Abstract
Complement activation is implicated in the pathogenesis of intestinal ischaemia–reperfusion injury (I/R), although the relative importance of individual complement components is unclear. A C3a receptor antagonist N(2)-[(2,2-diphenylethoxy)acetyl]-L-arginine (C3aRA) has been compared with a C5a receptor antagonist (C5aRA), AcF-[OPdChaWR], in a rat model of intestinal I/R.
C3aRA (IC50=0.15 μM) and C5aRA (IC50=0.32 μM) bound selectively to human polymorphonuclear leukocyte (PMN) C3a and C5a receptors, respectively. Effects on circulating neutrophils and blood pressure in the rat were also assessed.
Anaesthetised rats, subjected to intestinal ischaemia (30 min) and reperfusion (120 min), were administered intravenously with either (A) the C3aRA (0.1–1.0 mg kg−1); the C5aRA (1.0 mg kg−1); the C3aRA+C5aRA (each 1.0 mg kg−1); or vehicle, 45 min prior, or (B) the C3aRA (1.0 mg kg−1) or vehicle, 120 min prior to reperfusion.
The C3aRA and C5aRA, administered 45 min prior to reperfusion, displayed similar efficacies at ameliorating several disease markers (increased oedema, elevated ALT levels and mucosal damage) of rat intestinal I/R. The combination drug treatment did not result in greater injury reduction than either antagonist alone. However, doses of the C3aRA (0.01–10 mg kg−1) caused transient neutropaenia, and the highest dose (10 mg kg−1) also caused a rapid and transient hypertension.
The C3aRA (1.0 mg kg−1), delivered 120 min prior to reperfusion to remove the global effect of C3aRA-induced neutrophil sequestration, did not attenuate the markers of intestinal I/R, despite persistent C3aR antagonism at this time.
C3aR antagonism does not appear to be responsible for the anti-inflammatory actions of this C3aRA in intestinal I/R in the rat. Instead, C3aRA-mediated global neutrophil tissue sequestration during ischaemia and early reperfusion may account for the protective effects observed.
Keywords: C3a, C3a antagonist, C5a, C5a antagonist, intestinal ischaemia/reperfusion, neutropaenia
Introduction
Intestinal ischaemia/reperfusion (I/R) injury occurs when there is a reduction in, or cessation of, blood flow to the intestine, followed by the restoration of blood flow, or reperfusion of the tissue. Intestinal I/R manifests as damage to the intestinal mucosa, which is characterised by increased vascular permeability and intestinal oedema, increased mucosal permeability and barrier dysfunction, combined with haemodynamic and cardiovascular changes (Schoenberg & Beger, 1993; Khanna et al., 2001). The local effect in the intestine is coupled with injury to remote organs and in some cases the onset of sepsis and multiple organ failure (Poggetti et al., 1992; Harward et al., 1993; Moore et al., 1994; Turnage et al., 1996; Carden & Granger, 2000).
While the restoration of blood flow to ischaemic tissues is essential for survival, reperfusion will induce additional damage through induction of an inflammatory response (Parks & Granger, 1986; Carden & Granger, 2000). A number of chemical and cellular mediators, including reactive oxygen species (Zimmerman & Granger, 1994), platelet-activating factor (Kim et al., 1995; Sun et al., 2000), cytokines (tumour necrosis factor-α (TNF-α) and interleukin-6) (Sorkine et al., 1995; Yao et al., 1996; Sun et al., 1999), mucosal mast cells (Kanwar & Kubes, 1994; Kanwar et al., 1998) and polymorphonuclear leukocytes (PMNs) (Hernandez et al., 1987; Sisley et al., 1994; Koike et al., 1995) have been implicated in the pathogenesis of intestinal I/R. In addition, activation of the complement system, which results in production of the biologically active anaphylatoxins, complement factors 3a (C3a), 4a and 5a (C5a), has been demonstrated to play a significant role in the pathology of I/R (Riedemann & Ward, 2003).
Treatment with the soluble complement receptor type 1, or the anticomplementary agent K-76, alone or in combination with the nonselective serine protease inhibitor FUT-175, attenuates reperfusion-induced injury (Hill et al., 1992; Kimura et al., 1998; Eror et al., 1999; Andoh et al., 2001). Recently, C5a has been identified as one of the major complement factors responsible for induction of the reperfusion-associated inflammatory response. Treatment with either an anti-C5 antibody (Wada et al., 2001) or a large molecular weight C5a receptor antagonist (C5aRA) (Heller et al., 1999) attenuates I/R induced mucosal injury in the rat. A small molecule C5aRA, AcF-[OPdChaWR], has demonstrated efficacy in the treatment of experimental inflammatory conditions, including monoarticular arthritis and inflammatory bowel disease (Woodruff et al., 2002; 2003), sepsis (Huber-Lang et al., 2002) and renal I/R injury (Arumugam et al., 2003), implicating C5a as a pathogenic mediator in these conditions. In addition, C5aRA inhibits I/R induced neutrophil margination, intestinal oedema, serum enzyme elevation, and mucosal damage in the rat small intestine (Arumugam et al., 2002). A role for C3a in the pathogenesis of intestinal I/R has not yet been examined.
C5a and C3a are both involved in the mediation of immune responses and inflammatory processes (Ember & Hugli, 1997). While these anaphylatoxins have proinflammatory actions, C3a is responsible for mediating a more specialised set of inflammatory events in vitro. Compared to C5a, the biological actions of C3a in vivo and the role of C3a in disease have received limited attention. This situation has perhaps been, in part, due to the lack of potent and selective C3a receptor agonists and antagonists. Recently, Ames et al. (2001) have reported the discovery of a C3a receptor antagonist (C3aRA) that selectively blocked the C3a receptor (C3aR) and C3a activity in vitro and in vivo, but did not inhibit Ca2+ mobilisation in response to C5a, leukotriene B4, formyl-Met-Leu-Pro, platelet activating factor or chemokine (CXCR1 and CXCR2) activation of neutrophils.
In this study we set out to investigate some basic pharmacology of the C3aRA and then to utilise this C3aRA in a rat model of intestinal I/R and compare its effect with that of a C5aRA, which has previously been shown to attenuate injury in a rat model of intestinal I/R (Arumugam et al., 2002).
Methods
Synthesis of C3aRA and C5aRA
The C3aRA was synthesised and characterised by mass spectrometry and proton nuclear magnetic resonance spectroscopy. The C5aRA was synthesised by solution phase methods, purified by reversed phase HPLC, and characterised by mass spectrometry and proton nuclear magnetic resonance spectroscopy as described (Reid et al., 2003).
