Abstract
Vacuolar ATPase (V-ATPase) has been proposed as a drug target in lytic bone diseases. Studies of bafilomycin derivatives suggest that the key issue regarding the therapeutic usefulness of V-ATPase inhibitors is selective inhibition of osteoclast V-ATPase. Previous efforts to develop therapeutic inhibitors of osteoclast V-ATPase have been frustrated by a lack of synthetically tractable and biologically selective leads. Therefore, we tried to find novel potent and specific V-ATPase inhibitors, which have new structural features and inhibition selectivity, from random screening using osteoclast microsomes. Finally, a novel V-ATPase inhibitor, FR167356, was obtained through chemical modification of a parental hit compound.
FR167356 inhibited not only H+ transport activity of osteoclast V-ATPase but also H+ extrusion from cytoplasm of osteoclasts, which depends on the V-ATPase activity. As expected, FR167356 remarkably inhibited bone resorption in vitro.
FR167356 also showed inhibitory effects on other V-ATPases, renal brush border V-ATPase, macrophage microsome V-ATPase and lysosomal V-ATPase. However, FR167356 was approximately seven-fold less potent in inhibiting lysosomal V-ATPase compared to osteoclast V-ATPase. Moreover, LDL metabolism in cells, which depends on acidification of lysosome, was blocked merely at higher concentration than bone resorption, suggesting that FR167356 inhibits V-ATPase of osteoclast ruffled border membrane still more selectively than lysosome at the cellular level.
These results from the experiments seem to indicate that osteoclast V-ATPase may be different from lysosomal V-ATPase in respect of their structure.
FR167356 had a novel chemical structural feature as well as inhibitory characteristics distinctly different from any previously known V-ATPase inhibitor family. Therefore, FR167356 is thought to be a useful tool for estimating the essential characteristics of V-ATPase inhibitors for drug development.
Keywords: V-ATPase, osteoclast, bone resorption, lysosome, bafilomycin A1, FR167356
Introduction
Bone mass is maintained under a delicate balance between bone formation and bone resorption. Increase of bone resorption causes several lytic bone diseases such as osteoporosis, hypercalcemia, rheumatoid arthritis, tumor metastasis into bone, periodontitis and Paget's disease. Therefore, bone resorption inhibitors are valuable for treating these diseases. Osteoclasts, the multinucleated large cells that are derived from precursor cells of the monocytic/macrophage lineage, perform bone resorption under regulation by various hormones and cytokines. Bone resorption means that crystalline hydroxyapatite is dissolved and organic bone matrix rich in collagen fiber is degraded. Breakdown of both bone components requires the bone surface acidic microenvironment beneath the ruffled border, special plasma membrane of osteoclasts formed in face of bone, because hydroxyapatite is solubilized in acidic solution and collagen-digested protease, cathepsin K, is optimally active at acidic pH. Accordingly, disturbance of acid environment provided by osteoclasts is expected to lead to blockage of bone resorption.
Vacuolar ATPases (V-ATPases) are ATP-dependent proton pumps, which are highly expressed in the ruffled border membranes and function to acidify the resorption lacunae (Blair et al., 1989). In addition, suppression of gene expression of V-ATPases by antisense RNA and DNA molecules inhibits bone resorption (Laitala & Vaananen, 1994). Consequently, plasma membrane V-ATPase of osteoclasts (osteoclast V-ATPase) is thought to be a good molecular target for reducing osteoclast activity.
V-ATPase is one of three major classes of ATP driven cation pumps (V-, P- and F-ATPase). These three ATPases show the different sensitivity to inhibitors (Forgac, 1989). Bafilomycin A1 is a first potent and specific inhibitor of V-ATPase. Bafilomycin A1 inhibits V-ATPases at nanomolar concentration, while P-ATPases are affected only at micromolar concentration and F-ATPase is not inhibited at all (Bowman et al., 1988). V-ATPase is not only localized in the plasma membranes of osteoclasts but also the plasma membranes of renal intercalated cells and macrophages. Moreover, V-ATPases are also present on ubiquitous intracellular acidic compartment such as lysosomes, endosomes, the Golgi apparatus and secretory vesicles, and responsible for their acidification (Nishi & Forgac, 2002). Since bafilomycin A1 equally inhibits all of these V-ATPase activities leading to systemically undesirable alteration of cellular physiology when administered to animals, the characteristics of bafilomycin A1 is not suited for therapeutic application. In order to obtain safe V-ATPase inhibitors for the treatment of lytic bone diseases, we must discover the compounds that affect the activities of neither P- nor F-ATPase, and moreover, display selectivity with respect to inhibition of V-ATPases.
V-ATPases consist of a complex of many subunits. Recently, there is growing evidence for the presence of subunit diversity among V-ATPases and it is reported that they are expressed differently in organelles and tissues (Nishi & Forgac, 2002; Sun-Wada et al., 2003; Toyomura et al., 2003; Ueda et al., 2003). The existence of osteoclast-specific isoform is controversial (Chatterjee et al., 1992; van Hille et al., 1993,1995; Li et al., 1996; Scott & Chapman, 1998). However, recent report showed that the function of osteoclast V-ATPase can be depressed without influence on V-ATPase function in other cells. a3 subunit knockout mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification, whereas acidification of lysosomes and the proton transport in kidney microsomes are retained normally in these mice (Frattini et al., 2000). This fact suggests that it is possible to selectively inhibit osteoclast V-ATPase in vivo. However, it is not clear whether it can be achieved pharmacologically by agents. Recently, it was reported that bafilomycin A1 derivatives inhibited selectively osteoclast V-ATPase although this tissue selectivity was assessed in comparison to just one more tissue (adrenal gland or brain). However, chemical modification of bafilomycin is limited by its high complexity and low chemical stability. Accordingly, we tried to find novel potent and specific V-ATPase inhibitors, which have new chemical structures, by random screening using chicken osteoclast microsomes. Consequently, we obtained a novel specific V-ATPase inhibitor, FR167356. In this paper, pharmacological studies of FR167356 showed FR167356 had the inhibition profile distinctly different from any previously known V-ATPase inhibitor family.
