Abstract
Carbon monoxide (CO), one of the end products of heme catabolism by heme oxygenase, possesses antihypertensive and vasodilatory characteristics. We have recently discovered that certain transition metal carbonyls are capable of releasing CO in biological fluids and modulate physiological functions via the delivery of CO. Because the initial compounds identified were not water soluble, we have synthesized new CO-releasing molecules that are chemically modified to allow solubility in water. The aim of this study was to assess the vasoactive properties of tricarbonylchloro(glycinato)ruthenium(II) (CORM-3) in vitro and in vivo.
CORM-3 produced a concentration-dependent relaxation in vessels precontracted with phenylephrine, exerting significant vasodilatation starting at concentrations of 25–50 μM. Inactive CORM-3, which does not release CO, did not affect vascular tone.
Blockers of ATP-dependent potassium channels (glibenclamide) or guanylate cyclase activity (ODQ) considerably reduced CORM-3-dependent relaxation, confirming that potassium channels activation and cGMP partly mediate the vasoactive properties of CO. In fact, increased levels of cGMP were detected in aortas following CORM-3 stimulation.
The in vitro and in vivo vasorelaxant activities of CORM-3 were further enhanced in the presence of YC-1, a benzylindazole derivative which is known to sensitize guanylate cyclase to activation by CO. Interestingly, inhibiting nitric oxide production or removing the endothelium significantly decreased vasodilatation by CORM-3, suggesting that factors produced by the endothelium influence CORM-3 vascular activities.
These results, together with our previous findings on the cardioprotective functions of CORM-3, indicate that this molecule is an excellent prototype of water-soluble CO carriers for studying the pharmacological and biological features of CO.
Keywords: Carbon monoxide-releasing molecules (CO-RMs), nitric oxide (NO), vascular tone, guanylate cyclase
Introduction
Carbon monoxide (CO) is now regarded as a versatile signalling molecule having essential regulatory roles in a variety of physiological and pathophysiological processes (Marks et al., 1991; Motterlini et al., 2002b; Otterbein, 2002). Mammalian cells constantly generate CO gas via the endogenous degradation of heme by a family of constitutive (HO-2) and inducible (HO-1) heme oxygenase enzymes (Tenhunen et al., 1969; Maines, 1997). Endogenous CO possesses vasorelaxing properties (Coceani et al., 1997) through stimulation of soluble guanylate cyclase and potassium channels (Wang et al., 1997; Wang, 1998) and appears to modulate sinusoidal tone in the hepatic circulation (Suematsu et al., 1995). In addition, CO controls the proliferation of vascular smooth muscle cells (Morita et al., 1997; Durante & Schafer, 1998) and suppresses the rejection of transplanted hearts (Sato et al., 2001). Exogenously applied CO also exerts potent anti-inflammatory effects (Otterbein et al., 2000), prevents endothelial cell apoptosis (Brouard et al., 2000) and promotes protection against hyperoxic as well as ischemic lung injury (Otterbein et al., 1999; Fujita et al., 2001). These findings led to the idea of a therapeutic use of this gas for the treatment of vascular dysfunction and immuno-related diseases (Otterbein, 2002). To this end, the discovery of compounds capable of transporting and delivering CO to tissues and organs could accelerate and facilitate the use of CO as a pharmaceutical agent (Johnson et al., 2003; Motterlini et al., 2003).
Our group has recently reported that certain transition metal carbonyls possess the ability to liberate CO under appropriate conditions and function as CO-releasing molecules (CO-RMs) in biological systems. In particular, we have shown that CO-RMs induce vessel relaxation in isolated aortic tissue and prevent coronary vasoconstriction as well as acute hypertension in vivo (Motterlini et al., 2002a). Based on these data, we intensified our studies on the chemical features of carbonyl complexes in order to design new compounds that have different rates of CO release and are more compatible with biological systems. This was required since many carbonyl-based compounds described in the literature are soluble only in organic solvents, and physical (e.g. irradiating light) or chemical (e.g. strong ligands) stimuli are generally necessary to enable CO dissociation from these complexes (Motterlini et al., 2002b). Interestingly, the versatile chemistry of transition metals allows them to be effectively modified by coordinating biological ligands to the metal center in order to render the molecule less toxic, more water soluble and to modulate the release of CO. The initial studies have provided the first example of a pharmacologically active water-soluble metal carbonyl complex, tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), which was obtained by coordinating glycine onto the metal center. This compound liberates CO in vitro, ex vivo and in vivo biological models (Motterlini et al., 2003), and has been shown to protect myocardial cells and isolated rat hearts against ischemia–reperfusion injury as well as cardiac allograft rejection in mice (Clark et al., 2003). More recently, a study using a model of myocardial infarction in mice revealed the ability of CORM-3 to limit ischemia–reperfusion injury in vivo (Guo et al., 2004). These results prompted us to further investigate whether water-soluble CO-RMs can be effectively utilized to modulate specific vascular functions. Here we report our findings on the vasoactive properties of CORM-3 and describe its possible mechanism(s) of action as a regulator of vessel tone and blood pressure in vitro and in vivo.
