Abstract
In addition to its action as a fast inhibitory neurotransmitter, γ-aminobutyric acid (GABA) is thought to mediate excitatory action by activating cation currents in some cell types in invertebrates. However, to date no GABA receptor capable of mediating such action has been identified at the molecular level in insects. Using a systematic expression screening approach, we found that the Drosophila ligand-gated ion channel subunits GRD and LCCH3 combine to form cation-selective GABA-gated ion channels when coexpressed in Xenopus laevis oocytes. The heteromultimeric receptor is activated by GABA (EC50=4.5 μM), muscimol (EC50=4.8 μM) and trans-4-aminocrotonic acid (EC50=104.5 μM), and partially by cis-4-aminocrotonic acid (EC50=106.3 μM). Picrotoxin effectively blocked the GABA-gated channel (IC50=0.25 μM), but bicuculline, TPMTA, dieldrin and lindane did not. The benzodiazepines flunitrazepam and diazepam did not potentiate the GABA-evoked current. Our data suggest that heteromultimeric channels composed of GRD and LCCH3 subunits form GABA-gated cation channels in insects.
Keywords: GABA receptor, cation channel, ligand-gated ion channel, Xenopus laevis oocytes, functional genomics
Introduction
Ligand-gated ion channels (LGICs) mediate the fast responses of neuronal and muscle cells to neurotransmitters. They form a large family of anion and cation channels activated by various neurotransmitters (Ortells & Lunt, 1995). The widespread anion-selective γ-aminobutyric acid (GABA) receptors mediate rapid inhibitory neurotransmission in the nervous systems of both vertebrates and invertebrates (Lee et al., 2003). In addition, GABA can also act as an excitatory neurotransmitter (Beg & Jorgensen, 2003; Stein & Nicoll, 2003). One of several suggested mechanisms for this action is that in invertebrates GABA might mediate excitation via cation currents (Zhang et al., 1997; Swensen et al., 2000; Stein & Nicoll, 2003), and in Ceanorhabditis elegans a GABA-gated cation channel, named EXP-1, has been cloned (Beg & Jorgensen, 2003). Our knowledge about the structure and function of ionotropic GABA receptors in Drosophila is fragmentary; two GABA receptor subunits from Drosophila have been characterized by recombinant expression of cloned cDNA: the exhaustively investigated RDL receptor (resistance to dieldrin locus), which is an important target for insecticides (Ffrench-Constant et al., 1991), and the ß-subunit LCCH3 (LCCH3: ligand-gated chloride channel homolog 3) (Henderson et al., 1993). LCCH3 alters the pharmacological properties of the RDL receptor, when the subunits are coexpressed (Zhang et al., 1995), but it is not thought to function as a ß-subunit for RDL in vivo because the two subunits exhibit different spatial patterns (Hosie et al., 1997). Multiple types of pharmacologically distinct GABA receptors in invertebrates have been characterized in vivo (Sattelle et al., 1988), particularly in the arthropods, where they have been studied in more detail (Dudel & Hatt, 1976; Zhainazarov et al., 1997). It is probable that this variety of GABA receptors is coded by more than two subunit types. Apart from RDL and LCCH3, one additional subunit has been cloned in Drosophila, named GRD (GABA and glycine-like receptor of Drosophila), which exhibits considerable homology to vertebrate ionotropic GABA receptors (Harvey et al., 1994). Analysis of the Drosophila gene sequences predicts one additional candidate, AAF48539, which clearly fits into the GABA group (Witte et al., 2002). To address the question of whether ion channel proteins coded by GRD and AAF48539 may form functional GABA receptors, possibly in combination with the ß-subunit LCCH3, the in vitro-transcribed cRNAs of these subunits were expressed using the Xenopus laevis oocyte expression system. Here we report the functional expression and pharmacological characterization of a new type of heteromultimeric GABA-gated channel formed by GRD and LCCH3.
