Abstract
The effects of hydro-alcoholic extracts of Hypericum perforatum L on extracellular serotonin (5-HT), noradrenaline (NA) and dopamine (DA) levels and the acidic metabolites (3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxy-3-indoleacetic acid (5-HIAA)) were examined by in vivo microdialysis in the prefrontal cortex of awake rats. Thus, a single dose (60 mg kg−1 i.p. or 300 mg kg−1 p.o.) of H. perforatum increased DA concentrations to 165 and 140% of control values, respectively, and increased locomotor activity in nonhabituated rats. DOPAC and HVA levels were markedly reduced. 5-HT concentrations were elevated only moderately, while the NA levels were not affected by any treatment. The whole-tissue analysis revealed that hypericum increased, whereas the monoamine oxidase (MAO) A/B inhibitor phenelzine decreased DA and 5-HT turnover. The present data indicate that the mechanism of action of hypericum extract in vivo is more complex than the inhibition of monoamine reuptake or metabolism observed in vitro. The finding of preferential enhancement of DA transmission is in agreement with human studies measuring DA-mediated neuroendocrine responses.
Keywords: Serotonin, noradrenaline, reuptake inhibitors, depression, microdialysis, antidepressants
Introduction
The extracts from Hypericum perforatum L (St John's wort) have been shown to possess clinical efficacy in the therapy of mild to moderate depression (Lecrubier et al., 2002) although some negative reports exist as well (Davidson et al., 2002). Several in vitro studies have indicated that H. perforatum and its phloroglucinol constituent hyperforin act via a blockade of serotonin (5-HT), noradrenaline (NA) and possibly dopamine (DA) reuptake in a manner similar to most of the current antidepressants (Chatterjee et al., 1998; Muller et al., 1998; Singer et al., 1999). However, the detailed mechanism of action of St John's wort is not elucidated yet, in addition to the complex nature of the extract consisting of multiple active components (for review, see Butterweck, 2003; Muller, 2003). Surprisingly, only limited and rather sparse information is available on in vivo effects of H. perforatum on neurotransmitter release and metabolism. Thus, H. perforatum-CO2 extract given orally (p.o.) caused a modest increase in DA outflow in the nucleus accumbens and rat striatum measured by microdialysis, while it did not affect extracellular 5-HT and metabolite levels in the ventral hippocampus (Di Matteo et al., 2000). In another study, methanolic, as well as, hyperforin-rich extracts of H. perforatum given p.o. caused only a moderate increase in extracellular 5-HT and DA levels in the nucleus accumbens (Rommelspacher et al., 2001).
The purpose of the present study was to examine whether hydro-alcoholic extracts of H. perforatum given intraperitoneally (i.p.) or p.o. at pharmacologically relevant doses would affect the extracellular 5-HT, NA and DA levels and their acidic metabolites in the prefrontal cortex (PFC) of awake rats. This region of the brain has been implicated in the ethiology of depression and in the action of antidepressant drugs. The technique of in vivo microdialysis in combination with high-sensitive HPLC methods was used.
Methods
Male Sprague–Dawley rats (Charles River, Japan) (250–350 g) were used in the experiments. The rats (three animals per cage) were maintained on a 12-h light–dark cycle (light at 0700), room temperature 23±2°C and humidity 55–65%. Under pentobarbital anesthesia, the guide cannulae for microdialysis probes (Eicom Corp., Kyoto, Japan) were implanted into the PFC (AP +3.3 mm, L −2.8 mm, V −0.5 mm, from bregma and the dural surface, according to the stereotaxic atlas of Paxinos & Watson, 1997). The guide cannula was fixed firmly to the skull surface using dental cement. Following at least 5 days of recovery, a microdialysis probe (0.22 mm o.d., 3 mm membrane length with 50,000 Da cutoff, Eicom A-I) was inserted into the guide cannula of the awake rat and perfused with Ringer solution (NaCl, 147 mM; KCl, 4 mM; CaCl2, 2.3 mM) at a flow rate of 1 μl min−1. After the initial stabilization period of 2–3 h, the microdialysis samples were collected in 20-min intervals. First 2–4 samples were used for estimation of basal 5-HT, NA and DA levels. H. perforatum ethanolic extract (PSc 502 Ch. 039; WS 5572; Willmar-Schwabe, Germany) consisted of the following major constituents: hypericin 0.3%; hyperforin 4.1%; rutin 3.3%; hyperosid 1.8%; isoquercitrin 1.0%; quercetin 0.3% and biapigenin 0.3%. The dry extract was suspended in 0.3% w v−1 carboxymethylcellulose and injected at a dose of 60 mg kg−1 i.p. In separate groups of animals, H. perforatum suspension was given at a dose of 300 mg kg−1 p.o. using a gastro-esophageal gavage, or phenelzine sulfate (Sigma-Aldrich, St Louis, MO, U.S.A.) was given at a dose of 5 mg kg−1 i.p. All experiments were performed in accordance with the general recommendations of Japanese or Swedish animal protection legislation.
