Abstract
The present study tested the hypothesis that nerve growth factor (NGF) could affect presynaptic inhibition mediated by GABAA (GABA-sensitive ionotropic receptors) receptors on the afferents of nociceptive dorsal root ganglia (DRG) neurons, thus reducing the filtering of central nociceptive signals.
To investigate this issue, small-diameter, nociceptive DRG neurons were cultured for 48–72 h either in the normal medium or in the presence of NGF (50 ng ml−1). After 15 min washout, cells were patch clamped with Cs+ containing electrodes to block GABAB (GABA-sensitive metabotropic receptors) receptor-activated currents.
Chronically treated DRG neurons showed no difference in the peak amplitude of GABA-induced currents. However, NGF-treated cells exhibited increased fading of the response to continuous GABA application, with faster desensitization onset, decreased residual current at the end of agonist application and slower recovery from desensitization. Moreover, the deactivation phase after brief agonist pulses was also accelerated.
Unlike responses to GABA, chronic NGF treatment had no effect on the desensitization process to the excitatory transmitter ATP, as no difference in peak amplitude, fast and slow time constants of current decay was found.
Experimental tests indicated that the observed effects on GABA currents were not a reactive process triggered by washing out NGF after its long application. Acutely applied NGF did not change GABAA receptor-mediated responses.
NGF-treated neurons showed decreased sensitivity to the antagonist picrotoxin. The action of pentobarbitone, midazolam, bicuculline or gabazine was, however, unchanged.
These observations suggest that the modulation of GABAA receptor function of DRG nociceptors by NGF may contribute to the algogenic action of this neurotrophin.
Keywords: Desensitization, gabazine, bicuculline, picrotoxin, pentobarbitone, midazolam, ATP, pain
Introduction
The neurotrophin nerve growth factor (NGF) has several important effects on morphological and functional properties of neurons. Postnatally, NGF has a major, physiological role in survival, growth and development of ganglion neurons (Crowley et al., 1994; Patel et al., 2000) as, for example, newborn mice daily injected with anti-NGF antiserum show severe atrophy of sympathetic ganglia (Levi-Montalcini & Cohen, 1960). Although the same cells grown in culture can survive for several days in the absence of NGF, chronic NGF treatment promotes the formation of neuronal processes and allows long-term survival (Levi-Montalcini, 1964). Both high (TrkA)- and low (p75)-affinity receptors for NGF are expressed on dorsal root ganglion (DRG) sensory neurons (Bennett et al., 1996).
Another important action of NGF is facilitation of processing of nociceptive signals when levels of NGF grow dramatically in inflamed or damaged tissue (Donnerer et al., 1992; Woolf et al., 1994; Ueda et al., 2002). Furthermore, increased NGF retrograde transport by nociceptive neurons augments the expression of algogenic compounds like substance P and CGRP (Donnerer et al., 1992). Administration of NGF can produce hyperalgesia (Lewin et al., 1992; Woolf et al., 1994; Andreev et al., 1995), whereas neutralization of endogenous NGF reduces sensitivity to painful stimuli (McMahon et al., 1995; Koltzenburg et al., 1999). NGF administration enhances the sensitivity of DRG neurons to capsaicin and noxious heat (Winter et al., 1988; Rueff & Mendell, 1996; Bonnington & McNaughton, 2003).
A further action of NGF is concerned with the modulation of synaptic transmission. In fact, in animal models of inflammatory pain, NGF can also boost the efficiency of glutamatergic transmission at the spinal cord level, while anti-NGF antibodies decrease spinal glutamate release and attenuate algogenic behavior (Ishikawa et al., 1999). Moreover, while ATP is an important messenger in pain signaling in these neurons (Burnstock, 2001; North, 2004), chronic NGF treatment can increase the expression of P2X3 ATP receptors in sensory neurons (Ramer et al., 2001).
The processing of nociceptive inputs at the first relay synapse of the pain pathway in the spinal dorsal horn is modulated by GABA, which exerts an analgesic effect (Sivilotti & Woolf, 1994). GABA is believed to be the main transmitter for presynaptic inhibition in the spinal cord (Sivilotti & Nistri, 1991) due to primary afferent depolarization (PAD) that reduces presynaptic impulses and, thus, release of the excitatory transmitter (Rudomin & Schmidt, 1999). Owing to their key role in nociceptive information processing, the GABAA subclass of GABA-sensitive ionotropic receptors might be an important target in mediating the algogenic action of NGF. However, the difficulty of directly accessing such receptors on the fine central terminals of DRG neurons suggests the study of the action of NGF on somatic GABAA receptors normally expressed by the soma of these cells (Sivilotti & Nistri, 1991; Rudomin & Schmidt, 1999). On DRG neurons, GABA acts via GABAA receptors (sensitive to bicuculline (BIC) or picrotoxin (PTX)) to mediate a Cl−-dependent depolarization (Sivilotti & Nistri, 1991), as well as on GABA-sensitive metabotropic receptors (GABAB) that mediate a slowly developing K+ outward current (Gähwiler & Brown, 1985; Newberry & Nicoll, 1985). Since activation of GABAB receptors appears to play only a minor role in presynaptic inhibition of primary afferent terminals (Stuart & Redman, 1992), we focused on GABAA receptors. Hence, we used electrophysiological recording from DRG neurons in culture to investigate how acute or chronic NGF treatment might affect GABAA receptor-induced currents. To find out if chronic NGF treatment might also change the effects of an excitatory transmitter, we used the same cell preparation to examine how DRG neurons responded to ATP.
Methods
Cell culture preparation
Cultures of rat DRG neurons were prepared as described previously (Sokolova et al., 2004). In brief, rats (P11–18) of both sexes were deeply anesthetized by slowly raising levels of CO2 and were killed by decapitation, a procedure (including animal handling and care) in accordance with the Italian Animal Welfare Act and approved by the Local Authority Veterinary Service. Thoracic and lumbar ganglia were excised, enzymatically treated, plated on poly-L-lysine (5 mg ml−1) coated Petri dishes and cultured under an atmosphere containing 5% CO2. NGF (2.5S NGF; 50 ng ml−1) was added to half of the Petri dishes at the time of DRG neuron plating. Cells were used for recording from the 2nd to the 3rd day after plating. In order to minimize differences in responses between DRG neuron preparations, equivalent numbers of control (from Petri dishes without NGF) and NGF-treated neurons were used on each occasion.