Method
Animals
Female 1-day-old chickens were obtained from Sankyo Lab Service (Tokyo, Japan). Female Wistar,14 days pregnant rats, 8-week-old male Wistar rats and low Ca2+ (0.07%) diet for chicken were obtained from Clea Japan (Tokyo, Japan). Male ddY mice, 8 weeks old, and 13-week-old Japanese White rabbits were obtained from Nihon SLC (Hamamatsu, Japan). Male ICR mice, 8 weeks old, were obtained from Charles River Japan (Yokohama, Japan). Animals were housed in our animal care facility and kept under standard conditions (room temperature of 23±2°C, humidity of 55±5%, 12 : 12 light–dark cycle, free access to normal commercial diet (except chickens) and water). All experimental procedures have been subjected to evaluation and were approved by animal ethics committee of the Fujisawa Pharmaceutical Co.
Membrane preparation for H+-transport assay
We used tibiae of chickens fed low Ca2+ diet to obtain sufficient amounts of pure osteoclasts for random screening. Chicken osteoclasts were isolated from tibiae of 1-month-old chicks fed low Ca2+ diet (0.07%) for 1 month (Hunter et al., 1991) and their microsomes were prepared (Blair et al., 1989). Mouse osteoclasts were formed in the coculture of spleen cells obtained from ddY mice and ST2 cells (Riken, Tsukuba, Japan), murine bone marrow stromal cells, in the presence of 10 nM 1,25-dihydroxyvitamin D3 and 100 nM dexamethasone (Udagawa et al., 1989). After 1 week coculture, ST2 cells were eliminated by the treatment of 0.2% dispase. Remaining cells, which were mainly osteoclasts, were collected by cell scraper. Plasma membrane vesicles of murine osteoclasts were isolated (Koizumi et al., 1976). Lysosomes were isolated as tritosomes from ddY mice and Wistar rat liver. Then, membrane ghosts were prepared by osmotic lysis of tritosomes (Arai et al., 1993). Macrophages were harvested by peritoneal lavage from ddY mice injected with thioglycolate and their microsomes were fractionated. Murine (ddY mouse) and rat (Wistar rat) renal brush border membrane vesicles were isolated from renal cortical homogenate by Mg2+-aggregation method (Biber et al., 1981). Mitoplasts (inner membrane vesicle of mitochondria) were obtained from ddY mouse liver according to Wlodawer et al. (1966). Rabbit gastric microsomes were prepared according to Tomoi et al. (1988).
H+-transport assay
H+ transport by various membrane vesicles was assayed with a dual wavelength spectrophotometer (UV-3000, Shimadzu, Kyoto, Japan) by measuring uptake of acridine orange, in a reaction medium containing 10 μM acridine orange, 150 mM KCl, 2 mM MgCl2, 10 mM bis-tris-propane and 1 μM valinomycin, pH 7.0. (Blair et al., 1989). A reaction buffer for measuring of lysosomal V-ATPase activity was 10 μM acridine orange, 40 mM Bicine-TMAH, 0.1 M KCl, 0.2 M Sucrose, 1 μM valinomycin and 1 mM MgCl2, pH 8.5 (Moriyama et al., 1984). For measurement of V-ATPase activity, additionally 5 μg ml−1 oligomycin was added. The reaction was initiated by adding ATP (final concentration 1 mM).
Measurement of ATPase activity
ATPase activity of gastric H+,K+-ATPase was determined by measuring the release of inorganic phosphate (Tomoi et al., 1988). Macrophage V-ATPase was purified by immunoprecipitation using rabbit anti-bovine V-ATPase (first antibody) and mouse anti-rabbit IgG (second antibody). V-ATPase activity bound to protein A-bearing beads (pansorbin cells) was assayed (Gluck & Caldwell, 1987). The liberated phosphate was estimated by the malachite green method.
Measurement of acid extrusion
Previously glass bottom dishes (Asahi Technoglass, Funabashi, Japan) were coated with vitronectin by a 4 h incubation in 5 μg ml−1 bovine vitronectin at 37°C. Unfractionated bone cell suspension was prepared from tibiae and femora of 11- to 13-day-old ICR mice (Takada et al., 1992) and seeded on to the glass bottom dishes. The cells were incubated at 37°C in a CO2 incubator (5% CO2, 95% air) for 3 h. Then the glass bottom dishes were gently washed and added α-MEM supplemented with 10% FCS plus 10 nM 1,25-dihydroxyvitamin D3. The medium was changed every 2 days. After the incubation for 6- and 8-days, the pHi of cells was measured fluorometrically using the pH-sensitive probe, BCECF. Cells were bathed in a recording buffer (145 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 1 mM Na2SO4, 1.8 mM CaCl2, 30 mM HEPES, 1 mM MgCl2, 10 mM glucose, pH7.4) containing 40 mM NH4Cl for 15 min. After cytoplasmic acid loading accomplished by preincubating in 40 mM NH4Cl for 15 min, the pHi recovery rate was monitored (Fernandez & Malnic, 1998) in the presence of FR167356 plus 100 μM dimethylamiloride (Na+/H+-exchanger inhibitor) using the High speed [Ca2+] analyzing system (ARGUS/HiSCA Ver1.7, Hamamatsu Photonics, Hamamatsu, Japan). Multinucleated cells were determined to be osteoclasts.
Calvaria organ culture
Calvariae from neonatal Wistar rats were excised and cultured in 2 ml DMEM (10% FCS) supplemented with 10 nM hPTH fragment (1–34) in the presence of FR167356. After 6 days, the concentration of Ca2+ in the media was measured by the commercial kit (Wako Pure Chemical Industries) according to methylxylenol blue method.
Pit formation
Mouse bone marrow cells were obtained from tibiae of 9-week-old ddY mice. Mouse bone marrow cells were cultured on collagen gel-coated dishes in the presence of sRANKL (100 ng ml−1) and mM-CSF (25 ng ml−1) for 10 days. Then, cells were retrieved from dishes by treatment with collagenase. The cells, which consisted of mainly osteoclasts, were put on ivory slices and cultured in α-MEM containing 10% FCS in the presence of sRANKL (100 ng ml−1) and FR167356. After 24 h, the ivory slices were stained with acid hematoxylin and pit area at six fields (29.73 mm2) a slice was measured by image analyzer (LUZEX 3U, Nikon, Tokyo, Japan) (Udagawa et al., 1999).
Assay for cholesteryl ester formation
Murine peritoneal resident macrophages were isolated by peritoneal lavage from ddY mice and incubated with DMEM (10% FCS) for 20 h. Then the medium was changed to DMEM (10% FCS) containing [14C]-oleate-albumin, lipoprotein and FR167356. The cells were incubated for 4 h and then washed, and extracted with hexane/isopropanol. The extract was assayed for cholesteryl esters using thin-layer chromatography (Goldstein et al., 1983).
