443–451. doi: 10.1038/ki.1987.231. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., CONSTANTINO M.D., SEBASTIAO A.M., RIBEIRO J.A. Modification of A1 and A2a adenosine receptor binding in aged striatum hippocampus and cortex of the rat. NeuroReport. 1995;6:1583–1588. doi: 10.1097/00001756-199507310-00029. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., JOHANSSON B., CONSTANTINO M.D., SEBASTIAO A.M., FREDHOLM B.B. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H]-CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn–Schmiedeberg's Arch. Pharmacol. 1996;353:261–271. doi: 10.1007/BF00168627. [DOI] [PubMed] [Google Scholar]
- EMMANOUEL D.S., LINDHEIMAR M.D., KATS A.I. Mechanism of impaired water excretion in hypothyroid rat. J. Clin. Invest. 1974;54:926–934. doi: 10.1172/JCI107833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIDEU M.D., ARCE A., ESQUIFINO A.I, MIRAS-PORTUGAL T. Thyroid hormones modulate both adenosine transport and adenosine A1 receptors in rat brain. Am. J. Physiol. 1994;267:C1651–C1656. doi: 10.1152/ajpcell.1994.267.6.C1651. [DOI] [PubMed] [Google Scholar]
- FRANCO M., BOBADILLA N.A., SUAREZ J., TAPIA E., SANCHEZ L., HERRERA-ACOSTA J. Participation of adenosine in the renal hemodynamics abnormalities of hypothyroidism. Am. J. Physiol. 1996;270:F254–F262. doi: 10.1152/ajprenal.1996.270.2.F254. [DOI] [PubMed] [Google Scholar]
- HARRISON-BERNARD M.L., NAVAR L.G. Renal cortical and medullary microvascular blood flow autoregulation in the rat. Kidney Int. 1996;50 Suppl 57:S23–S29. [PubMed] [Google Scholar]
- HOLMES E.W., DISCALA V.A. Studies on the exaggerated natriuretic response to saline infusion in the hypothyroid rat. J. Clin. Invest. 1970;49:1224–1236. doi: 10.1172/JCI106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMAL Z., SAGGERSON D. Enzymes involved in adenosine metabolism in rat white and brown adipocytes. Effects of streptozotocin-diabetes, hypothyroidism, age and sex differences. Biochem. J. 1987;245:881–886. doi: 10.1042/bj2450881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H.T., CUOMO-SCHOLER A., BELLONI F.L., THOMPSON C.I. Regulation of renal A(1) adenosine receptors in vivo. Exp. Nephrol. 2002;10:43–50. doi: 10.1159/000049897. [DOI] [PubMed] [Google Scholar]
- LEE K.S., REDDINGTON M. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) inhibition of [3H]N-ethylcarboxamidoadenosine (NECA) binding allows the visualization of putative non-A1 adenosine receptors. Brain Res. 1986;368:394–398. doi: 10.1016/0006-8993(86)90589-5. [DOI] [PubMed] [Google Scholar]
- LEVENS N., BEIL M., SCHULTZ R. Intrarenal actions of the new adenosine agonist CGS21680, selective for the A2 receptor. J. Pharmacol. Exp. Ther. 1991;257:1013–1019. [PubMed] [Google Scholar]
- MARTÍNEZ F., FRANCO M., QUINTANA A., HERRERA-ACOSTA J. Sodium-dependent adenosine transport is diminished in brush border membrane vesicles from hypothyroid rat kidney. Pflügers Archiv. (Eur. J. Physiol.) 1997;433:269–275. doi: 10.1007/s004240050277. [DOI] [PubMed] [Google Scholar]
- MATIAS A., ZIMMER F.J., LORENZEN A., KEIL R., SCHUABE U. Affinity of central adenosine A1 receptors is decreased in spontaneously hypertensive rats. Eur. J. Pharmacol. 1993;244:223–230. doi: 10.1016/0922-4106(93)90147-2. [DOI] [PubMed] [Google Scholar]
- MAZURKIEWICS D., SAGGERSON D. Changes in the activities of adenosine-metabolizing enzymes in six regions of the rat brain on chemical induction of hypothyroidism. Biochem. J. 1989;261:667–672. doi: 10.1042/bj2610667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCOY D.E., BHATTACHARYA S., OLSON B.A., LEVIER D.G., AREND L.G., SPIELMAN W.S. The renal adenosine system: structure, function, and regulation. Semin. Nephrol. 1993;13:31–40. [PubMed] [Google Scholar]
- MICHAEL U.F., KELLEY J., ALPERT H., VAAMONDE C.A. Role of distal delivery of filtrate in impaired renal dilution on the hypothyroid kidney. Am. J. Physiol. 1976;230:699–705. doi: 10.1152/ajplegacy.1976.230.3.699. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO M., YAGIL Y., LARSON T., ROBERTSON C., JAMISON R.L. Effects of intrarenal adenosine and medullary blood flow in the rat. Am. J. Physiol. 1988;255:F1230–F1234. doi: 10.1152/ajprenal.1988.255.6.F1230. [DOI] [PubMed] [Google Scholar]
