Abstract
Using gene chip technology, we first identified that PGF2α (FP agonist) and Butaprost (EP2 agonist) induced about a five-fold upregulation of Nur77 mRNA expression in hFP-HEK 293/EBNA and hEP2-HEK293/EBNA cells. Northern Blot analysis revealed that PGF2α- and Butaprost-induced upregulation of Nur77 expression are dose- and time-dependent.
Both PGF2α and Butaprost upregulated Nur77 gene expression through the protein kinase C (PKC) pathway. These data are the first showing a link between EP2 receptor stimulation and protein kinase C activation. Calcineurin was found to be involved downstream of the PKC pathway in PGF2α-induced Nur77 expression, but not in Butaprost-induced Nur77 expression.
We also used Nur77 as a marker gene to compare the effects of PGF2α, Butaprost, and Bimatoprost (a prostamide) on Nur77 expression in human primary trabecular meshwork and ciliary smooth muscle (SM) cells, which are target cells for antiglaucoma drugs. The results showed that PGF2α and Butaprost, but not Bimatoprost, induced upregulation of Nur77 expression in human TM cells. PGF2α, but not Bimatoprost, dramatically induced upregulation of Nur77 mRNA expression in human ciliary SM cells, whereas Butaprost slightly upregulated Nur77 mRNA expression in SM cells.
Nur77 promoter deletion analysis indicated that PGF2α, but not Bimatoprost, activated Nur77 promoter-luciferase reporter in hFP-HEK 293/EBNA cells. Butaprost was less efficacious in inducing Nur77 promoter-luciferase reporter activity in hEP2-HEK293/EBNA cells relative to PGF2α in the comparable assay. The data for Nur77 promoter functional analysis were matched to the Northern blot analysis.
It appears that PGF2α and Butaprost activate Nur77 transcription mechanisms through the activation of FP and EP2 receptor-coupled signaling pathways, whereas Bimatoprost stimulates neither FP nor EP2 receptors.
Keywords: Transcription regulation, Nur77, prostaglandin F2α, butaprost, bimatoprost, gene expression
Introduction
Prostaglandin F2α (PGF2α) is a product of cyclooxygenase-catalyzed metabolism of arachidonic acid (Smith et al., 1991). It has been identified to be an endogenous ligand of prostaglandin FP receptors. Activation of FP receptors initiated by ligand binding triggers Gαq protein-coupled mechanisms involved Ca2+ signaling, IP3 turnover, and activation of protein kinase C (Toh et al., 1995). PGF2α has diverse physiological actions that include causing smooth muscle (SM) contraction (Horton & Poyser, 1976), stimulating DNA synthesis and cell proliferation, and cardiac myocyte hypertrophy (Adams et al., 1996). Importantly, PGF2α analogs have been used clinically to reduce ocular hypertension (Woodward et al., 1993a). Although the precise mechanisms involved remain unclear, the effects of PGF2α analogs on intraocular pressure (IOP) principally involve an increase in uveoscleral outflow of aqueous humor. These events involve secretion of metalloproteinases by ciliary SM cells and remodeling of the extracellular matrix with a resultant widening of intermuscular spaces (Gaton et al., 2001; Weinreb & Lindsey, 2002; Richter et al., 2003).
In contrast to prostaglandin F2α, Prostamides (prostaglandin ethanolamides) were recently identified as a new class of compounds that were formed from anandamide via metabolic transformation sequentially catalyzed by cyclooxygenase-2 (Yu et al., 1997). Although their physiological actions have not been fully investigated, a synthetic prostamide analog (Bimatoprost) has been shown to be very potent in reducing IOP by increasing both uveosceral and trabecular outflow of aqueous humor (Woodward et al., 2001). The activities of prostamides as endogenous ligands at prostaglandin receptor(s) have been investigated, but have been shown to exert no meaningful activity (Berglund et al., 1999; Woodward et al., 2001; Ross et al., 2002; Matias et al., 2004). Studies on their metabolic rate clearly demonstrate that prostamides and their synthetic analog Bimatoprost exert their in vitro pharmacological effects (Matias et al., 2004) and the ocular hypotensive effects as the intact molecule (Woodward et al., 2003). Experimental evidence suggests that prostamides may act as endogenous ligands at their own receptors (Woodward et al., 2001; Ross et al., 2002).