LDL metabolism
Human hepatocyte cell line, Hep G2 (ATCC, Manassas, VA, U.S.A.) was grown in monolayers in MEM (10% FCS). Then cells were switched to MEM (10% fetal calf lipoprotein-deficient serum) and incubated for 2 h in MEM (10% fetal calf lipoprotein-deficient serum) containing [125I]-LDL and FR167356, either in the absence or presence of excess unlabeled LDL. After incubation, specific binding, uptake and degradation of LDL were assayed according to Sparrow et al. (1989).
Materials
Bafilomycin A1 and anti bovine V-ATPase A subunit antibody (rabbit IgG) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Pansorbin cells were obtained from Calbiochem (La Jolla, CA, U.S.A.). Anti-rabbit IgG (mouse) was obtained from OEM Concepts (Toms River, NJ, U.S.A.). Acetoxymethyl ester of 2′,7′-biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) was obtained from Molecular Probe (Eugene, OR, U.S.A.). 1,25-dihydroxyvitamin D3 was obtained from Biomol Res. Lab. (Plymouth Meeting, PA, U.S.A.). Bovine vitronectin was purchased from Koken (Tokyo, Japan). Recombinant human soluble RANKL (sRANKL) was purchased from PeproTech EC (London, England). Dispase was purchased from Godo Shusei (Tokyo, Japan). Human PTH (1–34) fragment was purchased from Peptide Institute (Osaka, Japan). Triton WR-1339 was purchased from Nacalai tesque (Kyoto, Japan). Collagen gel solutions (Cellmatrix, type I-A) were obtained from Nitta Gelatin (Osaka, Japan). [14C]-oleic acid and [125I] were obtained from Amersham Biosciences (Piscataway, NJ, U.S.A.). Fetal calf serum (FCS) was obtained from Moregate (Bulimba, Australia). Tissue culture wares were obtained from Corning (Elmira, NY, U.S.A.) and Becton Dickinson (San Jose, CA, U.S.A.). All other reagents were purchased from Sigma (St Louis, MO, U.S.A.).
Data analysis
Results are expressed as mean±s.e.m. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons. Student's t-test (two-tailed) for independent samples was applied to examine the significance of the differences between the means of the two measurements. A 50% inhibitory concentration was obtained by fitting sigmoidal curve to data. When multiple experiments were repeated, we took an average from IC50 value of each experiment. For statistical comparisons, calculation of 50% inhibitory concentration and kinetics analysis, commercial Software (GraphPad Prism Ver 4, GraphPad Software, San Diego, CA, U.S.A.) was used. Differences were considered to be statistically significant at P<0.05.
Results
H+ transport
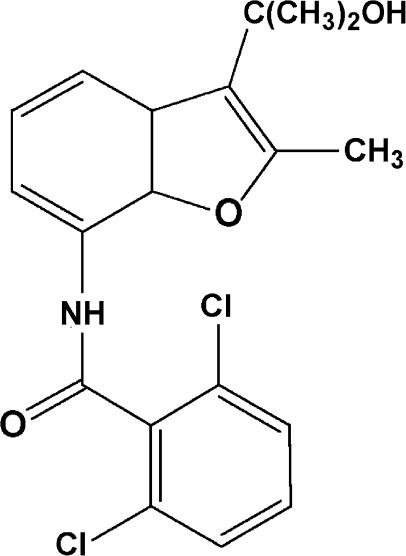
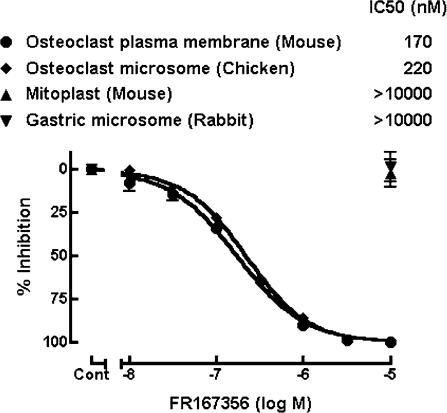
To discover novel inhibitors of osteoclast V-ATPase, we performed random screening by monitoring H+ transport activity of chicken osteoclast microsomes, which were inhibited by bafilomycin A1 and N-ethylmaleimide but not by oligomycin (F-ATPase inhibitor) or orthovanadate (P-ATPase inhibitor) (data not shown), suggesting that H+ transport in the osteoclast microsomes were driven by V-ATPase. FR167356 (Figure 1), 2,6-dichloro-N-[3-(1-hydroxy-1-methylethyl)-2-methyl-7-benzofuranyl]benzamide, was one of compounds which were obtained from chemical modifications of hit compounds. FR167356 inhibited H+ transport in plasma membrane vesicles of murine osteoclasts (IC50 170 nM) and chicken osteoclast microsomes (IC50 220 nM), whereas FR167356 exerted no effect on H+ transport of mitochondrial ATPase (F-ATPase) or gastric H+,K+-ATPase (P-ATPase) (Figure 2). Therefore FR167356 is thought to be a specific inhibitor of V-ATPase. Next we investigated the effect of FR167356 on H+ transport by other V-ATPases, which are derived from macrophage microsomes, renal brush border membranes and lysosomal membranes. FR167356 showed inhibitory effects on H+ transport activities of other V-ATPases (Table 1). The values of the inhibition (IC50) are 220 nM (murine macrophage), 370 nM (murine kidney) and 310 nM (rat kidney), respectively, which are almost similar to that determined for the inhibition of osteoclast V-ATPase. However, FR167356 displayed lesser potency (IC50 1200 nM) against murine and rat lysosomal V-ATPase, which raises the possibility that there is structural difference between lysosomal V-ATPase and osteoclast V-ATPase.
Figure 1.
Chemical structure of FR167356.
Figure 2.
Effect of FR167356 on ATP-dependent H+ transport activity of V-ATPase, F-ATPase and P-ATPase. H+ transport was assayed by monitoring absorption change of acridine orange at 492–540 nm (an index of the amount of acridine orange in free solution). The initial rate of change in the absorbance of acridine orange was used for calculation of IC50 value. V-ATPase (murine osteoclast plasma membrane vesicles and chicken osteoclast microsomes) was assayed in the presence of 5 μg ml−1 oligomycin. FR167356 did not inhibited H+ transport activity of F-ATPase (murine liver mitoplast) and P-ATPase (rabbit gastric microsomes) up to 10 μM. Each value represents the average of triplicated determinations. The bars represent s.e.m.
