Abstract
Acute promyelocytic leukaemia (APL) is characterized by a block in differentiation at the promyelocyte stage. Here, we describe the effects of auranofin (AF), a coordinated gold compound, on apoptosis and differentiation of APL cells.
Nucleosomal DNA fragmentation assay and Hoechst 33342 staining indicated that AF induced apoptosis in APL-derived NB4 cells at low concentrations (0.5–1.0 μM). The AF-induced apoptosis involved caspase-3 activation and specific cleavage of poly-ADP-ribose polymerase.
The AF-treated NB4 cells also produced reactive oxygen species (ROS) and cotreatment with N-acetyl-L-cysteine protected the NB4 cells from AF-induced apoptosis.
Expression of the CD11b cell surface marker and C/EBPɛ was increased when the cells were treated for 4 days with 0.3 μM AF and a physiological concentration of all-trans retinoic acid (ATRA, 5 nM). Treatment with AF in combination with ATRA markedly increased the number of cells with differentiated features, such as lobed or multiple nuclei and numerous granules and vacuoles. At these low concentrations, neither AF nor ATRA alone induced significant cell differentiation.
These findings suggest not only that AF induces caspase-3-dependent apoptosis via a mechanism involving ROS, but also that the combined treatment with AF and ATRA induces differentiation of NB4 cells. Our results demonstrate a novel characteristic of AF from which an effective drug treatment of APL might be developed.
Keywords: Acute promyelocytic leukaemia, apoptosis, auranofin, differentiation, retinoic acid, NB4 cell line
Introduction
Acute promyelocytic leukaemia (APL) is cytogenetically characterized by a reciprocal chromosomal translocation (t(15;17)) that juxtaposes the promyelocytic leukaemia (PML) gene on chromosome 15 and the retinoic acid receptor alpha (RARα) gene on chromosome 17. The protein resulting from the fusion of the genes for PML and RARα plays an important role in the pathogenesis of APL. APL presents as an abnormal accumulation of promyelocytic cells caused by a failure in cell differentiation. As pharmacologically high doses of all-trans retinoic acid (ATRA) can overcome this failure and induce neutrophilic differentiation of the APL cells both in vitro and in vivo, differentiation-inducing therapy with ATRA has been used in APL patients (Grignani et al., 1994; Mistry et al., 2003). ATRA therapy is more effective in inducing remission and has fewer complications than chemo-therapy. Moreover, combined treatment with ATRA and chemotherapy has markedly improved remission rates and survival in APL patients (Tallman et al., 2002). Despite this improved treatment regime, approximately 30% of patients relapsed within 4–5 years. Most of the relapsed APL patients are resistant to further treatment with ATRA (Gallagher, 2002).
Recent studies have reported a biphasic action of arsenic trioxide (As2O3) on ATRA-sensitive or -resistant APL cells, demonstrating that As2O3 induced apoptosis at relatively high concentrations (0.5–2 μM) and partial differentiation at lower concentrations (0.1–0.5 μM) (Chen et al., 1997). Although the mechanism of action of As2O3 is not fully understood, several studies have provided evidence that the reactive oxygen species (ROS) signalling pathways and PML-RARα degradation are involved in its effect (Shao et al., 1998; Jing et al., 1999; Davison et al., 2002). Based on these in vitro results, As2O3 has been effectively used to reinduce remission in relapsed APL patients (Shen et al., 1997; Agis et al., 1999; Niu et al., 1999; Soignet et al., 2001).
Auranofin (AF; 2,3,4,6-tetra-o-acetyl-1-thio-β-D-gluco-pyranosato-S-(triethyl-phosphine) gold) is a coordinated gold compound, which has been widely used for the treatment of rheumatoid arthritis, based on its anti-inflammatory and immunosuppressive properties (Blodgett, 1983; Snyder et al., 1987; Hashimoto et al., 1992). During our study of the anti-inflammatory properties of AF, interesting new findings emerged. It was demonstrated that 1.0 μM AF induced apoptosis and a combination of low concentrations of ATRA and AF (5 nM and 0.3 μM, respectively) induced cell differentiation in the APL-derived NB4 cell line.
AF is a thiol-reactive organometallic compound that changes the intracellular redox status (Gromer et al., 1998; Becker et al., 2000). As2O3 also interacts with redox enzymes that have thiol groups and depletes intracellular reduced glutathione (GSH) by forming a complex with GSH (Snow, 1992; Scott et al., 1993; Davison et al., 2002). Hence, it is possible that AF will act in a similar manner to As2O3 on apoptosis and differentiation.
In this paper, the dual effects of AF on apoptosis and differentiation of APL cells are described and the molecular bases for these effects are discussed. This is the first study to provide data, on the basis of which, AF alone, or in combination with ATRA, may be used as a novel APL therapy.