Human PMN isolation
Heparinised blood was layered over a double Ficoll-Hypaque gradient (Histopaque 1119 and 1077; 1 : 1; Sigma, U.S.A.) and centrifuged for 30 min (400 × g at 25°C). The top three layers of plasma, mononuclear cells and Histopaque were discarded and the PMN-rich layer collected, diluted with distilled water (4°C) and vigorously shaken for 40 s to lyse residual erythrocytes. Isotonicity was restored by addition of concentrated (× 10) Dulbecco's phosphate-buffered saline (Sigma, U.S.A.) and the cells were centrifuged (700 × g for 10 min at 4°C). After careful removal of the supernatant, cells were resuspended in Receptor Binding Assay buffer (3 ml; 50 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, 0.5% BSA, 0.1% bacitracin), counted on a haemocytometer and the volume adjusted to give a concentration of 4 × 106 cells ml−1.
Receptor binding assay
The C5a receptor (C5aR) binding assay was performed in Multiscreen Filtration plates (Millipore, Australia) as previously described in detail (Paczkowski et al., 1999). The C3aR binding assay was performed in 0.6 ml microcentrifuge tubes. Assay buffer and increasing concentrations of C3a (Calbiochem, U.S.A.) or receptor antagonists were added to tubes to a volume of 140 μl. [125I]-C3a (100 pM; Perkin Elmer, U.S.A.) and PMNs (2 × 105) were then added to each tube, to a final volume of 200 μl, and the tubes were incubated at 25°C for 60 min. After incubation, buffer (200 μl) was added to the tubes, which were immediately centrifuged (11,000 × g, 3 min at 25°C). The supernatant was gently removed in vacuo from the tube and the PMN pellet counted on a LKB gamma counter. For individual experiments, the data are expressed as percentage specific binding (nonspecific binding=100 nM C3a; typically 10–15% of total binding).
A nonlinear regression analysis (Graph Pad, U.S.A.) was performed on concentration–response curves and the IC50 and pIC50 values for each compound were determined. The pIC50 for each peptide was calculated from separate experiments and expressed as mean±s.e.m. IC50 values were expressed as a geometric mean.
Blood pressure measurements
Systolic blood pressure of female Wistar rats was recorded using a pressure transducer (ADI Instruments, Australia) and an inflatable tail cuff as previously described (Short et al., 1999). Briefly, a tail cuff was positioned on the base of the tail above a Pfiez finger pulse transducer (ADI Instruments, Australia). The tail cuff was connected to a pressure transducer and amplifier (J-RAK) and the signal was recorded (MacLab/8). The tail cuff was inflated until the pulse signal was lost and systolic blood pressure was recorded when the tail blood pressure exceeded cuff pressure and the pulse signal resumed. This was repeated ⩾3 times for each time point, and the mean value was recorded. Data are presented as a percentage of the resting blood pressure±s.e.m.
Pharmacokinetics
Female Wistar rats (200–220 g) were anaesthetised with an i.p. injection of zolazepam and tiletamine (Zoletil; 50 mg kg−1, Virbac, Australia) and xylazine (Ilium; 10 mg kg−1, Australia), and maintained throughout the experiment by periodic administration of zolazepam as required. Rats were infused with C3aRA (0.1, 0.3 and 1.0 mg kg−1 i.v. in 10% ethanol/saline) through the femoral vein and a heating pad was used to maintain the body temperature of the rats, while blood samples were taken over a period of 3 h. Blood samples were immediately added to tubes containing heparin (50 IU ml−1), centrifuged (11,000 × g for 3 min) and stored on ice. The plasma layer of each sample was removed, diluted with acetonitrile (HPLC grade), vortexed and centrifuged (11,000 × g for 3 min). The sample was evaporated to dryness and then resuspended and analysed on a PE Sciex Qstar Pulsar HPLC-Mass Spectrum. The concentration of C3aRA in experimental samples was calculated from integrated data using a standard curve and expressed as mean±s.e.m. Standard curves for the C3aRA, in both plasma and acetonitrile, were performed on each day of analysis and plotted as concentration versus peak area. Data were analysed with Rstrip software (Micromath, U.S.A.), and distribution and elimination half-lives were calculated and expressed as mean (range).
Model of intestinal I/R
Female Wistar rats (200–220 g) were fasted for 16–18 h preceding experimentation, but allowed access to water ad libitum. Rats were anaesthetised with an intraperitoneal injection of zolazepam and tiletamine (Zoletil; 50 mg kg−1) and xylazine (Ilium; 10 mg kg−1). Body temperature was maintained by placing the animals on a heating pad. Treated rats were administered C3aRA (0.1, 0.3 and 1.0 mg kg−1 i.v. in 10% ethanol/saline), C5aRA (1.0 mg kg−1 i.v. in 10% ethanol/saline) or the combination treatment (C3aRA and C5aRA, both at 1.0 mg kg−1 i.v.) through the isolated femoral vein, 45 min prior to induction of intestinal reperfusion. Alternatively, some rats received C3aRA (1.0 mg kg−1) or vehicle, 120 min prior to induction of reperfusion. Rats undergoing I/R alone or a sham operation received vehicle (10% ethanol/saline). Sham-operated drug-only controls were administered either drug (1.0 mg kg−1 i.v.), but did not undergo I/R.
A midline laparotomy was performed on all animals. I/R injury and drug-treated groups experienced 30 min of intestinal ischaemia, induced by nontraumatic occlusion of the superior mesenteric artery (SMA). During this period the abdomen was covered with saline-moistened gauze. Following 30 min of ischaemia the blood supply to the intestine was allowed to reperfuse for 120 min and the midline incision sutured.
Serum was collected after 150 min and stored at −20°C for alanine amino transferase (ALT) determination. Enzyme levels were assessed within 48 h of collection. For some animals serial blood samples were collected throughout the experiment into heparised tubes and the PMNs isolated and counted. After 150 min the rats were euthanased by cervical dislocation and tissue samples collected for assessment of intestinal oedema and histopathology. A section of the affected ileum was removed, blotted dry and weighed. Samples were then oven-dried overnight at 80°C and the tissue dry weight was determined. The intestinal wet to dry weight ratio was used as an assessment of intestinal oedema. Segments of harvested intestinal tissue were immediately fixed in 10% buffered formalin for histological study.
Neutropaenia
Blood samples (100 μl) were collected from the tail vein into heparinised tubes (50 IU ml−1), gently layered over Histopaque-1083 (200 μl; Sigma, U.S.A.), and centrifuged at 400 × g for 25 min at 25°C. The supernatant was removed and distilled water (4°C) was added to the remaining pellet and shaken for 40 s to lyse the red blood cells. Dulbecco's phosphate-buffered saline (10 ×) (Sigma, U.S.A.) was added to restore isotonicity before centrifugation (700 × g for 10 min at 4°C). The pellet was washed, centrifuged (700 × g for 10 min at 4°C), resuspended in saline, and cells were counted on a haemocytometer. PMN numbers were presented as mean percentage±s.e.m. of the values obtained immediately prior to administration of the test compound.
Alanine aminotransferase assay
Serum ALT concentrations were determined using a commercial kit (ALT/GPT, Sigma, U.S.A.) within 48 h of blood collection and according to the manufacturer's directions. Serum enzyme levels were derived from calibration curves and results were expressed as mean±s.e.m. in Sigma-Frankel (SF) units ml−1.
