Abstract
Studies were designed to examine the effects of α1 (α1AR)- plus β3-adrenoreceptor (β3AR) antagonists on 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy)-induced hyperthermia and measures of rhabdomyolysis (creatine kinase (CK)) and renal function (blood urea nitrogen (BUN) and serum creatinine (sCr)) in male Sprague–Dawley rats.
MDMA (40 mg kg−1, s.c.) induced a rapid and robust increase in rectal temperature, which was significantly attenuated by pretreatment with the α1AR antagonist prazosin (100 μg kg−1, i.p.) plus the β3AR antagonist SR59230A (5 mg kg−1, i.p.).
CK levels significantly increased (peaking at 4 h) after MDMA treatment and were blocked by the combination of prazosin plus SR59230A.
At 4 h after MDMA treatment, BUN and sCr levels were also significantly increased and could be prevented by this combination of α1AR- plus β3AR-antagonists.
The results from this study suggest that α1AR and β3AR play a critical role in the etiology of MDMA-mediated hyperthermia and subsequent rhabdomyolysis.
Keywords: 3,4-Methylenedioxymethamphetamine (MDMA); rhabdomyolysis; creatine kinase; UCP-3; thermogenesis
Introduction
One of the most life-threatening consequences of the abuse of phenethylamines such as, 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is hyperthermia (Logan et al., 1993), with maximum body temperature correlating with mortality (Gowing et al., 2002). MDMA-induced hyperthermia is commonly associated with skeletal muscle breakdown, rhabdomyolysis (Coore, 1996; Mallick & Bodenham, 1997; Walubo & Seger, 1999) and renal failure. A quantitative marker of rhabdomyolysis is serum creatine kinase (CK), an enzyme released from injured myocytes (Slater & Mullins, 1998). Renal failure often follows rhabdomyolysis, which accounts for 5–7% of all cases of acute renal failure in the United States (Slater & Mullins, 1998). Along with rhabdomyolysis, hyperthermia induced by MDMA has also been ostensibly linked to serotonergic neurotoxocity (Malberg et al., 1996), disseminated intravascular coagulation (Henry et al., 1992) and death (Dar & McBrien, 1996). Current treatment strategies for MDMA-induced hyperthermia are plagued by a lack of a thorough understanding of its thermogenic and myonecrotic mechanisms.
Mechanisms of MDMA-induced hyperthermia are complex and appear to involve a combination of (1) vasoconstriction (Pedersen & Blessing, 2001) and thermogenesis mediated through the activation of α1-adrenoreceptor (α1AR) (McDaid & Docherty, 2001; Sprague et al., 2003) and resultant blood pooling, (2) β3-adrenoreceptor (β3AR) activation resulting in skeletal muscle thermogenesis (Sprague et al., 2003) and (3) activation of the skeletal muscle thermogenic protein, uncoupling protein-3 (UCP3, Mills et al., 2003). We previously observed that the α1AR antagonist prazosin, when combined with the β3AR antagonist cyanopindolol, prevents hyperthermia induced by MDMA in rodents (Sprague et al., 2003). Unfortunately, a clear role for β3AR in this previous study was not established because cyanopindolol also antagonizes brain 5HT-1A/1B receptors and thus may block hyperthermia centrally (Hoyer et al., 1994).
Consistent with their well-described effects on oxygen consumption, respiratory exchange ratios and thermogenesis in animals, β3AR may regulate the levels and activity of mitochodrial UCP (Gong et al., 1997; Nakamura et al., 2001). Although its precise roles in physiological thermoregulation is controversial, UCP3 appears to play a critical role in the thermogenesis and death induced by MDMA (Mills et al., 2003). In particular, knockout mice deficient in UCP3 are protected against the hyperthermic and lethal effects of MDMA (Mills et al., 2003). UCP3 is highly expressed in skeletal muscle and has recently been associated with ex vivo skeletal muscle thermogenesis in transgenic mice overexpressing UCP3 (Curtin et al., 2002). Uncoupling proteins have also been associated with cell lysis or oncosis (Mills et al., 2002).