Besides prostaglandin F2α and Bimatoprost, Butaprost, an EP2 selective ligand, was also found to be very potent in lowering IOP in experimental animal models (Woodward et al., 1995). Interaction of Butaprost with EP2 receptors has been shown to initiate the cAMP/PKA pathway, which in turn modulates a variety of physiological processes, such as vasodilatation and uterus relaxation (Pierce et al., 1995). Despite the fact that FP and EP2 receptors are coupled to different signal transduction mechanisms, both receptors may ultimately share common intracellular pathways that mediate a reduction of intraocular pressure (IOP). For this reason, the effects of PGF2α, the prostamide analog Bimatoprost, and the EP2 agonist Butaprost were compared with respect to one PGF2α-sensitive pathway, namely upregulation of Nur77.
The immediate-early gene Nur77 (also called NGFI-B or TR3) encodes an orphan nuclear receptor, which is classified as a ligand-dependent transcriptional modulator protein (Mangelsdorf et al., 1995). It was originally cloned as a gene induced by Nerve Growth Factor (NGF) in the rat pheochromocytoma cell line PC12 (Milbrandt, 1988). A variety of stimuli involved in cell differentiation and proliferation were found to be capable of rapidly inducing Nur77 expression (Fahrner et al., 1990). The Nur77 binding element (NBRE) was identified by genetic selection in yeast (Wilson et al., 1991). Co-transfection of a reporter gene coupled to the NBRE demonstrated that Nur77 was a strong transcription activator in the cells examined (Davis et al., 1991; 1993; Paulsen et al., 1992). However, the exact function(s) of Nur77 in the regulation of physiological processes remains to be elucidated.
In this study, we have demonstrated that PGF2α and Butaprost upregulate Nur77 mRNA expression and activate the Nur77 promoter in hFP-HEK 293/EBNA and hEP2-HEK 293/EBNA cells. Using Nur77 as a marker gene, we further studied the differential mechanisms of PGF2α (FP receptor), Butaprost (EP2 agonist), and Bimatoprost (prostamide) in the regulation of Nur77 mRNA expression in human primary trabecular meshwork (TM) and ciliary SM cells. These are cellular targets for designing ocular hypotensive drugs. The information gained from this study will help us in the understanding of the molecular mechanisms of prostaglandin analogs (FP and/or EP2 agonist) and the prostamide analogs in the treatment of glaucoma.
Experimental procedures
Cell cultures
HEK 293/EBNA cells stably expressing the human FP receptor or EP2 receptor (hFP-HEK293/EBNA or hEP2-HEK 293/EBNA cells) were a gift from Dr John W. Regan (University of Arizona) (Fujino et al., 2000; 2002). Both hFP-HEK 293/EBNA and hEP2-HEK293/EBNA cell lines were routinely maintained in DMEM (Life Technology, Inc., Rockville, MD, U.S.A.) with 10% fetal bovine serum, 1% glutamine, 0.5% penicillin/ streptomycin, 250 μg ml−1 G418, and 200 μg ml−1 hygromycin, and were kept in humidified 5% CO2 and 95% air at 37°C.
Human ciliary SM cells were isolated from a 69-year-old male donor eye (NDRI, Philadelphia) and cultured in DMEM with 10% fetal bovine serum and 0.5% penicillin/streptomycin according to a method previously reported by Woldemussie et al. (1993).
Human TM cells were a gift from Dr J. Polansky (University of California, San Francisco, U.S.A.). Human TM cells were derived from a 30-year-old male donor eye and cultured in DMEM with 10% fetal bovine serum and 0.5% penicillin/streptomycin in humidified 8% CO2, 92% air at 37°C. Both human primary TM and SM cells were grown to confluence before addition of test compounds.
Stock solutions of PGF2α, Butaprost, and Bimatoprost were prepared in DMSO. The treated cells were incubated with graded concentrations of PGF2α, Butaprost or Bimatoprost, and the control cells received equivalent vehicle treatment.
Plasmids and luciferase reporter assay
A DNA fragment containing the Nur77 promoter (−960 bp to +67 bp; gene bank: U17590) (Uemura et al., 1995) was isolated from human genomic DNA, and subcloned into pGL3-luciferase vector (Promega, Inc) and a pGL3-N-960 plasmid was created. A series of deletion constructs of the Nur77 promoter (−960 to +67 bp) were created and are described as follows: pGL3-N-496 plasmid (−496 to +67 bp); pGL3-N-334 plasmid (−334 to +67 bp); pGL3-N-181 plasmid (−181 to +67 bp); and pGL3-N-114 plasmid (−114 to +67 bp). NFAT-Luciferase plasmids were purchased from Invitrogen, Inc.