Patch-clamp recording
Control or NGF-treated cells were continuously superfused (2 ml min−1) with physiological solution containing (in mM): 152 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES (pH adjusted to 7.4 with NaOH, osmolarity adjusted to 320 mOsm with glucose). This solution was applied for about 15 min to ensure full washout of the culture medium. Thereafter, single cells were patch clamped in the whole-cell configuration using pipettes with a resistance of ∼3–4 MΩ when filled (in mM) with 120 CsCl, 20 HEPES, 1 MgCl2, 3 Mg2ATP3 and 5 EGTA (pH adjusted to 7.2 with CsOH). The osmolarity of the pipette solution was 285 mOsm.
Currents were recorded from cells 15–30 μm in diameter (see also Figure 1b), thus considered to be small and medium-sized, nociceptive DRG neurons (North, 2004), which are very sensitive to the pain transmitter ATP (Grubb & Evans, 1999) and bind the nociceptor marker IB4 (Sokolova et al., 2004). DRG neurons of diameter up to 25 μm usually express the NK-1 receptor to the pain transmitter substance P (Li & Zhao, 1998), thus further characterizing them as nociceptors, although such expression can be found even in a minority of larger DRG cells. In most cells series resistance was compensated by 80%. Cells were voltage clamped at −70 mV (unless otherwise indicated). After whole-cell configuration was obtained, an equilibration period of 5 min was used for establishing adequate solution exchange between the patch pipette and the cell. Currents were filtered at 1 kHz and acquired by means of pClamp 8.2 software (Axon Instruments, Union City, CA, U.S.A.).
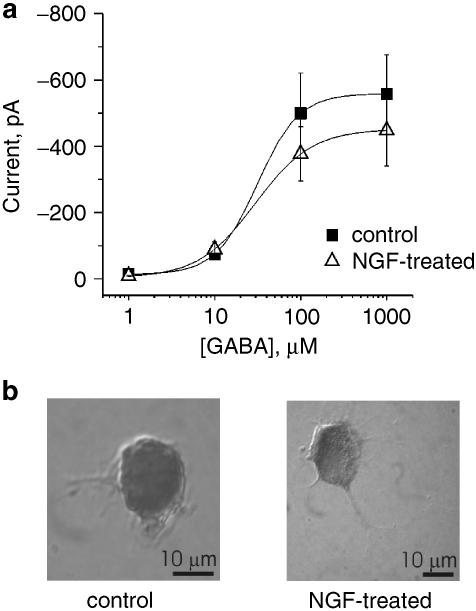
Figure 1.
Sensitivity of DRG neurons to GABA. (a) Log concentration–response curves for rapidly superfused GABA in control or chronically NGF-treated DRG neurons. Note the lack of difference between curves (n=14 and 15 for control and NGF-treated, respectively). (b) Example of cresyl violet-stained DRG neurons cultured for 48 h in the medium without (left) or with (right) NGF (50 ng ml−1).
Drug delivery and data analysis
GABA, antagonists and modulators of GABAA receptors, and ATP were diluted with extracellular solution to final concentration and applied by a rapid superfusion system (Rapid Solution Changer RSC-200, BioLogic Science Instruments, Grenoble, France) consisting in a multibarrel array of capillary tubes 100–150 μm from the recorded cell. The time for solution exchange was about 30 ms. In a separate set of experiments, GABA was applied by pressure application (15–20 psi) for 40 ms from glass micropipettes positioned approximately 15–25 μm away from the recorded cell (Di Angelantonio & Nistri, 2001). On some cells, NGF was acutely administered via pressure application (15 psi for 30 s) to pipettes containing a concentration of 150 ng ml−1.
During GABA application, the development of current fade from the early peak was quantified on the basis of its time constant (τON), which was best fitted monoexponentially (Clampfit program of the pCLAMP suite), with the exception of a few responses to 100 μM GABA that required two exponents and were discarded from further analysis. Current fade was also measured as the fractional residual current (r) left at the time of switching off GABA, as this value indicated the extent of activated GABA receptors prior to termination of agonist administration. In some experiments, the difference in r values between control and test conditions was expressed as Δr and allowed comparison of degree of fading (r) under various experimental conditions. GABA current deactivation was quantified as the monoexponential time constant of return to baseline (τOFF).
Paired-pulse experiments in which the second agonist pulse was delivered at different intervals were used to monitor recovery from desensitization. For this purpose, each pulse consisted of 25 μM GABA applied by superfusion for 2 s. The peak of GABA current generated by the second pulse was expressed as a fraction of the peak amplitude of the control response. Data were best fitted with a double hyperbola (SigmaPlot2001, SPSS, Chicago, IL, U.S.A.) and recovery from desensitization was expressed as the time needed to regain 90% of the control peak amplitude (t90).
Log concentration–response curves for GABA were fitted with the logistic equation (Origin 6.0, Microcal, Northampton, MA, U.S.A.). Each concentration of GABA was applied by superfusion for 2 s and repeated two to three times on each cell at a 3-min interval. The strong fading of the GABA responses plus the necessarily limited duration of whole-cell patch clamping (about 40 min) did not allow strictly quantitative tests for characterizing antagonism potency and nature. To circumvent this condition, tests to quantify the action of rapidly superfused blockers (BIC, PTX, gabazine, that is, SR 95531) were usually based on measuring peak currents elicited by a fixed GABA concentration (25 μM) close to the EC50 value for this agonist. Note that each antagonist was always present in the GABA solution to avoid sudden dilution of antagonist upon rapid application of the agonist. Several concentrations of each antagonist were cumulatively applied to determine their potency to block GABA-induced currents. GABA currents in the presence of antagonist were expressed as a fraction of the current amplitude obtained in the absence of antagonist. Data were then plotted with the logistic equation to express inhibitory potency in terms of IC50 values. When receptor antagonism was rapidly reversible (e.g. bicuculline or gabazine), tests with various antagonist concentrations were also repeated after intermediate washes and gave analogous results.
To assess the potentiating action of pentobarbitone (PB) (sodium salt) on 25 μM GABA, this agent was applied cumulatively in the range of 10–100 μM. The test GABA concentration was prepared in the corresponding PB solution. The GABA current in the presence of PB was expressed as a fraction of the control one and plotted with the logistic equation to obtain the EC50 value for PB. The limited availability of the water-soluble benzodiazepine midazolam HCl did not allow its systematic tests at various concentrations applied via rapid superfusion. Preliminary tests indicated that maximum facilitation was apparently observed with bath-applied 1 μM midazolam, which was then used in all other experiments. To minimize midazolam dilution by concomitant fast superfusion of GABA, GABA (30 μM) was applied via a puffer pipette for 40 ms. Previous experiments have indicated that this method produced an effective dose of the substance at cell membrane level three times lower than the pipette concentration (Di Angelantonio & Nistri, 2001).
In experiments on ATP-induced currents, ATP was rapidly superfused at a concentration of 10 μM for 2 s.
