Abstract
We examined reserpine-induced chemical denervation supersensitivity with special reference to alpha-1 adrenoceptor (AR) subtypes.
Chronic treatment with reserpine for 2 weeks depleted noradrenaline in the tail artery and spleen of rats. Noradrenaline in the thoracic aorta was negligible before and after reserpine treatment.
The treatment with reserpine produced supersensitivity in the contractile responses of the rat tail artery to phenylephrine, 5-HT and KCl, resulting in leftward shift of concentration–response curves (11.6-, 2.5- and 1.1-fold at EC50 value, respectively). These results suggest a predominant sensitization of the alpha-1 AR-mediated response by reserpine treatment.
BMY 7378 at a concentration (30 nM) specific for blocking the alpha-1D AR subtype, but not KMD-3213 at a concentration (10 nM) selective for blocking the alpha-1A AR subtype, inhibited the supersensitivity of the phenylephrine-induced response in the reserpine-treated artery. On the other hand, the response to phenylephrine in reserpine-untreated artery was selectively inhibited by the same concentration of KMD-3213, but not by BMY 7378. Prazosin, a subtype-nonselective antagonist, blocked the responses to phenylephrine with the same potency, regardless of reserpine treatment.
In the thoracic aorta and spleen, no supersensitivity was produced in the responses to phenylephrine by reserpine treatment.
In a tissue segment-binding study using [3H]-prazosin, the total density and affinity of alpha-1 ARs in the rat tail artery were not changed by treatment with reserpine. However, alpha-1D AR with high affinity for BMY 7378 was significantly detected in reserpine-treated tail artery, in contrast to untreated artery. Decreases in alpha-1A AR with high affinity for KMD-3213 and alpha-1B AR with low affinities for KMD-3213 and BMY 7378 were also estimated in reserpine-treated tail artery.
Alpha-1D AR mRNA in rat tail artery increased to three-folds by reserpine treatment, whereas the levels of alpha-1A and 1B mRNAs were not significantly changed.
The present results suggest that chronic treatment with reserpine affects the expression of alpha-1 AR subtypes of rat tail artery and that the induction of alpha-1D ARs with high affinity for catecholamines is in part associated with reserpine-induced supersensitivity.
Keywords: Supersensitivity, reserpine, chemical denervation, alpha-1 adrenoceptor (AR), intact tissue segment binding, mRNA, rat tail artery
Introduction
Supersensitivity is one of the classical and pharmacological phenomena and is defined as an increase in functional affinity (that is, a decreased EC50) for agonists (Fleming et al., 1973; Fleming, 1976; Westfall, 1981). Supersensitivity may be caused by a variety of procedures, including surgical and chemical denervation and chronic treatment with antagonists (Westfall, 1981; Insel, 1989). The one common feature of these treatments has been reported that they all cause chronic interruption of neuronal stimulation on target cells.
Reserpine depletes catecholamine in the adrenergic nerve due to inhibition of catecholamine reuptake by binding the pre-junctional storage vesicle for catecholamine (Berkowitz et al., 1971; Stitzel, 1976; Giachetti & Shore, 1978). Chronic treatment with reserpine is known to cause supersensitivity of the adrenergic and nonadrenergic responses in sympathetically innervated tissues, which has been respectively called specific and nonspecific supersensitivities (Fleming et al., 1973). In extensive studies, several mechanisms underlying the supersensitivity have been proposed; increases in the density or affinity of postjunctional receptors (Bito & Dawson, 1970; Colucci et al., 1981), changes in the resting membrane potential (Fleming & Westfall, 1975; Abel et al., 1981; Fleming, 1987) and alterations of calcium homeostasis (Hudgins & Harris, 1970; Carrier, 1975).
Recently, alpha-1 ARs have been classified into at least three subtypes, alpha-1A, -1B and -1D, in molecular and pharmacological evaluations (Minneman et al., 1994; Hieble et al., 1995; Michel et al., 1995; Piascik & Perez 2001). These subtypes distribute distinctly and play key roles in adrenergic functions in many tissues (McGrath & Wilson, 1988; Muramatsu et al., 1995; 1998; Graham et al., 1996; Daly et al., 2002). In rats, for example, the adrenergic contraction of the tail artery is predominantly mediated by the alpha-1A AR subtype (Lachnit et al., 1997; Murata et al., 1999; Gisbert et al., 2003), while the adrenergic contractions of the thoracic aorta and spleen are mainly through alpha-1D and alpha-1B AR subtypes, respectively (Burt et al., 1995; Muramatsu et al., 1995; Buckner et al., 1996; Saussy et al., 1996). Recent studies further developed various drugs specific for each subtype. Prazosin is a classical antagonist which shows no subtype selectivity (Muramatsu et al., 1995; Hancock, 1996). BMY 7378 and KMD-3213 are well characterized as highly selective antagonists for alpha-1D and alpha-1A AR subtypes, respectively (Goetz et al., 1995; Shibata et al., 1995; Saussy et al, 1996; Yamagishi et al., 1996; Murata et al., 1999). Furthermore, several agonists including phenylephrine show relatively high affinity for the alpha-1D subtype (Lomasney et al., 1991; Buckner et al., 1996; Graham et al., 1996; Piascik & Perez, 2001).
Stassen et al. (1998) evaluated the relationship between the presence of adrenergic nerves and the presence of alpha-1 ARs in the arterial trees of rat, and suggested that the presence of alpha-1 AR subtypes may be closely related to the sympathetic innervation. They also demonstrated that chemical sympathectomy of rats with 6-hydroxydopamine caused a distinct reduction in alpha-1A AR subtype, concomitant with a marked reduction of noradrenaline content. We hypothesized that the supersensitivity may in part reflect the changes in expression of alpha-1 AR subtypes. In the present study, we examined a possible relationship between reserpine-induced supersensitivity and alpha-1 AR subtypes. To this end, we analyzed the concentration–contractile response curve for phenylephrine, the binding of [3H]-prazosin, the effects of the alpha-1 AR subtype-selective ligands KMD-3213 and BMY 7378, and the mRNA levels of alpha-1 AR subtypes.
Methods
Animals and administration of reserpine
Male Wistar rats (Charles River Japan Inc., Tokyo, Japan) aged 7 weeks were used. The rats were weighed and intraperitoneally administered reserpine at a dose of 1 mg kg−1 once daily for 1 week and then 0.5 mg kg−1 for 1 week. The body weight of reserpine-treated rats (243±7 g, n=24) was significantly lower, compared with reserpine-untreated rats (346±7, n=23). A decrease in locomotor activity and diarrhea was also observed in reserpine-treated rats. After reserpine treatment for 2 weeks, rats were killed by exsanguination, and the thoracic aorta, spleen and tail artery were isolated carefully. The fat and connective tissues were removed from the arteries and spleen immediately after isolation in modified Krebs–Henseleit solution (NaCl: 120.7, KCl: 5.9, MgCl2: 1.2, CaCl2: 2.5, NaH2PO4: 1.2, NaHCO3: 25.5 and glucose: 11.5 mM, pH 7.4) at 4°C.