Methods
Synthesis of CORM-3 and chemicals
CORM-3 was synthesized as previously described (Clark et al., 2003). CORM-3 was freshly prepared before the experiments by dissolving the compound in distilled water. Inactive CORM-3 (iCORM-3) was obtained by leaving CORM-3 in Krebs–Henseleit buffer overnight at room temperature. This treatment produced a compound that does not release CO and, therefore, iCORM-3 was used as a negative control to assess the direct involvement of CO in the pharmacological actions of CORM-3. As already reported (Clark et al., 2003), approximately 1 mol of CO per mole of CORM-3 is liberated within 10 min after addition to Krebs–Henseleit buffer. 1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one (ODQ), glibenclamide and 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) were from Alexis Corporation (Nottingham, U.K.). All other chemicals used in the study were from Sigma, unless otherwise specified.
Aortic rings preparation
Transverse ring sections of aortas were obtained from male adult Sprague–Dawley rats (350 g) and suspended under 2 g tension in oxygenated Krebs–Henseleit buffer as previously described (Sammut et al., 1998; Clark et al., 2003). To establish the potential vasorelaxant effects of CORM-3, aortic rings were precontracted with phenylephrine (1 μM) before addition of CORM-3 at different concentrations (25–200 μM). CORM-3 was added three times at 6–10 min intervals. Additional experiments were conducted to examine the involvement of cGMP and ATP-dependent potassium channels (KATP) in the relaxation mediated by CORM-3. For these purposes, aortic rings were incubated with the inhibitor of guanylate cyclase ODQ (10 μM) or the KATP blocker glibenclamide (10 μM) 15 or 30 min prior to addition of phenylephrine, respectively. The effect of CORM-3 on vascular tone was also assessed in the presence of the stimulator of soluble guanylate cyclase, YC-1 (1 μM), which was incubated 30 min prior to addition of phenylephrine. Preliminary experiments were conducted in order to identify the concentration of YC-1 that did not significantly affect the response of aortic rings to pheny-lephrine. Indeed, in our experimental model concentrations of YC-1, higher than 1 μM reduced the phenylephrine-mediated contraction by various degrees. This is in line with previous studies showing that higher concentrations of phenylephrine were required to induce stable constriction in YC-1-pretreated rabbit vessels (Galle et al., 1999). To investigate the possible involvement of nitric oxide (NO) in the relaxation elicited by CORM-3, a separate group of experiments was performed by treating aortic rings with the NO synthase inhibitor NG-nitro-L-arginine-methyl ester (L-NAME, 100 μM) 30 min prior to addition of phenylephrine. Finally, CORM-3 was also tested in endothelium-denuded rings. For this purpose, the internal lumen of rings was subjected to gentle rubbing with a fine wooden stick, and failure to dilate upon addition of acetyl-choline was taken as a proof of successful endothelium removal.
Measurements of blood pressure in vivo
For the in vivo experiments, anesthetized adult Lewis rats (290–330 g) were chronically catheterized and blood pressure was continuously monitored prior to and after administration of various drugs as previously reported (Motterlini et al., 1998; 2002a). Animals were anesthetized using enfluorane as an inhalation anesthetic. Surgical anesthesia was maintained throughout the operation using oxygen in combination with enfluorane. The effect of CORM-3 on mean arterial pressure (MAP) was assessed following two consecutive injections (30 μmol kg−1, i.v.) given at 20 min intervals. Similar experiments were performed by administering YC-1 (1.2 μmol kg−1, i.v.) to animals 5 min prior to the first bolus addition of CORM-3.
Determination of aortic cGMP levels
Aortas were collected and allowed to recover for 1 h in oxygenated Krebs–Henseleit buffer before treatment with CORM-3. Aortas were freezed-clamped 8 min following one, two or three consecutive bolus additions of CORM-3 (100 μM), each addition given at 8 min intervals. This interval was chosen based on the time required by aortic rings to reach a stable relaxation upon addition of CORM-3. Levels of cGMP were measured in aortic tissue extracts using a commercial ELISA kit (Amersham) following the manufacturers' instructions. Four aortas per group were used for the assay. cGMP levels were expressed as fmol mg−1 of aortic tissue.