The chemicals and standards were purchased from Sigma-Aldrich, Wako Pure Chemical Co. (Osaka, Japan) or from Kisida Chemical Co. (Tokyo, Japan). Benzylamine hydrochloride was obtained from Tokyo Kasei Kogyo Co. (Tokyo, Japan) and was used after purification by recrystallization in absolute ethanol. Liquid chromatographic determination with fluorescence detection was based on precolumn derivatization of 5-HT, NA and 5-hydroxy-3-indoleacetic acid (5-HIAA) with benzylamine, as detailed elsewhere (Yoshitake et al., 2003). Determination of DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the dialysates was carried out by HPLC with electrochemical detection (ECD-300, Eicom, Japan). Briefly, a glassy carbon working electrode (WE-3G) operated at +450 mV vs Ag/AgCl reference electrode, and DA and metabolites were separated on an ODS column (150 × 2.1 mm i.d., Eicom). The mobile phase consisted of a mixture of methanol and 0.1 M phosphate buffer (pH 3.3) (1 : 9, v v−1) containing 1.1 mM octanesulfonic acid sodium salt and 0.013 mM disodium EDTA salt. The flow rate was 0.2 ml min−1. The limits of detection (signal-to-noise ratio=3) for 5-HT, NA, DA, 5-HIAA, DOPAC and HVA were 0.05, 0.035, 0.91, 0.055, 0.88 and 1.3 fmol, respectively, in 20 μl injected onto the column. HPLC determination of DA, 5-HT and their metabolites in acidic tissue extracts was carried out by use of the ESA Coulochem II HPLC equipment, on a 3 mm i.d. × 100 mm column, packed with C18 silica of particle size 5 μm. The elution buffer contained 10.25 g NaH2PO4, 185 mg EDTA, 100 mg octanesulfonic acid and 9% methanol, pH 3.7, in 1000 ml deionized water, pumped at a flow rate of 0.75 ml min−1. The activities of monoamine oxidase (MAO) A and MAO B in the homogenates from the rat cortex were measured as described elsewhere (Muller et al., 1997). Briefly, the resuspended pellets were incubated for 6 min with 14C-5-hydroxytryptamine (MAO A assay) or 14C-β-penylethylamine (MAO B) used as substrates. A computerized multicage motion detection system Scanet MV-10 (Toyo Sangyo, Toyama, Japan) was used to detect the horizontal activity (locomotion and motility) and vertical activity (rearing) in 10 min intervals. Locomotor activity was recorded during a period of 120 min, during which the rats habituated to the new environment. The microdialysis data are expressed as percentage of mean concentration values for each respective monoamine and the metabolites, taken as 100% at time 0 min. The data were examined using a repeated-measures analysis of variance (ANOVA), followed by Student–Newman–Keuls for post hoc comparison of different time intervals or Fisher's protected least significant difference test for comparison between the groups. A level of P<0.05 was accepted as evidence for a statistically significant effect.