All data are presented as the mean±s.e.m. (n=number of cells). The statistical significance of nonparametric data was assessed with Mann–Whitney rank-sum test for comparing two unmatched samples from two populations. For raw data their normal distribution was first assessed with SigmaStat (version 2.0, Jandel Scientific, San Rafael, CA, U.S.A.) and, if data met this requirement, were further analyzed with the unpaired Student's t-test. A P-value of <0.05 was accepted as indicative of a statistically significant difference.
Drugs
Chemicals for cell culture and recording were from Sigma (Milan, Italy); the culture media were obtained from Gibco BRL (Life Technologies, Milan, Italy). GABA and ATP were from Sigma (Milan, Italy), BIC, gabazine and PTX were from Tocris (Bristol, U.K.), PB (sodium salt) was a gift from Dr Laura Ballerini (University of Trieste), while midazolam HCl was donated by Professor J. Lambert (University of Dundee), Dr G. Puja (University of Modena) and Professor G. Biggio (University of Cagliari).
Results
Chronic NGF treatment does not significantly affect GABA sensitivity of DRG neurons
Since DRG neurons do not synthesize NGF as shown by the lack of mRNA signal (Wetmore & Olson, 1995), in the present study control conditions refer to cells bathed with standard culture medium (without NGF addiction). In this case, rapid superfusion of GABA evoked inward currents, the peak of which is plotted in Figure 1a (filled symbols) against varying concentration of GABA. On NGF-treated DRG neurons, peak amplitudes of GABA-evoked currents were slightly lower than in controls (Figure 1a, open symbols), although this difference was not statistically significant. The EC50 values (the concentration of GABA eliciting half-maximal current amplitude) obtained from these average plots were very similar between controls (31.4 μM, n=14) and NGF-treated cells (29.7 μM, n=15). Analogous results were obtained by averaging the log-transformed EC50 values obtained from each cell in control (34.5±3.9 μM, n=14) and after NGF treatment (26.9±5.1 μM, n=11). As all recorded cells had similar size regardless of NGF treatment (see examples in Figure 1b), this observation suggests that unequal GABA current responses were not masked by large differences in cell size. This fact was confirmed by the similar cell membrane capacitance between controls (28.5±1.4 pF; n=105) and NGF-treated cells (31.8±1.5 pF; n=68). Since there was no difference in GABA-induced responses between DRG neurons cultured for 48 and 72 h in the presence of NGF (nor between 48 and 72 h in controls), data from cells at the second and third day from plating were pooled.
NGF-mediated shaping of GABA-induced currents: desensitization degree and onset, deactivation phase and recovery from desensitization
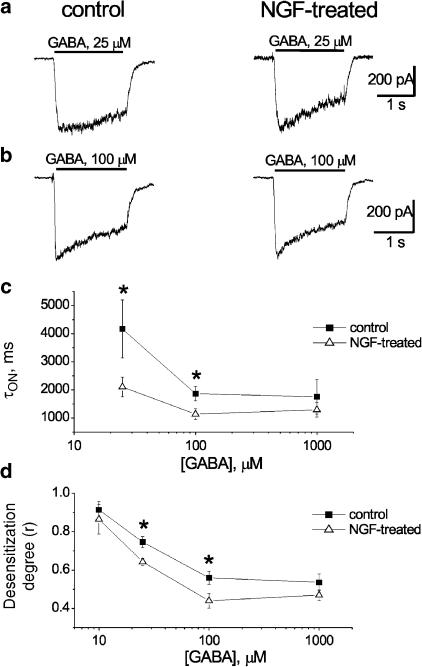
Despite lack of change in peak current amplitude, it appeared that chronic NGF treatment changed the time course of the decay of GABA-evoked currents. Analysis was performed on currents that presented clear fading during application of GABA, namely, those produced by 10, 25 (close to the dose inducing half-maximal responses), 100 and 1000 μM concentrations of this amino acid. Figure 2a and b shows sample traces obtained from control or NGF-treated DRG neurons using 25 or 100 μM GABA, respectively. For each agonist concentration used, care was taken to compare currents of virtually identical peak amplitude. Cells cultured in the presence of NGF showed faster fading of the response and smaller residual current at the end of GABA application. The differences in τON value for current fade and in the fractional residual current (r) are quantified in Figure 2c and d, and were statistically significant at 25 and 100 μM agonist concentration. Neither τON nor r values were affected by NGF treatment when obtained with 1000 μM GABA, which induced maximal response (Figure 2c and d). This observation suggests that NGF could facilitate current decay, but it could not augment it once it was maximally developed.
Figure 2.
Enhanced desensitization of GABAA receptor-mediated currents after chronic treatment with NGF. (a) Examples of amplitude-matched currents induced by 25 μM GABA applied for 2 s in control (left) or after NGF treatment (right). (b) Examples of amplitude-matched currents induced by 2 s application of 100 μM GABA as indicated in (a). Note enhanced fading of current with smaller residual response at the end of GABA application. (c) Plot of τon values for fading of GABA-induced currents versus log GABA concentrations from control or NGF-treated neurons. *P<0.05; n=10–14 cells. (d) Plot of desensitization degree (r) expressed as a fractional residual current versus log GABA concentrations for control or NGF-treated neurons. *P<0.05; n=11–13 cells.
The monoexponential time constant of current offset (τOFF) after interruption of 10, 25, 100 and 1000 μM GABA application was also measured. There was no difference in τOFF between traces obtained after 10 μM GABA from controls and NGF-treated cells (120±12 ms, n=9; 120±23 ms, n=5, respectively), nor after 25 μM (104±5 ms, n=19 for controls; 102±7 ms, n=7 for treated cells), 100 μM (111±8 ms, n=10 for controls; 104±10 ms, n=12 for treated cells) or 1000 μM GABA (204±28 ms, n=7 for controls; 170±13 ms, n=5 for treated cells).