Measurement of endogenous noradrenaline contents
The isolated rat tissues were weighed and homogenized in a polytron homogenizer (setting 3 for 40 s) at 4°C in 3.0 ml perchloric acid solution (0.4 mM) containing 1.3 mM Na2 EDTA and 5.3 mM Na2S2O5. The homogenates were then centrifuged (12 000 × g at 4°C for 15 min), and the supernatant (0.1 ml) was collected into a sample tube containing 0.1 ml internal standard solution (10 ng ml−1 of 3,4-dihydroxybenzylamine). Noradrenaline in the sample tubes was isolated using batch alumina chromatography and analyzed using high-performance liquid chromatography with electrochemical detection (Shinozuka et al., 2001).
Functional studies
Tail artery tissue was cut into rings of approximately 2 mm in length and then the endothelium was removed by gentle rubbing with enamel-coated stainless steel wire. The rings were suspended in a 2 ml micro-organ bath filled with modified Krebs–Henseleit solution gassed continuously with 95% O2 and 5% CO2 at 37°C. The aorta tissue was cut helically and the endothelium was removed by gentle rubbing with wet filter paper. The spleen was divided into two longitudinal parts. Aorta and spleen strips were mounted in a 20 ml of organ bath. The resting tensions were 0.5 × g for the tail artery and 1 × g for the aorta and spleen. After equilibration for at least 60 min, phenylephrine, 5-HT or KCl was added to the organ bath in a cumulative manner to obtain concentration–response curves for contraction. In general, a lack of endothelial cells was confirmed as a loss of acetylcholine-induced relaxation in the first concentration–response curve for agonist and the third concentration–response curve was used as control in each preparation. Reproducibility between the third and fourth concentration–response curves was confirmed in the parallel experiments with vehicle. Various alpha-1 AR antagonists were added to the organ bath 30 min before evaluating the fourth concentration–response curve for phenylephrine, 5-HT or KCl. The contractile responses were recorded as changes of isometric force via an isometric force transducer (model TB 612T, Nihon Kohden Co., Tokyo, Japan) and amplifier. The responses to phenylephrine were obtained in the presence of 1 μM propranolol, but 0.1 μM rauwolscine also added in the experiments with the tail artery tissue.
Tissue segment-binding study
Tissue segment binding of [3H]-prazosin was performed with the method described by Tanaka et al. (2004). Briefly, the isolated tail artery was cut lengthwise, yielding a rectangular sheet of vascular smooth muscle. Like functional study, the endothelium was removed by gentle rubbing with filter paper. Then, the tissues were cut into about 28–30 small pieces (approximately 3 mm in length) under a stereoscopic light microscope. The pieces were incubated for 10 h at 4°C in 1 ml of incubation buffer (50 mM Tris–HCl, 5 mM MgCl2, 100 mM NaCl, pH 7.4) with [3H]-prazosin (30–2000 pM for saturation experiments and 500 pM for competition experiments). After incubation, the pieces were gently blotted and rapidly rinsed by vortexing for 1 min with 1.5 ml of incubation buffer at 4°C. The pieces were then blotted and solubilized in 0.3 N NaOH solution to estimate the bound radioactivity and protein content. Nonspecific binding was determined in the presence of 30 μM phentolamine. The bound radioactivity was measured by liquid scintillation counting. The protein contents were determined by the method of Bradford (1976).
Measurement of alpha-1AR mRNA
Rat tail artery was rapidly removed, frozen in liquid nitrogen and then stored at −80°C before use. Total RNA was extracted using the QIAGEN RNeasy Mini-extraction kit (QIAGEN Ltd, Valencia, U.S.A.). Real-time quantitative PCR primers for alpha-1 ARs and beta-actin were designed with the software Primer Express™. The sequences of the PCR primer pairs used were as follows: alpha-1a AR: TGCACTCCGTGACTCACTACTACA, CCGCCCAGATATTGCAGAAC; alpha-1b AR: TTGGCGCTCCTCAGTGTGT, GGAGGAAAAGAGGGCGTAGAA; alpha-1d AR: GACCGCTACTAGGTTGGAAGGA, GGTAGAAGGAGCACACGGAAGA; beta-actin: ACCGTGAAAAGATGACCCAGAT, CAGTGGTACGACCAGAGGCATA. The cDNA template was synthesized from 1 μg of total RNA by the reverse transcriptase reaction, with the use of SuperScript™ First-Strand Synthesis System for RT–PCR kit (Invitrogen, Carlsbad, U.S.A.) by the manufacturer's method. The synthesized cDNA corresponding to 50 ng of total RNA was used for the real-time PCR. The real-time PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, U.S.A.) according to the manufacturer's method. The quantitative PCR reactions were set up in 1 × SYBR Green PCR Master mix, 400 nM each gene-specific primer and the template cDNA. Then, the SYBR Green PCR was performed as follows: initial incubations at 94°C for 10 min, subjected to 40 cycles of PCR (94°C for 30 s, 62°C for 15 s and 72°C for 30 s) using the ABI Prism 7700 Sequence detector (Applied Biosystems, Foster City, U.S.A.). The generation of PCR product was monitored at each cycle by increased SYBR Green fluorescence compared to an inert reference control. The resulted PCR products were confirmed to a single band by electrophoresis.
The beta-actin gene was used as an internal control for normalization of the data. The results were represented as a ratio of interest to beta-actin.
Data analysis
Data were analyzed using commercially available software (Graph Pad PRIZM®, Ver. 3.00, Graph Pad Software, San Diego, U.S.A.). The maximal contractions induced by phenylephrine, 5-HT and KCl in each preparation were taken as 100%, and nonlinear regression analyses were applied to sigmoid concentration–response curves for contractile agonists. The pKB value was determined for a single concentration of antagonist by the concentration-ratio method (Furchgott, 1972). Binding data were first fitted to a one- and then a two-site model, and if the residual sums of squares were statistically less for a two-site fit of the data than for a one-site, as determined by an F-test comparison, then the two-site model was accepted. P-values less than 0.05 were considered significant. Data are represented as the mean±s.e.m.
Drugs
The following drugs were used in this study: reserpine solution (Apoprone Inj. 1 mg) was from Daiichi Pharmaceutical Co., Ltd. (Tokyo, Japan). (−)-Phenylephrine hydrochloride, prazosin hydrochloride, 5-hydroxytryptamine creatinine sulfate (5-HT) and propranolol hydrochloride were from Sigma-Aldrich Co. (St Louis, U.S.A.). BMY 7378 and rauwolscine hydrochloride were from Research Biochemical Inc. (Natrick, U.S.A.). KMD-3213 was kindly provided from Kissei Pharmaceutical Co., Ltd (Matsumoto, Japan). [3H]-prazosin was from Dupont-New England Nuclear Inc. (Boston, U.S.A.). The stock solutions of prazosin and KMD-3213 were prepared with ethanol and dimethylsulfoxide, respectively, and then diluted with distilled water in functional experiments and with incubation medium in binding experiments.
