Abstract
The antioxidant properties of flavonols in vivo and their potential benefits in myocardial ischaemia/reperfusion (I/R) injury have been little investigated. We evaluated the ability of a synthetic flavonol, 3′,4′-dihydroxyflavonol (DiOHF) to scavenge superoxide in post-I/R myocardium and to prevent myocardial I/R injury.
Anaesthetized sheep were studied in four groups (n=5–6): control, ischaemic preconditioning (IPC), vehicle and DiOHF (before reperfusion, 5 mg kg−1, i.v.). The left anterior descending coronary artery was occluded distal to the second diagonal branch for 1 h followed by 2 h of reperfusion. Infarct size, myocardial function, NADPH-activated superoxide generation and biochemical markers of injury were measured.
DiOHF (10−8–10−4 M) incubated in vitro with post-I/R myocardium from the vehicle group suppressed superoxide production dose-dependently. DiOHF administered in vivo also significantly reduced superoxide generation in vitro.
DiOHF and IPC markedly reduced infarct size, which was 73±2% of the area at risk in vehicle, 50±4% in DiOHF, 75±5% in control and 44±4% in IPC. Post-I/R segment shortening within the ischaemic zone was greater in DiOHF (2.3±0.7%; P<0.01) and IPC (1.7±0.5%; P<0.01) than those in corresponding controls (−1.7±0.4; −2.1±0.4%).
DiOHF and IPC improved coronary blood flow to the ischaemic area and preserved higher levels of nitric oxide metabolites in the venous outflow from the ischaemic zone.
DiOHF attenuated superoxide production in post-I/R myocardium, and significantly reduced infarct size and injury following I/R in anaesthetized sheep. The extent of protection by DiOHF is comparable to that afforded by IPC. Thus, DiOHF has clinical potential for improving recovery from acute myocardial infarction and other ischaemic syndromes.
Keywords: Flavonoid, myocardial infarction, ischaemia, reperfusion, antioxidant, nitric oxide
Introduction
Reactive oxygen species (ROS) and other radicals derived from nitric oxide (NO) are generated in the heart after ischaemia/reperfusion (I/R). Indeed, an imbalance between the burst of ROS and endogenous antioxidant defences leads to oxidant stress, and ROS have a major role in the ensuing injury to the myocardium that limits myocardial salvage upon reperfusion (Lucchesi & Mullane, 1986; Dhalla et al., 2000; Ferdinandy & Schulz, 2003). Sources of ROS under these conditions include intracellular organelles (especially mitochondria), the invading neutrophils and macrophages that undergo a respiratory burst, and the coronary vascular endothelial cells and myocytes that are endowed with NADPH-oxidase (Lucchesi & Mullane, 1986; Bayraktutan et al., 2000; Bolli, 2001; Ferdinandy & Schulz, 2003). Various antioxidants have been used in attempts to improve recovery from myocardial I/R injury, but with mixed success (Ambrosio et al., 1986; Carrea et al., 1991; Petty et al., 1994; Dhalla et al., 2000). A particular difficulty is that exogenous superoxide dismutases cannot enter cells, where some of the damaging radical species originate. Antioxidants that are cell permeable may therefore be more useful for preventing I/R injury.
Flavonoids are a group of polyphenolic compounds synthesized by many plants. Epidemiological studies indicate that dietary flavonoids have beneficial effects against coronary heart disease (Renaud & De Lorgeril, 1992; Hertog et al., 1993), and many biological actions have been elucidated (Jiang & Dusting, 2003). Flavonoids have several properties that may potentially prevent myocardial I/R injury, such as vasodilator, antioxidant and anti-inflammatory actions (Pietta, 2000; Jiang & Dusting, 2003). An additional property of flavonoids that may contribute to cardioprotection is their high lipid solubility, which enables them to permeate biological membranes easily and scavenge intracellular ROS (Pietta, 2000). However, the antioxidant activity of flavonoids in vivo has been little studied, and their potential role in myocardial I/R injury has gained little attention.
Previous studies have established important structure–activity relationships for the antioxidant action of flavonoids. Pietta (2000) suggested that a catechol group in ring B and a 2,3-double bond conjugated with the 4-oxo group in ring C are the major determinants of radical-scavenging capability in flavonoids. Subsequent studies have shown that the presence of free hydroxyl groups at C3, C3′ and/or C4′ further enhances the antioxidant activity (Burda & Oleszek, 2001). The synthetic flavonol, 3′,4′-dihydroxyflavonol (DiOHF) has those structural characteristics, suggesting that it should be an effective antioxidant.
There has been surprisingly little investigation of the ability of flavonols to prevent myocardial ischaemic injury. Flavones and flavonols have been reported to prevent ischaemic injury in isolated hearts (Rump et al., 1994; Schussler et al., 1995; Liebgott et al., 2000; Lebeau et al., 2001; Brookes et al., 2002). However, in none of those studies were hearts perfused with blood thus excluding the role of leukocytes, important contributors to reperfusion injury. Furthermore, the treatment was usually applied before ischaemia (Liebgott et al., 2000; Lebeau et al., 2001; Brookes et al., 2002). In previous studies from our laboratory (Chan et al., 2003) we found that DiOHF was equally effective in reducing I/R injury in the rat hindquarters whether applied before ischaemia or just before reperfusion with blood. This indicates the potential for DiOHF to be used as an adjunct therapy to recanalization and not only as a preventative therapy. This study examines the ability of DiOHF to protect the myocardium in vivo when administered just before reperfusion.
The aim of this study was to test whether DiOHF has antioxidant action in the heart, and whether administration of DiOHF immediately before reperfusion reduces infarct size and injury due to myocardial I/R. To assess its efficacy, the antioxidant actions of DiOHF were investigated both in vitro and in vivo, and the cardioprotective effects of DiOHF were compared to ischaemic preconditioning (IPC), currently the most effective means known to prevent myocardial I/R injury. To evaluate indirectly the possible effect of DiOHF on regional NO, total plasma nitrate and nitrite (referred to as NOx) levels were determined in blood sampled from the coronary vein in the ischaemic zone.
Methods
All animals were treated humanely in compliance with the guidelines of the National Health and Medical Research Council of Australia, and the Animal Experimentation Ethics Committee at the Howard Florey Institute approved the present study. Sheep were chosen for this study as a large animal with coronary anatomy similar to that of humans. There are no native coronary collateral vessels and no collateral circulation develops after coronary occlusion in sheep heart (Maxwell et al., 1987; Markovitz et al., 1989). This is advantageous as the level of ischaemia is consistent in all experiments and no collateral vessels would confound the determination of infarct size.