Histopathology
Specimens fixed in 10% buffered formalin were embedded in paraffin wax, serially sectioned and stained with haematoxylin and eosin. Tissue sections were scored by a trained observer in a blinded manner using a graded scale developed to quantify the extent of mucosal damage (Arumugam et al., 2003b). The 5-point scale progresses from normal (0) through development of apical subepithelial space (1), epithelial lifting (2–3) and cellular infiltration (4), to disintegration of lamina propria, haemorrhage and ulceration (5). A blinded observer assigned all scores.
Statistical analysis
Results for intestinal oedema, ALT and histopathology studies were statistically analysed with a one-way ANOVA test coupled with a Dunnett's or Dunn's post-test. The levels of circulating neutrophils were compared statistically with a two-way ANOVA and subsequently with a one-way ANOVA coupled with a Dunn's post-test. In all cases, statistical significance was defined as P⩽0.05.
Results
Affinities of C3a, C3aRA, C5a and C5aRA for human PMN C3a and C5a receptors
C3a bound to C3aRs on isolated human PMNs with high affinity (IC50=0.2 nM, pIC50=9.64±0.10, n=3). The C3aRA binds to C3aRs (IC50=153 nM, pIC50=6.82±0.20, n=4), but not C5aRs (IC50>1 mM). The C5aRA did not bind to human PMN C3aRs (IC50>1 mM). C5a bound with high affinity to PMN C5aRs (IC50=0.8 nM, pIC50=9.10±0.01, n=14). The C5aRA had significantly lower affinity than C5a for the C5aR (IC50=320 nM, pIC50=6.49±0.07, n=16).
Pharmacology of C3a and C3aRA in vivo
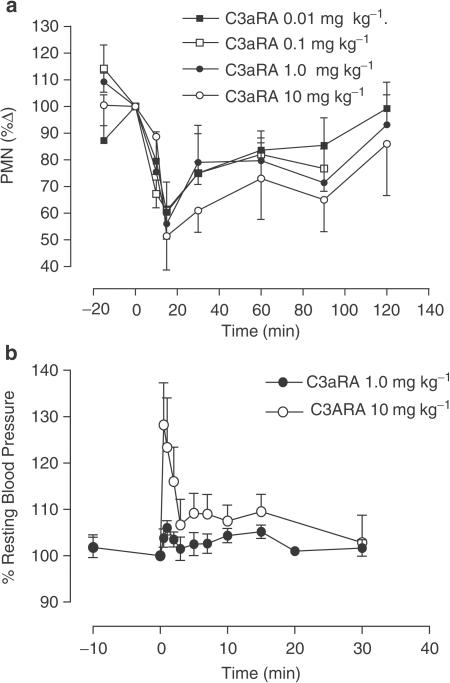
At doses of 0.01, 0.1, 1.0 and 10 mg kg−1 (n=6) the C3aRA induced a rapid neutropaenia that reached maximal levels (between 50 and 60% of predose numbers of circulating neutrophils) 15 min postadministration and recovered gradually over the following 2 h of the experiment (Figure 1a). A rapid, moderate and transient hypertension was observed following delivery of the C3aRA at 10 mg kg−1 i.v., but not at 1.0 mg kg−1 (n=4; Figure 1b).
Figure 1.
(a) Induction of neutropaenia in the rat by intravenous administration of C3aRA at 0.01, 0.1, 1.0 and 10 mg kg−1. PMN numbers are presented as percentage of predose numbers±s.e.m. (b) Effect of C3aRA (at 1.0 and 10 mg kg−1) on resting blood pressure in the rat. Values are expressed as mean percentage of predose blood pressure±s.e.m.
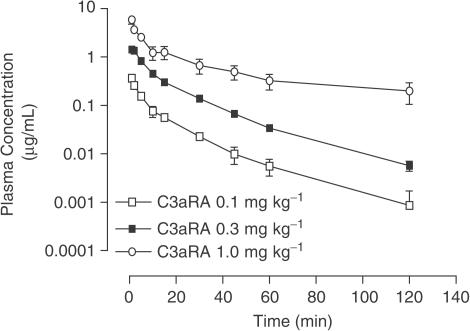
Pharmacokinetics of C3aRA
Following intravenous administration of C3aRA (0.1 and 0.3 mg kg−1 i.v., n=3; Figure 2) the C3aRA was rapidly distributed (distribution t1/2=2.19 (1.96–2.71) and 4.28 (3.25–5.52) min, respectively), and eliminated from the plasma within 3 h (elimination t1/2=34.68 (10.6–80.8) and 19.45 (17.25–23.25) min, respectively). In the case of the highest dose (1.0 mg kg−1 i.v., n=4), similar kinetics of distribution were observed (t1/2=7.56 (3.60–12.03) min). However, the elimination phase at this dose was considerably more prolonged (t1/2=195.01 (28.54–304.0) min; Figure 2). Moreover, when the C3aRA was infused at 1.0 mg kg−1 it completely blocked hypertension induced by a C3a agonist (WWGKKYRASKLGLAR, 30 μg kg−1) administered 2 h later (n=4; data not shown).
Figure 2.
Intravenous pharmacokinetics of C3aRA in the rat. Rats were anaesthetised and dosed at 0.1, 0.3 and 1.0 mg kg−1 i.v. Each point represents the mean plasma concentration at various time points and error bars indicate s.e.m.
Effect of drug treatment 45 min prior to reperfusion
Inhibition of I/R-induced intestinal oedema by C3aRA versus C5aRA
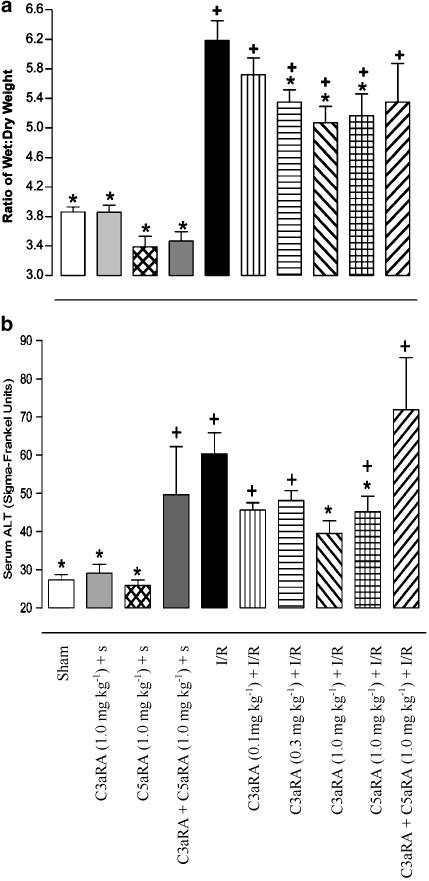
The intestines of sham and C3aRA or C5aRA-treated sham-operated animals displayed wet/dry weight ratios of 3.86±0.07, 3.86±0.09 and 3.31±0.14, respectively (n=6–11, Figure 3a). Marked intestinal oedema was evident in animals undergoing I/R (6.19±0.27, n=15). I/R-induced intestinal oedema was significantly attenuated by intravenous administration of either the C3aRA at 0.3 mg kg−1 (5.35±0.17, n=9) or 1.0 mg kg−1 (5.07±0.22, n=10), or the C5aRA at 1.0 mg kg−1 (5.16±0.30, n=10), 45 min prior to the induction of reperfusion (Figure 3a). The oedema observed in these treatment groups remained significantly greater than sham-operated animals. Administration of the C3aRA at 0.1 mg kg−1 (5.72±0.23, n=7) did not significantly affect I/R-induced intestinal oedema.