Results
Endogenous noradrenaline content
As shown in Table 1, the contents of noradrenaline varied among tail artery, thoracic aorta and spleen tissues in reserpine-untreated rats. In particular, the content of noradrenaline in the thoracic aorta was less than 1% of the values measured for the two other tissues. Reserpine treatment for 2 weeks markedly reduced the noradrenaline content in the tail artery and spleen and eliminated the amine in the thoracic aorta.
Table 1.
Tissue noradrenaline contents in tail artery, thoracic aorta and spleen of rats
| Tissues | Reserpine-untreated | Resperpine-treated |
|---|---|---|
| Trail artery | 69.8±2.0 | 0.07±0.02** |
| Aorta | 0.33±0.05 | 0.00±0.00 |
| Spleen | 44.4±5.1 | 0.38±0.25** |
P<0.01, Significant differences vs reserpine treated in unpaired t-test. pmol mg−1 wet weight, mean±s.e.m. of four independent experiments.
Effects of reserpine treatment on the contractile responses to phenylephrine and other drugs
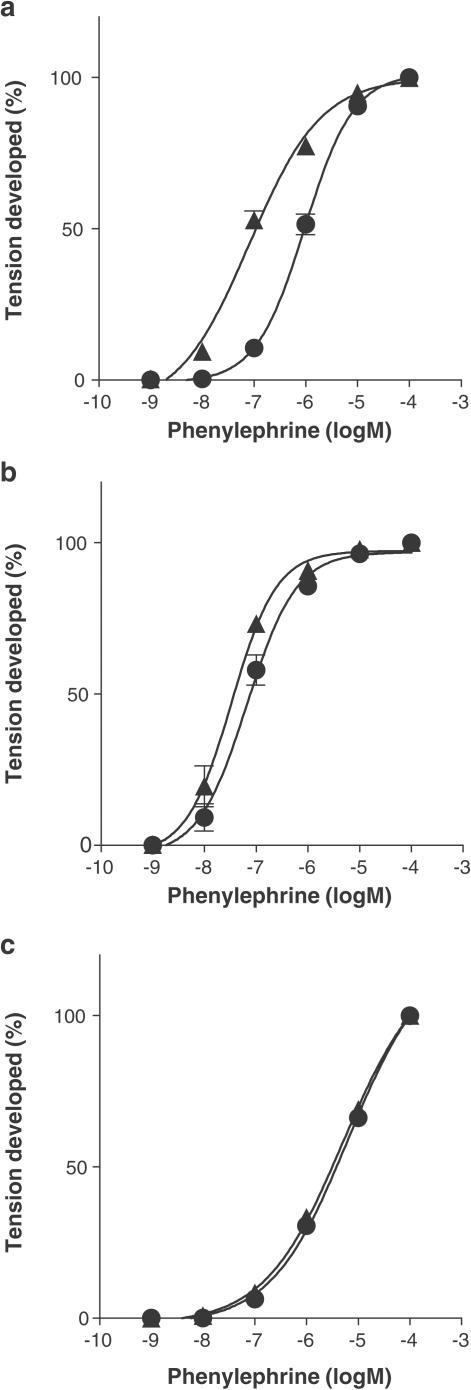
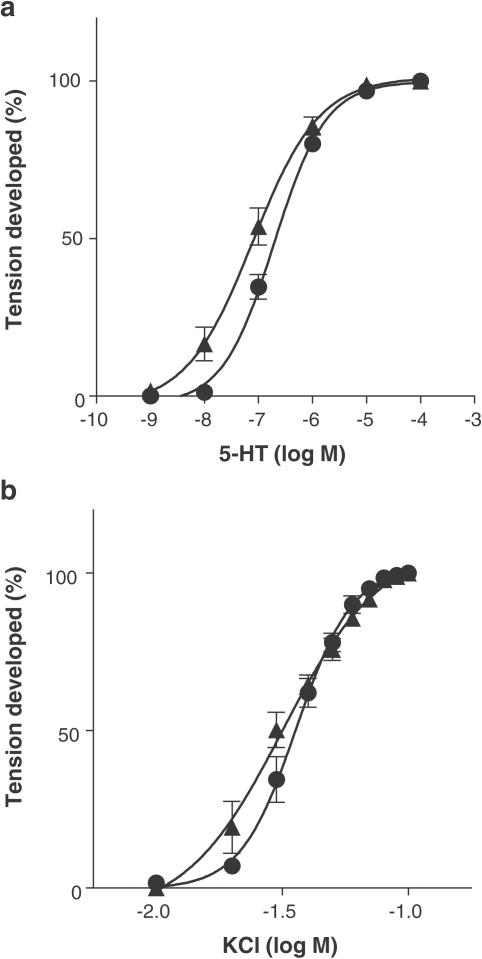
Phenylephrine produced concentration-dependent contractions in isolated tail artery, thoracic aorta and spleen of rats. Reserpine treatment caused a shift of concentration–response curve to the left in the tail artery, resulting in supersensitivity (Figure 1a). The pEC50 values for phenylephrine were, respectively, 7.1±0.1 and 6.0±0.1 in reserpine-treated and in -untreated arteries, causing a 11.6-fold shift in the sensitivity. Concentration–response curves for 5-HT and KCl were also shifted to the left, especially at the low concentrations (Figure 2). However, the shift of EC50 values (2.5-fold for 5-HT and 1.1-fold for KCl) was smaller than that of phenylephrine. The contractile responses to 5-HT and KCl were not inhibited by 0.1 μM prazosin, regardless of reserpine treatment.
Figure 1.
Effect of reserpine treatment on the contractile responses to phenylephrine in the tail artery (a), thoracic aorta (b) and spleen (c) of rats. Maximal contractions induced by phenylephrine in each preparation were taken as 100%. Circles: reserpine-untreated. Triangles: reserpine-treated. Data represent the mean±s.e.m. of 9–10 independent experiments.
Figure 2.
Effect of reserpine treatment on the contractile responses to 5-HT (a) and KCl (b) in the tail artery of rats. Maximal contractions induced by 5-HT and KCl in each preparation were taken as 100%. Circles: reserpine-untreated. Triangles: reserpine-treated. Data represent the means±s.e.m. of four independent experiments.
In the thoracic aorta and spleen, phenylephrine also produced concentration-dependent contractions (Figure 1b and c). The pEC50 value for phenylephrine in the thoracic aorta (7.2±0.1) was significantly higher than those in the tail artery and spleen (6.0±0.1 and 5.3±0.1, respectively) of reserpine-untreated rats, but was close to the pEC50 (7.1±0.1) measured for the reserpine-treated tail artery. No significant shift was produced by reserpine treatment in the concentration–response curves for phenylephrine for the thoracic aorta and spleen.