Determination of soluble guanylate cyclase activity
Guanylate cyclase activity was measured as previously described (Friebe et al., 1996). NO-sensitive guanylate cyclase was purified from bovine lung to apparent homogeneity by an immunoaffinity purification procedure as described (Humbert et al., 1990). Cyclase activity was measured by the conversion of [α-32P]GTP to [32P]cGMP at 37°C for 20 min in the presence of increasing concentrations (0–5000 μM) of CORM-3. Reaction mixtures contained 30 ng of purified guanylate cyclase, 3 mM Mg2+ as divalent metal ion, 3 mM dithiothreitol, 0.5 mg ml−1 bovine serum albumin, 1 mM cGMP, 500 μM GTP and 50 mM triethanolamine hydrochloride, pH 7.4, in a total volume of 0.1 ml. Reactions were stopped by ZnCO3 precipitation, and [32P]cGMP was isolated as described (Schultz & Bohme, 2004). Some experiments were performed by including YC-1 (200 μM) in the reaction mixture. All measurements were performed in duplicates and repeated five times. YC-1 was dissolved in DMSO. The final DMSO concentration in all samples did not exceed 2% (v v−1), a concentration that by itself did not influence guanylate cyclase activity. A 30 mM stock solution of CORM-3 was prepared by dissolving the compound in H2O. Further dilutions were prepared in 10 mM HCl.
Statistical analysis
Vasodilatory responses were expressed as percentage of the vasoconstriction induced by phenylephrine. Statistical analysis was performed using two-way ANOVA combined with Bonferroni test. Differences were considered to be significant at P<0.05.
Results
Effect of CORM-3 on vascular tone in vitro and blood pressure in vivo
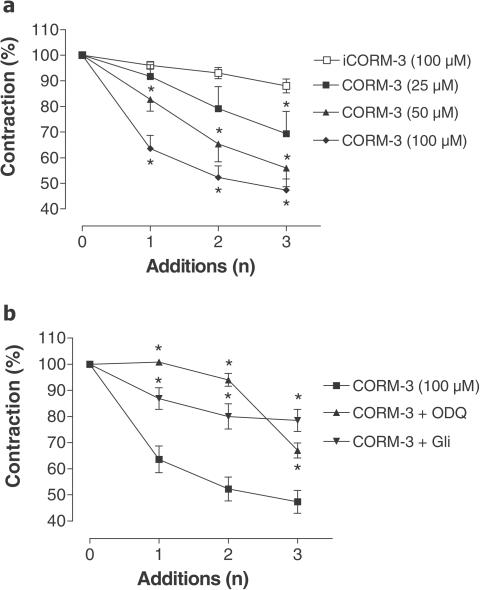
Figure 1 shows that CORM-3 relaxed precontracted aortic rings and Figure 2a confirms that this effect is concentration-dependent. In contrast, iCORM-3 did not elicit evident relaxation (see Figures 1 and 2a), demonstrating that CO released from CORM-3 is the factor involved in the modulation of vessel tone. CORM-3 also elicited vasodilatation of aortic rings incubated at low oxygen tension (data not shown). In addition, higher concentrations of phenylephrine (>10 μM as opposed to 1 μM) were necessary to contract aortas pretreated with CORM-3, suggesting that the compound has long-lasting effects. It is well known that CO activates guanylate cyclase to increase the production of cGMP (Utz & Ullrich, 1991; Morita et al., 1995; Sammut et al., 1998). Consistent with this notion, the vasorelaxant effect of CORM-3 was significantly inhibited in the presence of ODQ (Figure 2b). Interestingly, this inhibition was partially lost following the third addition of CORM-3. Similarly, we observed that glibenclamide, an inhibitor of KATP potassium channels, significantly prevented CORM-3-mediated vaso-relaxation. (Figure 2b). When rings were preincubated with the activator of soluble guanylate cyclase, YC-1 (1 μM), the vasoactivity of CORM-3 was markedly intensified at all the CORM-3 concentrations tested (Figure 3), further sustaining the direct contribution of cGMP in the effect elicited by CO. These data are in line with previous reports showing that YC-1 potentiates the vasorelaxation mediated by CO gas (McLaughlin et al., 2000). Administration of CORM-3 to rats in vivo produced a significant decrease in blood pressure (Figure 4), which was evident following the two bolus injections of CORM-3 administered at 20 min intervals. According to our recent measurements (Motterlini et al., 2003), the half-life of CORM-3 in plasma is very short (∼3.6 min in human plasma). Therefore, a 20 min interval after the first administration of CORM-3 should be an adequate period of time for complete dissociation of CO from CORM-3 prior to the second administration. However, it has to be pointed out that a short half-life does not necessarily imply that the vascular effects of CO liberated by CORM-3 are short-lasting. As observed with the aortic rings, pretreatment of animals with YC-1 markedly potentiated the decrease in MAP elicited by CORM-3 alone (Figure 4).
Figure 1.
Vasodilatation induced by CORM-3. Isometric recordings of aortic rings precontracted with phenylephrine (Phe, 1 μM) and subsequently subjected to a bolus addition of CORM-3 (100 μM) or iCORM-3 (100 μM). Typically, CORM-3 was added to the water bath containing aortic rings once phenylephrine had produced a stable contraction; CORM-3 caused relaxation within few minutes of addition whereas iCORM-3 was ineffective.
Figure 2.