Results
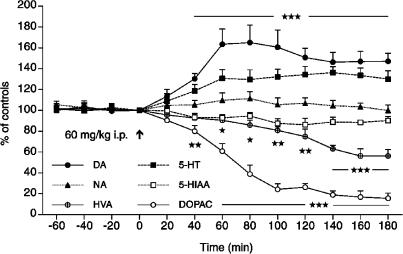
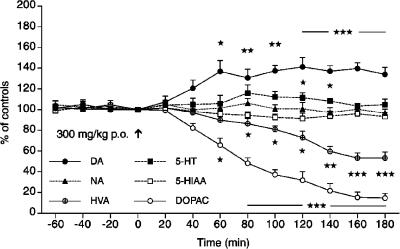
The basal levels of DA, 5-HT and NA concentrations in the dialysates from the PFC of awake rats were 9.2±0.41, 6.8±0.28 and 7.3±0.33 fmol in 20 μl (mean±s.e.m., n=10), respectively. The corresponding levels of acidic metabolites were 943.1±62.5 (DOPAC), 1645.9±93.9 (HVA) and 724.5±43.5 (5-HIAA), with all concentrations expressed in fmol 20 μl−1 (n=10). A single dose of suspended H. perforatum L extract (60 mg kg−1 i.p.) caused a gradual increase in the extracellular levels of DA within 20–60 min (P<0.005), reaching a maximal increase of 165% within 60–80 min after administration. This increase lasted at least for an additional 100 min (Figure 1). Extracellular 5-HT concentrations increased only moderately to about 135% of the control values (P<0.005), whereas NA levels remained unchanged. The most profound effect of H. perforatum was observed on the decrease of the DA metabolites HVA to 56–60% (P<0.005) and DOPAC to 16–20% (P<0.005) of the control (predrug levels at the end of the sampling period, 180 min), whereas the values of 5-HIAA were unaltered. H. perforatum given orally (300 mg kg−1 p.o.) caused also a significant increase in extracellular DA levels already at 40 min with a maximal elevation at 120–180 min after administration to about 140% (P<0.005) of the control values (Figure 2). The values of 5-HT were only marginally elevated to 110% at 120 and 140 min and the values of NA and 5-HIAA were not affected. However, the concentrations of metabolites HVA and DOPAC were strongly reduced to 53 and 15% of control levels (P<0.005), respectively, in a similar manner as seen after i.p. injection of hypericum.
Figure 1.
Effect of H. perforatum extract (60 mg kg−1 i.p.) given at time 0 min (arrow) on extracellular concentrations of DA, NA, 5-HT and metabolites DOPAC, HVA and 5-HIAA in the PFC of the awake rat. The data are expressed as percentage of control levels at 0 min; the values at individual time points were compared to the basal levels at −20 min; (★) P<0.05; (★★) P<0.01; (★★★) P<0.001, one-way ANOVA, Student–Newman–Keuls test, mean±s.e.m., n=5 rats.
Figure 2.
Effect of H. perforatum extract (300 mg kg−1 p.o.) given at time 0 min (arrow) on extracellular concentrations of DA, NA, 5-HT and metabolites DOPAC, HVA and 5-HIAA in the PFC of the awake rat. Statistical analysis was carried out as described in Figure 1.
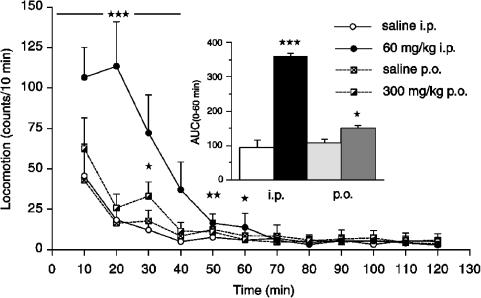
Studies of motor activity in nonhabituated rats revealed that H. perforatum at a high dose (60 mg kg−1 i.p.) caused a marked increase in locomotor activity during the initial 60 min compared to saline-injected rats, as shown in Figure 3 for individual 10-min measurement intervals and the total area-under-the-curve (AUC(0–60 min)) value (P<0.001, and ANOVA and Fisher's PLSD test). The peroral dose of H. perforatum caused a significant increase in locomotor activity only for the AUC(0–60 min) value (P<0.05). The effects of H. perforatum (300 mg kg−1 p.o.) and phenelzine (5 mg kg−1 i.p.) on tissue concentrations of DA, DOPAC, HVA, 5-HT and 5-HIAA, and on DA and 5-HT turnover are summarized in Table 1. As seen, neither hypericum nor phenelzine caused significant changes in tissue levels of monoamines and their metabolites compared to saline-treated rats. Hypericum caused significant increases in DA turnover in the PFC and 5-HT turnover in the striatum, whereas phenelzine caused a robust decrease of DA turnover in the striatum and 5-HT turnover in both striatum and PFC. Determination of MAO A and MAO B activities in vitro in cortical homogenates revealed that of hypericum concentrations (1, 10 and 100 μg ml−1) tested, only the highest concentration of the extract significantly inhibited MAO A and MAO B activities to 41±14 and 51±5% (P<0.05; mean±s.d., n=4), respectively.