Effects of alpha-1 AR antagonists in tail artery
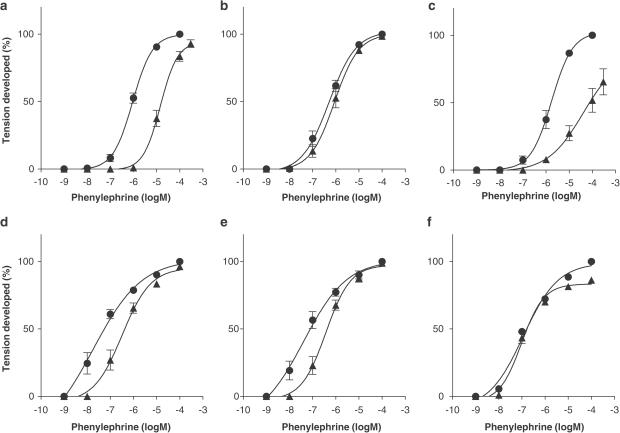
Since supersensitivity was more dominantly observed in the responses to phenylephrine, it was evaluated which alpha-1 AR subtypes contribute to the supersensitivity in the response to phenylephrine of reserpine-treated tail artery, by using three alpha-1 AR antagonists (prazosin, BMY 7378 and KMD-3213). Figure 3 shows the results obtained from the tail arteries of reserpine-untreated and -treated animals. Prazosin at 10 nM caused a parallel shift to the right in the concentration–response curve for phenylephrine, yielding in a pKB value of approximately 9 in both reserpine-treated and -untreated arteries (Table 2). BMY 7378 at 30 nM, a concentration specific for the alpha-1D AR subtype, had no effect on the response to phenylephrine in the untreated arterial tissue, but significantly inhibited the response in the reserpine-treated arterial tissue (pKB: 8.2±0.1). KMD-3213 at 10 nM, which is a selective concentration for the alpha-1A AR subtype, caused a rightward and downward shift of the concentration–response curve for phenylephrine in untreated arterial tissue, an apparent pKB value of 9.4±0.1. However, no shift was produced by 10 nM KMD-3213 in the responses to phenylephrine of the reserpine-treated arterial tissue, although the maximum contractions induced by high concentrations (10 and 100 μM) of phenylephrine were slightly attenuated (Figure 3). These results indicated that the leftward shift in the concentration–response curve for phenylephrine in the reserpine-treated artery were caused by a BMY 7378-sensitive but KMD-3213-insensitive component, which was not detected in the reserpine-untreated arterial tissues.
Figure 3.
Effects of alpha-1 AR antagonists on the contractile responses to phenylephrine in the tail artery isolated from reserpine-untreated (a–c) and -treated (d–f) rats. The maximum contraction induced by phenylephrine before treatment with antagonist was taken as 100%. Circles: control responses before treatment with antagonist. Triangles: responses after treatment with 10 nM prazosin (a and d), 30 nM BMY 7378 (b and e) and 10 nM KMD-3213 (c and f). Data represent the mean±s.e.m. of 4–5 independent experiments.
Table 2.
pKB values for alpha-1 AR antagonists in tail artery of rats
| Reserpine-untreated | Resperpine-treated | |||
|---|---|---|---|---|
| Drug (concentration) | −BMY 7378 | +BMY 7378 (30 nM) | −BMY 7378 | +BMY 7378 (30 nM) |
| Prazosin (10 nM) | 9.3±0.1 | 9.2±0.1 | 9.0±0.1 | 9.1±0.1 |
| BMY 7378 (30 nM) | NI | — | 8.2±0.1 | — |
| KMD-3213 (10 nM) | 9.4±0.1 | 9.1±0.1 | NI | 9.3±0.0 |
NI: No inhibition, mean±s.e.m. of 4–5 independent experiments.
The values represent pKB values in the absence (−) and presence (+) of BMY 7378.
Then, we examined the effects of prazosin and KMD-3213 in the continual presence of 30 nM BMY 7378 in the organ bath assay. In both reserpine-untreated and -treated arteries, prazosin at 10 nM shifted the concentration–response curve for phenylephrine, regardless of the presence of BMY 7378 in the organ bath, resulting in a pKB value close to that measured in the absence of BMY 7378 (Table 2). KMD-3213 (10 nM) also inhibited the responses to phenylephrine in the presence of 30 nM BMY 7378 with a comparable high affinity (pKB: 9.1 or 9.3) in both reserpine-untreated and -treated arteries, respectively.
Effects of alpha-1 AR antagonists in thoracic aorta and spleen
Concentration–response curves for phenylephrine in the rat thoracic aorta were inhibited by 10 nM prazosin or 30 nM BMY 7378. The inhibitory potencies were not affected by reserpine treatment (Table 3). The contractile responses to phenylephrine in the spleen of reserpine-untreated or -treated rats were inhibited by 10 nM prazosin, but not by 30 nM BMY 7378 (Table 3). The responses in both the thoracic aorta and spleen tissues were essentially unaffected by 10 nM KMD-3213.
Table 3.
pKB values for alpha 1-AR antagonists in isolated thoracic aorta and spleen of rats
| Tissues | Drugs | Reserpine-untreated | Reserpine-treated |
|---|---|---|---|
| Thoracic aorta | Prazosin (10 nM) | 10.2±0.3 | 10.1±0.4 |
| BMY 7378 (30 nM) | 8.6±0.0 | 8.7±0.1 | |
| KMD-3213 (10 nM) | 8.4±0.1 | NI | |
| Spleen | Prazosin (10 nM) | 9.5±0.2 | 9.4±0.2 |
| BMY 7378 (30 nM) | NI | NI | |
| KMD-3213 (10 nM) | NI | NI |
NI: No inhibition, mean±s.e.m. of 3–5 independent experiments.
[3H]-prazosin binding in rat tail artery segments
Ligand-specific [3H]-prazosin binding to the alpha-1 ARs of the rat tail artery segments was detected. The binding saturation isotherm revealed a high pKD value and a high density of receptors in the rat tail artery. No significant changes in these parameters were observed in the reserpine-treated artery segments (Table 4).
Table 4.
Pharmacological characterization of [3H]-prazosin-binding site in rat tail artery segements
| Reserpine-untreated | Reserpine-treated | |||
|---|---|---|---|---|
| Bmax (fmol mg−1 protein) | 524±19 | 526±11 | ||
| pKD | 9.5±0.1 | 9.4±0.2 | ||
| pKiHigh | pKiLow | pKiHigh | pKiLow | |
| KMD-3213 | 9.7±0.1 | 7.5±0.1 | 9.3±0.1 | 7.2±0.2 |
| (61±2%) | (39±2%) | (49±2%) | (51±2%) | |
| BMY 7378 | 6.4±0.1 | 9.0±0.2 | 6.2±0.1 | |
| (34±2%) | (66±2%) | |||
| Phenylephrine | 4.3±0.2 | 5.4±0.3 | ||
Mean±s.e.m. of 5–8 independent experiments. Numbers in parentheses show the proportion of the high- and low-affinity sites.