Effect of CORM-3 on vascular tone: involvement of cGMP and KATP channels. (a) Vasodilatory responses of aortic rings subjected to three consecutive bolus additions of CORM-3 at different concentrations (25–100 μM). iCORM-3 (100 μM), the negative control, did not cause any evident relaxation. Vasodilatation is expressed as percentage of the maximal preconstriction. Data represent the mean±s.e.m. of 6–8 independent experiments. *P<0.05 compared to iCORM-3. (b) Vasodilatory responses of aortic rings subjected to three consecutive bolus additions of CORM-3 in the presence of ODQ (10 μM) or glibenclamide (Gli, 10 μM). ODQ and glibenclamide were added to the water bath 15 or 30 min prior to the addition of phenylephrine, respectively. Dilatations are expressed as percentage of preconstriction. Data represent the mean±s.e.m. of 6–8 independent experiments. *P<0.05 compared to 100 μM CORM-3 alone.
Figure 3.
YC-1 potentiates CORM-3-mediated vasodilatation. (a) Isometric recordings of aortic rings pretreated with 1 μM YC-1 (30 min prior to phenylephrine). As shown, the presence of YC-1 potentiated the dilatory responses of CORM-3 (50 μM). (b–d) Vasodilatory responses of aortic rings subjected to three consecutive bolus additions of CORM-3 (25, 50 and 100 μM, respectively) in the presence of 1 μM YC-1. Data represent the mean±s.e.m. of 6–8 independent experiments. *P<0.05 compared to CORM-3 alone.
Figure 4.
Changes in MAP in vivo following administration of CORM-3 in the presence or absence of YC-1. Animals were anesthetized and chronically catheterized as described in Methods. CORM-3 (30 μmol kg−1, i.v.) caused a sustained decrease in MAP that was potentiated by YC-1 (1.2 μmol kg−1, i.v.). Data represent the mean±s.e.m. of three independent experiments. *P<0.05 compared to control; **P<0.05 compared to CORM-3 alone.
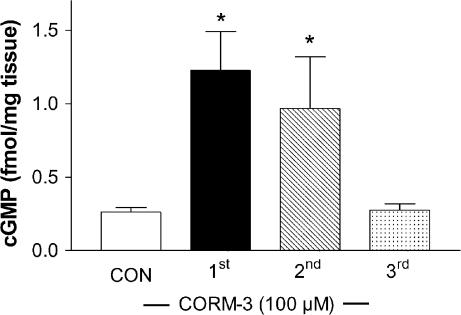
CORM-3 increases aortic cGMP levels
Consistent with the fact that ODQ was able to reduce the vascular relaxation elicited by CORM-3, we found that cGMP levels were elevated following the first and second addition of CORM-3 (100 μM) (Figure 5). cGMP significantly augmented from a basal level of 0.26±0.03 (control group, CON) to 1.23±0.3 and 0.97±0.4 fmol mg−1 tissue (P<0.05) after the first and second addition of CORM-3, respectively. In contrast, addition of a third bolus of CORM-3 did not change cGMP levels compared to control (0.27±0.02 fmol mg−1 tissue) (Figure 5).
Figure 5.
cGMP levels in aortas treated with CORM-3. Aortas were extracted and subjected to the same treatments with CORM-3 as for measurements of isometric tension. At 8 min after the first, second or third addition of CORM-3 (100 μM), aortas were snap-frozen and subsequently processed for cGMP assay. Control aortas (CON) were treated with vehicle (water). Data represent the mean±s.e.m. of four different aortas per group. *P<0.05 compared to control.
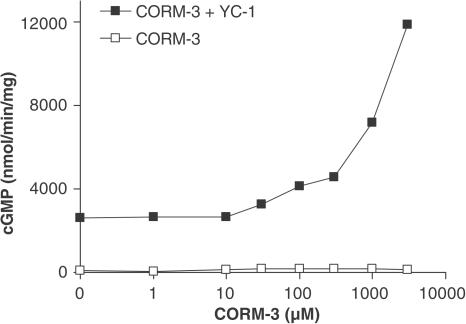
Activation of purified soluble guanylate cyclase by CORM-3
Since our results using the aortic ring model indicated that cGMP is involved in CORM-3-mediated relaxation, we wanted to determine the effect of CORM-3 on the activity of soluble guanylate cyclase. In contrast to the increase of cGMP levels in aortic tissue, CORM-3 per se did not stimulate the activity of purified soluble guanylate cyclase even when used at 3 mM concentrations (Figure 6). However, the presence of YC-1 sensitized guanylate cyclase to CORM-3 and the enzyme was concentration-dependently activated by the CO-releasing compound (Figure 6). A heme spectrum of guanylate cyclase in the presence of CORM-3 was performed and we observed a shift of the heme absorption to 423 nm (not shown); this confirms that CO is released from CORM-3 and binds to guanylate cyclase, since the same shift occurs with CO gas (Stone & Marletta, 1994).
Figure 6.