Surgical procedures
Adult merino whethers (40–58 kg) were used in this study. Anaesthesia was induced by intravenous thiopentone sodium (15 mg kg−1) and following tracheal intubation was maintained by isoflurane (1.5–2%). A catheter was inserted into the right facial artery for arterial sampling and monitoring arterial blood pressure. Serial blood gas analysis was performed and ventilatory parameters were adjusted if necessary to keep the arterial blood gases within the physiological range.
The heart was exposed through a left thoracotomy performed at the fourth intercostal space and suspended in a pericardial cradle. The left anterior descending coronary artery (LAD) was dissected from the epicardium immediately distal to its second diagonal branch, and a transit-time flow probe (2 mm, Transonic System Inc., U.S.A.) was placed around it to monitor the LAD blood flow. A silk suture was passed under the LAD proximal to the probe and both ends of the silk were threaded through a plastic tube to form a vascular snare.
Part I. Effects of DiOHF on superoxide production and neutrophil accumulation
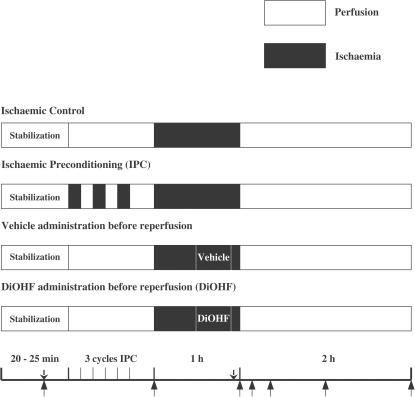
Following the surgical procedure, animals were allowed to stabilize for 15 min. The heart was then subjected to 1 h ischaemia and 2 h reperfusion with intravenous administration of vehicle or DiOHF (n=5–6). Ischaemia was obtained by tightening the snare, which was confirmed by a zero flow reading. DiOHF (Indofine Chemical Co., U.S.A.) dissolved in 4 ml dimethyl sulphoxide, then mixed with polyethylene glycol and water (1 : 1, total 40 ml) at a dose of 5 mg kg−1 was infused intravenously for 20 min (beginning after 30 min ischaemia and ending 10 min before reperfusion). This dose of DiOHF was estimated to achieve a plasma concentration of approximately 0.2 mM, which was similar to the concentration causing maximum reduction in superoxide levels generated by the myocardium in vitro (see below). The same volume of vehicle was administered over the same period. To reduce the incidence of ventricular arrhythmias, lidocaine (2 mg kg−1) was given as a bolus injection 10 min before ischaemia or IPC and 5 min before reperfusion. After prolonged I/R, tissues were collected from both the normal and ischaemic zones in the left ventricle to determine superoxide levels and neutrophil accumulation in coronary microvessels (Figure 1).
Figure 1.
Diagram of experimental protocol: ↑ indicates the time points for blood sampling; ↓ indicates the time points for lidocaine infusion. DiOHF or vehicle was infused intravenously for 20 min commencing 30 min after the onset of ischaemia and continuing until 10 min before reperfusion.
Superoxide production in postischaemic myocardium
To evaluate the antioxidant action of DiOHF in vitro, samples taken from the ischaemic zone in vehicle sheep were incubated with serial concentrations of DiOHF (10−4–10−8 M). To assess the free radical scavenging effect of DiOHF in vivo, superoxide levels were determined in myocardial tissues taken from both normally perfused and ischaemic areas in vehicle and DiOHF groups after prolonged I/R.
NADPH-activated superoxide generation in postischaemic myocardium was assayed by lucigenin-enhanced chemiluminescence using a method developed in our laboratory (Paravicini et al., 2002). Briefly, blood was washed from the harvested hearts with ice-cold HEPES-buffered Krebs solution (KHB); myocardium from the left ventricle was sliced into 2 × 2 mm segments and immersed in ice-cold KHB. All tissues were preincubated for 45 min before superoxide measurement. To prepare the preincubation solutions for in vitro study, DiOHF dissolved in dimethyl sulphoxide (stock solution 10−2 M) was diluted to the appropriate final concentrations with KHB to which NADPH (10 μM) and diethyldithiocarbamic acid trihydrate (DETCA, 3 mM) had been added. The latter was used to inactivate endogenous superoxide dismutase and maximize the detection of superoxide. Control preincubation solution contained 1% of dimethyl sulphoxide and the same concentrations of NADPH and DETCA. For the superoxide assay, after in vivo application of DiOHF or vehicle, tissues were preincubated in KHB with NADPH (10 μM) and DETCA (3 mM) only. To count background without tissues, an OptiPlate microplate (Packard Bioscience, Australia) containing all the above preincubation solutions (except DETCA), with the addition of 5 μM lucigenin, was inserted into the scintillation counter (Topcount, Packard Instrument Co., U.S.A.). Immediately after preincubation, one piece of myocardial tissue was added to each well in the lucigenin microplate, the assay was run again and the respective background was subtracted. All tissues were dried in a 65°C oven for 2–4 days and the results are expressed as photon emissions S−1 mg−1 of dry tissue weight.
Neutrophil accumulation in the coronary microcirculation
Neutrophil accumulation in microvessels was examined by modification of a previously described method (Ambrosio et al., 1989). Collected myocardial tissues were fixed in 4% buffered formalin and embedded in paraffin. Transverse sections (10 μM) were stained with haematoxylin–eosin and analysed under a light microscope at 400-fold magnification. A total of 100 capillaries were randomly selected in each section, and the vessels containing at least one neutrophil were determined. The neutrophils were counted only when the nucleus was clearly within a capillary lumen and distinguishable from the endothelial cells. Three different tissue sections were counted in each myocardial sample. The results were expressed as the percentage of the total 300 vessels.
Part II. Effects of DiOHF and IPC on myocardial I/R injury
As described above, a flow probe and vascular snare were placed around the LAD after exposure of the heart. In addition, in this series of experiments, a 4F catheter-tipped manometer (Mikro-tip, Millar Instruments, U.S.A.) was inserted through the left atrium into the left ventricle to measure left ventricular pressure. Another catheter was inserted into the left internal jugular vein then advanced into the great coronary vein, as close as possible to the LAD occlusion site, to collect blood samples for analysis of NOx. To assess regional myocardial contractility, one pair of ultrasonic crystals (Sonometrics Corporation, Canada) was embedded in the subendocardial layer within the ischaemic zone, and another pair was situated in a remote, control area. The percentage of myocardial regional segment shortening, defined as ((end diastolic regional length − end systolic regional length)/end diastolic regional length) × 100% (Lange et al., 1984), was calculated from the continuous recording of segment length in both regions.