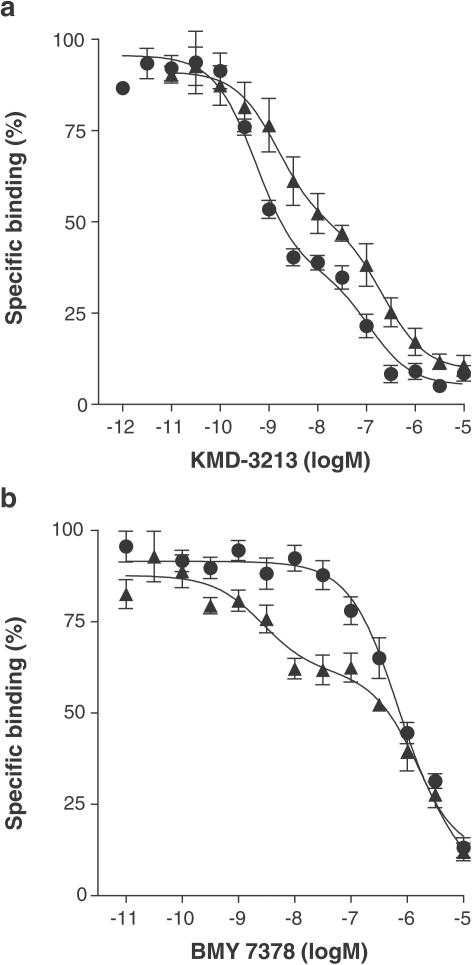
KMD-3213 biphasically inhibited [3H]-prazosin binding to alpha-1 ARs in both reserpine-untreated and -treated arteries (Figure 4a). However, the proportion of high-affinity sites was significantly lower (approximately 50%) in the reserpine-treated artery-segments than that observed (approximately 60%) in the untreated arterial tissue (Table 4). BMY 7378 monophasically inhibited [3H]-prazosin binding with a low affinity in the untreated artery, but yielded a biphasic binding competition curve for the artery segments obtained from reserpine-treated animals (Figure 4b). The proportion of high-affinity sites for BMY 7378 was 34% (Table 4). Phenylephrine showed a slightly but significantly higher affinity in the reserpine-treated artery than in the untreated artery.
Figure 4.
Competition curves for KMD-3213 (a) and BMY 7378 (b) against [3H]-prazosin binding to rat tail artery segments. Circles: reserpine-untreated rats. Triangles: reserpine-treated rats. Data represent the mean±s.e.m. of five independent experiments.
mRNA levels of alpha-1 AR subtypes in rat tail artery
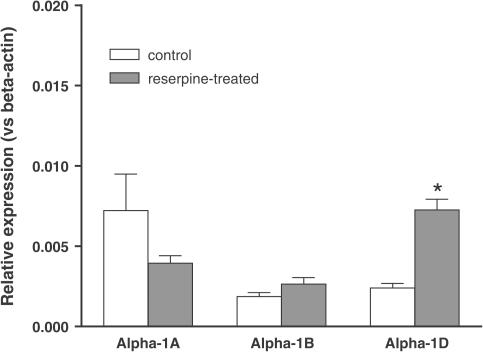
Figure 5 shows the mRNA levels of three alpha-1 AR subtypes in reserpine-untreated and -treated tail arteries, which were represented as ratios to beta-action mRNA (5.89±1.22 and 4.41±1.12 amol/50 ng total RNA, respectively, in reserpine-untreated and -treated arteries). Alpha-1D AR mRNA increased to approximately three-folds by reserpine treatment. The mRNA levels of alpha-1A and -1B AR subtypes were not significantly changed, although alpha-1A AR mRNA showed a tendency of decrease.
Figure 5.
Effects of reserpine treatment on mRNA levels of alpha-1 AR subtypes in rat tail arteries. Ordinate represents relative expression levels against beta-actin mRNA. Open column: reserpine-utreated rats (control). Shaded column: reserpine-treated rats. Data represent mean±s.e.m. of the results obtained from 6–7 rats. *Significantly different from reserpine-untreated rats.
Discussion
It has been well known that chronic treatment with reserpine causes supersensitivity in the tail artery (Nasseri et al., 1985; Duckles, 1991) and other tissues of rats (Pointon & Banerjee, 1979; Abel et al., 1985). The present study confirmed the development of supersensitivity in the responses to phenylephrine, 5-HT and KCl in the rat tail artery. However, the extent of supersensitivity varied between the agonists, a larger leftward shift (11.6-fold) being observed in the response to phenylephrine than in the responses to 5-HT (2.5-fold) and KCl (1.1-fold). The supersensitivity in the responses to 5-HT and KCl was evident at low concentrations of the agonists and was resistant to prazosin, an alpha-1 AR-selective antagonist (Hieble et al., 1995; Hancock, 1996), in contrast to the response to phenylephrine. These results suggest that reserpine treatment predominantly produces alpha-1 adrenergic supersensitivity, although the possible involvement of nonspecific supersensitivity cannot be ruled out (Fleming et al., 1973).
What mechanisms might be involved in the adrenergic supersensitivity induced in the reserpine-treated tail artery? Stassen et al. (1998) suggested that the presence of alpha-1A AR subtype was positively related to the sympathetic innervation in several arteries of rats. However, adrenergic supersensitivity has not yet been examined with special reference to alpha-1 AR subtypes. Then, we analyzed the alpha-1 AR subtypes responsible for the supersensitivity according to the current alpha-1 AR classification (Hieble et al., 1995), and with the use of both subtype-nonselective (prazosin) and subtype-selective antagonists (BMY 7378 for alpha-1D AR subtype, KMD-3213 for alpha-1A AR subtype), as mentioned in Introduction. Prazosin inhibited the contractile responses of tail arteries to phenylephrine with high pKB values irrespective of reserpine treatment, suggesting that the responses to phenylephrine are mediated through alpha-1 ARs having a high affinity for prazosin. The supersensitivity in the contractile response to phenylephrine was also inhibited by BMY 7378 at 30 nM, which is a specific concentration for blocking the alpha-1D AR subtype (Hussain & Marshall, 1997; Murata et al., 1999), while the response in reserpine-untreated artery failed to be inhibited by the same concentration of BMY 7378. Furthermore, the residual responses to phenylephrine of the reserpine-treated tail artery in the presence of 30 nM BMY 7378 showed the same sensitivities to prazosin and KMD-3213 as was observed in reserpine-untreated artery (Table 2). These results strongly suggest that the supersensitivity produced by low concentrations of phenylephrine is produced via the alpha-1D AR, while such a supersensitive response is absent in the untreated artery segments (present study; Lachnit et al., 1997; Murata et al., 1999), indicating apparent switching of functionally dominant alpha-1 AR from alpha-1A to alpha-1D subtype by reserpine treatment. In this regard, it is interesting to note that the alpha-1D AR subtype is highly sensitive to catecholamines and phenylephrine as compared with the alpha-1A and alpha-1B AR subtypes (Lomasney et al., 1991; Buckner et al., 1996; Graham et al., 1996). Additionally, the pEC50 value (7.1±0.1) for phenylephrine in the reserpine-treated tail artery was the same as the pEC50 value (7.2±0.1) in the thoracic aorta, where the adrenergic responses to phenylephrine have been known to be mainly mediated through alpha-1D ARs, irrespective of reserpine treatment (present study; Buckner et al., 1996; Muramatsu et al., 1998; Murata et al., 1999; Daly et al., 2002).