- MOREY T.E., BELARDINELLI L., DENNIS D.M. Validation of Furchgott's method to determine agonist-dependent A1-adenosine receptor reserve in guinea-pig atrium. Br. J. Pharmacol. 1998;123:1425–1433. doi: 10.1038/sj.bjp.0701747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLLER C.E. A1-adenosine receptor antagonists. Exp. Opin. Ther. Patents. 1997;7:419–440. [Google Scholar]
- MÜLLER C.E., SCIOR T. Adenosine receptors and their modulators. Pharmaceutica Acta. Helv. 1993;68:77–111. doi: 10.1016/0031-6865(93)90012-u. [DOI] [PubMed] [Google Scholar]
- OHISALO J.J., STONEHAM S., KESO L. Thyroid status and adenosine content of adipose tissue. Biochem. J. 1987;246:555–557. doi: 10.1042/bj2460555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. The role of structure in determine adenosine receptor activity. Pharmacol. Ther. 2000;85:55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- OSSWALD H., SCHMITZ H.J., KEMPER R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflüegers Arch. 1977;371:45–49. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- REINECK H.J., PARMA R. Effect of medullary tonicity on urine sodium excretion in the rat. J. Clin. Invest. 1982;69:971–978. doi: 10.1172/JCI110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVKEES S.A., BARBHAIYA H., IJZERMAN A.P. Identification of the adenine binding site of the human A1 adenosine receptor. J. Biol. Chem. 1999;274:3617–3621. doi: 10.1074/jbc.274.6.3617. [DOI] [PubMed] [Google Scholar]
- RUIZ A., SANZ J.M., GONZÁLEZ-CALERO G., FERNÁNDEZ A., ANDRÉS A., CUBERO A., ROS M. Desensitization and internalization of adenosine A1 receptors in rat brain by in vivo treatment with R-PIA: involvement of coated vesicles. Biochim. Biophys. Acta. 1996;1310:168–174. doi: 10.1016/0167-4889(95)00152-2. [DOI] [PubMed] [Google Scholar]
- SAGGERSON D.E. Sensitivity of adipocyte lipolysis to stimulatory and inhibitory agonists in hypothyroidism and starvation. Biochem. J. 1986;238:387–394. doi: 10.1042/bj2380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAURA C.A., MALLOL J., CANELA E.I., LLUIS C., FRANCO R. Adenosine deaminase and A1 adenosine internalize together following agonist-induced receptor desensitization. J. Biol. Chem. 1998;273:17610–17617. doi: 10.1074/jbc.273.28.17610. [DOI] [PubMed] [Google Scholar]
- SILLDORFF E.P., KREISBERG M.S., PALLONE T.L. Adenosine modulates vasomotor tone in outer medullary descending vasa recta of the rat. J. Clin. Invest. 1996;98:18–23. doi: 10.1172/JCI118764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J.A., WHITAKER E.M., BOWNER C.J., YATES M.S. Differential expression of renal adenosine A1 receptors induced by acute renal failure. Biochem. Pharmacol. 2000;59:727–732. doi: 10.1016/s0006-2952(99)00369-x. [DOI] [PubMed] [Google Scholar]
- SMITH P.K., KRHON R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZIANO I.D., FUJIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- SHO K., NARITA T., OKAJIMA F., KONDO Y. An adenosine receptor agonist-induced modulation of TSH-dependent cell growth in FRTL-5 thyroid cells mediated by inhibitory G protein, Gi. Biochimie. 1999;81:341–346. doi: 10.1016/s0300-9084(99)80079-0. [DOI] [PubMed] [Google Scholar]
- SPIELMAN W.S., AREND L.J. Adenosine receptors and signalling in the kidney. Hypertension. 1991;17:117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- SPIELMAN W.S., THOMPSON C.I. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am. J. Physiol. 1982;242:F423–F453. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- TOYA Y., UMEMURA S., IWAMOTO T., HIRAWA N., KIHARA M., TAKAGI N., ISHII M. Identification and characterization of adenosine A1 receptor cAMP system in human glomeruli. Kidney Int. 1993;43:928–993. doi: 10.1038/ki.1993.130. [DOI] [PubMed] [Google Scholar]