Sheep were randomly assigned into four groups (Figure 1, n=5–6): control, IPC, vehicle and DiOHF. Control sheep experienced 1 h ischaemia and 2 h reperfusion without treatment. Two cycles of 5 min ischaemia separated by 5 min reperfusion and one cycle of 5 min ischaemia followed by 10 min reperfusion were applied in the IPC group. DiOHF and vehicle groups used the same protocols as described above (Part I). Lidocaine (2 mg kg−1) was given to reduce ventricular arrhythmias. To reperfuse the myocardium, the snare was loosened, and during the first 15 min of reperfusion, the artery was partly occluded to restrict LAD flow to less than 15 ml min−1 in all groups. This procedure limited reperfusion hyperoemia, thus reducing the severity of reperfusion arrhythmias and the incidence of haemorrhagic myocardial infarction (Lucchesi et al., 1976). Myocardial infarct size was determined as described below at the end of experiment.
Digital data of LAD blood flow, segment shortening and haemodynamic indices were recorded on a computer equipped with a data acquisition program (SonoView 3.1.4, Sonometrics Corporation, Canada). Pressure transducers were calibrated before each experiment and data were collected under steady-state haemodynamic conditions. Mean arterial pressure (MAP) was calculated from the blood pressure recording. Heart rate, left ventricular systolic pressure, left ventricular end-diastolic pressure and the maximal positive value of the first derivative of left ventricular pressure were analysed from the left ventricular pressure recording.
Determination of the area at risk and infarct size
The area of myocardium at risk and infarct size were delineated by Evan's blue and triphenyltetrazolium chloride (TTC) staining as previously described (Loke & Woodman, 1998). After prolonged I/R, the LAD was reoccluded at the original occlusion site and cannulated distal to the occlusion, the left atrium was also cannulated. Immediately after intravenous injection of pentobarbitone (100 mg kg−1; Virbac, Australia) to arrest the heart, Evan's blue dye (1.5%, 40 ml, Sigma, Australia) and heparinized saline were simultaneously injected into the atrium and LAD respectively to define the myocardium at risk. The heart was rapidly removed and the left ventricle was sliced into transverse sections about 1 cm in width. The unstained risk area was traced onto transparencies. The sections were then incubated in 0.1 M sodium phosphate buffer containing 1% TTC (Sigma, Australia) for 20 min (37°C, pH 7.4). The viable area and infarcted area were traced onto transparencies. The area of myocardium at risk and infarct size were measured by computerized planimetry (MCID-M 2, Imaging Research Inc., Canada). The former was expressed as a percentage of total left ventricular volume and infarct size was expressed as a percentage of the area of myocardium at risk.
Biochemical assays
Arterial blood samples were withdrawn at baseline and at the end of reperfusion and collected in heparinized tubes to determine plasma levels of lactate dehydrogenase and creatine kinase using a CX5 Beckman Synchron Clinical System.
Blood samples collected throughout the study from the great coronary vein close to the LAD occlusion site were used to assay NOx levels in the venous outflow of the ischaemic zone. The method for NOx analysis has been described in detail (Giovannoni et al., 1997). Plasma was separated and samples were deproteinized by ultrafiltration, then a nitrate reductase enzyme system was applied to reduce nitrate to nitrite. Griess reagent was finally added to the mixture and measured at 550 nm. Duplicate assays were carried out and total NOx was expressed in μM.
Statistical analysis
All values are expressed as group mean±s.e.m. Data analysis was performed with SigmaStat (version 2.03). Differences in infarct size and superoxide levels were determined by one-way ANOVA. All other data were evaluated by one-way ANOVA with repeated measures followed, if appropriate, by Bonferroni's test for multiple comparisons. Differences were considered to be significant when P<0.05.
Results
Animal exclusion
Of 51 sheep (14 for part I and 37 for part II) used in this study, five sheep were excluded because of damage to the LAD during dissection, one was killed humanely because of diffuse pericardial adhesion and one was discarded due to poor signal quality. Three of eight sheep in the control group (n=5) and six of 18 sheep (three of nine in Part I and II) in the vehicle group (n=6) died due to irreversible ventricular fibrillation during prolonged ischaemia or early reperfusion. However, only one of seven sheep died in the IPC group (n=6) and none of 11 sheep died in the DiOHF group (n=5 in Part I and n=6 in Part II). Data from the remaining 34 sheep were analysed (n=5–6 per group).
Part I. Effects of DiOHF on superoxide production and neutrophil accumulation
Myocardial superoxide production
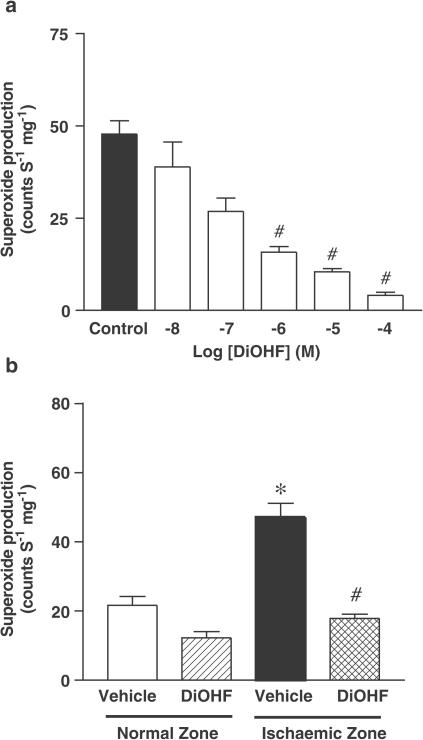
The level of NADPH-activated superoxide generated by the post-I/R myocardium (47.1±4.1 counts S−1 mg−1, P<0.01) was significantly greater in the vehicle group as compared to normally perfused myocardium (21.6±2.6 counts S−1 mg−1, Figure 2b). In vitro incubation of DiOHF with myocardial tissues taken from the ischaemic zone in the vehicle group reduced superoxide production in a concentration-dependent manner (Figure 2a). In addition, DiOHF administered in vivo before reperfusion significantly decreased the superoxide level detected in the post-I/R myocardium (17.8±1.2 versus 47.1±4.1 counts S−1 mg−1 in vehicle, P<0.01, Figure 2b).