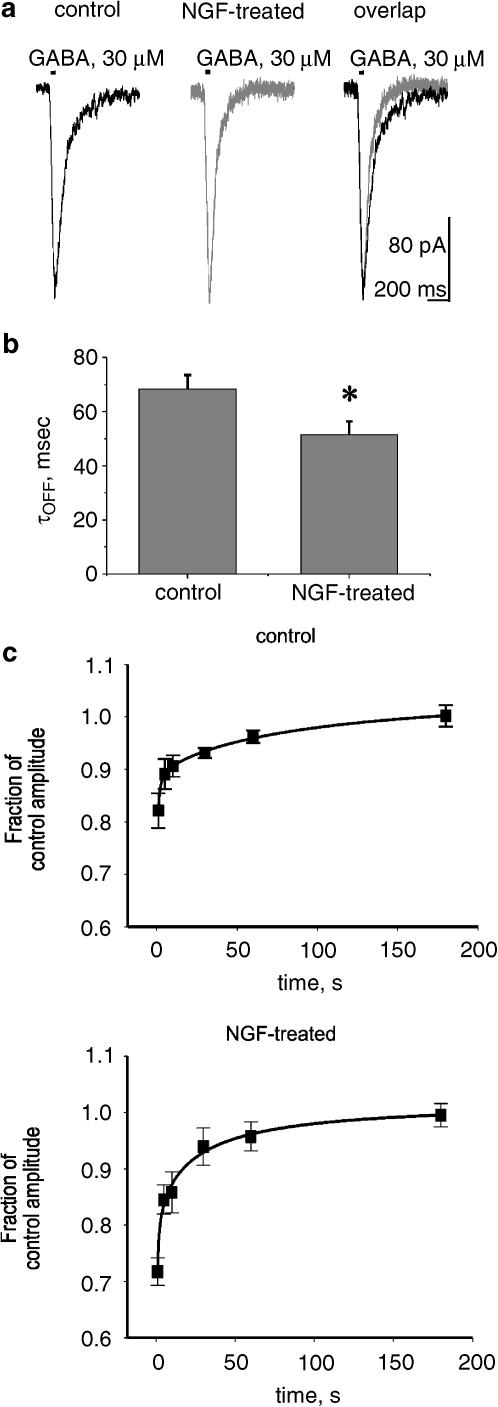
GABA current return to baseline after termination of the agonist application was a complex phenomenon presumably due to a combination of desensitization recovery, receptor deactivation and agonist removal, since the time course of receptor deactivation can be strongly affected by the duration of agonist application and the consequent desensitization (Jones & Westbrook, 1995). In order to examine deactivation of GABA currents less affected by desensitization, responses were induced with 40 ms pressure application of GABA (30 μM) from a micropipette close to the recorded cell. Figure 3a shows representative traces of similar amplitude obtained from a control cell and an NGF-treated cell using such an application of GABA. The deactivation phase, best fitted with a monoexponential function, became significantly shorter in NGF-treated neurons as indicated in Figure 3b.
Figure 3.
Chronic NGF treatment increases deactivation of GABAA receptor-mediated currents and modifies their recovery from desensitization. (a) Examples of amplitude-matched, inward currents evoked by brief (40 ms) pressure applications of GABA to single DRG neurons grown in control or NGF-containing medium. Note accelerated current decay (responses superimposed for clarity; right-hand side) after NGF treatment. (b) Histograms showing τOFF value of current decay for neurons grown in control or NGF-containing medium. *P<0.05; n=11–12 cells. (c) Plots of time course of recovery from desensitization of GABA currents for control (upper; n=10) and NGF-treated (lower; n=13) DRG neurons. Data are presented as fractional amplitude of the second response in the pair of pulses spaced at time intervals indicated on the abscissa. The difference between values at 1 s is statistically significant (P<0.05). Note initially slower recovery in chronically treated cells.
To further analyze the effects of chronic NGF treatment on desensitization of GABA-induced currents, paired-pulse experiments were performed. Figure 3c (upper) shows that fast recovery from desensitization, expressed as fractional amplitude of the second response in the pair, was best fitted by a hyperbolic function for control neurons with calculated t90 of 7.6 s. On NGF-treated neurons (Figure 3c, lower), the changes in fractional amplitude of the second response could also be fitted hyperbolically (calculated t90=19.7 s). This difference from control was due to the significantly (P<0.05) smaller second response at the first pulse interval (1 s), while subsequent data points were indistinguishable from control.
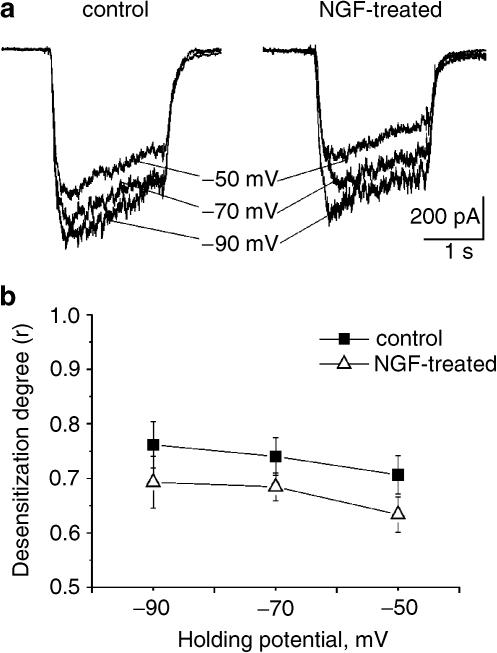
Current fade might be due to a combination of factors including agonist-induced channel block or desensitization. To explore the role of agonist-dependent channel block, 25 μM GABA was applied at three different holding potentials (−50, −70 and −90 mV) to control or NGF-treated cells. As exemplified by raw data in Figure 4a and quantified in Figure 4b, voltage dependence was very weak for both controls and NGF-treated DRG cells, suggesting that the observed fading was not primarily due to agonist-dependent channel block.
Figure 4.
GABA-evoked current decline is voltage insensitive. (a) Examples of GABA-evoked currents from control (left) or NGF-treated (right) neurons. In each panel currents induced by 25 μM GABA on the same cell held at –50 (uppermost), –70 and –90 mV are superimposed for comparison. (b) Plot of desensitization degree expressed as fractional residual current (r) induced by 25 μM GABA versus level of holding potential in control or NGF-treated neurons (n=10–12 cells).