Luciferase reporter plasmids were transfected into HEK 293/EBNA cells stably expressing human FP receptor (hFP-HEK293/EBNA) or human EP2 receptor (hEP2-HEK293/EBNA) using the Fugene 6 method (Roche Diagnostics Corp., Inc), according to manufacturer's instructions. In brief, the cells were plated in 24-well plates overnight, and then the 24-well plate cells were washed twice and resuspended in 1 ml of DMEM. In all, 0.2 μg of plasmid DNA in 100 μl of DMEM containing 0.6 μl Fugene 6 solution was mixed with the cell suspension and added into each well. The plates were cultured for 24 h at 37°C. PGF2α, Butaprost, or Bimatoprost at a concentration ranging from 10−11 to 10−6 M were added to the culture, and 6 h later the cells were harvested and lysed in 100 μl of 25 mM Tris-phosphate buffer (pH 7.5) containing 1% Triton X-100. A volume of 20 μl of soluble extracts was assayed for the luciferase activity. The luciferase assay was performed with a Promega assay kit (Promega, Inc.) at room temperature using an Autolumat LB 953 (EG&, Berthold). Luciferase content was measured by calculating the light emitted during the initial 10 s of the reaction. Relative luciferase activity was expressed as fold values of ratio compared to control. The luciferase assay results shown in the figures are representative of experiments independently repeated at least three times.
RNA isolation and Northern blot analysis
Total RNA was isolated from cells and human ocular tissues using RNeasy Kit (Qiagen, Inc) according to the manufacturer's instruction. RNA concentrations were determined by UV Spectrophotometry (Beckman DU640) at A 260 nM, and stored at −80°C.
10 μg of total RNA was denatured at 65°C in RNA loading buffer (Ambion, Inc.) for 15 min, and separated on 1.2% agarose gels containing 0.66 M formaldehyde. RNA loading was assessed by ethidium bromide staining of 28s and 18s ribosomal RNA bands. The relative intensities of the 28s and 18s ribosomal RNA bands were used as internal controls to normalize the hybridizations. Human 690 bp Nur77 (+1332 to +2022 bp; gene bank: L13740.1) specific DNA fragment was radiolabeled using α-32P dATP and Klenow (Ambion, Inc.). The blots were hybridized with gene-specific probes in 50% formamide, 4 × SSC, 1 × Denhardt's solution, 50 mM sodium phosphate, pH 7.0, 1% SDS, 50 μg ml−1 yeast tRNA, and 0.5 mg ml−1 sodium pyrophosphate at 42°C overnight, and washed with 2 × SSC and 0.1% SDS twice at 42°C and 0.1 × SSC and 0.1% SDS twice at 42°C. The hybridized blots were exposed to phosphor screens, and the exposed screens were analyzed in a PhosphorImager (Molecular Dynamics, Inc).
Results
Kinetics of PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression
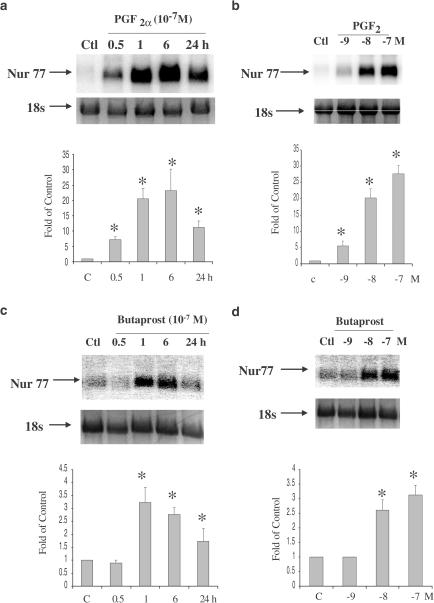
A kinetic study was performed to determine the time course and dose response of PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression in hFP- and hEP2-HEK 293/EBNA cells. For time course studies, the cells were treated with 10−7 M PGF2α or Butaprost at times ranging from 0.5 to 24 h. Dose response studies were determined by treating cells for 6 h with PGF2α or Butaprost at concentrations ranging from 10−9 to 10−7 M. PGF2α dramatically upregulated Nur77 mRNA expression in a time- and dose-dependent manner (Figure 1a, b). Nur77 mRNA induction reached a peak at 6 h after PGF2α treatment, and thereafter declined at 24 h when the final observation was made. Nur77 mRNA was induced to the maximum level by 10−7 M PGF2α. The pattern of Butaprost-induced Nur77 mRNA expression appeared to be similar to that of PGF2α, but it was less active (Figure 1c, d). Nur77 mRNA induction reached a peak at 1 h after Butaprost treatment, and declined to near basal levels at 24 h. A kinetic study was also performed using Bimatoprost treatment in hFP- and hEP2-HEK293/EBNA cells. Bimatoprost did not induce Nur77 mRNA expression in both hFP- and hEP2-HEK293/EBNA cell lines (data not shown).