Table 1.
Inhibition of ATP-dependent H+ transport activity of V-ATPase by FR167356
| IC50 (nM) | ||||||
|---|---|---|---|---|---|---|
| Mouse | Chicken | Mouse | Mouse | Rat | Mouse | Rat |
| osteoclast | osteoclast | Mϕ | kidney | kidney | tritosome | tritosome |
| 170 | 220 | 220 | 370 | 310 | 1200 | 1200 |
Mϕ, macrophage.
Values shown are averages from two independent experiments.
Inhibition kinetics
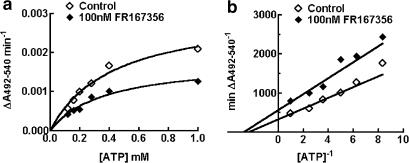
We determined the inhibition kinetics of FR167356 with respect to ATP. H+ transport as a function of ATP concentration was measured in the presence and absence of FR167356 (Figure 3a). A concentration of 100 nM FR167356 reduced the Vmax of H+ pumping of the macrophage microsomes from 0.00307 to 0.00178 A492–540 nm min−1, without remarkably altering the Km values (424 μM versus 364 μM ATP for control and 100 nM FR167356, respectively). The double reciprocal transformation (Figure 3b) reveals changes in both slope and the y-intercept of the resulting lines. Thus, the inhibition by FR167356 is of the noncompetitive type.
Figure 3.
Noncompetitive inhibition of H+ transport by FR167356. (a) An ATP-dependent activation curves of H+ transport in the absence and presence of 100 nM FR167356. The continuous lines are derived by fitting the data points to a Michaelis–Menten-type kinetics. (b), Lineweaver–Burk plot of the reciprocal rate of H+ transport versus the reciprocal of the different concentrations of ATP.
ATPase activity
V-ATPases translocate protons across membranes utilizing the energy of ATP hydrolysis. V-ATPases are composed of two functional domains, V1 and V0. V1, composed of eight subunits, is responsible for ATP hydrolysis. V0 domain contains five different types of subunits and is mainly responsible for proton translocation. We investigated whether FR167356 affected ATPase activity of V-ATPase. For measurement of ATPase activity, we used macrophage V-ATPase purified by immunoprecipitation because we could obtain sufficient amounts of uniform cells. FR167356 inhibited ATPase activity of macrophage microsome V-ATPase with an IC50 of 350 nM. Therefore, FR167356 may directly act on V-ATPase and inhibition of H+ transport by FR167356 seems to be attributed to the inhibition of ATPase activity. On the other hand, FR167356 did not affect ATPase activity of H+,K+-ATPase up to 10 μM.
H+ extrusion in acid-loaded osteoclasts
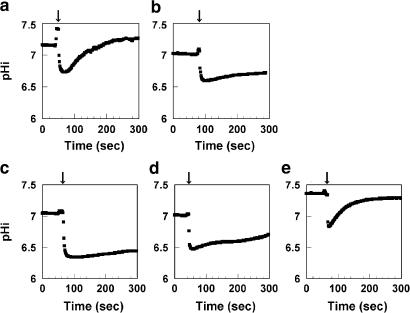
To examine the effect of FR167356 on V-ATPase activity at the cellular level, recovery from cytoplasmic acid loading was studied in murine osteoclasts. After 15 min exposure to 40 mM NH4Cl, NH4Cl removal caused a rapid acidification of pHi as a result of NH3 efflux. Then the pHi recovered to basal level in spite of the presence of 100 μM dimethylamiloride (inhibitor of Na+/H+-exchanger) (Figure 4a), whereas the pHi recovery in contaminating fibroblastic cells was not observed under the same experimental condition (data not shown). To confirm that V-ATPase residing in the plasma membrane of osteoclasts contributed to the dimethylamiloride-insensitive pHi recovery, the effect of bafilomycin A1 was examined. When osteoclasts were treated with bafilomycin A1 in the presence of dimethylamiloride, pHi recovery was inhibited (Figure 4b). It provided evidence that the dimethylamiloride-insensitive pHi recovery of acid-loaded osteoclasts was mediated by V-ATPase. FR167356 also inhibited pHi recovery with an IC50 of 220 nM, suggesting that FR167356 acted on V-ATPase residing in the plasma membrane of osteoclast at the cellular level (Figure 4c–e).
Figure 4.
Effect of FR167356 on pHi recovery of acid-loaded osteoclasts. pHi was measured fluorometrically using the probe BCECF. Cells were acid-loaded by the NH4+ prepulse method. Following the removal of NH4Cl (arrow), pHi decreases acutely due to rapid efflux of NH3. Then pHi recovery in a recording buffer containing 100 μM dimethylamiloride (a) was inhibited by additional presence of 200 nM bafilomycin A1 (b). Effect of adding FR167356 (10 μM (c), 1 μM (d), 100 nM (e)) in the presence of dimethylamiloride was investigated. Traces are representative of 5–11 experiments. The initial rate of pHi recovery was obtained by fitting tangent line and used for calculation of IC50 value.
Effect on bone resorption in vitro
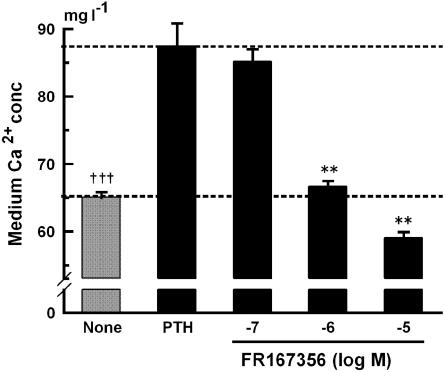
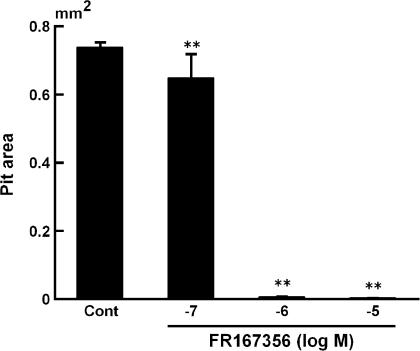
Next, we investigated whether inhibition of osteoclast V-ATPase by FR167356 consequently can cause blockade of bone resorption or not. Calvariae treated with parathyroid hormone (PTH) released more calcium into the culture media than did the control. An amount of 1 μM FR167356 inhibited almost completely the bone resorption induced by PTH (Figure 5). Its dose response (IC50 360 nM) appears to be similar to that observed in the effect on H+ extrusion (Figure 4c–e). Calvariae contain not only osteoclasts but also osteoblasts, bone marrow cells and fibroblasts. Therefore, it was not clear which cells were target of FR167356. Pit assay using purified murine osteoclasts showed that adding 1 μM FR167356 remarkably decreased the number and area of resorption lacunae. Figure 6 shows that pit area was inhibited by FR167356 in a dose-dependent manner (IC50 190 nM). It suggests that FR167356 directly acted on osteoclasts. The number of cells per slice did not change regardless of the presence of FR167356.