Figure 3.
Effect of H. perforatum extract (60 mg kg−1 i.p. and 300 mg kg−1 p.o.) given at time 0 min on locomotor activity in the nonhabituated rats. (★) P<0.05; (★★) P<0.01; (★★★) P<0.001, ANOVA, Fisher's PLSD test). Inset: The AUCs (0–60 min) for hypericum- and saline-treated groups. All data are expressed as mean±s.e.m., n=5 rats.
Table 1.
Effect of H. perforatum (300 mg kg−1 p.o.) and phenelzine (5 mg kg−1 i.p.) on tissue concentrations of DA, DOPAC, HVA, 5-HT and 5-HIAA, and on DA and 5-HT turnover in the PFC and striatum of the awake rat, 180 min after the drug administration
| DA | DOPAC | HVA | 5-HT | 5-HIAA | DA turnover | 5-HT turnover | |
|---|---|---|---|---|---|---|---|
| PFC | |||||||
| Vehicle | 62±9 | 27±3 | 66±4 | 1641±78 | 1429±90 | 1.585±0.164 | 0.874±.051 |
| Hypericum | 36±22 | 24±5 | 81±9 | 1507±105 | 1521±75 | 6.614±1.586** | 1.026±0.051 |
| Phenelzine | 58±4 | 11±2 | 52±4 | 2034±194 | 831±129 | 1.124±0.158 | 0.427±0.080*** |
| Striatum | |||||||
| Vehicle | 17838±2316 | 1634±214 | 1395±247 | 718±116 | 2247±306 | 0.168±0.007 | 3.177±0.104 |
| Hypericum | 16168±599 | 1817±93 | 1390±171 | 603±25 | 2455±128 | 0.199±0.015 | 4.098±0.246* |
| Phenelzine | 19006±591 | 626±219 | 582±136 | 879±57 | 1625±154 | 0.066±0.022*** | 1.947±0.322** |
The concentrations are expressed in ng g−1 wet weight, DA and 5-HT turnover as the ratio of (DOPAC+HVA)/DA and 5-HIAA/5-HT, respectively, mean±s.e.m., n=6 rats;
P<0.05, **P<0.01, ***P<0.001, ANOVA, Fisher's PLSD test.
Discussion
The present study demonstrates that a single dose of hydro-alcoholic extract of H. perforatum preferentially increased extracellular DA, stimulated locomotor activity and profoundly reduced acidic metabolites DOPAC and HVA in the PFC of awake rats. This is consistent with the observation that hypericum extracts can produce signs of DA-mediated stereotypies (Misane & Ögren, 2001). These results are also in line with clinical observations in healthy volunteers receiving a single dose of H. perforatum extract (300 mg containing 0.3% hypericin) and showing changes in neuroendocrine responses mediated by DA (Franklin et al., 1999). The moderate elevation of DA and the concomitant reduction of its metabolites would suggest an inhibitory effect of H. perforatum on MAO activity. Some early studies on the effects of H. perforatum have suggested that the extracts and hypericin may act via inhibition of metabolizing enzymes MAO A and MAO B and catechol-o-methyltranserase (COMT) (Suzuki et al., 1984; Bladt & Wagner, 1994). However, these findings were not confirmed in the later studies (Chatterjee et al., 1998) and currently, it is thought that only some flavonoids present in the extract may exert MAO inhibitory activity (for review, see Butterweck, 2003). In order to evaluate the MAO hypothesis, the same hypericum extract was tested under in vitro and in vivo conditions. Thus, in vitro test showed that hypericum could, in fact, inhibit MAO A and B activity in the rat cortical homogenates to about 40 and 50%, respectively, of the control values but only at the highest dose (100 μg ml−1). In a recent microdialysis study in awake rats (Yoshitake et al., 2004), we have shown that the MAO A/B inhibitor phenelzine (5 mg kg−1 i.p.) caused a gradual increase (during 120 min) in extracellular concentrations of DA up to 171%, 5-HT to 140% and NA to 121%, whereas 5-HIAA and DOPAC decreased to 83 and 41% of the basal levels, respectively. These data are similar to those reported here for the hypericum extract and would support a notion on MAO inhibitory effects. However, the ex vivo experiments, where the monoamines and their major metabolites were determined in striatal and PFC tissue extracts following single p.o. administration of hypericum or i.p. phenelzine, do not confirm this conclusion. Thus, hypericum increased DA turnover in the PFC and 5-HT turnover in the striatum, whereas phenelzine markedly reduced turnover of both monoamines. The content of DA, 5-HT and metabolites did not differ in saline- and drug-treated rats, which is in good agreement with previous data by Gobbi et al. (1999). The levels of 5-HT were only modestly elevated following an i.p. dose of hypericum, while the NA levels were not affected by either i.p. or p.o. dose. In conclusion, these findings do not support the initial view that H. perforatum extracts act via the inhibition of MAO or via the inhibition of 5-HT reuptake as it was suggested in some more recent in vitro studies (Chatterjee et al., 1998; Singer et al., 1999). Moreover, behavioral tests with the same extract as in this study showed that hypericum only weakly blocked the action of the 5-HT releasing compound p-chloramphetamine mediated via the 5-HT transporter (Misane & Ögren, 2001).