If alpha-1D ARs are actually involved in the adrenergic supersensitivity observed, we anticipated that the subtype should be also detected in the binding study. A previous study with microsomal fractions of rat tail artery demonstrated that the density and affinity of [125I]-BE-2245-binding sites did not change after treatment with reserpine (Nasseri et al., 1985). In the present study, we used a tissue segment-binding method which allows for a more efficient detection of receptors without a loss of receptor that has been observed upon homogenization and isolation of microsomal membranes (Tanaka et al., 2004). Using this method, we also observed that there were no significant changes in the density and affinity of [3H]-prazosin-binding sites after treatment with reserpine (Table 4). However, binding competition experiments clearly demonstrated an appearance (34% of total density in proportion) of high-affinity sites for BMY 7378 (most likely alpha-1D AR subtype) and a reduction (from 60 to 50%) of KMD-3213 high-affinity sites (alpha-1A AR subtype) by reserpine treatment. Since KMD-3213 low-affinity sites can be characterized as alpha-1B and/or alpha-1D AR subtypes, the low-affinity sites in reserpine-untreated tail artery (approximately 40% of total density) appeared to correspond to alpha-1B AR subtype, while the proportion in reserpine-treated artery (approximately 50% of total density) seemed to be composed of both alpha-1D AR subtype (34%, BMY 7378 high-affinity sites mentioned above) and the remaining alpha-1B AR subtype (approximately 16%), although this estimation must be confirmed by alpha-1B AR selective drugs. From these data, together with no change in total alpha-1AR density, it is likely that reserpine treatment causes an induction of alpha-1D AR subtype and conversely reduces the densities of both alpha-1A and -1B AR subtypes in the rat tail artery (Table 4). Stassen et al. (1998) reported that chemical sympathectomy with 6-hydroxydopamine caused a reduction of alpha-1A AR subtype and an increase in non-alpha-1A AR subtypes in the small or resistance-sized mesenteric arteries of rats. In agreement with these observations, alpha-1D AR mRNA increased to three-folds by reserpine treatment. However, mRNA levels of alpha-1A and alpha-1B ARs were not significantly changed after treatment with reserpine. Thus, it is likely that, although the observed upregulation of alpha-1D AR may be associated with its induction, the expression level of three alpha-1AR subtypes might be also regulated at post-transcriptional processes.
Recently, it has been demonstrated that alpha-1 ARs distribute in not only smooth muscle cells but also adventitial fibroblasts and other types of cells in rat thoracic aorta (Faber et al., 2001). Therefore, it is unlikely that [3H]-prazosin-binding sites and alpha-1 AR mRNAs detected in the tail artery segments reflect alpha-1 ARs occurring in the smooth muscle only (Tanaka et al., 2004). Thus, even though the density and mRNA level of alpha-1 ARs did not change, we must clarify in further studies whether the amount of alpha-1 ARs in smooth muscle is actually the same regardless of reserpine treatment.
Alpha-1D AR subtype is known to be constitutively active and undergo phosphorylation or sequestration, as compared with the alpha-1A and alpha-1B AR subtypes (Gisbert et al., 2000; McCune et al., 2000). From these data, together with the high affinity of the alpha-1D AR subtype for catecholamines, it is possible that most of the alpha-1D AR subtype is sequestered or downregulated under sympathetically innervated conditions and that this process may be reversed by sympathetic denervation, resulting in an upregulation of the alpha-1D AR. It is also interesting to note in this regard, that the alpha-1D AR subtype can be readily detected in both functional and ligand-binding studies using the rat thoracic aorta (Buckner et al., 1996; Muramatsu et al., 1998; Tanaka et al., 2004). Importantly, this tissue is not sympathetically innervated (Table 1 in the present study; Berkowitz et al., 1971; Stassen et al., 1998).
In the present study, reserpine-induced supersensitivity was observed in the tail artery but not in the thoracic aorta and spleen. As mentioned above, a lack of supersensitivity in the thoracic aorta might be anticipated, because the sympathetic innervation is negligible in the tissue and the alpha-1D AR subtype is already expressed. However, in contrast with the aorta but like the tail artery, the endogenous noradrenaline level in the spleen was high and was markedly reduced by reserpine treatment. Present and previous functional or binding studies have clearly demonstrated the exclusive presence of alpha-1B AR subtype in rat spleen, irrespective of reserpine treatment (Kenakin & Novak, 1988; Yang et al., 1997; Buckner et al., 2002; Zhang et al., 2002). Thus, it does not appear that noradrenaline depletion and/or lack of adrenergic input necessarily cause the changes of alpha-1 AR expression and supersensitivity in sympathetically innervated tissues all.
Three alpha-1 AR mRNAs have been detected in the rat thoracic aorta, tail artery and spleen (Rokosh et al., 1994; Graham et al., 1996; Phillips et al., 1997; Hrometz et al., 1999; Guimaraes & Moura, 2001), whereas all the receptor proteins are not ubiquitously distributed in such tissues. As mentioned above, it seems that different post-translational processes may affect the expression of each alpha-1 AR subtype in tissue-specific manners. Furthermore, induction of three alpha-1 AR subtypes has been recently shown to be regulated by individual promoter region, which would be differently affected under different neurogenic and hormonal conditions (Gao & Kunos, 1993; Day et al., 1999; Michelotti et al., 2003). Thus, the mechanisms underlying the tissue- and subtype-specific expression of alpha-1 AR and/or other receptors would merit further investigations for not only the maintenance of sympathetic neuroeffector processes but also some neurodegenerative disorders.
In conclusion, the present study shows that reserpine treatment produces significant changes in the expression of alpha-1 AR subtypes in rat tail artery, which may be in part associated with the adrenergic supersensitivity.
Acknowledgments
We greatly appreciate Professor M.D. Hollenberg (University of Calgary) for his critical reading and suggestion. This work was supported by Grant-in-Aid for Young Scientists (B), Exploratory Research, Scientific Research (B) and COE research from the Ministry of Education, Culture, Sports, Science and Technology, and by a grant from the Smoking Research Foundation.