Tests to rule out artifactual effects due to NGF
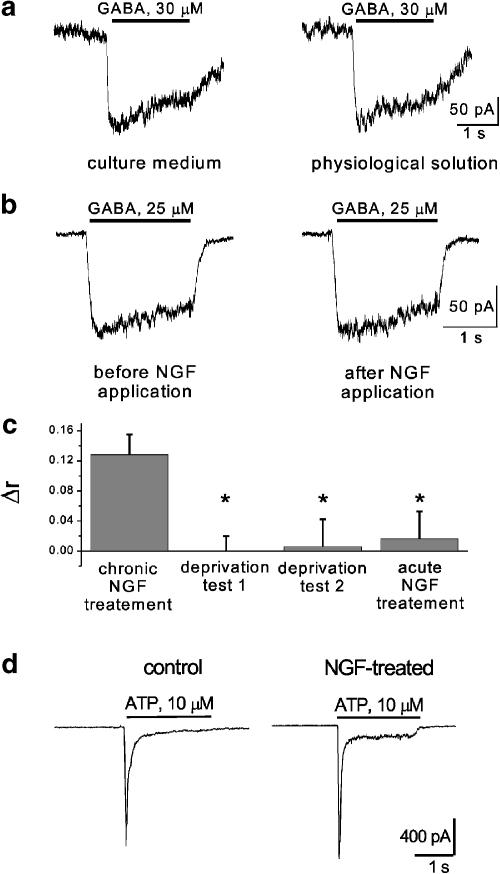
Since all electrophysiological recordings from DRG cells chronically exposed to NGF were performed in physiological solution after NGF washout (see Methods), we considered the possibility that increased current fading during agonist application might have been caused by acute NGF deprivation, rather than due to a long-lasting effect of NGF treatment. To examine this hypothesis, two different tests were performed on NGF-treated DRG neurons. In the first one (deprivation test 1), cells were patch clamped while still in the NGF-containing culture medium and tested for their response to pressure-applied (2 s) GABA (30 μM). The medium was then washed out while preserving patch-clamp recording and replaced with physiological solution. After 3 min, GABA was again applied to the same cell as described above. Comparing currents obtained in NGF-containing culture medium with those in physiological solution gave a good match of the two GABA-induced responses (see example in Figure 5a). To quantify this result, for each cell (n=5), we expressed, as Δr, the difference between fractional residual GABA currents (just prior to the end of GABA application) obtained in the culture medium and in physiological solution (Figure 5c). The Δr value for the deprivation test 1 was almost nil and was significantly different (P<0.05) from the Δr value obtained when comparing responses from control and chronically treated neurons in the standard protocol.
Figure 5.
Changes in GABA-evoked current fade are not due to sudden removal of NGF from neurons grown in the presence of this neurotrophin nor are accompanied by alterations in ATP-mediated responses. (a) Example of protocol (test 1) in which the same DRG neuron, grown in NGF-containing medium, is first tested for its response to pressure-applied (30 μM) GABA without washing out NGF (left), and then the same GABA application is repeated 3 min after washing out the NGF-containing medium (right). Note the lack of change in residual current at the end of the GABA response. (b) Example of protocol (test 2) in which the same neuron, grown in NGF-containing medium, is first tested in standard physiological solution for its response to rapidly superfused 25 μM GABA (left) and then the same GABA application is repeated 3 min after washing out NGF (150 ng ml−1) transiently applied from a pressure pipette for 30 s (right). Note again the lack of difference in residual current evoked by GABA. (c) Histograms summarizing differences in desensitization degree following GABA application. Left, values of desensitization degree (r) following 25 μM GABA application are compared between cells grown in control or NGF-containing medium (n=13): the difference between r data is expressed as Δr. Middle two histograms refer to Δr values obtained with the protocols exemplified as test 1 (n=5) or test 2 (n=5) in (a, b). Right histogram refers to Δr obtained by comparing 25 μM GABA-evoked responses before and after acute application of NGF (30 s) to neurons grown in the control medium (n=5). *P<0.05. (d) Examples of currents induced by 10 μM ATP in control (left) or NGF-treated (right) DRG neurons.
Furthermore, assuming that the stronger current fade might have been a consequence of NGF washout, a subsequent application of NGF should reverse, in part, this current fade. This possibility was assessed in our second test (deprivation test 2) when NGF was pressure applied for 30 s to chronically NGF-treated cells washed for 5 min with physiological solution. In detail, GABA (25 μM) was applied by rapid superfusion (2 s) thrice (3 min interval), then NGF was pressure applied and the cell retested with repeated GABA applications (see sample traces in Figure 5b). Also in this case, currents obtained from the same cells (n=5) before and after acute NGF application were almost identical. These data are quantified in Figure 5c, in which the Δr value from the deprivation test 2 was very small and significantly different when compared with Δr obtained with the standard protocol. Finally, we examined if 30 s NGF application to naïve, control cells could change their GABA (25 μM) responses. As shown by the right-hand side histogram of Figure 5c, this experimental condition was accompanied by a small Δr value significantly different (P<0.05) from the one obtained with standard protocol (left histogram). This observation demonstrated that short-term exposure of naïve DRG neurons to NGF was insufficient to modify their GABA-mediated responses.
In summary, these tests showed that increased fading of GABA-induced responses from NGF-treated DRG neurons was not due to abrupt NGF deprivation.
NGF-treated DRG neurons show differential drug sensitivity
NGF can turn on multiple intracellular messengers, leading to the activation of protein kinases and/or transcription factors (Kaplan & Miller, 2000). Thus, it seemed likely that the observed effects of chronically applied NGF on GABA-induced currents could be mediated by conformational changes of GABA receptors, due to post-translational modifications of native receptors (e.g. phosphorylation; Moss & Smart, 2001) or neosynthesis of receptors with different subunit composition. As a first approach to this issue, we took advantage of the fact that a number of GABA receptor ligands show preferential affinity for certain subunits (Hevers & Lüddens, 1998). Hence, if ligand pharmacology were changed after chronic NGF treatment, one might infer that the receptor subunit(s) binding such ligands were likely altered.
Table 1 shows the IC50 values for the competitive antagonists BIC, gabazine and the noncompetitive blocker PTX. While the potency of BIC or gabazine block was similar between NGF-treated cells and controls, PTX was less potent on NGF-treated cells. The potentiating action by PB was the same on control and NGF-treated neurons as indicated by similar EC50 values.
Table 1.
Effect of GABAA receptor ligands on GABA-mediated responses of control and NGF-treated DRG neurons
| IC50 (μM) | EC50 (μM) | % enhancement | ||||
|---|---|---|---|---|---|---|
| Control | NGF-treated | Control | NGF-treated | Control | NGF-treated | |
| BIC | 1.77±0.17 (n=2) | 2.24±0.21 (n=19) | ||||
| Gabazine | 0.55±0.12 (n=10) | 0.29±0.04 (n=11) | ||||
| PTX | 4.65±1.24 (n=11) | 9.84±3.07* (n=8) | ||||
| PB | 60.86±9.79 (n=12) | 50.53±7.50 (n=19) | ||||
| Midazolam | 80±18 (n=11) | 95±14 (n=10) | ||||
P<0.05.
Finally, application of 1 μM midazolam to NGF-treated or control DRG neurons indicated that, in both cell groups, GABA currents displayed the same sensitivity to the facilitatory action by this benzodiazepine (Table 1).