Figure 1.
Upregulation of Nur77 mRNA expression following PGF2α and Butaprost treatment in hFP- and hEP2-HEK 293/EBNA cells. hFP-HEK 293/EBNA cells treated with 10−7 M PGF2α for 0.5, 1, 6, 24 h (a), and treated with 10−9 to 10−7 M PGF2α for 6 h (b). hEP2-HEK293/EBNA cells treated with 10−7 M Butaprost for 0.5, 1, 6, 24 h (c), and treated with 10−9–10−7 M Butaprost for 6 h (d). Arrows indicate mRNA levels of Nur77 and 18s rRNA (a–d, top panels). The intensity of 18s rRNA bands was used as an internal control to normalize the RNA loading differences. The data represented mean±s.d. of three independent experiments (a–d, bottom panels). *P<0.01 vs Control.
PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression occurs via the activation of protein kinase C
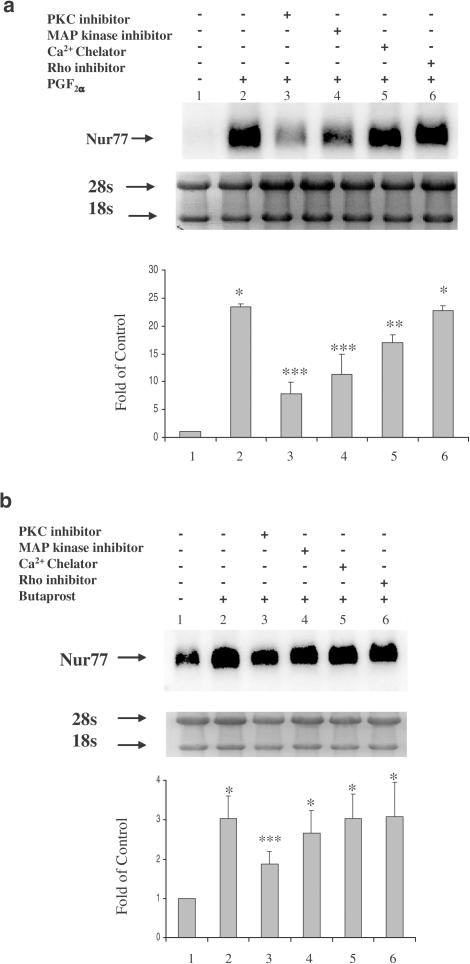
To clarify the signal transduction pathways involved in PGF2α- and Butaprost-induced Nur77 mRNA expression, pathway specific inhibitors were utilized to distinguish the intracellular mechanisms. Both hFP- and hEP2-HEK 293/EBNA cells were pretreated with each of these inhibitors (Toxin B, 100 ng ml−1, GF109203 X, 2.5 μM, PD 98059, 20 μM, BAPTA, 2.5 μM) for 30 min and the incubation was then continued in the presence of 10−7 M PGF2α or Butaprost for an additional 6 h. The PKC inhibitor (GF 109203) decreased both PGF2α- and Butaprost-induced Nur77 mRNA expression (Figure 2a, b). However, Butaprost-induced Nur77 expression appeared less dependent on the PKC pathway compared to PGF2α. The MAP kinase inhibitor (PD 98059) and the Ca2+ chelator (BAPTA) attenuated PGF2α-induced Nur77 mRNA expression. The Rho inhibitor (Toxin B) did not alter PGF2α- or Butaprost-induced Nur77 mRNA expression (Figure 2a, b). The protein kinase A (PKA) inhibitor (KT5720) was also used in this study and seemed to potentiate both PGF2α- and Butaprost-induced Nur77 mRNA expression (data not shown). These results suggested that both PGF2α- and Butaprost-induced Nur77 mRNA expression occurs via the PKC pathway.