Effect of CORM-3 on purified guanylate cyclase activity. The activity of purified guanylate cyclase was measured following addition of increasing concentrations of CORM-3 (10–3000 μM) in the presence or absence of YC-1 (200 μM). As shown, CORM-3 was able to activate the enzyme only in the presence of YC-1. Data represent the mean±s.e.m. of five independent experiments (s.e.m. not showing because values are smaller than the size of the symbols).
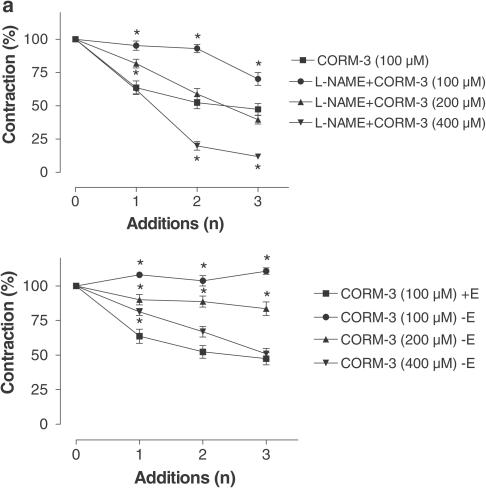
Effect of CORM-3 on vascular tone of aortic rings pretreated with a NO synthase inhibitor or endothelium-denuded rings
To assess whether CORM-3 could still vasodilate in the absence of endogenously generated NO, aortic rings were pretreated with L-NAME to block the NO synthase pathway. Surprisingly, the presence of L-NAME considerably prevented CORM-3-induced vasodilatation: for instance, following the first addition, CORM-3 caused approximately 37% relaxation but the presence of L-NAME in the organ bath reduced the extent of relaxation to 5% (P<0.05). Under these conditions, relaxation could be elicited by using much higher concentrations of CORM-3 (200 or 400 μM) (Figure 7a). These results prompted us to investigate whether the endothelium was a factor influencing CORM-3 vasoactivity. Intriguingly, also removal of the endothelium resulted in significant reduction of vasodilatation by CORM-3 and a small relaxation was only obtained with 200 or 400 μM of the compound (Figure 7b). These data together suggest that factors derived from the endothelium, including NO, may facilitate the action of CORM-3 and cooperate with CO in the regulation of aortic vessel tone.
Figure 7.
Blockade of NO production or removal of the endothelium reduces CORM-3-mediated dilatory responses. (a) Aortic rings were incubated with the inhibitor of NO synthase activity L-NAME (100 μM) for 30 min prior to contraction with phenylephrine. L-NAME markedly prevented relaxation caused by 100 μM CORM-3, and higher concentrations of the CO carrier (200 or 400 μM) were required to induce dilatation in the presence of L-NAME. Data represent the mean±s.e.m. of 6–8 independent experiments. *P<0.05 compared to 100 μM CORM-3 alone. (b) The endothelium of aortic rings was gently removed (−E) as described in Methods and the response to CORM-3 was compared to that of intact rings (+E). The absence of the endothelium markedly reduced CORM-3-mediated vasodilatation and higher CORM-3 concentrations were required to produce relaxation. Data represent the mean±s.e.m. of 6–8 independent experiments. *P<0.05 compared to 100 μM CORM-3 alone.
To examine the possibility that high concentrations of CORM-3 could exert vasodilatation via nonspecific effects, we also performed experiments in which endothelium-denuded aortas were exposed to 400 μM CORM-3 in the presence of glibenclimide (10 μM) or ODQ (10 μM). Considering that the percentage of contraction in endothelium-denuded aortas was 81±2.77, 67±4.15 and 51±4.45 following the first, second and third addition of 400 μM CORM-3, respectively, we found that glibenclamide reduced CORM-3-mediated vasorelaxation. In fact, the percentage of contraction in denuded vessels pretreated with glibenclamide was 99±0.028, 86±0.045 and 67±0.14 after the first, second and third addition of CORM-3, respectively. Blocking cGMP production also decreased the relaxation elicited by CORM-3 in denuded vessels, as the percentage of contraction in the presence of ODQ was 95±0.13, 74±0.17 and 52±0.13 following the first, second and third addition of CORM-3, respectively.
Discussion
The regulation of vascular tone is under the control of many factors, and NO appears to play a major role in physiological conditions. Activation of soluble guanylate cyclase, which as a heme-containing protein has a high affinity for NO, increases the production of cGMP and effectively transduces the NO signal into vasodilatation. There appear to exist also NO-independent mechanisms for relaxation, and CO, derived from the degradation of heme by heme oxygenase, possesses vasoactive properties (Motterlini et al., 1998; Sammut et al., 1998). The endothelium of vascular tissue expresses HO-2, the constitutive isoform of heme oxygenase, and CO derived from HO-2 likely affects vessel tone in normal conditions (Zakhary et al., 1996). In addition, HO-1 expression can be stimulated by a variety of stressful events with concomitant in-creased production of CO (Motterlini et al., 1998; Sammut et al., 1998); the most relevant effects observed in these non-physiological states are either prevention of vasoconstriction (Motterlini et al., 1998; 2002a; Sammut et al., 1998) or decrease in blood pressure (Yet et al., 1997; Wiesel et al., 2000). However, more investigations are required to define the role of CO in the control of vascular tone.