Methods
Construction of expression vectors
mRNA was isolated from adult Drosophila melanogaster using the Pharmacia QuickPrep Micro mRNA Purification Kit. cDNA was constructed using an oligo(dT)17 primer and MMLV reverse transcriptase (Invitrogen, Carlsbad, CA, U.S.A.). PCR products containing the coding region of LGICs were obtained in a PCR buffer containing 1.5 mM MgCl2, 0.2 mM of each dNTP, ∼10 ng Drosophila cDNA, 0.5 mM of the corresponding primer and 2.5 U Pfu polymerase (Stratagene, La Jolla, CA, U.S.A.). PCR amplification was performed according to the following schedule: 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, for 40 cycles. The resulting fragments were subcloned blunt end into pSGEM (courtesy of M. Hollmann) previously digested with EcoRV and SmaI, resulting in the plasmids pGEM-LCCH3 (P1–P2), pGEM-GRD (P3–P4) and pGEM-AAF48539 (P6–P7):
- P1:
gccaccatgacatgttttacgcgcgtcggag
- P2:
tcattccagaatataaaacagccagtagc
- P3:
gccaccatgtgcacaatgccagcaacaagag
- P4:
tcaggtgctcgcatccggcgtgttgg
- P5:
accatgggaccctcggcgattttaac
- P6:
catgactatgacttcatattggacc
Expression of receptor cRNA in Xenopus oocytes
RNAs were synthesized using the AmpliCap T7 high-yield message maker kit (Epicentre, Madison, WI, U.S.A.), according to the manufacturer's protocol, with PacI-linearized pSGEM plasmids as templates. X. laevis oocytes were prepared by standard methods. After 24 h, stage V–VI oocytes were injected with cRNA (about 50 ng per oocyte) and incubated at 18°C in Barth's solution (88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 2.4 mM NaHCO3, 5 mM Tris-HCl, pH 7.4) supplemented with 100 U ml−1 penicillin and 50 μg ml−1 streptomycin. Oocytes were tested for functional expression of LGICs after 3–7 days.
Electrophysiological recording
Two-electrode voltage-clamp recording was used to obtain current responses to applied substances. Agonists and antagonists were diluted to the concentrations indicated with Drosophila-Ringer solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, pH 7.2) and were applied by means of a multibarrel, single-tip superfusion device. Electrodes were pulled from borosilicate glass using a Kopf vertical pipette puller and were backfilled with 3 M KCl. The membrane potential was controlled and current signals were recorded with a two-electrode voltage-clamp amplifier (TURBO TEC-03, npi, Tamm, Germany) and pCLAMP software (Axon Instruments, Union City, CA, U.S.A.). The I/V curve and the ion selectivity experiments were performed essentially as described for the EXP-1 receptor (Beg & Jorgensen, 2003) and, to get comparable results, similar experimental conditions were used. Briefly, all points were obtained by a 10 s application of 5 μM GABA. The reversal potentials were determined in the following solution: 115 mM NaCl, 1.8 mM BaCl2, 10 mM HEPES, pH 7.2. For sodium exchange experiments, 50 or 75% of NaCl was replaced by Tris-HCl, and for chloride exchange, sodium gluconate was used. In the sodium-free potassium Ringer, NaCl was completely replaced by KCl. SigmaPlot 7.0 (SPSS Inc.) was used to analyze concentration–response data. A four-parameter logistic equation was used to fit curves to the averaged, normalized data. All data are±s.e.m.