In the present study, we sought to better understand the involvement of the sympathetic nervous system and the adrenergic receptor subtypes in MDMA-induced hyperthermia and rhabdomyolysis using specific inhibitors of adrenergic receptors with the aim of providing an experimental basis for a therapeutic intervention in psychostimulant-mediated hyperthermia and death. Here, we extend our previous results (Sprague et al., 2003) to show that pretreatment with a combination of an α1AR antagonist (prazosin) and a newly developed, peripherally selective β3AR-antagonist (SR59230A), markedly attenuates MDMA-induced hyperthermia. We also for the first time quantify MDMA-induced rhabdomyolysis and muscle breakdown in an animal model and further show that α1AR and β3AR antagonism also significantly protects against the development of muscle breakdown and rhabdomyolysis.
Methods
All of the studies were carried out in accordance with protocols approved by the Ohio Northern University Animal Care and Use Committee.
Animals
Adult, male, jugular vein cannulated (JVC), Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis) weighing 180–220 g were used in these experiments. All animals were individually housed and given access ad libitum to food and water. Housing conditions were maintained at a temperature of 22–24°C and a 12 : 12 light–dark cycle. JVC rats received complete cannula heparin (20 U ml−1) maintenance upon arrival.
Drugs and chemicals
SR59230A, prazosin and all other reagents were purchased from Sigma Chemical Company (St Louis, MO, U.S.A.) or VWR Scientific Products (Columbus, OH, U.S.A.). MDMA was a generous gift from Dr David E. Nichols (Purdue University, West Lafayette, IN, U.S.A.).
Core temperature
Core (rectal) temperatures were taken in all animals before administering MDMA or saline and 1, 2 and 3 h after treatment. Rectal temperatures were measured using a Thermalet TH-8 (Physitemp Instruments, Clifton, NJ, U.S.A.) temperature monitor with a (RET-2) rectal probe attached to the thermocouple and white petrolatum was applied to the probe before insertion. During the experiment, the rats were housed three per cage (size: 21.0 × 41.9 × 20.3 cm3) in cages fit with wire-top lids. The average room temperature during the experiments was 24.2±0.2°C.
Blood samples for CK, BUN and sCr assessment
JVC rats were administered MDMA (40 mg kg−1, s.c.; n=5), MDMA+prazosin (100 μg kg−1, i.p.) and SR59230A (5 mg kg−1, i.p.; n=5), or prazosin and SR59230A alone (n=2). The doses utilized in the present study were in accordance with those studied previously (Lenard et al., 2003; Sprague et al., 2003) and represent a severe human poisoning model because the degree change in temperature elevation correlates with human mortality rates of ∼45% (Gowing et al., 2002). Animals in the treatment group were given prazosin/SR59230 combination 30 min prior to MDMA administration. Animals given MDMA alone were injected with MDMA and simultaneously DMSO. At baseline, 4, 12 and 24 h post-MDMA administration for each group, 500 μl of blood was collected through the JVC cannula and replaced with an equal volume of saline. Collected blood was allowed to clot for 30 min at room temperature and centrifuged at 14,000 × g at 4°C. After serum was collected, the samples were immediately frozen at −80°C. CK levels were determined by using the Vitros analyzer (Johnson and Johnson), using Vitros CK slides. An 11 μl drop of sample was placed on the slide and distributed by the spreading layer to underlying layers. Reflection densities were monitored, with a 670 nm wavelength, during incubation (5 min at 37°C). The rate of change in reflection density was then converted to enzyme activity. The upper and lower detectable limits were 2000 and 20 IU l−1, respectively. Measurements of indicators of kidney function including sCr and BUN were obtained similarly using the Vitros analyzer by measuring the reaction product reflection densities spectrophotometrically at 670 nm. The dynamic range for serum BUN (mg dl−1) measurement is 2.0–120.0 and for sCr (mg dl−1) is 0.05–14.0.
Analysis
Rectal temperatures were compared within each treatment group with an ANOVA and a Dunnett's post hoc test to determine the significant differences from baseline levels. Between treatment groups, rectal temperatures and CK, BUN, sCr were compared with an ANOVA with a Student–Newman–Kuels post hoc test. A Student's t-test was used to compare changes between two groups. Statistical significance was set a priori at P⩽0.05.