Abbreviations
- AR
adrenoceptor
- BMY 7378
(8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride
- KMD-3213
(−)-1-(3-hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1H-indole-7-carboxamide
References
- ABEL P.W., JOHNSON R.D., MARTIN T.J., MINNEMAN K.P. Sympathetic denervation does not alter the density or properties of alpha-1 adrenergic receptors in rat vas deferens. J. Pharmacol. Exp. Ther. 1985;233:570–577. [PubMed] [Google Scholar]
- ABEL P.W., URQUILLA P.R., GOTO K., WESTFALL D.P., ROBINSON R.L., FLEMING W.W. Chronic reserpine treatment alters sensitivity and membrane potential of the rabbit saphenous artery. J. Pharmacol. Exp. Ther. 1981;217:430–439. [PubMed] [Google Scholar]
- BERKOWITZ B.A., TARVER J.H., SPECTOR S. Norepinephrine in blood vessels: concentration, binding, uptake and depletion. J. Pharmacol. Exp. Ther. 1971;177:119–126. [PubMed] [Google Scholar]
- BITO L.Z., DAWSON M.J. The site and mechanism of the control of cholinergic sensitivity. J. Pharmacol. Exp. Ther. 1970;175:673–684. [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUCKNER S.A., MILICIC I., DAZA A.V., MEYER M.D., ALTENBACH R.J., WILLIAMS M., SULLIVAN J.P., BRIONI J.D. ABT-866, a novel alpha(1A)-adrenoceptor agonist with antagonist properties at the alpha(1B)- and alpha(1D)-adrenoceptor subtypes. Eur. J. Pharmacol. 2002;449:159–165. doi: 10.1016/s0014-2999(02)01976-3. [DOI] [PubMed] [Google Scholar]
- BUCKNER S.A., OHEIM K.W., MORSE P.A., KNEPPER S.M., HANCOCK A.A. Alpha 1-adrenoceptor-induced contractility in rat aorta is mediated by the alpha 1D subtype. Eur. J. Pharmacol. 1996;297:241–248. doi: 10.1016/0014-2999(95)00755-5. [DOI] [PubMed] [Google Scholar]
- BURT R.P., CHAPPLE C.R., MARSHALL I. Evidence for a functional alpha 1A- (alpha 1C-) adrenoceptor mediating contraction of the rat epididymal vas deferens and an alpha 1B-adrenoceptor mediating contraction of the rat spleen. Br. J. Pharmacol. 1995;115:467–475. doi: 10.1111/j.1476-5381.1995.tb16356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRIER O., Jr Role of calcium in postjunctional supersensitivity. Fed. Proc. 1975;34:1975–1980. [PubMed] [Google Scholar]
- COLUCCI W.S., GIMBRONE M.A., Jr, ALEXANDER R.W. Regulation of the postsynaptic alpha-adrenergic receptor in rat mesenteric artery. Effects of chemical sympathectomy and epinephrine treatment. Circ. Res. 1981;48:104–111. doi: 10.1161/01.res.48.1.104. [DOI] [PubMed] [Google Scholar]
- DALY C.J., DEIGHAN C., McGEE A., MENNIE D., ALI Z., McBRIDE M., McGRATH J.C. A knockout approach indicates a minor vasoconstrictor role for vascular alpha-1B adrenoceptors in mouse. Physiol. Genomics. 2002;9:85–92. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- DAY H.E.W., CAMPEAU S., WATSON S.J., Jr, AKIL H. Expression of alpha-1b adrenoceptor mRNA in corticotrophin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J. Neurosci. 1999;19:10098–10106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCKLES S.P. Reserpine-induced supersensitivity in rat caudal artery: influence of age. J. Pharmacol. Exp. Ther. 1991;256:513–518. [PubMed] [Google Scholar]
- FABER J.E., YANG N., XIN X. Expression of alpha-adrenoceptor subtypes by smooth muscle cells and adventitial fibroblasts in rat aorta and in cell culture. J. Pharmacol. Exp. Ther. 2001;298:441–452. [PubMed] [Google Scholar]
- FLEMING W.W. Variable sensitivity of excitable cells: possible mechanisms and biological significance. Rev. Neurosci. 1976;2:43–90. [Google Scholar]
- FLEMING W.W. Membrane potential and vascular smooth muscle sensitivity. A minireview. Blood Vessels. 1987;24:108–112. doi: 10.1159/000158680. [DOI] [PubMed] [Google Scholar]
- FLEMING W.W., MCPHILLIPS J.J., WESTFALL D.P. Postjunctional supersensitivity and subsensitivity of excitable tissues to drugs. Ergeb. Physiol. 1973;68:55–119. doi: 10.1007/3-540-06238-6_5. [DOI] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P. Altered resting membrane potential in the supersensitive vas deferens of the guinea pig. J. Pharmacol. Exp. Ther. 1975;192:381–389. [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification on adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Handbuch der Experimentellen Pharmacologie 1972New York: Springer; 283–335.ed. Blaschko, H. & Muscholl, E. Vol. 3, pp [Google Scholar]
- GAO B., KUNOS G. Isolation and characterization of the gene encoding the rat alpha-1B adrenergic receptor. Gene. 1993;131:243–247. doi: 10.1016/0378-1119(93)90300-r. [DOI] [PubMed] [Google Scholar]
- GIACHETTI A., SHORE P.A. The reserpine receptor. Life Sci. 1978;10:89–92. doi: 10.1016/0024-3205(78)90254-0. [DOI] [PubMed] [Google Scholar]
- GISBERT R., MADRERO Y., SABINO V., NOGUERA M.A., IVORRA M.D., D'OCON P. Functional characterization of alpha(1)-adrenoceptor subtypes in vascular tissues using different experimental approaches: a comparative study. Br. J. Pharmacol. 2003;138:359–368. doi: 10.1038/sj.bjp.0705033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISBERT R., NOGUERA M.A., IVORRA M.D., D'OCON P. Functional evidence of a constitutively active population of alpha(1D)-adrenoceptors in rat aorta. J. Pharmacol. Exp. Ther. 2000;295:810–817. [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D., TRUE T.A., RIMELE T.J., SAUSSY D.L., Jr BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur. J. Pharmacol. 1995;16:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GRAHAM R.M., PEREZ D.M., HWA J., PIASCIK M.T. Alpha 1-adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ. Res. 1996;78:737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HANCOCK A.A. Alpha1 adrenoceptor subtypes: a synopsis of their pharmacology and molecular biology. Drug Dev. Res. 1996;39:54–107. [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R., JR International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HROMETZ S.L., EDELMANN S.E., McCUNE D.F., OLGES J.R., HADLEY R.W., PEREZ D.M., PIASCIK M.T. Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J. Pharmacol. Exp. Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- HUDGINS P.M., HARRIS T.M. Further studies on the effects of reserpine pretreatment on rabbit aorta: calcium and histologic changes. J. Pharmacol. Exp. Ther. 1970;175:609–618. [PubMed] [Google Scholar]