Methods
Cell treatment
The NB4 APL cell line was maintained in RPMI 1640 medium containing 10% foetal calf serum. To induce apoptosis, NB4 cells were plated at a density of 1 × 105/ml and treated with 1 μM AF for the periods indicated. To investigate effects on differentiation, the cells were treated with 0.3 μM AF and 5 nM ATRA in combination for 4 days.
Western blot analysis
The cells were washed twice with PBS, and then lysed in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethyl-sulphonyl fluoride, 100 μg ml−1 trypsin inhibitor, 50 μM leupeptin, 100 μM antipain, pH 8.0). After the protein concentration of each lysate had been determined, equal amounts of the samples were loaded onto a 10% SDS–polyacrylamide gel. Proteins were transferred to nitrocellulose membrane and detected with polyclonal antibodies against human caspase-3 and C/EBPɛ, or anti-PARP (poly-ADP-ribose polymerase) monoclonal antibody using an enhanced chemiluminescence detection kit.
FACS analysis
A direct immunofluorescence staining technique was used to detect the CD11b cell surface marker. Briefly, the cells that were treated with AF alone or a combination of AF and ATRA for 4 days were washed twice with buffer A (PBS, 0.1% sodium azide, 1% heat-inactivated foetal bovine serum). After being resuspended in buffer A, an aliquot of cells (1 × 106 cells, 50 μl) was incubated with R-phycoerythrin (RPE)-conjugated monoclonal mouse anti-human CD11b antibody (1 : 200) on ice for 30 min. For isotype control staining, an aliquot of cells was incubated with RPE-conjugated mouse IgG1. The incubated cells were washed again with buffer A, resuspended in 500 μl of buffer A containing propidium iodine, and then analysed with a FACScan flow cytometer (BD Biosciences, San Diego, CA, U.S.A.).
ROS determination
The production of ROS was detected using a previously described method (Royall & Ischiropoulos, 1993), with minor modifications. Briefly, 1 × 105/ml of NB4 cells were labelled with 0.5 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) for 1 h and then incubated in the absence of presence of 1 μM AF for 24 h. Within the cells, DCFH-DA is converted to DCFH, which can be oxidized to the fluorescent compound DCF in the presence of ROS. After incubation with AF, the cells were washed twice with PBS and analysed on a fluorescence microscope (excitation and emission wavelength: 495 and 525 nm, respectively) (Carl Zeiss, Oberkochen, Germany).
DNA fragmentation and cell morphology
Both treated and untreated (1 μM AF for 24 h) NB4 cells were washed twice with cold PBS. The cells were lysed overnight at 50°C in 500 μl of lysis buffer (50 mM Tris-HCl pH 7.6, 20 mM EDTA, 1% SDS) containing proteinase K (100 μg ml−1). To precipitate DNA, 0.2 ml of 5 M NaCl and an equal volume of isoamyl alcohol were added. The DNA pellet was dissolved in TE buffer, treated with 50 μg ml−1 of RNase A for 4 h at 37°C, and then electrophoresed on a 1.5% agarose gel.
For morphological observations, the AF-treated cells were incubated in Hoechst 33342 staining solution (10 μM) for 30 min at 37°C. After being spread onto slides by cytospin-centrifugation (700 r.p.m., 5 min), the stained cells were examined under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Quantification of apoptotic cells
DNA fragmentation caused by apoptosis was also quantified using a cellular DNA fragmentation ELISA kit (Boehringer Mannheim, Germany) according to the procedures recommended by the manufacturer. The cells (1 × 105/ml) were labelled with bromodeoxyuridine (BrdU) overnight and the labelled cells (1 × 104) were treated with various concentrations of AF for 6 h. Subsequently, the cells were lysed in lysis buffer and the BrdU-labelled DNA fragments were measured by an ELISA procedure.
Materials
The NB4 cell line was kindly provided by Dr Michel Lanotte (INSERM unit 301, Hospital Saint Louis, Paris, France), and AF was obtained from Dr Jue in The Catholic University of Korea. RPMI 1640 medium, ATRA, Nonidet P-40, sodium deoxycholate, phenylmethylsulphonyl fluoride, trypsin in-hibitor, leupeptin, antipain, proteinase K, propidium iodine, Hoechst 33342 and Giemsa stain were purchased from Sigma Chem. Co. (St Louis, MO, U.S.A.). RNase A and DNA fragmentation ELISA kits were obtained from Boehringer Mannheim (Mannheim, Germany). Foetal calf serum and chemiluminescence detection kit were from HyClone (Logan, UT, U.S.A.) and Amersham (Buckinghamshire, U.K.), respectively. Antibodies for caspase-3 and PARP were purchased from Pharmingen (San Diego, CA, U.S.A.), and anti-C/EBPɛ antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). RPE-conjugated antibodies (CD11b and mouse IgG1) were obtained from DAKO (Denmark). All other chemicals used in the study were of molecular biology grade.