Figure 5.
Effect of FR167356 on PTH-induced bone resorption of rat calvariae. Calvariae were incubated with FR167356 in the presence of 10 nM hPTH. In nontreated dishes (None), only distilled water was added. The bars represent the mean±s.e.m. for five dishes. The figure is representative of two independent experiments. Significantly different between None and PTH alone (PTH): †††P<0.001 (Student's t-test). Significantly different between treatment with FR167356 and PTH: **P<0.01 (Dunnett).
Figure 6.
Formation of resorption pits by isolated osteoclasts in the presence of FR167356. The osteoclast preparation obtained from bone marrow cultures treated with mM-CSF (10 ng ml−1) and hsRANKL (100 ng ml−1) was cultured on ivory slices in the presence of FR167356 and hsRANKL (100 ng ml−1). After culture for 24 h, ivory slices were stained with acid hematoxylin and pit area at six fields (29.73 mm2) a slice was measured by image analyzer. Data are expressed as mean±s.e.m. of triplicate cultures. Significantly different between treatment with FR167356 and Control (Cont): **P<0.01 (Dunnett).
Lysosomal function
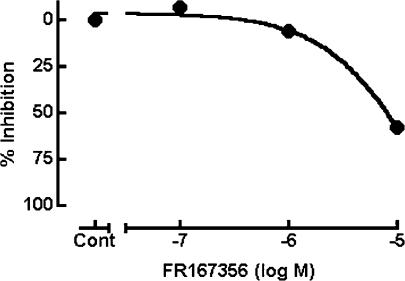
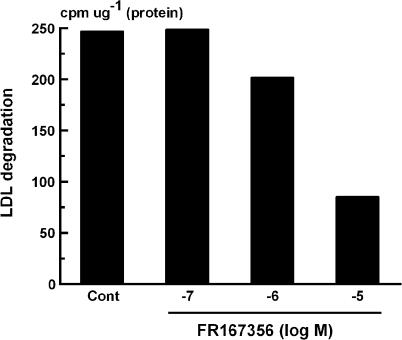
FR167356 inhibited lysosomal V-ATPase activity. However, FR167356 displayed lower inhibitory effect on lysosomal V-ATPase than osteoclast V-ATPase (Table 1). We examined the effect of FR167356 on lysosomal V-ATPase activity in living culture cells. Measurement of LDL metabolism was selected for the examination of the effect of FR167356 on lysosomal biological function, which depends on lysosomal pH. LDL took up by cells is hydrolyzed in lysosome, apolipoprotein being digested and free cholesterol being liberated. Then free cholesterol is effluxed into the cytoplasm, where much of it is re-esterified. As mentioned above, lysosome is an important organelle where the first step in LDL metabolism occurs, and moreover such functions operate under acidic pH. Based on these background, the effects of FR167356 on cholesteryl ester accumulation and LDL degradation were analyzed, both of which were inhibited by bafilomycin A1 (data not shown) (Naganuma et al., 1992). In all, 58% inhibition of accumulation of cholesteryl ester in macrophages was observed only at 10 μM FR167356 (IC50 7800 nM) (Figure 7). Degradation of internalized LDL was blocked by 66% in human hepatocytes treated with 10 μM (IC50 5200 nM) (Figure 8).
Figure 7.
Effect of FR167356 on cholesteryl ester accumulation in macrophages. After culture for 20 h, murine macrophages received [14C]-oleate in complex with albumin and the indicated concentrations of FR167356 in the presence of acetylated LDL. After incubation for 4 h, cholesteryl esters were determined. The value for control experiment was 347.7 PSL μg−1 (protein). Each value represents the average of duplicate determinations.
Figure 8.
Effect of FR167356 on the degradation of [125I]-LDL in hepatocyte Hep G2. Cells received 3 μg [125I]-LDL and FR167356 in the presence or absence of 120 μg unlabeled LDL. After incubation for 2 h, specific values for degraded [125I]-LDL were determined. Each value represents the average of duplicate determinations. The figure is representative of two independent experiments.
These results suggest that lysosomal V-ATPase (Figures 7 and 8) is less sensitive to FR167356 than V-ATPase of osteoclast ruffled border membrane (Figures 4,5 and 6) at the cellular level.
Discussion
Bone resorption process requires maintaining of acidic condition formed by osteoclast V-ATPase. Bafilomycin A1, the first specific V-ATPase inhibitor described (Bowman et al., 1988), remarkably inhibits bone resorption in vitro (Sundquist et al., 1990), which indicates that osteoclast V-ATPase plays a central role in the process of bone resorption. Inhibitors of osteoclast V-ATPase are expected to become therapeutic agents for treatment of several bone diseases that are caused by increase of bone resorption. The key issue regarding the therapeutic usefulness of V-ATPase inhibitors is their selectivity. V-ATPases are present not only in osteoclasts but also all eukaryotic cells where they are involved in many important cellular processes. Nonspecific inhibitor of V-ATPase can be expected to cause a multitude of effects, most of which are undesirable. Indeed, bafilomycin A1 is not used therapeutically, because it exhibits in vitro and in vivo toxic effect (Keeling et al., 1997). Recent studies indicated that isoforms of several subunits of V-ATPase are present and they are expressed differently in organelles and tissues (Nishi & Forgac, 2002; Sun-Wada et al., 2003; Toyomura et al., 2003; Ueda et al., 2003). Although the presence of osteoclast-specific isoform remains to be elucidated, it may be possible that only osteoclast V-ATPase activity can be depressed without influence on other cells and functions of acidic cellular compartments (Frattini et al., 2000). However, it is not clear whether chemical compounds can pharmacologically achieve it.