Figure 2.
Effect of DiOHF in vitro (a) and in vivo (b) on superoxide generation in postischaemic myocardium. In Figure 2a, ‘Control' represents myocardial tissues preincubated with the solution containing 1% dimethyl sulphoxide, without DiOHF but with the other reagents included (see details of incubation in Methods). The number of myocardial samples is n=6, #P<0.01 versus Control. In Figure 2b, n=6 in vehicle, n=5 in DiOHF. *P<0.01 versus vehicle (normal zone); #P<0.01 versus vehicle (ischaemic zone). All data are expressed as mean±s.e.m.
Microvascular neutrophil accumulation
In the vehicle group, there was an almost 20-fold increase in the number of capillaries containing accumulated neutrophils in the post-I/R myocardium (23.7±1.0%) in comparison to normally perfused myocardium (1.3±0.1%, P<0.01). Administration of DiOHF significantly reduced neutrophil accumulation in coronary microvessels after I/R (8.4±0.8%, P<0.01 versus vehicle).
Part II. Effects of DiOHF and IPC on myocardial I/R injury
Haemodynamic indices
Haemodynamic variables are summarized in Table 1 . Regional myocardial I/R had no significant effect on MAP, left ventricular systolic pressure and the maximal positive value of the first derivative of left ventricular pressure. Left ventricular end-diastolic pressure decreased shortly after IPC, but increased in all groups after the onset of ischaemia and reached its maximum 15 min later, then remained at this high level throughout the ischaemic period. During reperfusion, left ventricular end-diastolic pressure declined gradually but there was a significantly greater reduction in the DiOHF and IPC groups in comparison to their corresponding controls. Heart rate was unchanged during ischaemia but increased following reperfusion. By the end of reperfusion, the increase in heart rate in the DiOHF and IPC groups was significantly less than that in the vehicle and control groups.
Table 1.
Haemodynamic data
| Variables | Baseline | Post-IPC | 5 min Isc | 15 min Isc | 55 min Isc | 15 min Rep | 1 h Rep | 2 h Rep |
|---|---|---|---|---|---|---|---|---|
| HR (bpm) | ||||||||
| Control | 75±1.4 | 76±0.8 | 75±0.7 | 76±1.2 | 76±1.3 | 93±2.6 | 103±3.3 | 104±2.3 |
| IPC | 78±2.0 | 80±2.0 | 79±1.5 | 79±2.2 | 78±2.7 | 80±3.6* | 84±3.2‡ | 86±3.5‡ |
| Vehicle | 74±1.5 | 73±0.9 | 72±1.2 | 72±1.9 | 73±1.8 | 87±3.7 | 102±4.8 | 99±3.4 |
| DiOHF | 75±1.8 | 79±1.4 | 73±1.6 | 73±1.8 | 73±0.8 | 75±1.5 | 79±1.6† | 81±0.8† |
| MAP (mmHg) | ||||||||
| Control | 67±2.8 | 66±3.0 | 66±2.5 | 66±1.5 | 65±2.3 | 66±1.8 | 65±1.1 | 65±1.4 |
| IPC | 69±5.2 | 68±5.5 | 70±8.4 | 68±6.0 | 65±5.8 | 65±6.9 | 64±5.8 | 64±4.5 |
| Vehicle | 67±4.9 | 72±6.6 | 64±4.6 | 66±2.1 | 66±4.0 | 64±4.1 | 64±3.9 | 62±2.2 |
| DiOHF | 65±3.3 | 67±3.9 | 65±5.1 | 65±6.2 | 64±4.8 | 65±3.0 | 62±3.1 | 62±3.7 |
| LVSP (mmHg) | ||||||||
| Control | 74±3.0 | 76±3.7 | 72±2.6 | 75±1.7 | 74±4.2 | 74±3.9 | 73±3.7 | 72±3.8 |
| IPC | 72±4.5 | 76±2.4 | 77±5.7 | 74±2.3 | 73±4.3 | 70±3.1 | 69±5.3 | 69±4.7 |
| Vehicle | 75±2.8 | 73±4.1 | 72±2.5 | 75±4.3 | 77±3.5 | 75±4.8 | 75±3.4 | 75±4.6 |
| DiOHF | 76±5.5 | 74±3.4 | 76±3.9 | 76±5.0 | 77±6.7 | 75±3.1 | 74±2.5 | 72±4.2 |
| LVEDP (mmHg) | ||||||||
| Control | 5.2±0.5 | 5.3±0.4 | 7.4±0.6 | 11.4±1.2 | 11±1.0 | 11.6±0.8 | 11.4±0.7 | 10.9±0.7 |
| IPC | 5.9±0.6 | 4.0±0.4* | 6.1±0.4 | 9.1±0.6 | 10.3±0.7 | 10.8±0.8 | 9.2±0.4‡ | 8.5±0.5‡ |
| Vehicle | 5.6±0. 7 | 5.8±0.6 | 7.2±0.5 | 11.9±0.9 | 11.2±0.6 | 12.2±1.0 | 11.3±0.7 | 10.3±0.9 |
| DiOHF | 5.4±0.6 | 5.5±0.7 | 7.6±0.5 | 11.4±0.7 | 11.7±0.8 | 11.5±0.8 | 9.1±0.6† | 8.2±0.5† |
| dP/dtmax (mmHg S −1) | ||||||||
| Control | 1437±58 | 1429±75 | 1392±43 | 1408±56 | 1445±55 | 1415±26 | 1423±49 | 1405±24 |
| IPC | 1466±91 | 1489±82 | 1338±64 | 1461±98 | 1512±74 | 1464±107 | 1462±83 | 1464±75 |
| Vehicle | 1529±80 | 1506±66 | 1412±77 | 1556±95 | 1500±96 | 1489±86 | 1441±63 | 1487±40 |
| DiOHF | 1473±62 | 1563±88 | 1437±51 | 1524±45 | 1555±69 | 1498±58 | 1523±87 | 1496±55 |
Post-IPC=the time point of the end of IPC or sham treatment; Isc=ischaemia; Rep=reperfusion. Data are mean±s.e.m., n=5 in control, n=6 in the left groups.
P<0.05 versus control,
P<0.01 versus control;
P<0.01 versus vehicle.