Results
α1- and β3-adrenergic regulation of MDMA-induced hyperthermia and rhabdomyolysis
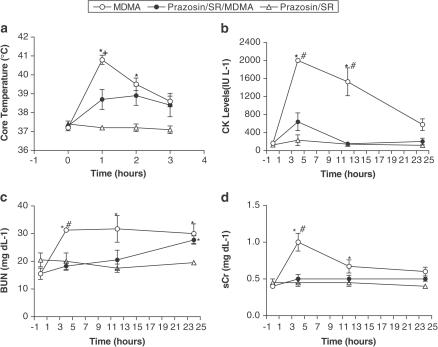
To determine the role of α1AR and β3AR in mediating the hyperthermia and rhabdomyolysis associated with MDMA, we treated rats with prazosin (100 μg kg−1, i.p.), α1AR antagonist, and SR59230A (5 mg kg−1, i.p.), β3AR antagonist, 30 min before MDMA (40 mg kg−1, s.c.). MDMA-treated animals had significantly higher core temperatures at 1 and 2 h post-MDMA administration compared to baseline. The combination of prazosin plus SR59230A significantly attenuated the peak rise in core temperature seen 1 h after treatment with MDMA (Figure 1a).
Figure 1.
α1- and β3-adrenergic regulation of MDMA-induced hyperthermia and rhabdomyolysis. (a) Core body temperature in rats treated with MDMA (40 mg kg−1, s.c.) or MDMA and a combination of prazosin (100 μg kg−1, i.p.) and SR59230A (5 mg kg−1, i.p.) 30 min before MDMA. Each value is the mean±s.e.m. (n=5). *Significantly different from baseline (P<0.001). +Significantly different from all other treatment groups (P<0.01). The effects of prazosin and SR59230A on MDMA-induced changes in the markers of rhabdomyolysis were assessed in (b) CK levels, (c) BUN and (d) sCr. Each value is the mean±s.e.m. (n=5). *Significantly different than baseline (P<0.05). #Significantly different than corresponding 4 or 12 h prazosin/SR59230A+MDMA (P<0.02). MDMA was administered at time zero.
We assessed renal function by measuring blood urea nitrogen (BUN) and serum creatinine (sCr) levels. MDMA induced a greater than 10-fold increase in CK levels 4 h after treatment. The CK levels demonstrated a monophasic decline over the 24 h monitoring period. Prazosin plus SR59230A significantly blocked the rise in CK levels (Figure 1b). Accompanying this rise in CK, BUN and sCr also significantly rose following MDMA treatment. As was seen with temperature and CK levels, combining prazosin with SR59230A blocked the rise in these measures of renal function (Figures 1c and d, respectively).
Discussion
Hyperthermia, a complication of MDMA use, is many times accompanied by rhabdomyolysis, which may ultimately lead to death (Dar & McBrien, 1996; Mallick & Bodenham, 1997). This study demonstrates that by using a combination of prazosin, an α1AR-antagonist, and SR59230A, a selective β3AR-antagonist, MDMA-induced hyperthermia was significantly attenuated (Figure 1a). MDMA-induced hyperthermia can lead to skeletal muscle breakdown in humans (Fahal et al., 1992; Screaton et al., 1992; Murthy et al., 1997), which increases serum myoglobin and creatinine kinase levels. In turn, myoglobinuria can lead to rhabdomyolysis and acute renal failure (Slater & Mullins, 1998). In the present study, MDMA induced a robust increase in core temperatures and serum CK levels, both of which were markedly attenuated by blocking α1AR and β3AR with prazosin plus SR59230A prior to MDMA treatment. Furthermore, prazosin plus SR59230A blunted MDMA-induced derangements in the serum levels of BUN and blocked the changes seen in sCr. Curiously, BUN rose over the 12–24 h time frame in the prazosin plus SR59230A plus MDMA treatment group. This phenomenon may be the result of repeated blood draws and not renal damage because sCr did not change over that same time frame.