The present study reports findings on the vasorelaxant properties of CORM-3, a newly synthesized CO-RM that has been specifically designed to render it water soluble by coordinating a biologically compatible ligand (glycine) onto the metal center (Clark et al., 2003; Johnson et al., 2003; Motterlini et al., 2003). The water solubility is a significant advancement in the design of CO-RMs, as the previously identified CO-RMs were only soluble in organic solvents such as DMSO, posing justified doubts about the potential use of these compounds in vivo. Furthermore, it appears that both physiological pH and strong ligands present in the biological environment would enable dissociation of CO from the metal center; in contrast, tricarbonyldichlororuthenium(II) dimer (CORM-2) requires DMSO to liberate CO (Motterlini et al., 2003). We found that CORM-3 vasorelaxed precontracted aortic rings in a concentration-dependent manner and this effect was evident starting at 25 μM CORM-3. The magnitude of vasorelaxation was similar to that obtained with CORM-2, a DMSO-soluble CO-RM originally described in our previous publications (Motterlini et al., 2002a; 2003). In fact, we observed a 45% decrease in vessel contractility after the first addition of 200 μM CORM-3 (data not shown) compared to ∼50% with 222 μM CORM-2 (Motterlini et al., 2002a). The inactive form of the compound, iCORM-3, which does not release CO according to the myoglobin assay, did not elicit relaxation. The results strongly imply that CO released from CORM-3 is responsible for the significant decrease in tension observed in our model of isolated aortic rings. In addition, our data obtained with a blocker of KATP channels and an inhibitor of guanylate cyclase activity indicate that KATP channels and cGMP partially mediated this vasodilatory effect. The involvement of cGMP was also emphasized by increased levels of cGMP measured in aortas following the addition of CORM-3. Our data confirm previous observations obtained in other laboratories using CO gas (Hussain et al., 1997; Wang et al., 1997). The vasodilatory action mediated by CORM-3 in isolated vessels is supported by our results in vivo showing a hypotensive effect by this water-soluble metal carbonyl. The fact that YC-1, which sensitizes guanylate cyclase to CO and NO (Friebe et al., 1996; 1998), markedly enhanced CORM-3-induced vasorelaxation in vivo and ex vivo points again to guanylate cyclase as an important intermediary of CORM-3 vasoactivities. These findings also highlight that in the presence of YC-1, lower concentrations of CORM-3 are necessary for substantial vessel dilatation and the combination of the two compounds could be of interest from a pharmacological perspective. Based on these data, it is therefore intriguing that CORM-3 (even at 5 mM) did not activate purified soluble guanylate cyclase in in vitro experiments and only the presence of YC-1 allowed for activation of the enzyme by the CO carrier. In contrast, vasodilatation was observed in aortic rings with 25–200 μM and increased aortic cGMP levels were seen with 100 μM CORM-3 (lower concentrations of CORM-3 were not tested with the cGMP assay). Considering that the amount of CO released from CORM-3 is approximately equimolar (Clark et al., 2003), we can deduce that 100 μM CORM-3 will release ∼100 μM CO, a concentration of the gas sufficient to stimulate cGMP production in aortas. A reasonable explanation for this discrepancy is that the interaction of CO with soluble guanylate cyclase is different in intact tissue or cells compared to that reported with the purified enzyme (Friebe et al., 1996; Hussain et al., 1997; Sammut et al., 1998). Interestingly, the nitrovasodilator nitroglycerin also appears to be a more potent activator of soluble guanylate cyclase in intact cells than in cell-free preparations (Kleschyov et al., 2003).
A surprising finding of this study is that the relaxant properties of CORM-3 are markedly reduced when NO synthase activity is blocked or in endothelium-denuded vessels. Some possible reasons underlying these effects include the following: (1) the possibility that CO liberated from CORM-3 stimulates the displacement of NO from an intracellular storage pool as previously suggested by Thorup et al. (1999) in a study conducted in perfused renal resistance arteries; (2) the production in the endothelium of substances that synergize with CO or facilitate its dilatory activities (among these unidentified products, NO appears to be a major player); (3) endothelium-derived factors, such as NO, may coordinate with ruthenium and weaken the bond between carbonyl groups and the transition metal of the CORM-3 molecule, thus favoring the release of CO and allowing the pharmacological action of CORM-3. The last hypothesis is relevant since ruthenium complexes have a high affinity for NO and effectively scavenge NO in biological systems (Mosi et al., 2002). Furthermore, metal carbonyls are used in inorganic chemistry as starting materials for the preparation of metal nitrosyl complexes, taking advantage of the higher affinity of the metal for NO compared to carbonyl groups (Miranda et al., 1997). It has to be noted, however, that the vasodilatation elicited by YC-1 in rabbit aorta is also more pronounced in rings with intact endothelium compared with endothelium-denuded vessels (Galle et al., 1999), suggesting that endothelial cells have the ability to assist the relaxant effect of dilatory substances that act via the cGMP pathway. The fact that the vasorelaxation caused by 400 μM CORM-3 in denuded vessels was reduced by glibenclamide and ODQ suggests that guanylate cyclase and KATP channels still partially mediate the effects obtained with high concentrations of CORM-3.