Results
AF induces apoptosis of NB4 cells through caspase-3 activation
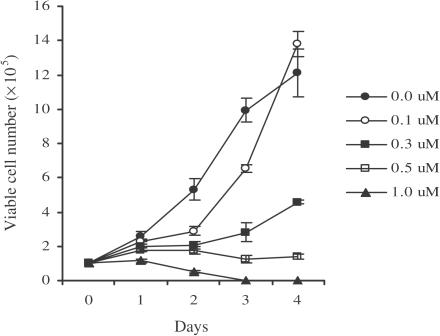
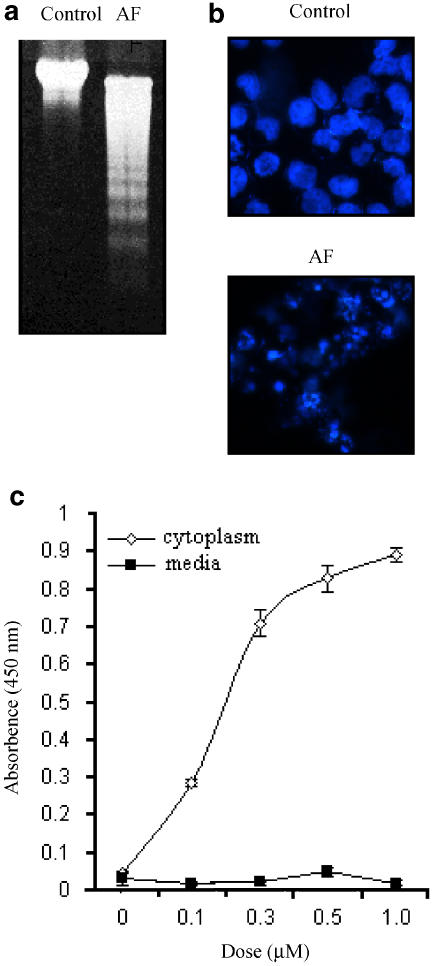
The effect of AF on NB4 cell growth is shown in Figure 1. Cell growth was retarded at low concentrations of AF (0.3 μM), while concentrations greater than 0.5 μM of AF were cytotoxic. To confirm whether the AF-induced cell death was due to apoptosis, DNA fragmentation was analysed in AF-treated NB4 cells. As shown in Figure 2a and b, an apparent DNA ladder and fragmented nuclei were observed when the cells were treated with 1 μM AF. In addition, the BrdU-labelled DNA fragments were detected only in the cytoplasm of cells incubated with AF, but not in the culture media (Figure 2c), indicating that AF-induced cell death is not the result of cell lysis due to the loss of membrane integrity. These findings suggest that AF-induced cell death was caused by apoptosis and not by a mechanism involving necrosis.
Figure 1.
Inhibition of the growth of NB4 cells by AF. NB4 cells (1 × 105/ml) were incubated for 1–4 days in culture media containing different concentrations of AF (0.1–1 μM). At the indicated times after AF treatment, the number of viable cells at each concentration was determined in triplicate by use of a haemocytometer.
Figure 2.
AF-induced DNA fragmentation in NB4 cells. (a) The NB4 cells were incubated with medium alone (control) or 1 μM AF for 24 h. Genomic DNAs were isolated and analysed on a 1% agarose gel. (b) The AF-treated cells were stained with Hoechst 33342 (10 μM) for 30 min, and then observed under a fluorescence microscope equipped with a DAPI filter. (c) BrdU-labelled NB4 cells (1 × 104 cells) were incubated for 6 h in various concentrations of AF (0–1 μM). The BrdU-labelled DNA fragments in the cytoplasm or culture media were quantitated using cellular DNA fragmentation ELISA kit (Roche, Germany). Experiments were carried out in triplicate and the results represent means±s.d.
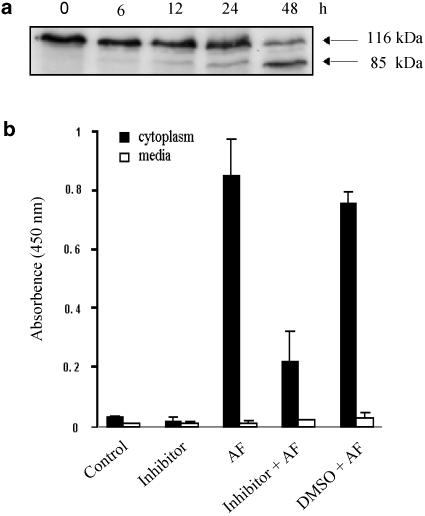
To examine whether the AF-induced apoptosis was mediated by activation of caspase-3, the cleavage of PARP, which is known to be a substrate of caspase-3, was elucidated by Western blot analysis. Whereas only the 116 kDa intact PARP was observed in untreated control cells, an additional 85 kDa cleavage fragment was detected in AF-treated cells (Figure 3a). Preincubation for 1.5 h with z-DEVD.fmk, a specific inhibitor of caspase-3, markedly inhibited AF-induced apoptosis (Figure 3b), suggesting that caspase-3 activation is involved in AF-induced apoptosis.