Myocardial regional function
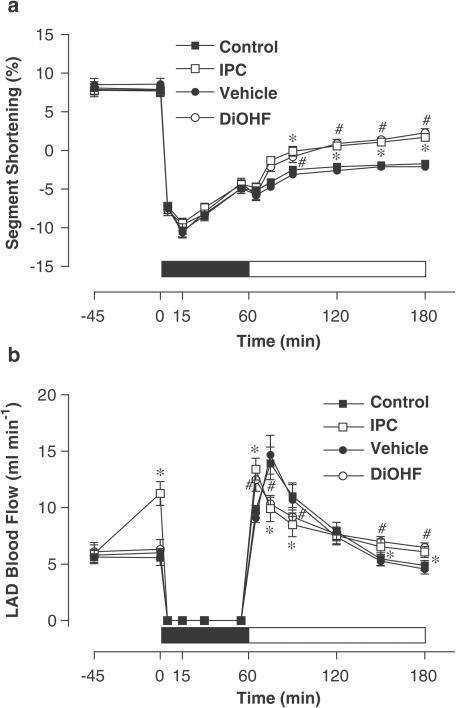
Baseline levels of segment shortening were similar in all groups. Ischaemia induced a rapid and marked systolic bulging evidenced by a negative segment shortening in the ischaemic zone, which was maximal after 15 min of ischaemia. Subsequently, there was incomplete recovery of systolic function over the remaining experimental period in all groups. However, segment shortening significantly improved after 2 h of reperfusion in the DiOHF and IPC groups in comparison to that in their corresponding controls (Figure 3a).
Figure 3.
Effect of I/R on regional myocardial contractility (a) and LAD blood flow (b) in the ischaemic zone among groups at different time points. The solid bar indicates the period of ischaemia and the open bar represents reperfusion. Results are illustrated as mean±s.e.m., n=5 in control, n=6 in vehicle, IPC and DiOHF groups. *P<0.01 versus control; #P<0.01 versus vehicle.
LAD blood flow
IPC increased LAD blood flow transiently (11.4±1.0. versus 5.7±0.7 ml min−1 in control, P<0.01). During the early stage of reperfusion, coronary hyperperfusion occurred in all sheep. However, the time-dependent regression of LAD blood flow was more rapid in the control and vehicle groups, and more than 30% of LAD blood flow augmentation was achieved at the end of reperfusion in the DiOHF and IPC groups as compared to vehicle and control groups (Figure 3b).
Myocardial infarct size
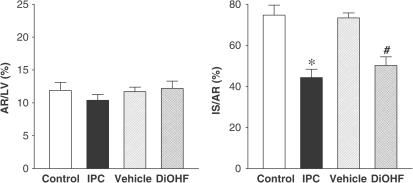
The mass of myocardium at risk, the major determinant of infarct size, was similar in all groups after prolonged ischaemia and reperfusion (Figure 4). DiOHF (50±4%; P<0.01). and IPC (44±4%; P<0.01) caused a marked reduction of infarct size (as a percentage of the myocardium at risk) in comparison to vehicle (73±2%) and control (75±5%).
Figure 4.
Effect of DiOHF and IPC on infarct size. LV indicates the volume of left ventricle. Area at risk (AR) is expressed as the percentage of LV and infarct size (IS) is expressed as a percentage of AR. Results are presented as mean±s.e.m. *P<0.01 versus control; #P<0.01 versus vehicle, n=5 in control, n=6 in vehicle, IPC and DiOHF groups.
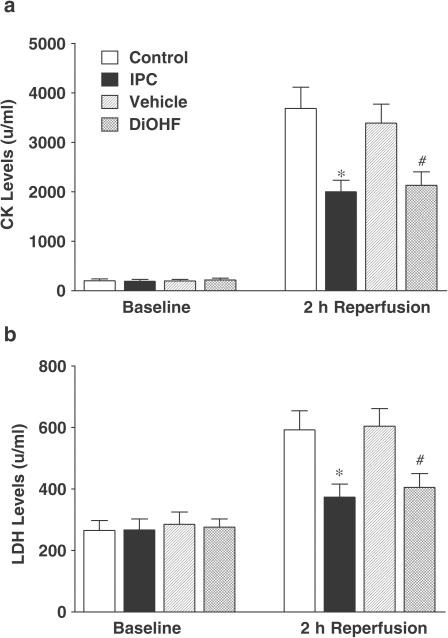
Plasma lactate dehydrogenase and creatine kinase activity
The baseline levels of lactate dehydrogenase and creatine kinase were similar among all groups (Figure 5). In the vehicle and control groups, prolonged I/R significantly increased lactate dehydrogenase (604±58, 593±61 U, respectively) and creatine kinase (3390±381, 3684±426 U, respectively) activities. However, in the DiOHF and IPC groups, the levels of lactate dehydrogenase (405±45, 373±43 U, respectively; P<0.01) and creatine kinase (2131±273, 1998±234 U, respectively; P<0.01) were significantly lower in comparison to their appropriate controls.
Figure 5.
Creatine kinase and lactate dehydrogenase levels before ischaemia and after reperfusion. Baseline readings were made after 10–15 min stabilization following surgery and reperfusion readings were obtained 2 h after reperfusion. Data are expressed as mean±s.e.m. *P<0.05 versus control; #P<0.05 versus vehicle, n=5 in control, n=6 in vehicle, IPC and DiOHF groups.