Chronic NGF treatment does not affect the amplitude and desensitization of ATP-induced currents
The ATP-sensitive ionotropic receptors P2X are involved in direct excitation of primary afferent DRG neurons (Bland-Ward & Humphrey, 1997; Hamilton & McMahon, 2000). Sensory neurons express different levels of P2X2, P2X3 and P2X2/3 receptors, characterized by different time courses of current decay during agonist application (Burgard et al., 1999). In the present study, application of 10 μM ATP for 2 s elicited inward currents with different decay time courses (see Burgard et al., 1999; Sokolova et al., 2001), presumably reflecting the presence of various P2X receptor subtypes (see Figure 5d). Since the P2X3 receptor seems to be the main subtype involved in nociception (North, 2004), we focused on cells expressing ATP responses with biphasic current decay, a hallmark of the presence of P2X3 receptors (Burgard et al., 1999). In particular, we looked at the fast (τfast) and slow (τslow) components of current decay to characterize desensitization of ATP-induced currents in control cells or after chronic NGF application. No statistically significant difference was found in peak amplitude of ATP-evoked currents between NGF-treated neurons (−1226±210 pA, n=11) and controls (−935±118 pA, n=24). Analysis of ATP-induced desensitization, performed by fitting current decay with a double exponential function, showed no difference in either τfast (40.5±2.4 ms, n=25 controls; 44.7±7.8 ms, n=11 NGF-treated neurons) or τslow (532.9±105.5 ms, n=25 controls; 302.8±58.6 ms, n=11 treated cells).
Discussion
The principal finding of the present study is the novel demonstration of increased decline of GABAA receptor-mediated currents of nociceptive DRG neurons grown in the presence of NGF. This phenomenon could be manifested in two ways, namely, neurotrophin-enhanced desensitization of sustained responses mediated by GABAA receptors, and accelerated deactivation of short responses evoked by brief GABA pulses. This action of NGF might be important for understanding the mechanism of pain induction and/or intensification by NGF, since increased NGF levels during inflammation or injury could change the effectiveness of GABA-mediated inhibition of excitatory transmitter release from afferents of DRG neurons in the spinal cord.
Fading of GABA-evoked currents
In symmetrical Cl− concentrations, GABA evoked inward currents readily blocked by bicuculline or gabazine. Since these currents reverse at ∼0 mV (Sokolova et al., 2001), they are clearly due to the activation of GABAA receptors. Furthermore, inclusion of Cs+ in the patch pipette solution prevented activation of GABAB receptors (Otis et al., 1993).
A decline in the GABA-evoked currents during continuous exposure to the agonist could have been due to receptor desensitization, agonist-induced channel block or GABA uptake. Our observations that current fade was little sensitive to membrane potential would make channel block unlikely. Furthermore, although DRG neurons can take up GABA applied for several minutes (Hosli & Hosli, 1979), fade of GABA-induced responses during its sustained application is not affected in Na+-free media (Adams & Brown, 1975; Deschenes et al., 1976; Gallagher et al., 1978), indicating that Na+-dependent GABA uptake played a minimal role in the membrane response to this amino acid. Hence, the current fade reported in the present study was probably due to receptor desensitization, which is a prominent property of such receptors (Sivilotti & Nistri 1991; Bormann, 2000).
Current fade following NGF treatment
When DRG neurons were grown in the presence of NGF, applied at the standard concentration (50 ng ml−1) optimized for ganglion culture (Levi-Montalcini & Angeletti, 1963), they generated GABA currents characterized by faster and stronger fade. Although concentrations up to 200 ng ml−1 have been used to investigate changes in DRG neuronal responses (Winter et al., 1988), the concentration of NGF used in the present study is largely in excess of the one normally present in the extracellular fluid of young rats (about 0.6 ng ml−1; Xia et al., 2000), and thus it more closely corresponds to the substantial increases in local NGF levels typically found during tissue inflammation and injury (Ueda et al., 2002). Hence, the present experiments suggest that the changes in DRG cells responses to GABA should be considered as a model of what might happen to such cells in the presence of pathological amounts of this neurotrophin.
NGF treatment could not by itself bring about GABAA receptor desensitization when current responses were small and associated with minimal fade, nor could it intensify the strong fade associated with maximal responses. The role of NGF therefore seemed to be a modulatory one of the desensitization process once it has developed. An earlier report describing reduction in GABA current amplitude only after >1 week of NGF exposure (50–200 ng ml−1; Bevan & Winter 1995) accords with our data on the lack of change in the peak amplitude of GABA currents after 2–3 days of NGF treatment.
Accelerated fading and smaller residual current had no significant influence on the return of the response to baseline after 2 s application, as this process was probably due to a combination of agonist removal, receptor deactivation and recovery from desensitization with complex dependence on NGF treatment. It is, however, worth noting that NGF treatment accelerated the deactivation time course of currents induced by brief pulses of GABA.
Recovery from GABA current desensitization, tested with the paired pulse protocol, was rapid in control cells, enabling full reattainment of cell responses within 3 min. NGF-treated cells also recovered fully from desensitization within 3 min, although at the earliest measured interval (1 s) their ability to regain responsiveness was significantly less.
The change in GABA current fade took place before the development of neuronal processes and was not associated with apparent morphological or cell capacitance changes in the time frame of 48–72 h. Thus, the effect resulting from NGF treatment was a novel action distinct from the well-known effect of promoting neuronal growth.
Since DRG neurons do not synthesize NGF as they lack mRNA for this substance (Wetmore & Olson, 1995), effects induced by NGF could only be generated by exogenous application of this neurotrophin. It was therefore important to demonstrate in the present study that augmented current fade of GABA currents after NGF chronic exposure was not a consequence of sudden removal of this neurotrophin. In addition, acute application of NGF to naïve cells had no effect on GABA-induced currents.
Despite the effects of NGF treatment on the inhibitory GABA-induced currents in DRG neurons, our data suggest that this neurotrophin could not induce any significant change in the response of these neurons to the excitatory transmitter ATP. The lack of difference in peak amplitude of ATP-induced currents is consistent with a previous report based on 1 to 2-week NGF treatment of DRG neurons (Bevan & Winter, 1995). Although it is unknown if the desensitization process is altered by 2-week NGF treatment, the present results can exclude that this neurotrophin might modulate the desensitization of ATP currents in the time frame of 2–3 days.