Figure 3.
The involvement of caspase-3 in AF-induced apoptosis. (a) Western blot analysis of PARP in NB4 cells treated with AF. After treatment with 1 μM AF, the cells were lysed and 40 μg of protein was electrophoresed on a 4–20% gradient SDS–polyacrylamide gel. Note that an 85 KDa fragment, which was the product of PARP cleavage by caspase-3, could be detected in AF-treated cells. (b) Inhibition of AF-induced apoptosis by a caspase-3 inhibitor. The BrdU-labelled NB4 cells were preincubated for 1.5 h with z-DEVD (200 μM) or DMSO (0.4%), which was used as a solvent for the inhibitor, before exposure to AF (1 μM). The DNA fragment was detected by the same method as in Figure 2(c).
Involvement of ROS production in AF-induced apoptosis
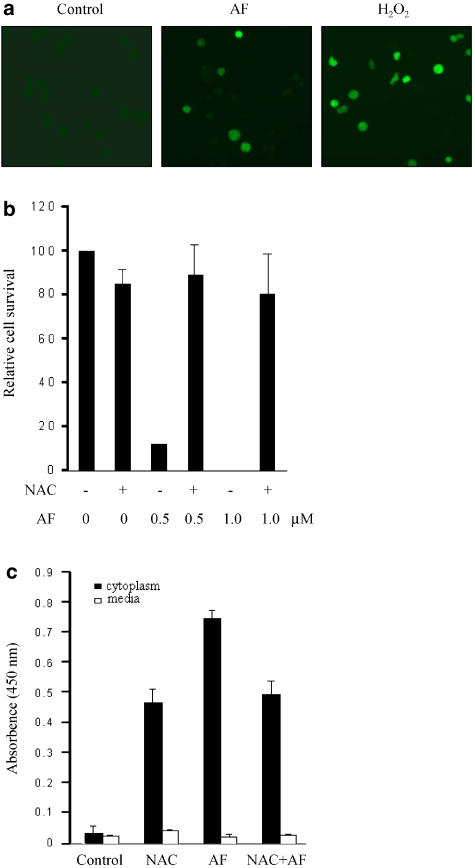
Previous evidence has suggested that ROS is a mediator of apoptosis of APL cells and AF is able to induce ROS generation. Hence, we investigated whether the production of ROS is associated with the AF-induced apoptosis of NB4 cells. After being labelled with DCFH-DA, the NB4 cells were treated with 1 μM AF and then observed with a fluorescence microscope. DCFH-DA is converted to DCFH by nonspecific esterases in cells and the resultant DCFH can be oxidized to a fluorescent compound, DCF, in the presence of ROS. Therefore, ROS-generating cells can be identified with a fluorescence microscope. As shown in Figure 4a, a fluorescent signal was detected in the cells incubated with AF. Even though the number of fluorescent cells was lower in the AF-treated group than in the H2O2 (50 μM)-treated positive control group, a significant difference was obtained between the AF-treated group and the untreated negative control group. To confirm the involvement of ROS, we tested the effect of the antioxidant N-acetyl-L-cysteine (NAC) on AF-induced apoptosis. Treatment with AF in the presence of 10 mM NAC attenuated AF-inhibited cell growth by 80% and significantly protected against cell death (Figure 4b and c). These results indicate that a ROS-dependent pathway is involved in the apoptosis induced by AF.
Figure 4.
AF-induced apoptosis mediated via the ROS signalling pathway. (a) Exponentially growing cells (1 × 105/ml) were labelled with 0.5 μM DCFH-DA for 1 h and then incubated with 1 μM AF for 24 h. Treatment with 50 μM H2O2 for 1 h was used as a positive control. After being washed with PBS, cells were analyzed by a fluorescence microscope (excitation and emission wavelength: 495 and 525 nm, respectively). (b) For recovery of growth inhibition by NAC, NB4 cells were treated with AF (0.5 or 1 μM) alone or together with 10 mM NAC. After treatment for 53 h, the number of viable cells was determined in triplicate by counting with a haemocytometer. The results are expressed as cell survival relative to the control treatment. (c) Effect of NAC on AF-induced DNA fragmentation; the NB4 cells were incubated for 6 h with AF (0.5 μM) alone or together with 10 mM NAC. DNA fragmentation was quantified using a cellular DNA fragmentation ELISA kit. The results represent means±s.d. of data from three experiments.