Results
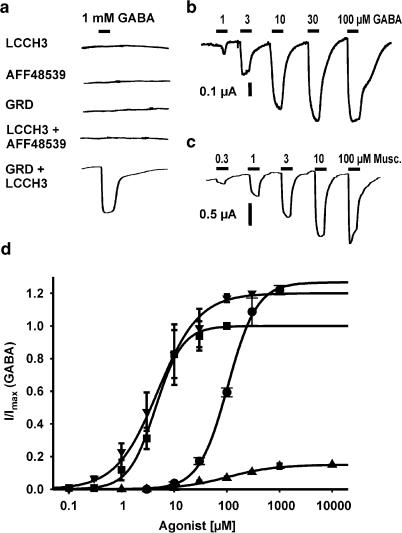
In order to investigate the function of the cloned cDNAs, RNA was in vitro transcribed from the linearized plasmids pGEM-LCCH3, pGEM-GRD and pGEM-AAF48539 and microinjected individually or in combination into the cytoplasm of Xenopus oocytes. After 3–7 days, oocytes were recorded in the two-electrode voltage-clamp configuration and tested for responses to agonists. To test the functional expression of the GABA receptors, we applied GABA and several other neurotransmitters including glycine, acetylcholine, histamine and serotonin (1 mM, n>30 for each combination). Only oocytes co-injected with cRNAs for LCCH3 and GRD exhibited a detectable current evoked by GABA (Figure 1a). Oocytes coexpressing LCCH3 and GRD responded to GABA with a concentration-dependent inward current, when clamped at −80 mV (Figure 1a). The rapidly developing current had amplitudes typically in the range 0.5–4 μA at a holding potential (Vh) of −80 mV. From analysis of concentration–response data, an EC50 value of 4.3±0.4 μM (n=7) was estimated for the heteromultimeric receptor (Figure 1b and d). The Hill coefficient was 1.73±0.26.
Figure 1.
(a) Oocytes were voltage clamped (Vh=−80 mV) 3–7 days after injection of 25 ng of various cRNA mixes. GABA applied at 1 mM for 10 s produced inward currents only in oocytes coexpressing GRD and LCCH3. The duration of application of agonists is indicated by bars. (b, c) Currents evoked by GABA and muscimol in oocytes co-injected with cRNA encoding GRD and LCCH3 subunits voltage clamped at –60 mV. (d) Agonist profile of oocytes coexpressing GRD and LCCH3. Recordings were performed as in (b, c). Each point represents the average value of four to seven oocytes. Data were normalized to the maximum GABA response (100 μM) seen in each oocyte. The concentration–response curve was fitted to the logistic equation.
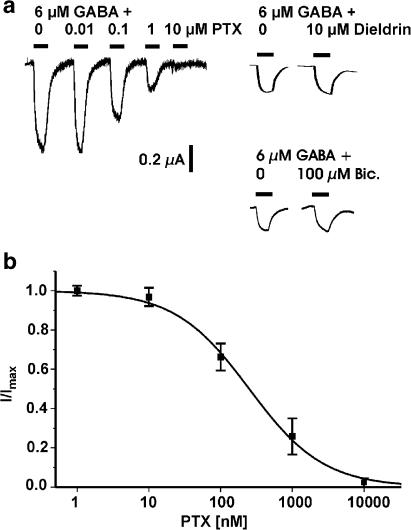
Next we characterized the pharmacological properties of the heteromultimeric GRD/LCCH3 receptors. The agonists muscimol and trans-4-aminocrotonic acid (TACA) activated about 120% of the maximal GABA-induced current (Figure 1c and d) (EC50 for muscimol=4.8±1.1 μM, n=4; EC50 for TACA=104.5±5.3 μM, n=4), whereas cis-4-aminocrotonic acid (CACA) was a weak partial agonist (EC50 for CACA=106.3±17 μM, n=4) (Figure 1d). Bicuculline, an antagonist for GABAA receptors, and for some populations of GABA receptors in insects (Hosie et al., 1997), did not antagonize the current evoked by 6 μM GABA at a concentration as high as 100 μM. The same was found for the GABAC antagonist (Bormann, 2000) 1,2,5,6-tetrahydropyridine-4-yl (TPMPA, 100 mM; data not shown). γ-Hexachlorohexane (γ-HCH, lindane), a ligand related to cyclodienes that interacts with the picrotoxin (PTX)/cyclodiene binding site and blocks the RDL receptor (Zhang et al., 1994), had no effect at 10 μM concentrations (Figure 2a). Dieldrin too was not an effective blocker at concentrations up to 100 μM. The antagonist PTX, known to be an effective noncompetitive blocker for both vertebrate and invertebrate GABA receptors, virtually completely blocked the GABA-induced current at concentrations above 10 μM (Figure 2a and b). The heteromultimeric channels were highly sensitive to PTX; the IC50 for PTX was 250±24 nM. The low Hill coefficient of 0.84±0.06 is in good agreement with a simple single-site blocking mechanism (Figure 2b). Numerous allosteric modulators, including benzodiazepines, are known to regulate GABAA receptor function. To test if benzodiazepines potentiated the GABA-evoked current, we co-applied a near-EC10 concentration of GABA (1 μM) with 30 μM flunitrazepam or 30 μM diazepam and found no enhancement of the evoked current (data not shown).