Mechanisms underlying increased fade of GABA currents by NGF
Desensitization and deactivation kinetics of the current mediated by GABAA receptors has been shown to depend on GABAA receptor subunit combination (Gingrich et al., 1995; Haas & Macdonald, 1999). While rat DRG neurons at 2–3 postnatal weeks express α2, α3, β2, β3 and γ2 subunits (having lost the expression of the embryonic α5 subunit; Ma et al., 1993), the precise combination of GABAA subunits to form single receptors in DRG neurons remains unclear and cannot exclude the contribution by more recently discovered subunits like θ and ɛ subunits (Barnard et al., 1998; Hevers & Lüddens, 1998). Since chronic NGF treatment did not modify the dose–response curve for GABA, it seems likely that there was no significant change in the interaction of GABA with its binding site at the interface between α and β subunits (Smith & Olsen, 1995). As a first approach to outline potential GABAA receptor sites modified by NGF, we investigated whether the effect of certain subunit-selective ligands might have been altered by chronic NGF. In particular, we used gabazine or bicuculline to test changes in the competitive antagonist binding sites believed to be partially overlapping the GABA binding one (Sigel et al., 1992; Holden & Czajkowski, 2002). This experiment was also advantageous to confirm the observed similarity in GABA sensitivity between control and NGF-treated cells. Furthermore, we tested the potency of PTX that binds a specific site within the Cl− pore of the transmembrane domain 2 region (Ffrench-Constant et al., 1993; Gurley et al., 1995), PB that predominantly binds the β3 subunit (Davies et al., 1997; Wooltorton et al., 1997) and midazolam that binds the α2 and α3 subunits at their interface with the γ2 subunit (Barnard et al., 1998).
The lack of change in the potency of the competitive antagonists gabazine and bicuculline suggests their binding site to be essentially unaltered by NGF. PTX was significantly less potent after NGF treatment, indicating that its binding region was a target for the action of the neurotrophin. In fact, site-directed mutagenesis of motifs corresponding to the PTX binding site can strongly affect desensitization and deactivation of GABAA receptors (Scheller & Forman, 2002). The lack of change in PB or midazolam activity after NGF treatment suggests that the PB binding site on the β3 subunit as well as the midazolam binding site at the interface between α2 and (or) α3 subunits with the γ2 subunit were insensitive to neurotrophin exposure. In summary, it seems feasible that chronic application of NGF led to a modification in the GABAA receptor region inside the Cl− pore itself. It should be emphasized that validation of the proposed molecular targets on the GABAA receptor for the action of NGF will require further investigations based on single-cell RT–PCR to identify subunit changes, and on single-channel recordings to characterize the precise mechanisms underlying alterations in GABA-mediated responses. While NGF can upregulate sodium and calcium channels (Baldelli et al., 2000; Vidaltamayo et al., 2002), its long-term action on GABAA receptor channels remains unclear.
Pathophysiological implications
By activating GABAA receptors on sensory neurons endings, GABA generates presynaptic inhibition of primary afferent inputs (Rudomin & Schmidt, 1999). Presynaptic inhibition can be induced only by high-frequency stimulation of GABA-releasing fibers, causing a sustained reduction in excitability lasting hundreds of milliseconds (Frank & Fuortes, 1955). This slow and long-lasting effect of GABA is different from its more rapid transmitter role in other areas of the brain and can be adequately modeled by the method of agonist application employed in the present study.
The results obtained in our work suggest that increased levels of NGF, like those occurring after tissue damage or inflammation (see Introduction), might modify GABA-induced currents in sensory neurons decreasing the total amount of current entering their central terminals, thus reducing local depolarization and its effects on action potential propagation.
Notwithstanding the need for further investigation to clarify the molecular mode of action of NGF, GABAA receptor modulation by NGF should be viewed within a wide framework of regulatory activity exerted by neurotrophins on afferent inputs, especially during chronic pain states. In particular, since NGF can facilitate BDNF expression in DRG neurons (Michael et al., 1997) and BDNF can increase the release of GABA (Pezet et al., 2002), the whole process of pain signaling may comprise self-regulating mechanisms to compensate pain (Lever et al., 2003) at least in early stages of chronic inflammation.
Acknowledgments
Midazolam was kindly donated by Professor J. Lambert (University of Dundee), Dr G. Puja (University of Modena) and Professor G. Biggio (University of Cagliari). Pentobarbitone was a generous gift from Dr Laura Ballerini (University of Trieste). We are most grateful to Professor Rashid Giniatullin for his suggestions to start this project and help with preliminary results, Dr Elena Sokolova for her assistance with initial experiments on DRG neurons and Dr Massimo Righi for his help with cell cultures. This work was supported by grants from MIUR (FIRB).
Abbreviations
- BIC
bicuculline methochloride
- DRG
dorsal root ganglion
- GABAA
subclass of GABA-sensitive ionotropic receptors
- GABAB
GABA-sensitive metabotropic receptors
- NGF
nerve growth factor
- PAD
primary afferent depolarization
- PTX
picrotoxin
References
- ADAMS P.R., BROWN D.A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J. Physiol. 1975;250:85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREEV N.Y., DIMITRIEVA N., KOLTZENBURG M., MCMAHON S.B. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995;63:109–115. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- BALDELLI P., FORNI P.E., CARBONE E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur. J. Neurosci. 2000;12:4017–4032. doi: 10.1046/j.1460-9568.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid-A receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- BENNETT D.L., AVERILL S., CLARY D.O., PRIESTLEY J.V., MCMAHON S.B. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur. J. Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- BEVAN S., WINTER J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J. Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAND-WARD P.A., HUMPHREY P.P.A. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br. J. Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNINGTON J.K., MCNAUGHTON P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMANN J. The ‘ABC' of GABA receptors. Trends Pharmacol. Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- BURGARD E.C., NIFORATOS W., VAN BIESEN T., LYNCH K.J., TOUMA E., METZGER R.E., KOWALUK E.A., JARVIS M.F. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J. Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- CROWLEY C., SPENCER S.D., NISHIMURA M.C., CHEN K.S., PITTS-MEEK S., ARMANINI M.P., LING L.H., MCMAHON S.B., SHELTON D.L., LEVINSON A.D., PHILLIPS H.S. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- DAVIES P.A., KIRKNESS E.F., HALES T.G. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br. J. Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESCHENES M., FELTZ P., LAMOUR Y. A model for an estimate in vivo of the ionic basis of synaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976;118:486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- DI ANGELANTONIO S., NISTRI A. Calibration of agonist concentrations applied by pressure pulses or via rapid solution exchanger. J. Neurosci. Methods. 2001;110:155–161. doi: 10.1016/s0165-0270(01)00437-x. [DOI] [PubMed] [Google Scholar]