Enhancement of cell differentiation by the combined treatment with AF and ATRA
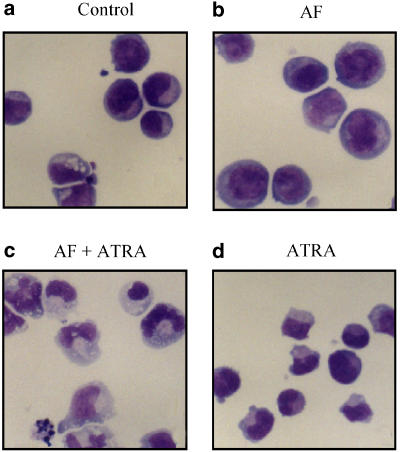
As low concentrations (0.3 μM) of AF retarded cell growth (Figure 1), we determined whether the inhibition of cell growth was accompanied by cell differentiation. To verify that AF could induce cell differentiation, the NB4 cells were incubated with 0.3 μM AF for 4 days. After incubation, the cells were stained with Giemsa solution and the morphological changes were examined. As shown in Figure 5b, no significant changes were observed following a single treatment of AF. However, a low concentration of AF (0.3 μM) combined with a low concentration ATRA (5 nM) markedly increased the number of cells with differentiated features, such as lobed or multiple nuclei accompanied by numerous granules and vacuoles, compared to those cells treated with AF or ATRA alone (Figure 5a–d). It was not possible to test the effects of ATRA in combination with AF at concentrations of AF higher than 0.3 μM, as at higher concentrations it has a cytotoxic effect.
Figure 5.
Induction of cell differentiation by the combined treatment with AF and ATRA at low doses. The cells were incubated with AF (0.3 μM) alone, ATRA (5 nM) alone or a combination of AF (0.3 μM) with ATRA (5 nM) for 4 days. To evaluate cell differentiation, the treated cells were stained with Giemsa solution and photographed (× 400).
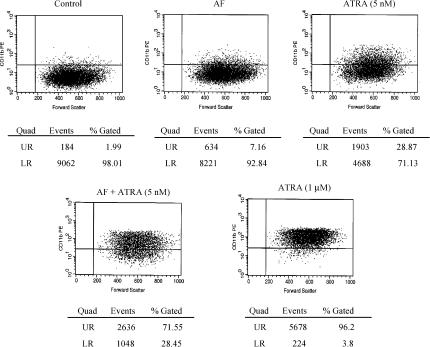
We also investigated the effect of the combination of AF and ATRA on the expression of the differentiation marker CD11b by flow cytometric analysis. Only 7.2 or 28.9% of the cells treated with AF (0.3 μM) or ATRA (5 nM) alone, respectively, were positively stained by the RPE-conjugated monoclonal antibody against human CD11b. However, when the cells were treated with the combination of AF and ATRA, 72% of the cells were positively stained. The expression pattern with the combined treatment was similar to that obtained after treatment with 1 μM ATRA (96%), which induced differentiation of all NB4 cells (Figure 6). Isotype controls with RPE-conjugated mouse IgG1 exhibited no positively stained cells (data not shown).
Figure 6.
Flow cytometric analysis of CD11b expression in NB4 cells treated with AF and ATRA. Cells were incubated with AF (0.3 μM) alone, ATRA (5 nM) alone or a combination of AF (0.3 μM) and ATRA (5 nM). After 4 days, the cells were collected, stained with antibody against human CD11b and analysed by a flow cytometer. Control represents untreated cells. Also, the cells treated with 1 μM of ATRA were used as a positive control. The results shown here are representative of two separate experiments.
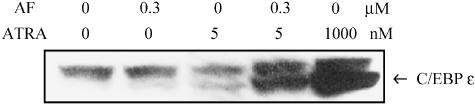
C/EBPɛ, which is a potential retinoid target gene, plays a crucial role in the granulocytic differentiation of PML cells (Morosetti et al., 1997). Therefore, the upregulation of C/EBPɛ was investigated as another indicator of cell differentiation. Figure 7 shows that the combined treatment with AF (0.3 μM) and ATRA (5 nM) increased C/EBPɛ expression to the same level as the treatment with 1 μM ATRA, which was used as a positive control. If these results are taken together, it is evident that the combination of low doses of AF and ATRA induces differentiation of NB4 cells, even though at these low doses neither AF nor ATRA alone elicited marked cell differentiation.
Figure 7.
Induction of C/EBPɛ in differentiated NB4 cells. The NB4 cells were incubated for 4 days with AF or ATRA and then 50 μg of protein was subjected to Western blot analysis to detect the expression of C/EBPɛ. The experiments were performed three times and the results from the three experiments were similar.