A possible explanation for the marked dopaminergic effect of hypericum could be an inhibitory effect of H. perforatum extract and, particularly, of its constituent hyperforin on synaptosomal uptake of DA measured in vitro (Muller et al., 1998; Gobbi et al., 1999; Singer et al., 1999). In fact, for the methanolic extract of hypericum, the IC50 value for DA (0.85 μg ml−1) is three times lower than for 5-HT and more than five times lower than for NA (Muller et al., 1997; Chatterjee et al., 1998), and even pure hyperforin is almost twice more potent to inhibit DA than 5-HT reuptake (Muller, 2003). However, the selective DAT inhibitor GBR12909 failed to alter extracellular DA levels in the PFC of awake rats although it increased DA levels in the striatum (Mazei et al., 2002). H. perforatum did not elevate extracellular NA, while it still increased the DA levels in the PFC, suggesting that hypericum does not act via a blockade of the NA transporter, which itself is capable of reuptaking DA (Carboni et al., 1990). Rather a ‘reserpine-like mechanism' implicating a depletion of storage vesicles (Gobbi et al., 1999) or a blockade of vesicular uptake mediated via H+-ATPase (Roz & Rehavi, 2003) could explain the accumulation of extracellular DA as a result of reduced demand of DA as the NA precursor for vesicular storage. Further in vivo studies are needed to elucidate the mechanisms behind hypericum-induced reduction of extracellular DOPAC and HVA values and a moderate increase in extracellular DA outflow in the PFC and other neuroanatomical areas with relevance to depression and mood control.
In conclusion, the present microdialysis data in the PFC of awake rats suggest that the mechanism of action of H. perforatum extract does not primarily involve the inhibition of 5-HT or NA reuptake but rather hypericum exerts its action via a moderate stimulation of DA function. The activation of DA system can underlie the mechanisms of proposed antidepressant activity of H. perforatum extracts, implementing the role of DA in diminishing anhedonia associated with depression, stimulating vigilance and reward seeking behavior.
Acknowledgments
We thank Dr M. Nöldner from Willmar-Schwabe GmbH & Co, Karlsruhe, Germany for kind supply of H. perforatum L extracts. Dr Yoshitake was supported by The Swedish Foundation for International Cooperation in Research and Higher Education, STINT.