Several tissue-selective V-ATPase inhibitors were reported. Salicylihalamide A had the selectivity for mammalian versus fungal V-ATPase, although there was not selectivity among tested human V-ATPases (kidney, liver and osteoclast) (Boyd et al., 2001). H362/48 was approximately six-fold less potent against brain V-ATPase as opposed to bone V-ATPase (Keeling et al., 1998). SB242784 inhibited osteoclast V-ATPase at 1000-fold lower concentration than V-ATPases in other evaluated tissues (liver, kidney and brain) (Visentin et al., 2000). However, in these experiments the inhibitory activity was determined by measuring bafilomycin-sensitive ATPase activity of tissue membranes without the purification steps. As variable amount of Mg+-dependent ATPase activities were contaminated in these assays, these V-ATPase activities were calculated as difference of the ±bafilomycin A1 treatment. Accordingly, percentage of inhibition by tested compounds completely depended on the inhibition by bafilomycin treatment (control value). Moreover bafilomycin-sensitive ATPase activity occupied only a small proportion of total Mg+-dependent ATPase activities, which allows percentage of inhibition to fluctuate easily. Additionally, if tested compounds inhibited other Mg+-dependent ATPase activities contaminating in these assays than V-ATPase activity, the inhibition of Mg+-dependent ATPase could not be excluded from total inhibition by the compounds. After all, the IC50 value seems to be variable and not accurate in these assays.
There are some reports described about tissue selective V-ATPase inhibitors using H+ transport assay. Vanadate, which is known as a P-ATPase inhibitor, could inhibit specifically osteoclast H+ pump among other V-ATPases (Chatterjee et al., 1992). Tiludronate also had a significant degree of selectivity for osteoclast V-ATPase relative to kidney V-ATPase (David et al., 1996). However, these results of two compounds were not repeatable by other laboratories (Blair et al., 1989; Keeling et al., 1997). Therefore, it seems that only bafilomycin A1 derivatives had certainly selectivity. Gagliardi et al. (1998) reported that two of derivatives were three- or six-fold less potent against adrenal gland as opposed to bone and oppositely two of derivatives were five- or 50-fold less potent against bone. Other bafilomycin A1 derivative, (2Z,4E)-5-(5,6-dichloro-2-indolyl)-2-methoxy-N-[4-(2,2,6,6-tetramethyl)piperidinyl]-2,4-pentadienamide, was reported to be seven-fold more potent in inhibiting bone V-ATPase compared to brain V-ATPase (Mattsson et al., 2000).
Since chemical modification of bafilomycin is limited by its high complexity and low chemical stability, we tried to obtain novel potent and specific V-ATPase inhibitors, which have new structural features, from random screening using osteoclast microsomes. The structure of a hit compound was imidazopyridine and subsequently good structure–activity relationships were observed in chemical modification. Consequently, FR167356 was synthesized through replacement of imidazopyridine of a parental hit compound by benzofuran. FR167356 has potent inhibitory activity on V-ATPase and simple structure. Therefore, FR167356 derivatives seem to be more suitable for study of selective V-ATPase inhibitor.
FR167356 is the first V-ATPase inhibitor that can discriminate between osteoclast plasma membrane V-ATPase and lysosomal V-ATPase. In addition, FR167356 is the first compound that could distinguish between V-ATPases at cellular level. At cellular level, FR167356 also showed the selectivity between lysosomal function (LDL metabolism), which depends on lysosomal V-ATPase and osteoclast V-ATPase activities (H+ extrusion and bone resorption). Lysosomes play many critical roles in biological functions, including digestion of exogenous or endogenous macromolecules, receptor recycling, processing of bioactive peptide substances and antigen presentation, with acidic environment. Therefore, the inhibitory profile of V-ATPase by FR167356, distinction between inhibition of osteoclast V-ATPase and inhibition of lysosomal V-ATPase, is noteworthy. The risk of undesirable alteration of cellular physiology may be decreased when FR167356 is administered to animals compared with other V-ATPase inhibitors.
The inhibitory selectivity of FR167356 suggests that there are differences in isoform expression between osteoclast and lysosomal V-ATPase. The presence of osteoclast-specific isoform is controversial. There is a report that described the presence of osteoclast-specific isoform in A subunit (van Hille et al., 1993), but the specificity was denied after that (van Hille et al., 1995). Recently, a3 was reported to be an osteoclast-specific isoform in a subunit (Li et al., 1996). a3 subunit-deficient mice exhibited severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification, whereas acidification of lysosomes was retained normally in these mice (Frattini et al., 2000). However, a3 was also reported to be ubiquitously expressed in man, and is found in all tissues studied using the polymerase chain reaction (Scott & Chapman, 1998). Toyomura et al. (2003) reported that a3 isoform was transported from the lysosome to the plasma membrane during osteoclast differentiation. Accordingly, inhibition profile of FR167356 may predict the presence of novel isoforms, which may differentiate osteoclast V-ATPase from lysosomal V-ATPase.
Osteoclasts contain not only plasma membrane V-ATPase but also lysosomal V-ATPase. It is very interesting to compare the effect of FR167356 on plasma membrane V-ATPase with intracellular lysosomal V-ATPase, both of which osteoclasts contain. However, it remains to be elucidated because it is difficult to isolate pure lysosomes from osteoclasts.
At first, we aimed to obtain the compound that inhibits nothing but osteoclast V-ATPase. However, FR167356 inhibited macrophage V-ATPase and kidney V-ATPase with a similar potency to inhibition of osteoclast V-ATPase. In macrophage, mechanism of pHi regulation includes not only V-ATPase but also Na+/H+ exchanger and HCO3−/Cl− exchanger (Swallow et al., 1989). We actually observed that in acid-loaded macrophages H+ extrusion did not affect by FR167356 but dimethylamiloride (Na+/H+ exchanger inhibitor) (data not shown). Kidney secretes H+ for maintenance of acid–base balance. Multiple mechanisms (Na+/H+ exchanger, H+,K+-ATPase) involved for H+ secretion with the exception of V-ATPase (Wang et al., 2002). Accordingly, the inhibitory effects of FR167356 on macrophage V-ATPase and kidney V-ATPase may not emerge in vivo.