Discussion
ATRA has been used as a therapeutic treatment of APL due to its ability to induce differentiation of the leukaemic cells into mature granulocytes. Many patients treated with ATRA alone have a relapse and acquire resistance to ATRA, therefore, some combined treatments with chemotherapy have been tried to prevent early relapse and improve long-term survival (Grignani et al., 1994; Tallman et al., 2002). The present study is the first to demonstrate the ability of AF to induce apoptosis as well as differentiation of APL cells, on the basis of which AF could be developed as a novel drug for the treatment of APL.
AF generated intracellular ROS, and the treatment of cells with AF in combination with NAC protected against the AF-induced apoptosis (Figure 4). These findings suggest that ROS signalling is involved in the induction of apoptosis by AF. NAC has been widely used as an antioxidant reagent. However, NAC can also react directly with thiol-reactive compounds, since it has a thiol group. Indeed, Hayakawa et al. (2003) demonstrated that NAC blocked tumour necrosis factor (TNF)-stimulated NF-κB activation by inhibiting the binding of TNF to its receptors. These authors hypothesized that NAC changes the structure of the TNF receptor, as the extracellular domain of the TNF receptor has four conserved cysteine-rich repeats that are involved in the formation of disulphide linkages. In this study, a high concentration of NAC (10 mM) was used and the NAC was added to the cells together with AF. Therefore, it is possible that the NAC reacts with the thiol-reactive AF. If so, the protective effect of NAC on apoptosis is due to a structural change in the thiol-reactive AF rather than the elimination of ROS. Further studies with nonthiol antioxidant reagents are required to elucidate the mechanism of this effect of NAC.
Caspase-dependent apoptosis, in certain cell types, involves processes that disrupt mitochondrial transmembrane potential and release cytochrome c; antiapoptotic Bcl-2 family proteins prevent this effect. Very recently, it was reported that AF could also induce mitochondrial permeability transition (McKeage, 2002; Rigobello et al., 2002). In addition, we confirmed that cytochrome c is released from mitochondria into cytosol in the AF-induced apoptotic cells (data not shown). Therefore, it is likely that the mechanism of AF-induced apoptosis involves an increase in mitochondrial permeability, cytochrome c release, caspase-3 activation, PARP cleavage and DNA fragmentation. To elucidate the mechanism in more detail, we are studying signalling pathways including those involved in AF-induced programmed cell death.
AF is an antirheumatic drug with immunosuppressive and anti-inflammatory properties and it is known to inhibit NF-κB activation by blocking IκB-kinase (Jeon et al., 2000). NF-κB is important in cell survival, acting as a transcription factor to induce the expression of antiapoptotic IAP genes (Lee & Collins, 2001). If AF inhibits NF-κB activation in NB4 cells, it may induce apoptosis. However, NF-κB activation has also been shown to induce apoptotic cell death (Dirsch et al., 1998). Therefore, the effect of AF on NF-κB activation should be elucidated in NB4 leukaemic cells.
Recently, Akahoshi et al. (2000) studied the effect of AF on neutrophil apoptosis. They showed that 1 μM AF prolonged neutrophil survival by delaying apoptosis. On the basis of this finding, the dose of AF used in our study could be employed to treat APL with the knowledge that it would not have any toxic effects on normal blood cells.
Subeffective concentrations of AF (0.3 μM) and ATRA (5 nM) applied as a combined treatment resulted in an enhancement of NB4 cell differentiation (Figures 5, 6 and 7). Other leukaemic HL-60 cells, where the PML-RARα fusion protein is missing, were also shown to have differentiated morphology when the cells were incubated for 5 days in a medium containing AF and ATRA, although slightly higher doses of AF (0.5 μM) and ATRA (10 nM) were required than in the case of NB4 cells (data not shown). However, so far, the mech-anisms of AF-enhanced cell maturation remain unclear and to discern them we are, at present, investigating the signalling pathways and the degradation/localization of PML-RARα.
In several studies it has been demonstrated that HL-60 and U937 cells require a higher dose of As2O3 for induction of apoptosis than NB4 cells, and that this is due to increased amounts of glutathione Peroxidase (GPx) and catalase in these cells (Jing et al., 1999; Cai et al., 2000). In our system, the HL-60 cells were slightly resistant to AF (for apoptosis, the effective concentrations of AF were 0.5–1.0 μM in NB4 cells and 1.0–2.0 μM in HL-60 cells), but the U937 cells were as sensitive as the NB4 cells to AF, even though U937 cells have much higher concentrations of GPx and catalase than the NB4 cells (data not shown). Therefore, it is likely that AF induces apoptosis, in part, via different mechanisms from As2O3.
AF is a lipophilic organometallic complex that has been widely used for the treatment of rheumatoid arthritis. For the management of rheumatoid arthritis in adults, the recommended initial daily dose of AF is 6 mg. Unlike other available gold compounds, this drug can be given by mouth, and is relatively well absorbed from the GI tract. Following oral administration of multiple doses of AF in patients, steady-state blood gold concentrations are usually attained after 8–12 weeks; these are approximately 0.5–0.7 μg ml−1 (0.7–1.0 μM) in patients receiving 6 mg daily, and 0.9–1.0 μg ml−1 (1.3–1.5 μM) in patients receiving 9 mg daily. At these doses, AF has been used with favourable results, and with no adverse toxic side effects, for the management of rheumatoid arthritis (McEvoy et al., 2002).