Figure 3.
Effect of drug treatment 45 min prior to intestinal reperfusion. (a) I/R-induced intestinal oedema. Pretreatment with the C3aRA, at 0.3 or 1.0 mg kg−1 i.v. and C5aRA at 1.0 mg kg−1 i.v., significantly attenuated I/R-induced oedema in the rat intestine. Data are shown as mean±s.e.m. *P⩽0.05 when compared to I/R animals, + P⩽0.05 when compared to sham animals. (b) Effect of antagonists on elevation of serum ALT following I/R. Elevation of serum ALT following intestinal I/R is attenuated by treatment with a C3aRA (1.0 mg kg−1) or C5aRA (1.0 mg kg−1). Administration of both the C3aRA and C5aRA (both 1.0 mg kg−1 i.v.) to sham-operated animals resulted in an elevation in ALT that was not associated with I/R. Data are expressed as mean±s.e.m. * P⩽0.05 when compared to I/R animals. +P⩽0.05 when compared to sham-operated animals. S, sham.
Effect of drug treatment on I/R-induced elevation of ALT levels
Following intestinal I/R, ALT levels (60.3±5.5 SF units, n=12) rose significantly over those detected in sham (27.3±1.4 SF units, n=8) and C3aRA- or C5aRA-treated sham operated animals (29.2±2.3 and 25.9±1.4 SF units respectively; n=7–9, Figure 3b). The C3aRA administered at 1.0 mg kg−1 i.v. (39.5±3.3 SF units, n=9) 45 min prior to reperfusion attenuated I/R induced elevation of ALT (P⩽0.05; Figure 3b). Similarly, the C5aRA (1.0 mg kg−1, 45.2±4.1 SF units, n=10) also significantly reduced ALT levels in rats that underwent I/R. When both the C3aRA and C5aRA were administered concomitantly to sham-operated animals (49.6±12.6 SF units, n=9), there was a rise in ALT to a level that was not significantly different to I/R animals. Consequently, pretreatment of I/R animals with the combination treatment (71.9±13.7 SF units, n=7) resulted in ALT levels similar to that with I/R injury (Figure 3b).
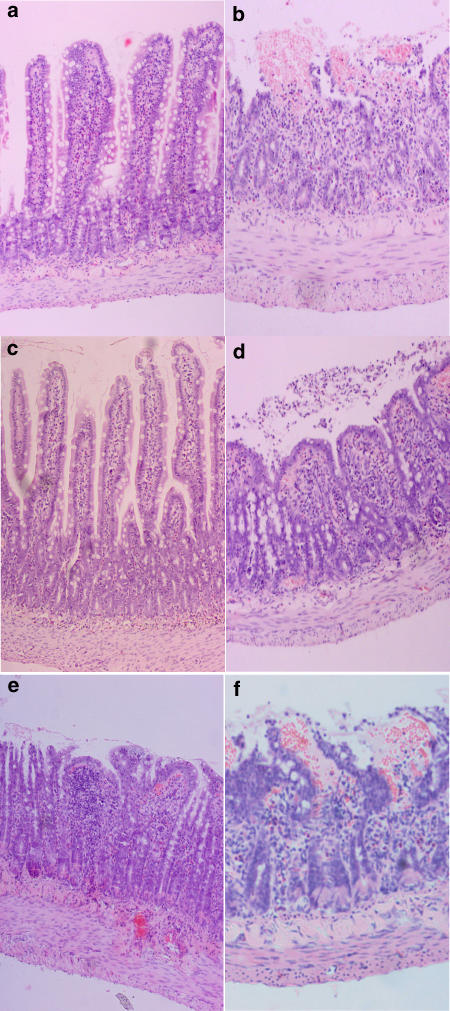
Histopathology
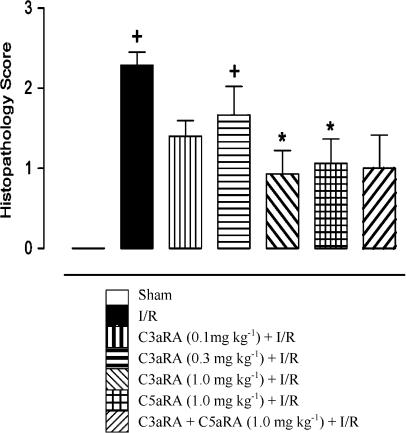
The progressive mucosal damage that occurs following intestinal I/R has been comprehensively characterised and individual scores for all groups are shown in Figure 4. Normal mucosal structures (0) were observed in animals in sham and drug-treated sham groups (Figure 5a). Massive epithelial lifting along the sides of villi, denuded tips and some haemorrhage were characteristic in I/R animals (2.28±0.16; n=14; Figure 5b). The response to the C3aRA was variable, either being highly protective or minimally effective (Figures 5c and d, respectively). Overall, the C3aRA (1.0 mg kg−1 i.v; 0.92±0.3; n=14; Figure 5c and d) and C5aRA (1.0 mg kg−1 i.v.; 1.06±0.31; n=9) ameliorated I/R-induced mucosal damage when delivered 15 min before occlusion of the SMA. The protective effect of the C3aRA was dose-dependent with a lesser reduction in damage following administration of 0.1 mg kg−1 (1.40±0.19; n=10) and 0.3 mg kg−1 i.v (1.67±0.35; n=9). Concurrent treatment with both antagonists (1.00±0.41; n=8) also resulted in reduction of I/R-mediated intestinal mucosal damage (Figure 5e), but the combination drug treatment was not more effective than either drug given alone.
Figure 4.
Effect of antagonists on mucosal damage following I/R. Administration of the C3aRA (1.0 mg kg−1) or C5aRA (1.0 mg kg−1) significantly attenuated I/R-induced mucosal damage to the intestine. Combination treatment (both antagonist 1.0 mg kg−1) was also protective against mucosal injury.
Figure 5.