Abbreviations
- CAPS
3-(cyclohexylamino)-1-propanesulfonic acid
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- 5-HIAA
5-hydroxy-3-indoleacetic acid
- HVA
homovanillic acid
- 5-HT
serotonin
- NA
noradrenaline
- PFC
prefrontal cortex
References
- BLADT S., WAGNER H. Inhibition of MAO by fractions and constituents of hypericum extract. J. Geriatr. Psychiatry Neurol. 1994;7:S57–S59. doi: 10.1177/089198879400700115. [DOI] [PubMed] [Google Scholar]
- BUTTERWECK V. Mechanism of action of St John's wort in depression: what is known. CNS Drugs. 2003;17:539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- CARBONI E., TANDA G.L., FRAU R., DI CHIARA G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE S.S., BHATTACHARYA S.K., WONNEMANN M., SINGER A., MULLER W.E. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998;63:499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J.R.T., Hypericum Depression Trial Study Group Effect of Hypericum perforatum (St John's wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287:1807–1814. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- DI MATTEO V., DI GIOVANNI G., DI MASCIO M., ESPOSITO E. Effect of acute administration of Hypericum perforatum-CO2 extract on dopamine and serotonin release in the rat central nervous system. Pharmacopsychiatry. 2000;33:14–18. doi: 10.1055/s-2000-8449. [DOI] [PubMed] [Google Scholar]
- FRANKLIN M., CHI J., MCGAVIN C., HOCKNEY R., REED A., CAMPLING G., WHALE R.W., COWEN P.J. Neuroendocrine evidence for dopaminergic actions of hypericum extract (LI 160) in healthy volunteers. Biol. Psychiatry. 1999;46:581–584. doi: 10.1016/s0006-3223(99)00102-x. [DOI] [PubMed] [Google Scholar]
- GOBBI M., VALLE F.D., CIAPPARELLI C., DIOMEDE L., MORAZZONI P., VEROTTA L., CACCIA S., CERVO L., MENNINI T. Hypericum perforatum L. extract does not inhibit 5-HT transporter in rat brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:262–269. doi: 10.1007/s002109900073. [DOI] [PubMed] [Google Scholar]
- LECRUBIER Y., CLERC G., DIDI R., KIESER M. Efficacy of St- John's wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am. J. Psychiatry. 2002;159:1361–1366. doi: 10.1176/appi.ajp.159.8.1361. [DOI] [PubMed] [Google Scholar]
- MAZEI M.S., PLUTO C.P., KIRKBRIDE B., PEHEK E.A. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- MISANE I., ÖGREN S.O. Effects of Hypericum perforatum (St John's wort) on passive avoidance in the rat: evaluation of potential neurochemical mechanisms underlying its antidepressant activity. Pharmacopsychiatry. 2001;34:S89–S97. doi: 10.1055/s-2001-15449. [DOI] [PubMed] [Google Scholar]
- MULLER W.E. Current St John's wort research from mode of action to clinical efficacy. Pharmacol. Res. 2003;47:101–109. doi: 10.1016/s1043-6618(02)00266-9. [DOI] [PubMed] [Google Scholar]
- MULLER W.E., ROLLI M., SCHAFER C., HAFNER U. Effects of Hypericum extract (LI 160) in biochemical models of antidepressant activity. Pharmacopsychiatry. 1997;30:S102–S107. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- MULLER W.E., SINGER A., WONNEMANN M., HAFNER U., ROLLI M., SCHAFER C. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31:S16–S21. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates. New York: Academic Press; 1997. [Google Scholar]
- ROMMELSPACHER H., SIEMANOWITZ B., MANNEL M. Acute and chronic actions of a dry methanolic extract of Hypericum perforatum and a hyperforin-rich extract on dopaminergic and serotonergic neurones in rat nucleus accumbens. Pharmacopsychiatry. 2001;34:S119–S126. doi: 10.1055/s-2001-15441. [DOI] [PubMed] [Google Scholar]
- ROZ N., REHAVI M. Hyperforin inhibits vesicular uptake of monoamines by dissipating pH gradient across synaptic vesicle membrane. Life Sci. 2003;73:461–470. doi: 10.1016/s0024-3205(03)00295-9. [DOI] [PubMed] [Google Scholar]
- SINGER A., WONNEMANN M., MULLER W.E. Hyperforin, a major antidepressant constituent of St John's Wort, inhibits serotonin uptake by elevating free intracellular Na+1. J. Pharmacol. Exp. Ther. 1999;290:1363–1368. [PubMed] [Google Scholar]
- SUZUKI O., KATSUMATA Y., OYA M., BLADT S., WAGNER H. Inhibition of monoamine oxidase by hypericin. Planta Med. 1984;50:272–274. doi: 10.1055/s-2007-969700. [DOI] [PubMed] [Google Scholar]
- YOSHITAKE T., FUJINO K., KEHR J., ISHIDA J., NOHTA H., YAMAGUCHI M. Simultaneous determination of norepinephrine, serotonin, and 5-hydroxyindole-3-acetic acid in microdialysis samples from rat brain by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal. Biochem. 2003;312:125–133. doi: 10.1016/s0003-2697(02)00435-9. [DOI] [PubMed] [Google Scholar]
- YOSHITAKE T., KEHR J., YOSHITAKE S., FUJINO K., NOHTA H., YAMAGUCHI M.Determination of serotonin, noradrenaline, dopamine and their metabolites in rat brain extracts and microdialysis samples by column liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine J. Chromatogr. B 2004(in press) [DOI] [PubMed]