Figure 2.
(a) Antagonist profile of oocytes coexpressing GRD and LCCH3. Oocytes were voltage clamped (Vh=−80 mV) and 6 μM GABA was co-applied with various concentrations of PTX, dieldrin or bicuculline. (b) Concentration–block curve for PTX. Each point represents the average value of three to five oocytes. Concentration–response curves were fitted to the logistic equation.
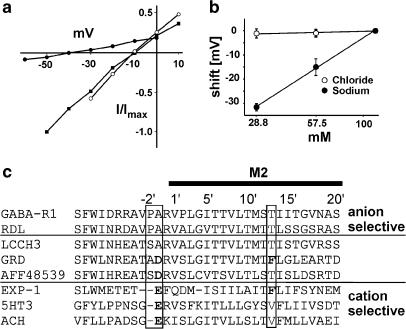
The current exhibited a linear current–voltage relationship (Figure 3a). The GABA-dependent current reversed at −11.2±1.6 mV (n=9). Under the ionic conditions of the experiments, the reversal potentials are roughly −22 mV for a chloride and ∼0 mV for mixed K+–Na+ currents in Xenopus oocytes, provided typical intracellular concentrations of 108 mM K+, 10.8 mM Na+ and 40 mM Cl− are present. These data are consistent with the hypothesis that the channel could conduct chloride, but the shift in the reversal potential to more positive values suggests that other ions may also permeate the channel. To identify the permeating ions, we determined the reversal potential in different extracellular solutions. The reversal potential was virtually independent of the external chloride concentration (n=4), an indication that chloride ions make no significant contribution to the current (Figure 3b). In contrast to this, variation of the external sodium concentration shifted the reversal potential to more negative values. The shift of –31.6±1.6 mV (n=4) in 25% sodium Ringer is comparable to the theoretical shift of –34.3 mV predicted by the Nernst equation for the given ionic conditions. This proves that sodium permeates through the channels. To test if the channel also conducted potassium, we replaced sodium Ringer with potassium Ringer and found a shift in the reversal potential of 7.1±0.7 mV (n=5) to more positive values (data not shown), an indication that potassium permeated the ion channel pore even more effectively; the estimated selectivity of sodium over potassium was 0.75, assuming no other ions were involved. A possible reason for the cation selectivity of this channel was discovered by analyzing the amino-acid sequence of the pore-forming M2 region, which determines the ion selectivity of LGICs. Three residues at positions −2′, −1′ and 13′ have been identified by mutation studies as being critical for ion selectivity (Wotring et al., 2003). The M2 region of the GRD subunit differs from a typical chloride-selective pore in all three critical positions: a proline is absent at position −2′, a phenylalanine is located at position 13′ and , importantly, a charged aspartic acid is found at position −1′ (Figure 2c). In LCCH3 subunits, the proline at position −2′ is absent.
Figure 3.