I/R induced mucosal injury in the rat small intestine. Histological images of small intestine sections representative of (a) vehicle and all drug-treated sham-operated animals; (b) I/R injury animals, treatment 45 min prior to reperfusion; (c) C3aRA (1.0 mg kg−1)+I/R (low score); (d) C3aRA (1.0 mg kg−1)+I/R (high score); (e) C3aRA (1.0 mg kg−1)+C5aRA (1.0 mg kg−1)+I/R, treatment 120 min prior to reperfusion; (f) C3aRA (1.0 mg kg−1)+I/R.
Effect of C3aRA treatment 120 min prior to reperfusion
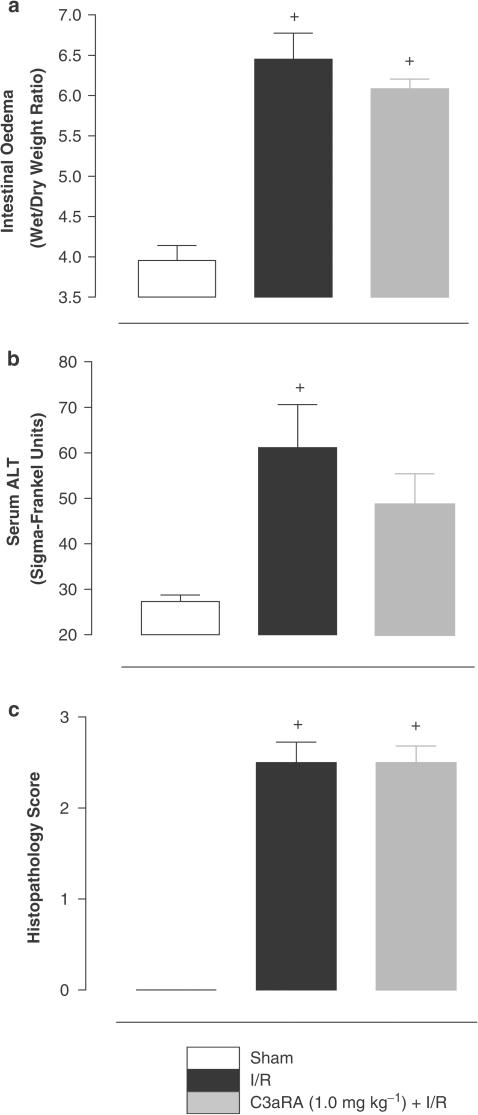
I/R-induced intestinal oedema
Intestinal I/R (6.45±0.32, n=8) produced a significant degree of intestinal oedema compared to sham-operated animals (3.96±0.19, n=4). Intravenous infusion of the C3aRA (1.0 mg kg−1) 90 min prior to induction of I/R did not significantly attenuate intestinal oedema arising from I/R (6.08±0.12, n=6; Figure 6a).
Figure 6.
Effect of drug treatment 120 min prior to intestinal reperfusion. (a) I/R-induced intestinal oedema. Pretreatment with the C3aRA, 1.0 mg kg−1, was not protective against I/R-induced intestinal oedema. Data are shown as mean±s.e.m. *P⩽0.05 when compared to I/R animals, + P⩽0.05 when compared to sham animals. (b) Effect of antagonists on elevation of serum ALT following I/R. Elevation of serum ALT following intestinal I/R was not significantly reduced by treatment with a C3aRA (1.0 mg kg−1). Data are expressed as mean±s.e.m. *P⩽0.05 when compared to I/R animals. +P⩽0.05 when compared to sham-operated animals. (c) Effect of antagonists on mucosal damage following I/R. No protection against I/R-induced mucosal injury was afforded by treatment with the C3aRA (1.0 mg kg−1).
Elevation of ALT levels by intestinal I/R
Following intestinal I/R, serum levels of ALT in vehicle-treated animals were significantly elevated (74.8±15.7, n=6) above sham-operated animals (27.33±1.39, n=8). Administration of the C3aRA (1.0 mg kg−1; 48.6±6.8; n=6) 120 min prior to induction of reperfusion did not significantly attenuate I/R-induced ALT elevation (Figure 6b).
Histopathology
Similar levels of mucosal damage were attained in vehicle-treated I/R (2.50±0.22; n=6) and sham-operated (0) animals in the second stage of the study. Pretreatment of animals with the C3aRA (2.50±0.19; n=6; Figure 5f) 120 min prior to reperfusion did not significantly inhibit I/R-induced intestinal mucosal damage (Figure 6c).
I/R-induced neutropaenia
Due to the considerable neutropaenia induced by the C3aRA the effect of I/R on circulating neutrophil numbers could not be assessed when the C3aRA was delivered 15 min prior to induction of ischaemia. However, when the C3aRA is administered 120 min prior to reperfusion numbers of circulating neutrophils have returned to ∼80–90 % of resting levels by intestinal reperfusion, allowing the affect of C3aRA on I/R-induced neutropaenia to be assessed. Upon occlusion of the SMA in vehicle-treated animals, the levels of circulating neutrophils dropped by 39±7.39 % (n=10) and remained depressed for the remainder of the experimental period (Figure 7). Neutrophil numbers remained unchanged throughout the experiment in sham-treated animals (n=4). The levels of circulating neutrophils in C3aRA-treated rats were 89±7.18 % (n=7) of pretreatment numbers immediately prior to induction of ischaemia. Upon induction of ischaemia, neutrophil numbers decreased by a further 24±6.20% before recovering to preischaemia levels after 120 min of reperfusion (Figure 7).
Figure 7.
Effect of I/R on circulating neutrophil levels in the rat. Intestinal ischaemia induced a neutropaenia that did not recover completely during the remainder of the experiment. The C3aRA did not alter I/R-induced changes in neutrophil levels. PMN numbers are presented as percentage of predose numbers±s.e.m.
Discussion
A previous study (Ames et al., 2001) reported the discovery of a nonpeptidic C3aRA with high affinity for the human C3aR. This compound, N(2)-[(2,2-diphenylethoxy)acetyl]-L-arginine, antagonised C3a-induced calcium mobilisation, smooth muscle contraction and attenuated paw oedema in a rat model of adjuvant-induced arthritis (Ames et al., 2001). Given the in vivo anti-inflammatory activities reported, and because to date the effects of this C3aRA have only been reported in one in vitro study (Mollnes et al., 2002), we decided to investigate its pharmacology in more detail and compare its actions with a small molecule C5aRA, which has been well characterised (Strachan et al., 2000; 2001; Arumugam et al., 2002; 2003; Woodruff et al., 2002; 2003).
We have confirmed that the C3aRA binds with high affinity and high selectivity to C3aRs compared with C5aRs on human PMNs and that it exhibits in vivo anti-inflammatory activity. The in vivo activity investigated in this study was inhibition of intestinal I/R injury, using a rat model in which a C5aRA (AcF-[OPdChaWR]) is efficacious in reducing cellular injury as well as tissue injury (Arumugam et al., 2002).