Our study showed that AF-induced apoptosis of NB4 cells at a concentration of 1.0 μM (0.7 μg ml−1) and the combined use of 0.3 μM (0.2 μg ml−1) AF and 5 nM ATRA induced differentiation in vitro. These doses are widely employed for the management of rheumatoid arthritis without any side effects, and therefore may be used for treating APL without the risk of serious toxicity.
Acknowledgments
We are indebted to Dr Michel Lanotte (Hospital Saint Louis, Paris, France) for the NB4 cell line. We thank Professor Dae-Myung Jue (Department of Biochemistry, The Catholic University of Korea) for the generous gift of auranofin and kind advice throughout this study. We also thank Dr JM Kang (Department of clinical pathology, Holy family hospital, The Catholic University of Korea) for evaluating the cytology specimens. This work was supported by grant no (R04-2002-000-20121-0) from the Basic Research Program of the Korea Science & Engineering Foundation.
Abbreviations
- AF
auranofin
- APL
acute promyelocytic leukaemia
- ATRA
all-trans retinoic acid
- BrdU
bromodeoxyuridine
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- NAC
N-acetyl-L-cysteine
- PARP
poly-ADP-ribose polymerase
- PML
promyelocytic leukaemia
- RARα
retinoic acid receptor alpha
- RAS
retinoic acid syndrome
- ROS
reactive oxygen species
- RPE
R-phycoerythrin
- TNF
tumour necrosis factor
References
- AGIS H., WELTERMANN A., MITTERBAUER G., THALHAMMER R., EDELHAUSER M., SEEWANN H.L., VALENT P., LECHNER K., FONATSCH C., GEISSLER K. Successful treatment with arsenic trioxide of a patient with ATRA-resistant relapse of acute promyelocytic leukemia. Ann. Hematol. 1999;78:329–332. doi: 10.1007/s002770050523. [DOI] [PubMed] [Google Scholar]
- AKAHOSHI J.L.T., NAMAI R., MATSUI T., KONDO H. Effect of auranofin, an antirheumatic drug, on neutrophil apop-tosis. Inflamm. Res. 2000;49:445–451. doi: 10.1007/s000110050615. [DOI] [PubMed] [Google Scholar]
- BECKER K., GROMER S., SCHIRMER R.H., MÜLLER S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur. J. Biochem. 2000;267:6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- BLODGETT R.C. Auranofin: experience to date. Am. J. Med. 1983;75:86–89. doi: 10.1016/0002-9343(83)90480-1. [DOI] [PubMed] [Google Scholar]
- CAI X., SHEN Y.-L., ZHU Q., JIA P.-M., YU Y., ZHOU L., HUANG Y., ZHANG J.-W., XIONG S.-M., CHEN S.-J., WANG Z.-Y., CHEN Z., CHEN G.-Q. Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000;14:262–270. doi: 10.1038/sj.leu.2401650. [DOI] [PubMed] [Google Scholar]
- CHEN G.Q., SHI X.G., TANG W., XIONG S.M., ZHU J., CAI X., HAN Z.G., NI J.H., SHI G.Y., JIA P.M., LIU M.M., HE K.L., NIU C., MA J., ZHANG P., ZHANG T.D., PAUL P., NAOE T., KITAMURA K., MILLER W., WAXMAN S., WANG Z.Y., DE THE H., CHEN S.J., CHEN Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- DAVISON K., MANN K.K., MILLER W.H., JR Arsenic trioxide: mechanisms of action. Semin. Hematol. 2002;39:3–7. doi: 10.1053/shem.2002.33610. [DOI] [PubMed] [Google Scholar]
- DIRSCH V.M., GERBES A.L., VOLLMAR A.M. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor κB. Mol. Pharmacol. 1998;53:402–407. doi: 10.1124/mol.53.3.402. [DOI] [PubMed] [Google Scholar]
- GALLAGHER R.E. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–1958. doi: 10.1038/sj.leu.2402719. [DOI] [PubMed] [Google Scholar]
- GRIGNANI F., FAGIOLI M., ALCALAY M., LONGO L., PANDOLFI P.P., DONTI E., BIONDI A., LO COCO F., GRIGNANI F., PELICCI P.G. Acute promyelocytic leukemia: from genetics to treatment. Blood. 1994;83:10–25. [PubMed] [Google Scholar]