(a) Current–voltage relationship. The averaged peak currents of the I/V curves were determined under varying external ionic conditions (normalized to the response in 100% Na+, 100% Cl− Ringer): 100% Na+, 100% Cl− Ringer (n=9); 25% Na+, 100% Cl− Ringer (n=4); 100% Na+, 25% Cl− Ringer (n=4). (b) Reversal potential shifts for ionic substitution experiments. Reversal potentials were measured in solutions with varying amounts of sodium (n=4) or chloride ions (n=4). (c) The pore-forming M2 domain of GRD (CAA55144) contains the molecular determinants for cation selectivity and differs in the three critical positions −2′, −1′ and 13′ (Wotring et al., 2003) from the ligand-gated chloride channel RDL (AAA28556) and the rat ρ1-GABA receptor (P50572) and resembles ligand-gated cation channels such as EXP-1 (AY383563), the rat 5HT3 receptor (NP_038589) and the rat nicotinic α7 ACh receptor (NP_036964) at these positions. AFF48539 and LCCH3 (AAB27090) show these differences to a lesser extent.
Discussion
Pharmacologically distinct classes of invertebrate GABA-gated ion channels existing in vivo have been reported by many groups (Dudel & Hatt, 1976; Sattelle et al., 1988; Zhainazarov et al., 1997), but nearly all investigations of recombinantly expressed channels have focused, in contrast, on chloride channels such as the RDL receptor and its homologs. There are indications from physiological studies in invertebrates that GABA mediates excitation by activating cation currents (Zhang et al., 1997; Swensen et al., 2000). Recently, a homomultimeric GABA-gated cation channel in C. elegans, named EXP-1 (Beg & Jorgensen, 2003), was cloned that belongs to the superfamily of LGICs. Our data show the existence of a second, distinct type of heteromultimeric GABA receptor occurring in Drosophila, made up of LCCH3 and GRD subunits, which also form cation-selective channels when coexpressed in Xenopus oocytes. LCCH3 and GRD are not receptors of the EXP-1 type. Their homology to EXP-1 is weak (17–21% identical amino acids). LCCH3 has been described as a clear homologue of vertebrate GABAA ß-subunits (Hosie et al., 1997) and GRD is similar to the vertebrate GABA and glycine α-subunits (Harvey et al., 1994). The high homology of AAF48539 with GRD suggests that it could be an additional potential α-subunit. However, AAF48539 alone or in combination with GRD or LCCH3 did not form any detectable GABA receptors when expressed in oocytes. It should be noted that the cDNA used for expression of AAF48539 was generated by RT–PCR based on the genome sequence data only. Hence we cannot rule out the possibility that the cDNA was incomplete and thus coded for a nonfunctional protein.
What causes the cation selectivity of the ion channel formed by GRD/LCCH3? GRD differs from GABA-gated chloride channels at three positions, −2′, −1′ and 13′, that are critical for the ion selectivity of the pore (Wotring et al., 2003). At these positions, GRD shows more similarity to ligand-gated channels with cation selectivity. Changes at these positions are sufficient to change the ion selectivity of the pore of LGICs from chloride to cation selective (Wotring et al., 2003). GRD and EXP-1 share more or less the same differences to chloride-selective channels in the pore region. The importance of these amino-acid exchanges is discussed for EXP-1 in detail by Beg and Jorgensen (2003). LCCH3, which can function as a subunit with RDL in chloride channels (Zhang et al., 1995) and with GRD in cation channels, lacks proline at position −2′ but displays no charged amino acid at position −1′. Therefore, the GRD subunit seems to be the molecular determinant of the ion selectivity, analogous to findings that, in GABAA receptors, the M2 region of the GABAA ß-subunit alone can determine ion selectivity in a heteromultimeric channel (Zhang et al., 1997).