In conclusion, our study shows that CORM-3 delivers CO and exerts pharmacological actions by modulating vessel tone ex vivo and blood pressure in vivo. It appears that CORM-3 vasoactivities are mediated by the same pathways (cGMP and potassium channels) utilized by endogenously formed CO and exogenously applied CO gas (Hussain et al., 1997; Sammut et al., 1998), emphasizing that CO-RMs could be an excellent research tool for the identification of cellular targets and molecular mechanisms that characterize the signalling properties of CO. In addition, our findings support the notion that water-soluble metal carbonyls could be utilized as prototypic chemicals in the development of pharmacologically active compounds capable of delivering CO for the control of vascular functions and prevention of hypertension.
Acknowledgments
We thank the Dunhill Medical Trust for financial support. This study was partially supported by a grant from the British Heart Foundation (FS/02/027). Brian E. Mann and Roberto Motterlini have financial interest with hemoCORM Ltd.
Abbreviations
- CO
carbon monoxide
- CO-RMs
carbon monoxide-releasing molecules
- HO-1
heme oxygenase-1
- KATP
ATP-dependent potassium channels
- L-NAME
NG-nitro-L-arginine-methyl ester
- MAP
mean arterial pressure
- NO
nitric oxide
- ODQ
1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one
- YC-1
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole
References
- BROUARD S., OTTERBEIN L.E., ANRATHER J., TOBIASCH E., BACH F.H., CHOI A.M., SOARES M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK J.E., NAUGHTON P., SHUREY S., GREEN C.J., JOHNSON T.R., MANN B.E., FORESTI R., MOTTERLINI R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- COCEANI F., KELSEY L., SEIDLITZ E., MARKS G.S., MCLAUGHLIN B.E., VREMAN H.J., STEVENSON D.K., RABINOVITCH M., ACKERLEY C. Carbon monoxide formation in the ductus arteriosus in the lamb: implications for the regulation of muscle tone. Br. J. Pharmacol. 1997;120:599–608. doi: 10.1038/sj.bjp.0700947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURANTE W., SCHAFER A.I. Carbon monoxide and vascular cell function (review) Int. J. Mol. Med. 1998;2:255–262. doi: 10.3892/ijmm.2.3.255. [DOI] [PubMed] [Google Scholar]
- FRIEBE A., MULLERSHAUSEN F., SMOLENSKI A., WALTER U., SCHULTZ G., KOESLING D. YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol. Pharmacol. 1998;54:962–967. doi: 10.1124/mol.54.6.962. [DOI] [PubMed] [Google Scholar]
- FRIEBE A., SCHULTZ G., KOESLING D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- FUJITA T., TODA K., KARIMOVA A., YAN S.F., NAKA Y., YET S.F., PINSKY D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- GALLE J., ZABEL U., HUBNER U., HATZELMANN A., WAGNER B., WANNER C., SCHMIDT H.H. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO Y., STEIN A.B., WU W.J., TAN W., ZHU X., LI Q.H., DAWN B., MOTTERLINI R., BOLLI R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMBERT P., NIROOMAND F., FISCHER G., MAYER B., KOESLING D., HINSCH K.D., GAUSEPOHL H., FRANK R., SCHULTZ G., BOHME E. Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur. J. Biochem. 1990;190:273–278. doi: 10.1111/j.1432-1033.1990.tb15572.x. [DOI] [PubMed] [Google Scholar]
- HUSSAIN A.S., MARKS G.S., BRIEN J.F., NAKATSU K. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ) inhibits relaxation of rabbit aortic rings induced by carbon monoxide, nitric oxide, and glyceryl trinitrate. Can. J. Physiol. Pharmacol. 1997;75:1034–1037. [PubMed] [Google Scholar]
- JOHNSON T.R., MANN B.E., CLARK J.E., FORESTI R., GREEN C.J., MOTTERLINI R. Metal carbonyls: a new class of pharmaceuticals. Angew. Chem. Int. Ed. Engl. 2003;42:3722–3729. doi: 10.1002/anie.200301634. [DOI] [PubMed] [Google Scholar]
- KLESCHYOV A.L., OELZE M., DAIBER A., HUANG Y., MOLLNAU H., SCHULZ E., SYDOW K., FICHTLSCHERER B., MULSCH A., MUNZEL T. Does nitric oxide mediate the vasodilator activity of nitroglycerin. Circ. Res. 2003;93:e104–e112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- MAINES M.D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- MARKS G.S., BRIEN J.F., NAKATSU K., MCLAUGHLIN B.E. Does carbon monoxide have a physiological function. Trends Pharmacol. Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN B.E., CHRETIEN M.L., CHOI C., BRIEN J.F., NAKATSU K., MARKS G.S. Potentiation of carbon monoxide-induced relaxation of rat aorta by YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole] Can. J. Physiol. Pharmacol. 2000;78:343–349. [PubMed] [Google Scholar]