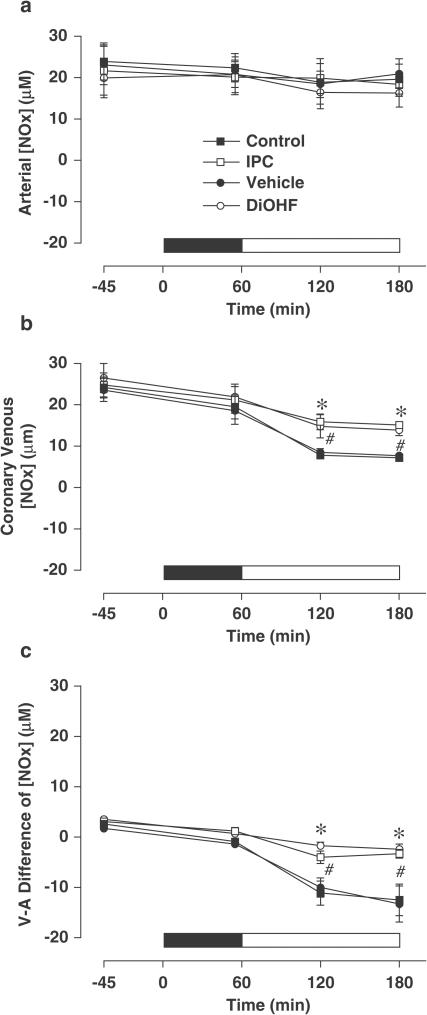
Plasma NOx levels
Arterial levels of plasma NOx did not change significantly throughout the study (Figure 6a). Coronary venous levels of NOx were comparable among all groups up to the end of the ischaemic period, but declined significantly upon reperfusion (Figure 6b). Venous-arterial NOx difference, which before ischaemia did not differ between groups, declined to negative values in all groups after reperfusion, indicating net extraction of NOx across the ischaemic zone (Figure 6c). Importantly, plasma NOx extraction increased much less (and venous NOx level declined much less) in the DiOHF and IPC groups than in vehicle and control groups. Thus, by the end of reperfusion, coronary venous NOx levels in DiOHF and IPC groups (13.9±0.7 and 15.1±0.8 μM, respectively; P<0.01) were significantly higher than those in the vehicle and control groups (7.7±0.7 and 7.2±0.6 μM, respectively).
Figure 6.
Plasma levels of nitrite and nitrate (NOx) before and during ischaemia and after reperfusion in arterial and coronary venous samples and the calculated venous−arterial (V−A) difference. The solid bar indicates the period of ischaemia and the open bar represents reperfusion. All values are mean±s.e.m. *P<0.01 versus control; #P<0.01 versus vehicle, n=5 in control, n=6 in vehicle, IPC and DiOHF groups.
Discussion
The main finding of this study is that DiOHF, a synthetic flavonol with a structure predicted to enhance antioxidant activity over that of natural flavonols, significantly reduced infarct size and the injury associated with myocardial I/R in anaesthetized sheep. This protection was evident when DiOHF was administered during ischaemia, immediately before reperfusion. These beneficial actions were achieved at least partly through antioxidant activity, for DiOHF reduced superoxide generated during the early stages of reperfusion, and it may prevent NO inactivation by superoxide and thus the formation of peroxynitrite. Reduced superoxide and better maintenance of NO during reperfusion were also accompanied by improved restoration of myocardial perfusion within the ischaemic zone after I/R injury. The extent of protection was comparable to that afforded by IPC, currently the most effective method to prevent myocardial I/R injury.
Although flavonoids have long been recognized to possess antioxidant properties (Pietta, 2000; Burda & Oleszek, 2001; Chan et al., 2003; Jiang & Dusting, 2003), most studies have focused on their beneficial effects in the prevention of atherosclerosis and coronary heart disease and little attention has been paid to the potential therapeutic benefit in myocardial I/R injury. In the present study, in vitro incubation with DiOHF markedly reduced NADPH-activated superoxide production, in a concentration-dependent manner, in myocardial tissue from the ischaemic zone in the vehicle treated sheep after reperfusion. DiOHF administered in vivo also significantly decreased superoxide generation in vitro in postischaemic myocardium, as compared to vehicle. These data indicate that DiOHF is an effective suppressor of superoxide production both in vitro and in vivo in the myocardium after I/R injury. Flavonoids, such as DiOHF, are capable of scavenging ROS directly (as demonstrated in this study) by donating hydrogen atoms to free radicals (Pietta, 2000). Flavonoids (over a concentration range of 0.1–1 mM) have also been shown to inhibit several enzyme systems responsible for superoxide production, including xanthine oxidase, NADPH-oxidase and lipoxygenase (de Groot & Rauen, 1998) and the estimated plasma concentration of DiOHF in this study (0.2 mM) falls within that range. In addition, DiOHF is highly lipid soluble and being a small molecule, may enter myocytes easily. Thus, DiOHF has potential to prevent myocardial I/R injury. Indeed, DiOHF administered shortly before reperfusion markedly reduced infarct size and I/R injury in terms of animal mortality, tachycardia, myocardial enzyme release and regional myocardial contractility in our study. Importantly, the level of protection achieved by DiOHF was similar to that afforded by IPC. Thus, DiOHF could be a useful adjunct therapy in the treatment of acute myocardial infarction, particularly when this compound is administered shortly before blocked coronary arteries are reopened by thrombolysis, coronary angioplasty or bypass surgery.
In this study, DiOHF reduced infarct size by approximately 40% after 1 h ischaemia and 2 h reperfusion, an effect similar to that achieved by IPC. It is well recognized that the collateral circulation is an important determinant of infarct size (Maxwell et al., 1987). As ovine hearts are known to lack coronary collateral vessels, even after coronary occlusion (Markovitz et al., 1989) we limited the size of the area at risk. However, a large proportion (>70%) of the area at risk was infarcted in the untreated groups. This is in contrast to other species with larger risk area but more abundant coronary collateral circulation where infarction occurs in only 30–50% of the area at risk (Auchampach et al., 1991; Deodato et al., 1999; Jones et al., 1999). Thus, it is unlikely that the relatively small area at risk would confound our assessment of infarct size. Furthermore, other measurements, including animal mortality and the biochemical markers, also consistently supported the cardioprotective and infarct limiting effect of DiOHF.
In our experiments, during reperfusion in all groups we observed a marked decrease of coronary venous NOx levels draining the ischaemic zone, and the cardiac tissue changed from being a net producer of NOx to extracting NOx. Very similar changes in plasma NOx have been observed across the whole heart with the development of chronic heart failure in dogs (Recchia et al., 1999). The metabolism of NO, nitrite and nitrate in the ischaemic heart is complex (Zweier et al., 1999), and it is likely that the micromolar levels of NOx detected in plasma reflect high tissue levels largely derived from the diet. It is difficult to ascertain the proportion of NOx produced enzymatically from NO synthases, but it is likely to be very small (Ishibashi et al., 1999; Zweier et al., 1999), and we have not attempted to do so. Indeed, Recchia et al. (1999) attributed the change in plasma NOx in heart failure to redistribution of nitrate from plasma into erythrocytes. The exchange of nitrate (for chloride ion) from plasma to erythrocytes is enhanced with reduced PO2, and so would increase in ischaemic tissue, and conversely be relatively attenuated in a reduced ischaemic zone (Recchia et al., 1999). Clearly, our finding that at the end of reperfusion, venous NOx was much higher in the DiOHF and IPC groups than it was in the vehicle and control groups is consistent with this hypothesis. It is likely that because the infarct size is smaller in the treated groups, the venous plasma NOx levels are maintained higher than in the other groups, and this can be seen as another reflection of increased myocardial salvage. What is crucial for myocardial salvage during reperfusion is the relative levels of free NO and peroxynitrite produced (Ferdinandy & Schulz, 2003), and it could be inferred that in the DiOHF group the latter would be much reduced because superoxide is removed. However, this is unlikely to make a major contribution to the plasma NOx levels. Therefore, to summarize, three mechanisms could contribute to the myocardial protection by DiOHF. First, reduction in the levels of superoxide and other ROS could be expected to reduce the direct myocardial damage induced by these radical species. Second, NO bioavailability might be increased, partly by increased conversion of myocardial nitrite to NO under the acidic and reducing conditions in the ischaemic myocardium (Zweier et al., 1999) and NO may be cardioprotective in its own right (Wang et al., 2002; Ferdinandy & Schulz, 2003). Third, and perhaps most important, NO conversion to the cytotoxic peroxynitrite derivatives would be limited by removal of the superoxide reactant in this setting (Cheung et al., 2000).