- HUSSAIN M.B., MARSHALL I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br. J. Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSEL P.A. Structure and function of alpha-adrenergic receptors. Am. J. Med. 1989;87:12S–18S. doi: 10.1016/0002-9343(89)90108-3. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., NOVAK P.J. Classification of phenoxybenzamine/prazosin-resistant contractions of rat spleen to norepinephrine by Schild analysis: similarities and differences to postsynaptic alpha-2 adrenoceptors. J. Pharmacol. Exp. Ther. 1988;244:206–212. [PubMed] [Google Scholar]
- LACHNIT W.G., TRAN A.M., CLARKE D.E., FORD A.P.D.W. Pharmacological characterization of an alpha 1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of rat. Br. J. Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMASNEY J.W., COTECCHIA S., LEFKOWITZ R.J., CARON M.G. Molecular biology of alpha-adrenergic receptors: implications for receptor classification and for structure–function relationships. Biochim. Biophys. Acta. 1991;1095:127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- McCUNE D.F., EDELMANN S.E., OLGES J.R., POST G.R., WALDROP B.A., WAUGH D.J., PEREZ D.M., PIASCIK M.T. Regulation of the cellular localization and signaling properties of the alpha(1B)- and alpha(1D)-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- MCGRATH J., WILSON V. Alpha-adrenoceptor subclassification by classical and response-related methods: same question, different answers. Trends Pharmacol. Sci. 1988;9:162–165. doi: 10.1016/0165-6147(88)90030-2. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., KENNY B., SCHWINN D.A. Classification of alpha 1-adrenoceptor subtypes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:1–10. doi: 10.1007/BF00169183. [DOI] [PubMed] [Google Scholar]
- MICHELOTTI G.A., BAUMAN M.J., SMITH M.P., SCHWINN D.A. Cloning and characterization of the rat alpha-1a adrenergic receptor gene promoter. J. Biol. Chem. 2003;278:8693–8705. doi: 10.1074/jbc.M211986200. [DOI] [PubMed] [Google Scholar]
- MINNEMAN K.P., THEROUX T.L., HOLLINGER S., HAN C., ESBENSHADE T.A. Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. Mol. Pharmacol. 1994;46:929–936. [PubMed] [Google Scholar]
- MURAMATSU I., MURATA S., ISAKA M., PIAO H.L., ZHU J., SUZUKI F., MIYAMOTO S., OSHITA M., WATANABE Y., TANIGUCHI T. Alpha1-adrenoceptor subtypes and two receptor systems in vascular tissues. Life Sci. 1998;62:1461–1465. doi: 10.1016/s0024-3205(98)00090-3. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., HASHIMOTO S., OSHITA M. Functional subclassification of vascular alpha1 adrenoceptors. Pharmacol. Commun. 1995;6:23–28. [Google Scholar]
- MURATA S., TANIGUCHI T., MURAMATSU I. Pharmacological analysis of the novel, selective alpha1-adrenoceptor antagonist, KMD-3213, and its suitability as a tritiated radioligand. Br. J. Pharmacol. 1999;127:19–26. doi: 10.1038/sj.bjp.0702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASSERI A., BARAKEH J.F., ABEL P.W., MINNEMAN K.P. Reserpine-induced postjunctional supersensitivity in rat vas deferens and caudal artery without changes in alpha adrenergic receptors. J. Pharmacol. Exp. Ther. 1985;234:350–357. [PubMed] [Google Scholar]
- PHILLIPS J.K., VIDOVIC M., HILL C.E. Variation in mRNA expression of alpha-adrenergic, neurokinin and muscarinic receptors amongst four arteries of the rat. J. Auton. Nerv. Syst. 1997;12:85–93. doi: 10.1016/s0165-1838(96)00114-2. [DOI] [PubMed] [Google Scholar]
- PIASCIK M.T., PEREZ D.M. Alpha1-adrenergic receptors: new insights and directions. J. Pharmacol. Exp. Ther. 2001;298:403–410. [PubMed] [Google Scholar]
- POINTON S.E., BANERJEE S.P. Alpha- and beta-adrenergic receptors of the rat salivary gland. Elevation after chemical sympathectomy. Biochim. Biophys. Acta. 1979;584:231–241. doi: 10.1016/0304-4165(79)90267-8. [DOI] [PubMed] [Google Scholar]
- ROKOSH D.G., BAILEY B.A., STEWART A.F., KARNS L.R., LONG C.S., SIMPSON P.C. Distribution of alpha 1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with alpha 1B and alpha 1D. Biochem. Biophys. Res. Commun. 1994;200:1177–1184. doi: 10.1006/bbrc.1994.1575. [DOI] [PubMed] [Google Scholar]
- SAUSSY D.L., JR, GOETZ A.S., QUEEN K.L., KING H.K., LUTZ M.W., RIMELE T.J. Structure activity relationships of a series of buspirone analogs at alpha-1 adrenoceptors: further evidence that rat aorta alpha-1 adrenoceptors are of the alpha-1D-subtype. J. Pharmacol. Exp.Ther. 1996;278:136–144. [PubMed] [Google Scholar]
- SHIBATA K., FOGLAR R., HORIE K., OBIKA K., SAKAMOTO A., OGAWA S., TSUJIMOTO G. KMD-3213, a novel, potent, alpha 1a-adrenoceptor-selective antagonist: characterization using recombinant human alpha 1-adrenoceptors and native tissues. Mol. Pharmacol. 1995;48:250–258. [PubMed] [Google Scholar]
- SHINOZUKA K., TANIOKA Y., KWON Y.M., TANAKA N., KUBOTA Y., NAKAMURA K., KUNITOMO M. Characterization of prejunctional purinoceptors inhibiting noradrenaline release in rat mesenteric arteries. Jpn. J. Pharmacol. 2001;85:41–46. doi: 10.1254/jjp.85.41. [DOI] [PubMed] [Google Scholar]
- STASSEN F.R., MAAS R.G., SCHIFFERS P.M., JANSSEN G.M., Demey J.G. A positive and reversible relationship between adrenergic nerves and alpha-1A adrenoceptors in rat arteries. J. Pharmacol. Exp. Ther. 1998;284:399–405. [PubMed] [Google Scholar]
- STITZEL R.E. The biological fate of reserpine. Pharmacol. Rev. 1976;28:179–208. [PubMed] [Google Scholar]
- TANAKA T., ZHANG L., SUZUKI F., MURAMATSU I. Alpha-adrenoreceptor: evaluation of receptor subtype binding kinetic in intact arterial tissue strips. Br. J. Pharmacol. 2004;141:468–476. doi: 10.1038/sj.bjp.0705627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTFALL D.P.Supersensitivity of smooth muscle Smooth Muscle: An Assessment of Current Knowledge 1981London: Butlerand Tanner Ltd; 285–309.ed. Bulbring, E., Brading, A.F., Jones, A.W. & Tomita, T. pp [Google Scholar]
- YAMAGISHI R., AKIYAMA K., NAKAMURA S., HORA M., MASUDA N., MATSUZAWA A., MURATA S., UJIIE A., KURASHINA Y., IIZUKA K., KITAZAWA M. Effect of KMD-3213, an alpha 1a-adrenoceptor-selective antagonist, on the contractions of rabbit prostate and rabbit and rat aorta. Eur. J. Pharmacol. 1996;315:73–97. doi: 10.1016/s0014-2999(96)00589-4. [DOI] [PubMed] [Google Scholar]
- YANG M., VERFURTH F., BUSCHER R., MICHEL M.C. Is alpha1D-adrenoceptor protein detectable in rat tissues. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:438–446. doi: 10.1007/pl00004966. [DOI] [PubMed] [Google Scholar]
- ZHANG L., TANIGUCHI T., TANAKA T., SHINOZUKA K., KUNITOMO M., NISHIYAMA M., KAMATA K., MURAMATSU I. Alpha-1 adrenoceptor up-regulation induced by prazosin but not KMD-3213 or reserpine in rats. Br. J. Pharmacol. 2002;135:1757–1764. doi: 10.1038/sj.bjp.0704639. [DOI] [PMC free article] [PubMed] [Google Scholar]