The pharmacological characteristics of the Drosophila receptor are similar to the excitatory muscimol II type GABA receptor found in the v-LCDN giant neurons of the African giant snail Achatina fulica (Zhang et al., 1997). At both receptors, GABA, muscimol, TACA and CACA are agonists, TACA activating the receptor with greater efficacy and CACA with lesser efficacy than GABA. Furthermore, both receptors are insensitive to diazepam. In some neurons of the stomatogastric ganglion of the crab Cancer borealis, a GABA-evoked excitatory response occurs (Swensen et al., 2000) that shares the muscimol and PTX sensitivity of the Drosophila receptor. Therefore, it seems possible that the GRD/LCCH3 receptor is the prototype of this excitatory type of muscimol-activated GABA receptor. The nematode EXP-1 channel (Beg & Jorgensen, 2003) differs from the Drosophila receptor in several properties: for example, the EXP-1 receptor is homomultimeric and not antagonized by PTX, whereas the heteromultimeric Drosophila receptor is highly sensitive to PTX.
Recently, it has been shown that LGICs with a complex subunit composition exist in Drosophila, such that glutamate and GABA receptors are both found in the same heteromultimeric ion channel (Ludmerer et al., 2002). This fits with the finding that the LCCH3 subunit can co-assemble with both RDL and GRD subunits. The ß-subunit LCCH3 alters the pharmacological properties of the RDL receptor when they are coexpressed. In RDL/LCCH3 heteromultimers, PTX sensitivity is drastically reduced, by nearly three orders of magnitude, from 1 to 500 μM, compared to RDL homo-oligomeres (Zhang et al., 1995). In contrast, heteromultimeric GRD/LCCH3 channels are approximately as sensitive to PTX as are RDL receptors, so that the possibility that LCCH3 alone is sufficient to obstruct PTX binding can be excluded. The fact that γ-HCH does not block the GRD/LCCH3 receptor is surprising because PTX and γ-HCH are thought to bind to the same site in insecticide-resistant insect strains; a mutation in the RDL-GABA receptor influences sensitivity to the antagonists PTX and γ-HCH in the same manner. γ-HCH and PTX also have similar blocking properties at GABA receptors in other invertebrates (Zufall et al., 1989). In contrast, GRD/LCCH3 receptors are highly sensitive to block by PTX but not by γ-HCH. This difference may be caused by the altered pore structure.
The identification of heteromultimeric GABA receptors with cation selectivity broadens our view of GABA receptors in insects. It can be speculated that heteromultimeric receptors composed of proteins with homology to GRD and LCCH3 form the molecular basis of the excitatory action of GABA in several invertebrates. Functional expression studies will enable us to characterize the pharmacological properties of this new class of GABA receptor and their potential use as targets for new kinds of GABA-receptor insecticides.
Acknowledgments
We acknowledge the excellent technical assistance of A. Saras, H. Bartel, W. Grabowsky and A. Stoeck and thank M. Hollmann for the gift of the pSGEM vector. We are especially indebted to the excellent reviewers whose suggestions for further experiments led to the discovering of essential new properties of the ion channel.
Abbreviations
- CACA
cis-4-aminocrotonic acid
- GABA
γ-aminobutyric acid
- γ-HCH
γ-hexachlorohexane
- LGIC
ligand-gated ion channel
- PTX
picrotoxin
- RDL
resistance to dieldrin locus
- TACA
trans-4-aminocrotonic acid
- TPMPA
1,2,5,6-tetrahydropyridine-4-yl
References
- BEG A.A., JORGENSEN E.M. EXP-1 is an excitatory GABA-gated cation channel. Nat. Neurosci. 2003;6:1145–1152. doi: 10.1038/nn1136. [DOI] [PubMed] [Google Scholar]