- GROMER S., ARSCOTT L.D., WILLIAMS C.H., JR, SCHIRMER R.H., BECKER K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., WHITEHURST C.E., MATSUBARA T., HIROHATA K., LIPSKY P.E. Immunomodulatory effects of therapeutic gold compounds. J. Clin. Invest. 1992;89:1839–1848. doi: 10.1172/JCI115788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAKAWA M., MIYASHITA H., SAKAMOTO I., KITAGAWA M., TANAKA H., YASUDA H., KARIN M., KIKUGAWA K. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEON K.I., JEONG J.Y., JUE D.M. Thiol-reactive metal compounds inhibit NF-κB activation by blocking IκB kinase. J. Immunol. 2000;164:5981–5989. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- JING Y., DAI J., CHALMERS-REDMAN R.M.E., TATTON W.G., WAXMAN S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- LEE R.T., COLLINS T. Nuclear factor-kappaB and cell survival: IAPs call for support. Circ. Res. 2001;88:262–264. doi: 10.1161/01.res.88.3.262. [DOI] [PubMed] [Google Scholar]
- MCEVOY G.K., MILLER J.L., LITVAK K., DEWEY D.R., FONG P.A., MELTON G.S., O'ROURKE A., DOUGLAS P.M., SHANNON E.P., PETERSON J.A., SCHNELL N.C., KESTER L., ZIGLER K.M. AHFS Drug Information. Bethesda: American Society of Health System Pharmacists Inc; 2002. pp. 2880–2885. [Google Scholar]
- MCKEAGE M.J. Gold opens mitochondrial pathways to apoptosis. Br. J. Pharmacol. 2002;136:1081–1082. doi: 10.1038/sj.bjp.0704822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY A.R., PEDERSEN E.W., SOLOMON E., GRIMWADE D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003;17:71–97. doi: 10.1016/s0268-960x(02)00075-9. [DOI] [PubMed] [Google Scholar]
- MOROSETTI R., PARK D.J., CHUMAKOV A.M., GRILLIER I., SHIOHARA M., GOMBART A.F., NAKAMAKI T., WEINBERG K., KOEFFLER H.P. A novel, myeloid transcription factor, C/EBPɛ, is upregulated during granulocytic, but not monocytic, differentiation. Blood. 1997;90:2591–2600. [PubMed] [Google Scholar]
- NIU C., YAN H., YU T., SUN H.-P., LIU J.-X., LI X.-S., WU W., ZHANG F.-O., CHEN Y., ZHOU L., LI J.-M., ZENG X.-Y., YANG R.-R.O., YUAN M.-M., REN M.-Y., GU F.-Y., CAO O., GU B.-W., SU X.-Y., CHEN G.-O., XIONG S.-M., ZHANG T.-D., WAXMAN S., WANG Z.-Y., CHEN Z., HU J., SHEN Z.-X., CHEN S.-J. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- RIGOBELLO M.P., SCUTARI G., BOSCOLO R., BINDOLI A. Induction of mitochondrial permeability transition by auranofin, a gold(I)-phosphine derivative. Br. J. Pharmacol. 2002;136:1162–1168. doi: 10.1038/sj.bjp.0704823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYALL J.A., ISCHIROPOULOS H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- SCOTT N., HATLELID K.M., MACKENZIE N.E., CARTER D.E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem. Res. Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- SHAO W., FANELLI M., FERRARA F.F., RICCIONI R., ROSENAUER A., DAVISON K., LAMPH W.W., WAXMAN S., PELICCI P.G., LO COCO F., AVVISATI G., TESTA U., PESCHLE C., GAMBACORTI-PASSERINI C., NERVI C., MILLER W.H., JR Arsenic trioxide as an inducer of apoptosis and loss of PML/RARα protein in acute promyelocytic leukemia cells. J. Natl. Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- SHEN Z.X., CHEN G.Q., NI J.H., LI X.S., XIONG S.M., QIU Q.Y., ZHU J., TANG W., SUN G.L., YANG K.Q., CHEN Y., ZHOU L., FANG Z.W., WANG Y.T., MA J., ZHANG P., ZHANG T.D., CHEN S.J., CHEN Z., WANG Z.Y. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- SNOW E.T. Metal carcinogenesis: mechanistic implications. Pharmacol. Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- SNYDER R.M., MIRABELLI C.K., CROOKE S.J. The cellular pharmacology of auranofin. Semin. Arthritis Rheum. 1987;17:71–80. doi: 10.1016/0049-0172(87)90017-5. [DOI] [PubMed] [Google Scholar]
- SOIGNET S.L., FRANKEL S.R., DOUER D., TALLMAN M.S., KANTARJIAN H., CALLEJA E., STONE R.M., KALAYCIO M., SCHEINBERG D.A., STEINHERZ P., SIEVERS E.L., COUTRE S., DAHLBERG S., ELLISON R., WARRELL R.P., JR United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J. Clin. Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- TALLMAN M.S., NABHAN C., FEUSNER J.H., ROWE J.M. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]