- MIRANDA K.M., BU X., LORKOVIC I., FORD P.C. Synthesis and structural characterization of several ruthenium porphyrin nitrosyl complexes. Inorg. Chem. 1997;36:4838–4848. doi: 10.1021/ic970065b. [DOI] [PubMed] [Google Scholar]
- MORITA T., MITSIALIS S.A., KOIKE H., LIU Y.X., KOUREMBANAS S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J. Biol. Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- MORITA T., PERRELLA M.A., LEE M.E., KOUREMBANAS S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSI R., SEGUIN B., CAMERON B., AMANKWA L., DARKES M.C., FRICKER S.P. Mechanistic studies on AMD6221: a ruthenium-based nitric oxide scavenger. Biochem. Biophys. Res. Commun. 2002;292:519–529. doi: 10.1006/bbrc.2002.6685. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., CLARK J.E., FORESTI R., SARATHCHANDRA P., MANN B.E., GREEN C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 2002a;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., FORESTI R., GREEN C.J.Studies on the development of carbon monoxide-releasing molecules: potential applications for the treatment of cardiovascular dysfunction Carbon Monoxide and Cardiovascular Functions 2002bBoca Raton, FL: CRC Press; 249–271.ed. Wang, R. pp [Google Scholar]
- MOTTERLINI R., GONZALES A., FORESTI R., CLARK J.E., GREEN C.J., WINSLOW R.M. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ. Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., MANN B.E., JOHNSON T.R., CLARK J.E., FORESTI R., GREEN C.J. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr. Pharm. Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid. Redox. Signal. 2002;4:309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., BACH F.H., ALAM J., SOARES M., TAO LU H., WYSK M., DAVIS R.J., FLAVELL R.A., CHOI A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., MANTELL L.L., CHOI A.M.K. Carbon monoxide provides protection against hyperoxic lung injury. Am. J. Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- SAMMUT I.A., FORESTI R., CLARK J.E., EXON D.J., VESELY M.J.J., SARATHCHANDRA P., GREEN C.J., MOTTERLINI R. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haem oxygenase-1. Br. J. Pharmacol. 1998;125:1437–1444. doi: 10.1038/sj.bjp.0702212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO K., BALLA J., OTTERBEIN L., SMITH R.N., BROUARD S., LIN Y., CSIZMADIA E., SEVIGNY J., ROBSON S.C., VERCELLOTTI G., CHOI A.M., BACH F.H., SOARES M.P. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- SCHULTZ G., BOHME E.Guanylate cyclase. GTP pyrophosphate-lyase (cyclizing), E. C. 4.6.1.2 Methods of Enzymatic Analysis 2004Weinheim, Germany: Verlag Chemie; 379–389.ed. Bergmeyer, H.U., Bergmeyer, J. & Gra ß l, M. pp [Google Scholar]
- STONE J.R., MARLETTA M.A. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- SUEMATSU M., GODA N., SANO T., KASHIWAGI S., EGAWA T., SHINODA Y., ISHIMURA Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J. Clin. Invest. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENHUNEN R., MARVER H.S., SCHMID R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- THORUP C., JONES C.L., GROSS S.S., MOORE L.C., GOLIGORSKY M.S. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am. J. Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- UTZ J., ULLRICH V. Carbon monoxide relaxes ileal smooth muscle through activation of guanylate cyclase. Biochem. Pharmacol. 1991;41:1195–1201. doi: 10.1016/0006-2952(91)90658-r. [DOI] [PubMed] [Google Scholar]
- WANG R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can. J. Physiol. Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- WANG R., WANG Z.Z., WU L.Y. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br. J. Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL P., PATEL A.P., DIFONZO N., MARRIA P.B., SIM C.U., PELLACANI A., MAEMURA K., LEBLANC B.W., MARINO K., DOERSCHUK C.M., YET S.F., LEE M.E., PERRELLA M.A. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- YET S.F., PELLACANI A., PATTERSON C., TAN L., FOLTA S.C., FOSTER L., LEE W.S., HSIEH C.M., PERRELLA M.A. Induction of heme oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J. Biol. Chem. 1997;272:4295–4301. doi: 10.1074/jbc.272.7.4295. [DOI] [PubMed] [Google Scholar]
- ZAKHARY R., GAINE S.P., DINERMAN J.L., RUAT M., FLAVAHAN N.A., SNYDER S.H. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]