Since bafilomycin A1 exerts cell death in parallel with inhibition of V-ATPase (Xu et al., 2003), we have never been able to judge whether both cell death and inhibition of H+ secretion contribute to inhibition of bone resorption in vitro. As the number of cells per slice did not change regardless of the presence of FR167356 in pit formation assay, FR167356 clearly demonstrated that inhibition of H+ secretion by V-ATPase inhibitors solely mediated inhibition of osteoclastic bone resorption.
In conclusion, the pharmacological studies of a novel V-ATPase inhibitor, FR167356, showed FR167356 had selectivity for osteoclast V-ATPase relative to lysosomal V-ATPase, suggesting that novel isoforms differentiate osteoclast V-ATPase from lysosomal V-ATPase. As FR167356 is available for oral administration, FR167356 may be a useful tool for indicating the direction of improvement for therapeutic use of V-ATPase inhibitor.
Abbreviations
- BCECF
2′,7′-biscarboxyethyl-5(6)-carboxyfluorescein
- LDL
low-density lipoprotein
- M-CSF
macrophage colony-stimulating factor
- PTH
parathyroid hormone
- sRANKL
soluble receptor activator of NF-κB ligand
- V-ATPase
vacuolar ATPase
References
- ARAI K., SHIMAYA A., HIRATANI N., OHKUMA S. Purification and characterization of lysosomal H(+)-ATPase. An anion-sensitive v-type H(+)-ATPase from rat liver lysosomes. J. Biol. Chem. 1993;268:5649–5660. [PubMed] [Google Scholar]
- BIBER J., STIEGER B., HAASE W., MURER H. A high yield preparation for rat kidney brush border membranes different behaviour of lysosomal markers. Biochim. Biophys. Acta. 1981;647:169–176. doi: 10.1016/0005-2736(81)90243-1. [DOI] [PubMed] [Google Scholar]
- BLAIR H.C., TEITELBAUM S.L., GHISELLI R., GLUCK S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- BOWMAN E.J., SIEBERS A., ALTENDORF K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD M.R., FARINA C., BELFIORE P., GAGLIARDI S., KIM J.W., HAYAKAWA Y., BEUTLER J.A., MCKEE T.C., BOWMAN B.J., BOWMAN E.J. Discovery of a novel antitumor benzolactone enamide class that selectively inhibits mammalian vacuolar-type (H+)-ATPases. J. Pharmacol. Exp. Ther. 2001;297:114–120. [PubMed] [Google Scholar]
- CHATTERJEE D., CHAKRABORTY M., LEIT M., NEFF L., JAMSA-KELLOKUMPU S., FUCHS R., BARON R. Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton pump from other vacuolar H+ ATPases. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6257–6261. doi: 10.1073/pnas.89.14.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID P., NGUYEN H., BARBIER A., BARON R. The bisphosphonate tiludronate is a potent inhibitor of the osteoclast vacuolar H(+)-ATPase. J. Bone Miner. Res. 1996;11:1498–1507. doi: 10.1002/jbmr.5650111017. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ R., MALNIC G. H+ ATPase and Cl− interaction in regulation of MDCK cell pH. J. Membr. Biol. 1998;163:137–145. doi: 10.1007/s002329900378. [DOI] [PubMed] [Google Scholar]
- FORGAC M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 1989;69:765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- FRATTINI A., ORCHARD P.J., SOBACCHI C., GILIANI S., ABINUN M., MATTSSON J.P., KEELING D.J., ANDERSSON A.K., WALLBRANDT P., ZECCA L., NOTARANGELO L.D., VEZZONI P., VILLA A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- GAGLIARDI S., GATTI P.A., BELFIORE P., ZOCCHETTI A., CLARKE G.D., FARINA C. Synthesis and structure–activity relationships of bafilomycin A1 derivatives as inhibitors of vacuolar H+-ATPase. J. Med. Chem. 1998;41:1883–1893. doi: 10.1021/jm9707838. [DOI] [PubMed] [Google Scholar]
- GLUCK S., CALDWELL J. Immunoaffinity purification and characterization of vacuolar H+ ATPase from bovine kidney. J. Biol. Chem. 1987;262:15780–15789. [PubMed] [Google Scholar]
- GOLDSTEIN J.L., BASU S.K., BROWN M.S. Receptor-mediated endocytosis of low density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- HUNTER S.J., ROSEN C.J., GAY C.V. In vitro resorptive activity of isolated chick osteoclasts: effects of carbonic anhydrase inhibition. J. Bone Miner. Res. 1991;6:61–66. doi: 10.1002/jbmr.5650060111. [DOI] [PubMed] [Google Scholar]
- KEELING D.J., HERSLOF M., MATTSSON J.P., RYBERG B. Tissue-selective inhibition of vacuolar acid pumps. Acta Physiol. Scand. 1998;163:195–201. [PubMed] [Google Scholar]
- KEELING D.J., HERSLOF M., RYBERG B., SJOGREN S., SOLVELL L. Vacuolar H(+)-ATPases. Targets for drug discovery. Ann. N.Y. Acad. Sci. 1997;834:600–608. doi: 10.1111/j.1749-6632.1997.tb52329.x. [DOI] [PubMed] [Google Scholar]
- KOIZUMI K., ITO Y., KOJIMA K., FUJII T. Isolation and characterization of the plasma membranes from rat ascites hepatomas and from normal rat livers, including newborn, regenerating, and adult livers. J. Biochem. 1976;79:739–748. doi: 10.1093/oxfordjournals.jbchem.a131126. [DOI] [PubMed] [Google Scholar]
- LAITALA T., VAANANEN H.K. Inhibition of bone resorption in vitro by antisense RNA and DNA molecules targeted against carbonic anhydrase II or two subunits of vacuolar H(+)-ATPase. J. Clin. Invest. 1994;93:2311–2318. doi: 10.1172/JCI117235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y.P., CHEN W., STASHENK O.P. Molecular cloning and characterization of a putative novel human osteoclast-specific 116-kDa vacuolar proton pump subunit. Biochem. Biophys. Res. Commun. 1996;218:813–821. doi: 10.1006/bbrc.1996.0145. [DOI] [PubMed] [Google Scholar]