As only limited pharmacological data on the C3aRA is currently available, we also examined some other basic pharmacological actions of the drug. As shown here, the C3aRA affected circulating numbers of neutrophils in the rat. The C3aRA (10 μg kg−1–1.0 mg kg−1) induced neutropaenia, and the highest dose (10 mg kg−1) also caused a rapid and transient hypertension. Whether these effects are related to activity at C3aRs, or at different receptors, is not yet known. However, a rapid but transient hypertension, and neutropaenia followed by neutrophilia, has been observed following intravenous infusion of C3a to guinea pigs (Hoffman et al., 1988; Regal & Klos, 2000).
The C3aRA significantly attenuates paw oedema in a rat model of adjuvant-induced arthritis (Ames et al., 2001). This effect was observed when the C3aRA was administered at the high dose of 30 mg kg−1 i.p. b.i.d. throughout the 20-day study. Our pharmacokinetic studies in the rat showed that the C3aRA concentration did not fall below pharmacologically relevant levels for at least 2 h following i.v. administration at 0.1 and 0.3 mg kg−1 and even longer at 1.0 mg kg−1. Thus, for the purposes of the acute study, which lasted 2.5 h, the C3aRA was administered intravenously at 0.1, 0.3 or 1.0 mg kg−1.
Evidence that complement activation, which occurs during intestinal I/R, is critical for the induction of tissue damage can be found in a number of recent studies that have targeted various levels of the complement cascade for inhibition (Hill et al., 1992; Eror et al., 1999; Heller et al., 1999; Andoh et al., 2001; Wada et al., 2001; Arumugam et al., 2002). A crucial role for C5a in the mediation of I/R injury has been confirmed by several studies (Heller et al., 1999; Wada et al., 2001; Arumugam et al., 2002). In the first section of the present study we now report that a C3aRA, administered 45 min prior to reperfusion, attenuated I/R-induced intestinal oedema, elevation of ALT and mucosal damage.
The local consequences of intestinal I/R include increased vascular permeability and resultant interstitial oedema, increased mucosal permeability and decreased barrier function. Significant protection against I/R-induced intestinal oedema was afforded by pretreatment (45 min prereperfusion) with the C3aRA and the C5aRA. However, no greater protection was seen when both antagonists were delivered concurrently.
Elevated serum levels of intracellular enzymes can be detected following hepatic injuries, including experimental intestinal I/R, and is indicative of recent tissue and organ damage (Thompson et al., 1990; Amacher, 1998; Caglayan et al., 2002). Elevated serum levels of ALT can reflect a disruption of liver parenchymal cell membrane integrity and acute hepatocellular injury (Amacher, 1998). The C3aRA or C5aRA, administered 45 min prior to reperfusion, significantly attenuated serum ALT indicating protection of liver from intestinal I/R. Infiltration of activated PMNs into the liver and the subsequent release of inflammatory mediators may be responsible for liver damage (Turnage et al., 1991; Bion, 1999). Thus, it is possible that C5a- and C3a-induced activation of PMNs, directly or indirectly, may mediate this effect of I/R-induced liver damage. However, delivery of the combined treatment to sham animals resulted in a significant rise in ALT levels when compared to single drug or vehicle-treated sham animals, indicating that combination treatment produces some degree of hepatotoxicity. An interaction between the different drug metabolic pathways may be involved and further studies are required to elucidate the mechanism of this effect. Unfortunately, this phenomenon confounded interpretation of the effect of combination treatment on I/R-induced ALT elevation.
The local consequences of intestinal I/R include considerable damage to the mucosal layer of the intestine. Preservation of the mucosal microvilli is essential for maintenance of the intestine's fundamental roles in nutrient absorption and as a physiological, mechanical and immunological barrier (Kraehenbuhl et al., 1997). Both the C3aRA and C5aRA were protective against I/R-induced mucosal damage when administered intravenously at 1.0 mg kg−1 45 min prior to induction of reperfusion. Interestingly, administration of both antagonists together did not provide greater protection than either alone.
While our results appear to indicate a pathogenic role for both C3a and C5a in intestinal I/R, a pronounced neutropaenic side effect of the C3aRA, apparently not associated with C3aR antagonism, precludes any firm conclusions from this part of our study. This is particularly true as the neutropaenia, resulting in global sequestration or tissue margination in the animal, may be indirectly related to the anti-inflammatory effects of the C3aRA in intestinal I/R. Studies employing strategies that deplete neutrophils directly (Hernandez et al., 1987) or inhibit their adhesion to the local tissue endothelium (Hernandez et al., 1987; Koike et al., 1995) report attenuation of intestinal I/R. These results strongly implicate the accumulation of neutrophils, activated by numerous mediators including C5a, in the microvasculature and their subsequent infiltration into the intestinal tissue and other sites (e.g. lung) in the pathophysiology of intestinal I/R.
Consequently, the second part of this study sought to examine the role of C3aRA-induced neutropaenia on C3aRA-mediated protection from intestinal I/R. The neutropaenia induced by the C3aRA administered at 1.0 mg kg−1 is essentially resolved within 120 min of administration. Pharmacologically relevant levels of the C3aRA are present for at least 2–3 h following i.v. dosing at 1.0 mg kg−1 and C3a agonist-mediated transient hypertension in the rat remained completely inhibited 2 h following C3aRA treatment. These experiments demonstrated effective block of the C3aR beyond the period of neutropaenia by the C3aRA. Accordingly, we administered the C3aRA 120 min prior to reperfusion, to substantially eliminate the effect of neutrophil tissue sequestration and allow clearer assessment of C3aR antagonism in intestinal I/R.
Under these conditions, delivery of the C3aRA at 1.0 mg kg−1 120 min prior to reperfusion afforded no protection against I/R-induced oedema, ALT levels and mucosal damage. Also, the C3aRA did not attenuate the neutropaenia observed immediately following I/R. These experiments indicate that antagonism of C3aRs does not protect against these markers of intestinal I/R-induced injury in the rat and suggest that C3a does not play a major role in the pathogenesis of intestinal I/R.
The in vivo pharmacology of this C3aRA is clearly complex and not simply explained by antagonism of C3aRs. Indeed, it seems likely that this compound (a dipeptide analogue) is simply too small and insufficiently functionalised to be particularly receptor selective. C3aR antagonism is certainly one property of this agent, but does not appear to be the property, responsible for the anti-inflammatory actions observed in this study. C3aRA-mediated neutrophil global tissue sequestration during ischaemia and much of the reperfusion period could account for some protective effects observed, but this does not shed any light upon the underlying mechanism of C3aRA-mediated neutrophil effects. It is possible that other receptors, currently unidentified, may also be involved in the protective effects of the C3aRA. These facets complicate the use of this C3aRA as a specific pharmacological tool. Our results indicate that determination of the relative roles of C3a and C5a in immune and inflammatory disorders, including intestinal I/R, requires the development of a more selective C3a antagonist devoid of confounding effects as we have described.
Acknowledgments
We thank Mr Paul Addison for his excellent technical assistance and Dr Sandra Pollitt for assistance in the preparation of this manuscript. This work was supported by a grant from the National Health and Medical Research Council (NHMRC) in Australia. All animal research performed in this study was conducted in accordance with NHMRC guidelines.