- BORMANN J. The ‘ABC' of GABA receptors. Trends Pharmacol. Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- DUDEL J., HATT H. Four types of GABA receptors in crayfish leg muscles characterized by desensitization and specific antagonist. Pflugers Arch. 1976;364:217–222. doi: 10.1007/BF00581758. [DOI] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., MORTLOCK D.P., SHAFFER C.D., MACINTYRE R.J., ROUSH R.T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R.J., SCHMITT B., HERMANS-BORGMEYER I., GUNDELFINGER E.D., BETZ H., DARLISON M.G. Sequence of a Drosophila ligand-gated ion-channel polypeptide with an unusual amino-terminal extracellular domain. J. Neurochem. 1994;62:2480–2483. doi: 10.1046/j.1471-4159.1994.62062480.x. [DOI] [PubMed] [Google Scholar]
- HENDERSON J.E., SODERLUND D.M., KNIPPLE D.C. Characterization of a putative gamma-aminobutyric acid (GABA) receptor beta subunit gene from Drosophila melanogaster. Biochem. Biophys. Res. Commun. 1993;193:474–482. doi: 10.1006/bbrc.1993.1648. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., ARONSTEIN K., SATTELLE D.B., FFRENCH-CONSTANT R.H. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- LEE D., SU H., O'DOWD D.K. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J. Neurosci. 2003;23:4625–4634. doi: 10.1523/JNEUROSCI.23-11-04625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDMERER S.W., WARREN V.A., WILLIAMS B.S., ZHENG Y., HUNT D.C., AYER M.B., WALLACE M.A., CHAUDHARY A.G., EGAN M.A., MEINKE P.T., DEAN D.C., GARCIA M.L., CULLY D.F., SMITH M.M. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both gamma-aminobutyric acid-gated Rdl and glutamate-gated GluCl alpha chloride channel subunits. Biochemistry. 2002;41:6548–6560. doi: 10.1021/bi015920o. [DOI] [PubMed] [Google Scholar]
- ORTELLS M.O., LUNT G.G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- SATTELLE D.B., PINNOCK R.D., WAFFORD K.A., DAVID J.A. GABA receptors on the cell-body membrane of an identified insect motor neuron. Proc. R. Soc. Lond. B. 1988;232:443–456. doi: 10.1098/rspb.1988.0006. [DOI] [PubMed] [Google Scholar]
- STEIN V., NICOLL R.A. GABA generates excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- SWENSEN A.M., GOLOWASCH J., CHRISTIE A.E., COLEMAN M.J., NUSBAUM M.P., MARDER E.GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis J. Exp. Biol. 20002032075–2092.(Part 14) [DOI] [PubMed] [Google Scholar]
- WITTE I., KREIENKAMP H.J., GEWECKE M., ROEDER T. Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion. J. Neurochem. 2002;83:504–514. doi: 10.1046/j.1471-4159.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- WOTRING V.E., MILLER T.S., WEISS D.S. Mutations at the GABA receptor selectivity filter: a possible role for effective charges. J. Physiol. 2003;548:527–540. doi: 10.1113/jphysiol.2002.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAINAZAROV A.B., WACHOWIAK M., BOETTCHER A., ELENES S., ACHE B.W. Ionotropic GABA receptor from lobster olfactory projection neurons. J. Neurophysiol. 1997;77:2235–2251. doi: 10.1152/jn.1997.77.5.2235. [DOI] [PubMed] [Google Scholar]
- ZHANG H.G., FFRENCH-CONSTANT R.H., JACKSON M.B.A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms J. Physiol. 199447965–75.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H.G., LEE H.J., ROCHELEAU T., FFRENCH-CONSTANT R.H., JACKSON M.B. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol. Pharmacol. 1995;48:835–840. [PubMed] [Google Scholar]
- ZHANG W., HAN X.Y., WONG S.M., TAKEUCHI H. Pharmacologic characteristics of excitatory gamma-amino-butyric acid (GABA) receptors in a snail neuron. Gen. Pharmacol. 1997;28:45–53. doi: 10.1016/s0306-3623(96)00152-8. [DOI] [PubMed] [Google Scholar]
- ZUFALL F., FRANKE C., HATT H. Similarities between the effects of lindane (gamma-HCH) and picrotoxin on ligand-gated chloride channels in crayfish muscle membrane. Brain Res. 1989;503:342–345. doi: 10.1016/0006-8993(89)91688-0. [DOI] [PubMed] [Google Scholar]