- MATTSSON J.P., LI X., PENG S.B., NILSSON F., ANDERSEN P., LUNDBERG L.G., STONE D.K., KEELING D.J. Properties of three isoforms of the 116-kDa subunit of vacuolar H+-ATPase from a single vertebrate species. Eur. J. Biochem. 2000;267:4115–4126. doi: 10.1046/j.1432-1327.2000.01445.x. [DOI] [PubMed] [Google Scholar]
- MORIYAMA Y., TAKANO T., OHKUMA S. Proton translocating ATPase in lysosomal membrane ghosts. Evidence that alkaline Mg2+-ATPase acts as a proton pump. J. Biochem. 1984;95:995–1007. doi: 10.1093/oxfordjournals.jbchem.a134726. [DOI] [PubMed] [Google Scholar]
- NAGANUMA S., KUZUYA N., SAKAI K., HASUMI K., ENDO A. Inhibition of the accumulation of lipid droplets in macrophage J774 by bafilomycin B1 and destruxin E. Biochim. Biophys. Acta. 1992;1126:41–48. doi: 10.1016/0005-2760(92)90214-g. [DOI] [PubMed] [Google Scholar]
- NISHI T., FORGAC M. The vacuolar (H+)-ATPases: nature's most versatile proton pumps. Nat. Rev. Mol. Cell. Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- SCOTT B.B., CHAPMAN C.G. The putative 116 kDa osteoclast specific vacuolar proton pump subunit has ubiquitous tissue distribution. Eur. J. Pharmacol. 1998;346:R3–R4. doi: 10.1016/s0014-2999(98)00163-0. [DOI] [PubMed] [Google Scholar]
- SPARROW C.P., PARTHASARATHY S., STEINBERG D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J. Biol. Chem. 1989;264:2599–2604. [PubMed] [Google Scholar]
- SUNDQUIST K., LAKKAKORPI P., WALLMARK B., VAANANEN K. Inhibition of osteoclast proton transport by bafilomycin A1 abolishes bone resorption. Biochem. Biophys. Res. Commun. 1990;168:309–313. doi: 10.1016/0006-291x(90)91709-2. [DOI] [PubMed] [Google Scholar]
- SUN-WADA G.H., YOSHIMIZU T., IMAI-SENGA Y., WADA Y., FUTAI M. Diversity of mouse proton-translocating ATPase:presence of multiple isoforms of the C, d and G subunits. Gene. 2003;302:147–153. doi: 10.1016/s0378-1119(02)01099-5. [DOI] [PubMed] [Google Scholar]
- SWALLOW C.J., ROTSTEIN O.D., GRINSTEIN S. Mechanisms of cytoplasmic pH recovery in acid-loaded macrophages. J. Surg. Res. 1989;46:588–592. doi: 10.1016/0022-4804(89)90025-5. [DOI] [PubMed] [Google Scholar]
- TAKADA Y., KUSUDA M., HIURA K., SATO T., MOCHIZUKI H., NAGAO Y., TOMURA M., YAHIRO M., HAKEDA Y., KAWASHIMA H., MUMEGAWA M. A simple method to assess osteoclast-mediated bone resorption using unfractionated bone cells. Bone Mineral. 1992;17:347–359. doi: 10.1016/0169-6009(92)90785-c. [DOI] [PubMed] [Google Scholar]
- TOMOI M., ITOH H., UEDA S., ONO T., SHIBAYAMA F. Effects of omeprazole and famotidine on (H+–K+) ATPase and acid secretion in rabbit gastric glands. Folia Pharmacol. Japan. 1988;92:105–111. doi: 10.1254/fpj.92.105. [DOI] [PubMed] [Google Scholar]
- TOYOMURA T., MURATA Y., YAMAMOTO A., OKA T., SUNWADA G.H., WADA Y., FUTAI M. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J. Biol. Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- UDAGAWA N., TAKAHASHI N., AKATSU T., SASAKI T., YAMAGUCHI A., KODAMA H., MARTIN T.J., SUDA T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- UDAGAWA N., TAKAHASHI N., JIMI E., MATSUZAKI K., TSURUKAI T., ITOH K., NAKAGAWA N., YASUDA H., GOTO M., TSUDA E., HIGASHIO K., GILLESPIE M.T., MARTIN T.J., SUDA T. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- UEDA T., UGAWA S., SHIMADA S. A novel putative M9.2 isoform of V-ATPase expressed in the nervous system. Neuroreport. 2003;14:25–30. doi: 10.1097/00001756-200301200-00005. [DOI] [PubMed] [Google Scholar]
- VAN HILLE B., RICHENER H., EVANS D.B., GREEN J.R., BILBE G. Identification of two subunit A isoforms of the vacuolar H(+)-ATPase in human osteoclastoma. J. Biol. Chem. 1993;268:7075–7080. [PubMed] [Google Scholar]
- VAN HILLE B., RICHENER H., GREEN J.R., BILBE G. The ubiquitous VA68 isoform of subunit A of the vacuolar H(+)-ATPase is highly expressed in human osteoclasts. Biochem. Biophys. Res. Commun. 1995;214:1108–1113. doi: 10.1006/bbrc.1995.2400. [DOI] [PubMed] [Google Scholar]
- VISENTIN L., DODDS R.A., VALENTE M., MISIANO P., BRADBEER J.N., ONETA S., LIANG X., GOWEN M., FARINA C. A selective inhibitor of the osteoclastic V-H+-ATPase prevents bone loss in both thyroparathyroidectomized and ovariectomized rats. J. Clin. Invest. 2000;106:309–318. doi: 10.1172/JCI6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG T., GIEBISCH G., ARONSON P.S. Use of transgenic animals to study renal acid–base transport. J. Nephrol. 2002;15 Suppl 5:S151–160. [PubMed] [Google Scholar]
- WLODAWER P., PARSONS D.F., WILLIAMS G.R., WOJTCZAK L. Morphological changes in isolated rat-liver mitochondria during swelling and contraction. Biochim. Biophys. Acta. 1966;128:34–47. doi: 10.1016/0926-6593(66)90139-1. [DOI] [PubMed] [Google Scholar]
- XU J., FENG H.T., WANG C., YIP K.H., PAVLOS N., PAPADIMITRIOU J.M., WOOD D., ZHENG M.H. Effects of bafilomycin A1: an inhibitor of vacuolar H (+)-ATPases on endocytosis and apoptosis in RAW cells and RAW cell-derived osteoclasts. J. Cell. Biochem. 2003;88:1256–1264. doi: 10.1002/jcb.10477. [DOI] [PubMed] [Google Scholar]