Abbreviations
- ALT
alanine aminotransferase
- C3aR
C3a receptor
- C3aRA
C3a receptor antagonist
- C5aR
C5a receptor
- C5aRA
C5a receptor antagonist
- I/R
ischaemia/reperfusion
- PMN
polymorphonuclear leukocyte
- SMA
superior mesenteric artery
References
- AMACHER D.E. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul. Toxicol. Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- AMES R.S., LEE D., FOLEY J.J., JUREWICZ A.J., TORNETTA M.A., BAUTSCH W., SETTMACHER B., KLOS A., ERHARD K.F., COUSINS R.D., SULPIZIO A.C., HIEBLE J.P., MCCAFFERTY G., WARD K.W., ADAMS J.L., BONDINELL W.E., UNDERWOOD D.C., OSBORN R.R., BADGER A.M., SARAU H.M. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J. Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- ANDOH A., FUJIYAMA Y., ARAKI Y., KIMURA T., TSUJIKAWA T., BAMBA T. Role of complement activation and mast cell degranulation in the pathogenesis of rapid intestinal ischemia/reperfusion injury in rats. Digestion. 2001;63 Suppl 1:103–107. doi: 10.1159/000051920. [DOI] [PubMed] [Google Scholar]
- ARUMUGAM T.V., SHIELS I.A., STRACHAN A.J., ABBENANTE G., FAIRLIE D.P., TAYLOR S.M. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63:134–142. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- ARUMUGAM T.V., SHIELS I.A., WOODRUFF T.M., REID R.C., FAIRLIE D.P., TAYLOR S.M. Protective effect of a new C5a receptor antagonist against ischemia–reperfusion injury in the rat small intestine. J. Surg. Res. 2002;103:260–267. doi: 10.1006/jsre.2002.6369. [DOI] [PubMed] [Google Scholar]
- BION J.F. Is the gut responsible for multiple organ failure. Schweiz Med. Wochenschr. 1999;129:1600–1604. [PubMed] [Google Scholar]
- CAGLAYAN F., CAGLAYAN O., GUNEL E., ELCUMAN Y., CAKMAK M. Intestinal ischemia–reperfusion and plasma enzyme levels. Pediatr. Surg. Int. 2002;18:255–257. doi: 10.1007/s003830100666. [DOI] [PubMed] [Google Scholar]
- CARDEN D.L., GRANGER D.N. Pathophysiology of ischemia–reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- EMBER J.A., HUGLI T.E. Complement factors and their receptors. Immunopharmacology. 1997;38:3–15. doi: 10.1016/s0162-3109(97)00088-x. [DOI] [PubMed] [Google Scholar]
- EROR A.T., STOJADINOVIC A., STARNES B.W., MAKRIDES S.C., TSOKOS G.C., SHEA-DONOHUE T. Antiinflammatory effects of soluble complement receptor type 1 promote rapid recovery of ischemia/reperfusion injury in rat small intestine. Clin. Immunol. 1999;90:266–275. doi: 10.1006/clim.1998.4635. [DOI] [PubMed] [Google Scholar]
- HARWARD T.R., BROOKS D.L., FLYNN T.C., SEEGER J.M.Multiple organ dysfunction after mesenteric artery revascularization J. Vasc. Surg. 199318459–467.discussion 467–9 [DOI] [PubMed] [Google Scholar]
- HELLER T., HENNECKE M., BAUMANN U., GESSNER J.E., ZU VILSENDORF A.M., BAENSCH M., BOULAY F., KOLA A., KLOS A., BAUTSCH W., KOHL J. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J. Immunol. 1999;163:985–994. [PubMed] [Google Scholar]
- HERNANDEZ L.A., GRISHAM M.B., TWOHIG B., ARFORS K.E., HARLAN J.M., GRANGER D.N. Role of neutrophils in ischemia–reperfusion-induced microvascular injury. Am. J. Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- HILL J., LINDSAY T.F., ORTIZ F., YEH C.G., HECHTMAN H.B., MOORE F.D., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J. Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- HOFFMANN T., BOTTGER E.C., BAUM H.P., MESSNER M., HADDING U., BITTER-SUERMANN D. In vivo effects of C3a on neutrophils and its contribution to inflammatory lung processes in a guinea-pig model. Clin. Exp. Immunol. 1988;71:486–492. [PMC free article] [PubMed] [Google Scholar]
- HUBER-LANG M.S., RIEDEMAN N.C., SARMA J.V., YOUNKIN E.M., MCGUIRE S.R., LAUDES I.J., LU K.T., GUO R.F., NEFF T.A., PADGAONKAR V.A., LAMBRIS J.D., SPRUCE L., MASTELLOS D., ZETOUNE F.S., WARD P.A. Protection of innate immunity by C5aR antagonist in septic mice. FASEB. J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- KANWAR S., HICKEY M.J., KUBES P. Postischemic inflammation: a role for mast cells in intestine but not in skeletal muscle. Am. J. Physiol. 1998;275:G212–G218. doi: 10.1152/ajpgi.1998.275.2.G212. [DOI] [PubMed] [Google Scholar]
- KANWAR S., KUBES P. Ischemia/reperfusion-induced granulocyte influx is a multistep process mediated by mast cells. Microcirculation. 1994;1:175–182. doi: 10.3109/10739689409148272. [DOI] [PubMed] [Google Scholar]
- KHANNA A., ROSSMAN J.E., FUNG H.L., CATY M.G. Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J. Surg. Res. 2001;99:114–119. doi: 10.1006/jsre.2001.6103. [DOI] [PubMed] [Google Scholar]
- KIM F.J., MOORE E.E., MOORE F.A., BIFFL W.L., FONTES B., BANERJEE A. Reperfused gut elaborates PAF that chemoattracts and primes neutrophils. J. Surg. Res. 1995;58:636–640. doi: 10.1006/jsre.1995.1100. [DOI] [PubMed] [Google Scholar]
- KIMURA T., ANDOH A., FUJIYAMA Y., SAOTOME T., BAMBA T. A blockade of complement activation prevents rapid intestinal ischaemia–reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin. Exp. Immunol. 1998;111:484–490. doi: 10.1046/j.1365-2249.1998.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIKE K., MOORE E.E., MOORE F.A., FRANCIOSE R.J., FONTES B., KIM F.J.CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion J. Trauma 19953923–27.discussion 27–8 [DOI] [PubMed] [Google Scholar]
- KRAEHENBUHL J.P., PRINGAULT E., NEUTRA M.R.Review article: intestinal epithelia and barrier functions Aliment Pharmacol. Ther. 199711Suppl 33–8.discussion 8–9 [DOI] [PubMed] [Google Scholar]