One mechanism whereby elevated NO could assist the recovery of myocardium is by reducing the ‘no-reflow' phenomenon. It is well accepted that after myocardial I/R injury, ‘no reflow' may exacerbate irreversible tissue injury and reduced filling of the microvascular network causes infarct expansion (Ambrosio & Tritto, 1999). In this study, administration of DiOHF significantly improved LAD blood flow by approximately 50% in comparison to vehicle, and this was accompanied by better recovery of myocardial contractility and a smaller infarct size. Although regional coronary flow at the tissue level was not measured in this study, DiOHF significantly decreased neutrophil accumulation in the coronary microcirculation. Neutrophil plugging, which may mechanically obstruct microvessels, is regarded as the principal cause of the no-reflow phenomenon (Ambrosio & Tritto, 1999). Therefore, reduction of this pathophysiological condition during postischaemic reperfusion may also contribute to the protection afforded by DiOHF. Indeed, previous studies have reported that both flavonoids and NO prevent expression of adhesion molecules and inhibit neutrophil activation as well as inhibiting platelet adhesion and aggregation (de Groot & Rauen, 1998; Gewaltig & Kojda, 2002), all of which may facilitate the relief of the no-reflow phenomenon. Further studies are required to assess whether DiOHF promotes microvascular reperfusion in the ischaemic zone.
In conclusion, we have demonstrated that DiOHF reduces superoxide production both in vitro and in vivo in postischaemic myocardium. DiOHF administered during ischaemia, shortly before reperfusion, significantly reduced myocardial I/R injury and provided a level of protection similar to that afforded by IPC. DiOHF enhanced NOx levels in venous blood collected from the ischaemic zone, improved the restoration of coronary blood flow and reduced neutrophil accumulation in the coronary microcirculation within the postischaemic myocardium. The beneficial effect of DiOHF found in this study indicates that it has potential as an adjunctive therapeutic agent for reperfusion therapy in the management of acute myocardial infarction.
Acknowledgments
We are indebted to Alan McDonald for his assistance with surgery, to Dr Grant Drummond for instruction regarding the lucigenin assay, to Angela Gibson for biochemical analysis and to Dr Qian Sang for histological study. This study was supported by a block grant from National Health and Medical Research Council of Australia (NHMRC) to the Howard Florey Institute and a NHMRC project grant to Dr Owen L. Woodman.
Abbreviations
- DETCA
diethyldithiocarbamic acid trihydrate
- DiOHF
3′,4′-dihydroxyflavonol
- IPC
ischaemic preconditioning
- I/R
ischaemia/reperfusion
- KHB
HEPES-buffered Krebs solution
- LAD
the left anterior descending coronary artery
- NOx
total plasma nitrate and nitrite
- ROS
reactive oxygen species
- TTC
triphenyltetrazolium chloride
References
- AMBROSIO G., BECKER L.C., HUTCHINS G.M., WEISMAN H.F., WEISFELDT M.L. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986;74:1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- AMBROSIO G., TRITTO I. Reperfusion injury: experimental evidence and clinical implications. Am. Heart. J. 1999;138:S69–S75. doi: 10.1016/s0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- AMBROSIO G., WEISMAN H.F., MANNISI J.A., BECKER L.C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989;80:1846–1861. doi: 10.1161/01.cir.80.6.1846. [DOI] [PubMed] [Google Scholar]
- AUCHAMPACH J.A., MARUYAMA M., CAVERO I., GROSS D.J. The new K+ channel opener Aprikalim (RP 52891) reduces experimental infarct size in dogs in the absence of hemodynamic changes. J. Pharmacol. Exp. Ther. 1991;259:961–967. [PubMed] [Google Scholar]
- BAYRAKTUTAN U., BLAYNEY L., SHAH A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- BOLLI R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J. Mol. Cell. Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- BROOKES P.S., DIGERNESS S.B., PARKS D.A., DARLEY-USMAR V. Mitochondrial function in response to cardiac ischaemia–reperfusion after oral treatment with quercetin. Free. Radical Biol. Med. 2002;32:1220–1228. doi: 10.1016/s0891-5849(02)00839-0. [DOI] [PubMed] [Google Scholar]
- BURDA S., OLESZEK W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- CARREA F.P., LESNEFSKY E.J., REPINE J.E., SHIKES R.H., HORWITZ L.D. Reduction of canine myocardial infarct size by a diffusible reactive oxygen metabolite scavenger. Efficacy of dimethylthiourea given at the onset of reperfusion. Circ. Res. 1991;68:1652–1659. doi: 10.1161/01.res.68.6.1652. [DOI] [PubMed] [Google Scholar]
- CHAN E.C., DRUMMOND G.R., WOODMAN O.L. 3′,4′-dihydroxyflavonol enhances nitric oxide bioavailability and improves vascular function after ischemia and reperfusion injury in the rat. J. Cardiovasc. Pharmacol. 2003;42:727–735. doi: 10.1097/00005344-200312000-00006. [DOI] [PubMed] [Google Scholar]
- CHEUNG P.Y., WANG W., SCHULZ R. Glutathione protects against myocardial ischemia–reperfusion injury by detoxifying peroxynitrite. J. Mol. Cell Cardiol. 2000;32:1669–1678. doi: 10.1006/jmcc.2000.1203. [DOI] [PubMed] [Google Scholar]