Recent evidence suggests that α1AR activation potentiates the thermogenic effects of β3AR receptor agonists in brown adipose (Zhao et al., 1997), suggesting that α1AR and β3AR antagonism may attenuate MDMA-induced activation of candidate thermogenic molecules such as UCP3. Although expressed in relatively low concentrations, skeletal muscle contains both α1AR (Martin et al., 1990) and β3AR (Sillence et al., 1993; Ye et al., 1995; Chamberlain et al., 1999). As UCP3 plays a major role in MDMA-induced hyperthermia (Mills et al., 2003), we suggest that part of the protective effects seen in the present study may be the result of α1AR- and β3AR-dependent regulation of UCP3 activity in skeletal muscle.
The results of the present study suggest that the antagonism of α1AR and β3AR not only reduce the hyperthermic effects of MDMA but also attenuate the pathological sequela of muscle breakdown. As such, the hyperthermia induced by MDMA and other sympathomimetic agents such as ephedrine, methamphetamine and cocaine may be responsive to pharmacological agents that antagonize the α1AR and β3AR.
Acknowledgments
We are grateful for the generous gift of MDMA from Dr David E. Nichols. We also thank the laboratory of Animal Medicine and Surgery for their editorial comments and assistance in serum chemistry measurements.
Abbreviations
- α1AR
α1-adrenoreceptor
- β3AR
β3-adrenoreceptor
- BUN
blood urea nitrogen
- CK
creatine kinase
- JVC
jugular vein cannulated
- MDMA
3,4-methylenedioxymethamphetamine
- sCr
serum creatinine
- UCP
uncoupling protein
References
- CHAMBERLAIN P.D., JENNINGS K.H., PAUL F., CORDELL J., BERRY A., HOLMES S.D., PARK J., CHAMBERS J., SENNITT M.V., STOCK M.J., CAWTHORNE M.A., YOUNG P.W., MURPHY G.J. The tissue distribution of the human beta3-adrenoceptor studied using a monoclonal antibody: direct evidence of the beta3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int. J. Obes. Relat. Metab. Disord. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- COORE J.R. A fatal trip with Ecstasy: a case of 3,4-methylenedioxymethamphet amine/3,4-methylenedioxyamphetamine toxicity. J. Br. Soc. Med. 1996;89:51–52. doi: 10.1177/014107689608900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIN N.A., CLAPHAM J.C., BARCLAY C.J. Excess recovery heat production by isolated muscles from mice overexpressing uncoupling protein-3. J. Physiol. 2002;542:231–235. doi: 10.1113/jphysiol.2002.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAR K.J., MCBRIEN M.E. MDMA induced hyperthermia: report of a fatality and review of current therapy. Intens. Care Med. 1996;22:995–996. doi: 10.1007/BF02044131. [DOI] [PubMed] [Google Scholar]
- FAHAL I.H., SALLOMI D.F., YAQOOB M., BELL G.M. Acute renal failure after Ecstasy. BMJ. 1992;305:29. doi: 10.1136/bmj.305.6844.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONG D., HE Y., KARAS M., REITMAN M. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, β3-adrenergic agonists, and leptin. J. Biol. Chem. 1997;272:24129–24132. doi: 10.1074/jbc.272.39.24129. [DOI] [PubMed] [Google Scholar]
- GOWING L., HENRY-EDWARDS S., IRVINE R., ALI R. The health effects of Ecstasy: a literature review. Drug Alcohol Rev. 2002;21:53–63. doi: 10.1080/09595230220119363. [DOI] [PubMed] [Google Scholar]
- HENRY J.A., JEFFREYS K.J., DAWLING S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (‘Ecstasy') Lancet. 1992;340:384–387. doi: 10.1016/0140-6736(92)91469-o. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- LENARD N.R., GETTYS T.W., DUNN A.J. Activation of β3 and β3-adrenergic receptors increases brain tryptophan. J. Pharmacol. Exp. Ther. 2003;305:653–659. doi: 10.1124/jpet.102.048249. [DOI] [PubMed] [Google Scholar]