- MOLLNES T.E., BREKKE O.L., FUNG M., FURE H., CHRISTIANSEN D., BERGSETH G., VIDEM V., LAPPEGARD K.T., KOHL J., LAMBRIS J.D. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- MOORE E.E., MOORE F.A., FRANCIOSE R.J., KIM F.J., BIFFL W.L., BANERJEE A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J. Trauma. 1994;37:881–887. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- PACZKOWSKI N.J., FINCH A.M., WHITMORE J.B., SHORT A.J., WONG A.K., MONK P.N., CAIN S.A., FAIRLIE D.P., TAYLOR S.M. Pharmacological characterization of antagonists of the C5a receptor. Br. J. Pharmacol. 1999;128:1461–1466. doi: 10.1038/sj.bjp.0702938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKS D.A., GRANGER D.N. Contributions of ischemia and reperfusion to mucosal lesion formation. Am. J. Physiol. 1986;250:G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- POGGETTI R.S., MOORE E.E., MOORE F.A., KOIKE K., BANERJEE A. Gut ischemia/reperfusion-induced liver dysfunction occurs despite sustained oxygen consumption. J. Surg. Res. 1992;52:436–442. doi: 10.1016/0022-4804(92)90308-m. [DOI] [PubMed] [Google Scholar]
- REGAL J.F., KLOS A. Minor role of the C3a receptor in systemic anaphylaxis in the guinea pig. Immunopharmacology. 2000;46:15–28. doi: 10.1016/s0162-3109(99)00152-6. [DOI] [PubMed] [Google Scholar]
- REID R.C., ABBENANTE G., TAYLOR S.M., FAIRLIE D.P. A convergent solution-phase synthesis of the macrocycle Ac-Phe-[Orn-Pro-d-Cha-Trp-Arg], a potent new antiinflammatory drug. J. Org. Chem. 2003;68:4464–4471. doi: 10.1021/jo034228r. [DOI] [PubMed] [Google Scholar]
- RIEDEMANN N.C., WARD P.A. Complement in ischemia reperfusion injury. Am. J. Pathol. 2003;162:363–367. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOENBERG M.H., BEGER H.G. Reperfusion injury after intestinal ischemia. Crit. Care. Med. 1993;21:1376–1386. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- SHORT A.J., PACZKOWSKI N.J., VOGEN S.M., SANDERSON S.D., TAYLOR S.M. Response-selective C5a agonists: differential effects on neutropenia and hypotension in the rat. Br. J. Pharmacol. 1999;128:511–514. doi: 10.1038/sj.bjp.0702847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISLEY A.C., DESAI T., HARIG J.M., GEWERTZ B.L. Neutrophil depletion attenuates human intestinal reperfusion injury. J Surg Res. 1994;57:192–196. doi: 10.1006/jsre.1994.1130. [DOI] [PubMed] [Google Scholar]
- SORKINE P., SETTON A., HALPERN P., MILLER A., RUDICK V., MARMOR S., KLAUSNER J.M., GOLDMAN G. Soluble tumor necrosis factor receptors reduce bowel ischemia-induced lung permeability and neutrophil sequestration. Crit. Care Med. 1995;23:1377–1381. doi: 10.1097/00003246-199508000-00011. [DOI] [PubMed] [Google Scholar]
- STRACHAN A.J., SHIELS I.A., REID R.C., FAIRLIE D.P., TAYLOR S.M. Inhibition of immune-complex mediated dermal inflammation in rats following either oral or topical administration of a small molecule C5a receptor antagonist. Br. J. Pharmacol. 2001;134:1778–1786. doi: 10.1038/sj.bjp.0704417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRACHAN A.J., WOODRUFF T.M., HAAIMA G., FAIRLIE D.P., TAYLOR S.M. A new small molecule C5a receptor antagonist inhibits the reverse-passive Arthus reaction and endotoxic shock in rats. J. Immunol. 2000;164:6560–6565. doi: 10.4049/jimmunol.164.12.6560. [DOI] [PubMed] [Google Scholar]
- SUN Z., WANG X., DENG X., LASSON A., SOLTESZ V., BORJESSON A., ANDERSSON R. Beneficial effects of lexipafant, a PAF antagonist on gut barrier dysfunction caused by intestinal ischemia and reperfusion in rats. Dig. Surg. 2000;17:57–65. doi: 10.1159/000018801. [DOI] [PubMed] [Google Scholar]
- SUN Z., WANG X., LASSON A., BORJESSON A., LEVEAU P., HARALDSEN P., ANDERSSON R. Roles of platelet-activating factor, interleukin-1beta and interleukin-6 in intestinal barrier dysfunction induced by mesenteric arterial ischemia and reperfusion. J. Surg. Res. 1999;87:90–100. doi: 10.1006/jsre.1999.5746. [DOI] [PubMed] [Google Scholar]
- THOMPSON J.S., BRAGG L.E., WEST W.W. Serum enzyme levels during intestinal ischemia. Ann. Surg. 1990;211:369–373. doi: 10.1097/00000658-199003000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNAGE R.H., BAGNASCO J., BERGER J., GUICE K.S., OLDHAM K.T., HINSHAW D.B. Hepatocellular oxidant stress following intestinal ischemia–reperfusion injury. J. Surg. Res. 1991;51:467–471. doi: 10.1016/0022-4804(91)90166-j. [DOI] [PubMed] [Google Scholar]
- TURNAGE R.H., KADESKY K.M., MYERS S.I., GUICE K.S., OLDHAM K.T. Hepatic hypoperfusion after intestinal reperfusion. Surgery. 1996;119:151–160. doi: 10.1016/s0039-6060(96)80163-2. [DOI] [PubMed] [Google Scholar]
- WADA K., MONTALTO M.C., STAHL G.L. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology. 2001;120:126–133. doi: 10.1053/gast.2001.20873. [DOI] [PubMed] [Google Scholar]
- WOODRUFF T.M., ARUMUGAM T.V., SHIELS I.A., REID R.C., FAIRLIE D.P., TAYLOR S.M. A potent human C5a receptor antagonist protects against disease pathology in a rat model of inflammatory bowel disease. J. Immunol. 2003;171:5514–5520. doi: 10.4049/jimmunol.171.10.5514. [DOI] [PubMed] [Google Scholar]
- WOODRUFF T.M., STRACHAN A.J., DRYBURGH N., SHIELS I.A., REID R.C., FAIRLIE D.P., TAYLOR S.M. Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis Rheum. 2002;46:2476–2485. doi: 10.1002/art.10449. [DOI] [PubMed] [Google Scholar]
- YAO Y.M., BAHRAMI S., REDL H., SCHLAG G. Monoclonal antibody to tumor necrosis factor-alpha attenuates hemodynamic dysfunction secondary to intestinal ischemia/reperfusion in rats. Crit. Care Med. 1996;24:1547–1553. doi: 10.1097/00003246-199609000-00020. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN B.J., GRANGER D.N. Oxygen free radicals and the gastrointestinal tract: role in ischemia–reperfusion injury. Hepatogastroenterology. 1994;41:337–342. [PubMed] [Google Scholar]