- DE GROOT H., RAUEN U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam. Clin. Pharmacol. 1998;12:249–255. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- DEODATO B., ALTAVILLA D., SQUADRITO G., CAMPO G.M., ARLOTTA M., MINUTOLI L., SAITTA A., CUCINOTTA D., CALAPAI G., CAPUTI A.P., MIANO M., SQUADRITO F. Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia–reperfusion injury. Br. J. Pharmacol. 1999;128:1683–1690. doi: 10.1038/sj.bjp.0702973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHALLA N.S., ELMOSELHI A.B., HATA T., MAKINO N. Status of myocardial antioxidants in ischemia–reperfusion injury. Cardiovasc. Res. 2000;47:446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEWALTIG M.T., KOJDA G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc. Res. 2002;55:250–260. doi: 10.1016/s0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- GIOVANNONI G., LAND J.M., KEIR G., THOMPSON E.J., HEALES S.J.Adaptation of the nitrate reductase and Griess reaction methods for the measurement of serum nitrate plus nitrite levels Ann. Clin. Biochem. 199734193–198.Part 2 [DOI] [PubMed] [Google Scholar]
- HERTOG M.G., FESKENS E.J., HOLLMAN P.C., KATAN M.B., KROMHOUT D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- ISHIBASHI T., YOSHIDA J., NISHIO M. Evaluation of NOx in the cardiovascular system: relationship to NO-related compounds in vivo. Jpn. J. Pharmacol. 1999;81:317–323. doi: 10.1254/jjp.81.317. [DOI] [PubMed] [Google Scholar]
- JIANG F., DUSTING G.J. Natural phenolic compounds as cardiovascular therapeutics: potential role of their anti-inflammatory effects. Curr. Cardiovasc. Pharmacol. 2003;1:135–156. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- JONES S.P., GIROD W.G., PALAZZO A.J., GRANGER D.N., GRISHAM M.B., HEUIL D.J., HUANG P.L., LEFER D.J. Myocardial ischemia–reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 1999;276:H1567–H1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- LANGE R., WARE J., KLONER R.A. Absence of a cumulative deterioration of regional function during three repeated 5 or 15 minute coronary occlusions. Circulation. 1984;69:400–408. doi: 10.1161/01.cir.69.2.400. [DOI] [PubMed] [Google Scholar]
- LEBEAU J., NEVIERE R., COTELLE N. Beneficial effects of different flavonoids, on functional recovery after ischemia and reperfusion in isolated rat heart. Bioorg. Med. Chem. Lett. 2001;11:23–27. doi: 10.1016/s0960-894x(00)00589-8. [DOI] [PubMed] [Google Scholar]
- LIEBGOTT T., MIOLLAN M., BERCHADSKY Y., DRIEU K., CULCASI M., PIETRI S. Complementary cardioprotective effects of flavonoid metabolites and terpenoid constituents of Ginkgo biloba extract (EGb 761) during ischemia and reperfusion. Basic Res. Cardiol. 2000;95:368–377. doi: 10.1007/s003950070035. [DOI] [PubMed] [Google Scholar]
- LOKE K.E., WOODMAN O.L. Preconditioning improves myocardial function and reflow, but not vasodilator reactivity, after ischaemia and reperfusion in anaesthetized dogs. Clin. Exp. Pharmacol. Physiol. 1998;25:552–558. doi: 10.1111/j.1440-1681.1998.tb02250.x. [DOI] [PubMed] [Google Scholar]
- LUCCHESI B.R., BURMEISTER W.E., LOMAS T.E., ABRAMS G.D. Ischemic changes in the canine heart as affected by the dimethyl quaternary analog of propranolol, UM-272 (SC-27761) J. Pharmacol. Exp. Ther. 1976;199:310–328. [PubMed] [Google Scholar]
- LUCCHESI B.R., MULLANE K.M. Leukocytes and ischemia-induced myocardial injury. Annu. Rev. Pharmacol. Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- MARKOVITZ L.J., SAVAGE E.B., RATCLIFFE M.B., BAVARIA J.E., KREINER G., IOZZO R.V., HARGROVE W.C., III, BOGEN D.K., EDMUNDS L.H., JR Large animal model of left ventricular aneurysm. Ann. Thorac. Surg. 1989;48:838–845. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- MAXWELL M.P., HEARSE D.J., YELLON D.M. Species variation in the coronary collateral circulation during regional myocardial ischemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc. Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- PARAVICINI T.M., GULLUYAN L.M., DUSTING G.J., DRUMMOND G.R. Increased NADPH oxidase activity, gp91phox expression, and endothelium-dependent vasorelaxation during neointima formation in rabbits. Circ. Res. 2002;91:54–61. doi: 10.1161/01.res.0000024106.81401.95. [DOI] [PubMed] [Google Scholar]
- PETTY M.A., LUKOVIC L., GRISAR J.M., DOW J., BOLKENIUS F.N., DE JONG W. Myocardial protection by a cardioselective free radical scavenger. Eur. J. Pharmacol. 1994;255:215–222. doi: 10.1016/0014-2999(94)90100-7. [DOI] [PubMed] [Google Scholar]
- PIETTA P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- RECCHIA F.A., MCCONNELL P.I., LOKE K.E., XU X., OCHOA M., HINTZE T.H. Nitric oxide controls cardiac substrate utilization in the conscious dog. Cardiovasc. Res. 1999;44:325–332. doi: 10.1016/s0008-6363(99)00245-x. [DOI] [PubMed] [Google Scholar]
- RENAUD S., DE LORGERIL M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- RUMP A.F., SCHUSSLER M., ACAR D., CORDES A., THEISOHN M., ROSEN R., KLAUS W., FRICKE U. Functional and antiischemic effects of luteolin-7-glucoside in isolated rabbit hearts. Gen. Pharmacol. 1994;25:1137–1142. doi: 10.1016/0306-3623(94)90129-5. [DOI] [PubMed] [Google Scholar]
- SCHUSSLER M., HOLZL J., RUMP A.F., FRICKE U. Functional and antiischaemic effects of Monoacetyl-vitexinrhamnoside in different in vitro models. Gen. Pharmacol. 1995;26:1565–1570. doi: 10.1016/0306-3623(95)00051-8. [DOI] [PubMed] [Google Scholar]
- WANG Q.D., PERNOW J., SJOQUIST P.O., RYDEN L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc. Res. 2002;55:25–37. doi: 10.1016/s0008-6363(02)00261-4. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., SAMOUILOV A., KUPPUSAMY P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]