- LOGAN A.S., STICKLE B., O'KEEFE N., Hewitson H. Survival following ‘Ecstasy' ingestion with a peak temperature of 42°C. Anaesthesia. 1993;48:1017–1018. [PubMed] [Google Scholar]
- MALBERG J.E., SABO K.E., SEIDEN L. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J. Pharmacol. Exp. Ther. 1996;278:258–267. [PubMed] [Google Scholar]
- MALLICK A., BODENHAM A.R. MDMA induced hyperthermia: a survivor with an initial body temperature of 42.9°C. J. Accid. Emerg. Med. 1997;14:336–338. doi: 10.1136/emj.14.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN H., TOLLEY T.K., SAFFITZ J.E. Autoradiographic delineation of skeletal muscle α1-adrenergic receptor distribution. Am. J. Physiol. 1990;259:H1402–H1408. doi: 10.1152/ajpheart.1990.259.5.H1402. [DOI] [PubMed] [Google Scholar]
- MCDAID J., DOCHERTY J.R. Vascular actions of MDMA involve alpha1 and alpha 2-adrenoreceptors in the anaesthetized rat. Br. J. Pharmacol. 2001;135:170–180. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS E.M., BANKS M.L., SPRAGUE J.E., FINKEL T. Uncoupling the agony from Ecstasy. Nature. 2003;426:403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- MILLS E.M., XU D., FERGUSSON M.M., COMBS C.A., XU Y., FINKEL T. Regulation of cellular oncosis by uncoupling protein 2. J. Biol. Chem. 2002;277:27385–27392. doi: 10.1074/jbc.M111860200. [DOI] [PubMed] [Google Scholar]
- MURTHY B.V.S., ROBERTS N.B., WILKES R.G. Biochemical implications of Ecstasy toxicity. Ann. Clin. Biochem. 1997;34:442–445. doi: 10.1177/000456329703400421. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y., NAGASE I., ASANO A., SASAKI N YOSHIDA T., UMEKAWA T., SAKANE N., SAITO M. β3-adrenergic agonist up-regulates uncoupling proteins 2 and 3 in skeletal muscle of the mouse. J. Vet. Med. Sci. 2001;63:309–314. doi: 10.1292/jvms.63.309. [DOI] [PubMed] [Google Scholar]
- PEDERSEN N.P., BLESSING W.W. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J. Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCREATON G.R., SINGER M., CAIRNS H.S., THRASHER A., SARNER M., COHEN S.L. Hyperpyrexia and rhabdomyolysis after MDMA (‘Ecstasy') abuse. Lancet. 1992;339:677–678. doi: 10.1016/0140-6736(92)90834-p. [DOI] [PubMed] [Google Scholar]
- SILLENCE M.N., MOORE N.G., PEGG G.G., LINDSAY D.B. Ligand binding properties of putative beta-3 adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br. J. Pharmacol. 1993;109:1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER M.S., MULLINS R.J. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. Am. Coll. Surg. 1998;186:693–716. doi: 10.1016/s1072-7515(98)00089-1. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J.E., BANKS M.L., COOK V.J., MILLS E.M. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in the hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) J. Pharmacol. Exp. Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- WALUBO A., SEGER D. Fatal multi-organ failure after suicidal overdose with MDMA, ‘Ecstasy': case report and review of literature. Hum. Exp. Toxicol. 1999;18:119–125. doi: 10.1177/096032719901800209. [DOI] [PubMed] [Google Scholar]
- YE J.M., CLARK M.G., COLQUHOUN E.Q. Constant-pressure perfusion of rat hindlimb shows alpha- and beta-adrenergic stimulation of oxygen consumption. Am. J. Physiol. 1995;269:E960–E968. doi: 10.1152/ajpendo.1995.269.5.E960. [DOI] [PubMed] [Google Scholar]
- ZHAO J., CANNON B., NEDERGAARD J. β3-adrenergic stimulation potentiates the thermogenic action of β3-adrenoreceptor-generated cAMP in brown fat cells. J. Biol. Chem. 1997;272:32847–32856. doi: 10.1074/jbc.272.52.32847. [DOI] [PubMed] [Google Scholar]