- DONNERER J., SCHULIGOI R., STEIN C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., ROCHELEAU T.A., STEICHEN J.C., CHALMERS A.E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature (Lond.) 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M.G. Potentials recorded from the spinal cord with microelectrodes. J. Physiol. 1955;130:625–654. doi: 10.1113/jphysiol.1955.sp005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÄHWILER B.H., BROWN D.A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc. Natl. Acad. Sci. U.S.A. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLAGHER J.P., HIGASHI H., NISHI S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J. Physiol. 1978;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINGRICH K.J., ROBERTS W.A., KASS R.S. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure–function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUBB B.D., EVANS R.J. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur. J. Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- GURLEY D., AMIN J., ROSS P.C., WEISS D.S., WHITE G. Point mutations in the M2 region of the alpha, beta, or gamma subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- HAAS K.F., MACDONALD R.L. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. (Lond.) 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON S.G., MCMAHON S.B. ATP as a peripheral mediator of pain. J. Auton. Nerv. Syst. 2000;81:187–194. doi: 10.1016/s0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- HEVERS W., LÜDDENS H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- HOLDEN J.H., CZAJKOWSKI C. Different residues in the GABA(A) receptor alpha 1T60-alpha 1K70 region mediate GABA and SR-95531 actions. J. Biol. Chem. 2002;277:18785–18792. doi: 10.1074/jbc.M111778200. [DOI] [PubMed] [Google Scholar]
- HOSLI L., HOSLI E. Autoradiographic studies on the cellular localization of GABA and beta-alanine uptake by neurones and glia in tissue culture. Adv. Exp. Med. Biol. 1979;123:205–218. doi: 10.1007/978-1-4899-5199-1_12. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA T., NAKANISHI O., FUNATSU N., KAMEYAMA H. Nerve growth factor inducer, 4-methyl catechol, potentiates central sensitization associated with acceleration of spinal glutamate release after mustard oil paw injection in rats. Cell. Mol. Neurobiol. 1999;19:587–596. doi: 10.1023/a:1006928317312. [DOI] [PubMed] [Google Scholar]
- JONES M.V., WESTBROOK G.L. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- KAPLAN D.R., MILLER F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- KOLTZENBURG M., BENNETT D.L., SHELTON D.L., MCMAHON S.B. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur. J. Neurosci. 1999;11:1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- LEVER I., CUNNINGHAM J., GRIST J., YIP P.K., MALCANGIO M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R. The nerve growth factor 35 years later. Science. 1964;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P.U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev. Biol. 1963;7:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., COHEN S. Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann. N. Y. Acad. Sci. 1960;85:324–341. doi: 10.1111/j.1749-6632.1960.tb49963.x. [DOI] [PubMed] [Google Scholar]
- LEWIN G.R., RITTER A.M., MENDELL L.M. On the role of nerve growth factor in the development of myelinated nociceptors. J. Neurosci. 1992;12:1896–1905. doi: 10.1523/JNEUROSCI.12-05-01896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H.S., ZHAO Z.Q. Small sensory neurons in the rat dorsal root ganglia express functional NK-1 tachykinin receptor. Eur. J. Neurosci. 1998;10:1292–1299. doi: 10.1046/j.1460-9568.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- MA W., SAUNDERS P.A., SOMOGYI R., POULTER M.O., BARKER J.L. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J. Comp. Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- MCMAHON S.B., BENNETT D.L., PRIESTLEY J.V., SHELTON D.L. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA–IgG fusion molecule. Nat. Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- MICHAEL G.J., AVERILL S., NITKUNAN A., RATTRAY M., BENNETT D.L., YAN Q., PRIESTLEY J.V. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS S.J., SMART T.G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- NEWBERRY N.R., NICOLL R.A. Comparison of the action of baclofen with γ-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J. Physiol. Lond. 1985;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. P2X3 receptors and peripheral pain mechanisms. J. Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTIS T.S., DE KONINCK Y., MODY I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J. Physiol. 1993;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL T.D., JACKMAN A., RICE F.L., KUCERA J., SNIDER W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- PEZET S., CUNNINGHAM J., PATEL J., GRIST J., GAVAZZI I., LEVER I.J., MALCANGIO M. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol. Cell. Neurosci. 2002;21:51–62. doi: 10.1006/mcne.2002.1166. [DOI] [PubMed] [Google Scholar]
- RAMER M.S., BRADBURY E.J., MCMAHON S.B. Nerve growth factor induces P2X(3) expression in sensory neurons. J. Neurochem. 2001;77:864–875. doi: 10.1046/j.1471-4159.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- RUDOMIN P., SCHMIDT R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- RUEFF A., MENDELL L.M. Nerve growth factor NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J. Neurophysiol. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- SCHELLER M., FORMAN S.A. Coupled and uncoupled gating and desensitization effects by pore domain mutations in GABA(A) receptors. J. Neurosci. 2002;22:8411–8421. doi: 10.1523/JNEUROSCI.22-19-08411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGEL E., BAUR R., KELLENBERGER S., MALHERBE P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992;11:2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIVILOTTI L., NISTRI A. GABA receptor mechanisms in the central nervous system. Prog. Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- SIVILOTTI L., WOOLF C. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J. Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- SMITH G.B., OLSEN R.W. Functional domains of GABAA receptors. Trends Pharmacol. Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- SOKOLOVA E., NISTRI A., GINIATULLIN R. Negative cross talk between anionic GABAA and cationic P2X ionotropic receptors of rat dorsal root ganglion neurons. J. Neurosci. 2001;21:4958–4968. doi: 10.1523/JNEUROSCI.21-14-04958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOLOVA E., SKORINKIN A., FABBRETTI E., MASTEN L., NISTRI A., GINIATULLIN R. Agonist-dependence of recovery from desensitization of P2X3 receptors provides a novel and sensitive approach for their rapid up or down regulation. Br. J. Pharmacol. 2004;141:1048–1058. doi: 10.1038/sj.bjp.0705701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUART G.J., REDMAN S.J. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J. Physiol. 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEDA M., HIROSE M., TAKEI N., IBUKI T., NARUSE Y., AMAYA F., IBATA Y., TANAKA M. Nerve growth factor induces systemic hyperalgesia after thoracic burn injury in the rat. Neurosci. Lett. 2002;328:97–100. [Google Scholar]
- VIDALTAMAYO R., SANCHEZ-SOTO M.C., HIRIART M. Nerve growth factor increases sodium channel expression in pancreatic beta cells: implications for insulin secretion. FASEB J. 2002;16:891–892. doi: 10.1096/fj.01-0934fje. [DOI] [PubMed] [Google Scholar]
- WETMORE C., OLSON L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J. Comp. Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- WINTER J., FORBES C.A., STERNBERG J., LINDSAY R.M. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J., SAFIEH-GARABEDIAN B., MA Q.P., CRILLY P., WINTER J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- WOOLTORTON J.R., MOSS S.J., SMART T.G. Pharmacological and physiological characterization of murine homomeric beta3 GABA(A) receptors. Eur. J. Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- XIA Y.X., IKEDA T., XIA X.Y., IKENOUE T. Differential neurotrophin levels in cerebrospinal fluid and their changes during development in newborn rat. Neurosci. Lett. 2000;280:220–222. doi: 10.1016/s0304-3940(00)00782-5. [DOI] [PubMed] [Google Scholar]