Figure 2.
Activation of PKC is involved in PGF2α and Butaprost-induced upregulation of Nur77 mRNA expression. hFP-HEK 293/EBNA cells or hEP2-HEK 293/EBNA cells were pretreated with a Rho inhibitor (Clostridium difficile Toxin B, 100 ng ml−1), a PKC inhibitor (GF109203 X, 2.5 μM), an MAPK inhibitor (PD 98059, 20 μM), or a Ca2+ chelator (BAPTA, 2.5 μM) for 30 min, followed by continued incubation with 10−7 M PGF2α (a) or Butaprost (b) for 6 h. Arrows indicate Nur77 mRNA levels and 28s and 18s rRNA (a and b, top panels). The intensity of 28s and 18s rRNA bands was used as an internal control to normalize RNA loading differences. The data represent mean±s.d. of three independent experiments (a and b, bottom panels). *P<0.01 vs control; **P<0.05 vs PGF2α alone; ***P<0.01 vs PGF2α or Butaprost alone.
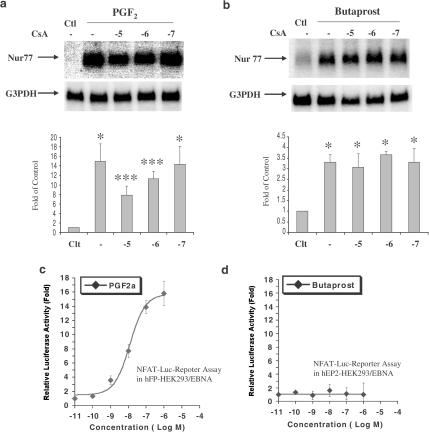
To further differentiate downstream of PKC pathways after the treatment with PGF2α and Butaprost, cyclosporin A (CsA) was used as inhibitor of calcineurin (Clipstone & Crabtree, 1992), a PKC downstream mediator. Both hFP- and hEP2-HEK 293/EBNA cells were pretreated with CsA at concentrations ranging from 10−7 to 10−5 M for 30 min and the incubation was then continued in the presence of 10−7 M PGF2α or Butaprost for an additional 6 h. CsA attenuated PGF2α-induced upregulation of Nur77 mRNA expression in a dose-dependent manner, but did not attenuate Butaprost-induced Nur77 mRNA expression (Figure 3a, b). This result was consistent with the NFAT luciferase reporter (Invitrogen, Inc) assay. NFAT is a substrate of calcineurin: de-phosphorylation of NFAT by calcineurin initiates NFAT nuclear translocation, and activates transcription (Crabtree, 2001). PGF2α activated NFAT-luciferase reporter in hFP-HEK 293/EBNA in a dose-dependent manner, whereas Butaprost did not activate NFAT-luciferase reporter in hEP2-HEK293/EBNA (Figure 3c, d). These data further supported calcineurin involvement in PGF2α-induced Nur77 mRNA expression. Calcineurin did not appear to be involved in Butaprost-induced Nur77 mRNA expression. These data suggest that PGF2α and Butaprost may activate different isoforms of protein kinase C through which they differentially regulate Nur77 upregulation.
Figure 3.
Calcinurin is involved in PGF2α-induced upregulation of Nur77 mRNA expression, but not in Butaprost-induced upregulation of Nur77 mRNA expression. hFP-HEK293/EBNA cells or hEP2-HEK293/EBNA cells were pretreated with cyclosporine A (CsA) at concentrations ranging from 10−7 to 10−5 M for 30 min, followed by continued incubation with 10−7 M PGF2α (a) or 10−7 M Butaprost (b) for an additional 6 h. Arrows indicate mRNA levels of Nur77 and 18s rRNA (a and b, top panels). The intensity of 18s rRNA bands was used as an internal control to normalize the RNA loading difference. The data represent mean±s.d. of three independent experiments (a and b, bottom panels). *P<0.01 vs control; **P<0.05 vs control. NFAT-luciferase reporter plasmids were transfected into hFP-HEK293/EBNA cells (c) or hEP2-HEK293/EBNA cells (d). The transfected cells were treated with PGF2α or Butaprost at a concentration ranging from 10−11 to 10−6 M. Relative luciferase activity was expressed as fold changes of ratio compared with control. The luciferase assay results shown in figures are representative of experiments independently repeated at least three times.
Novel transcript synthesis is required for PGF2α- and Butaprost-induced Nur77 mRNA expression
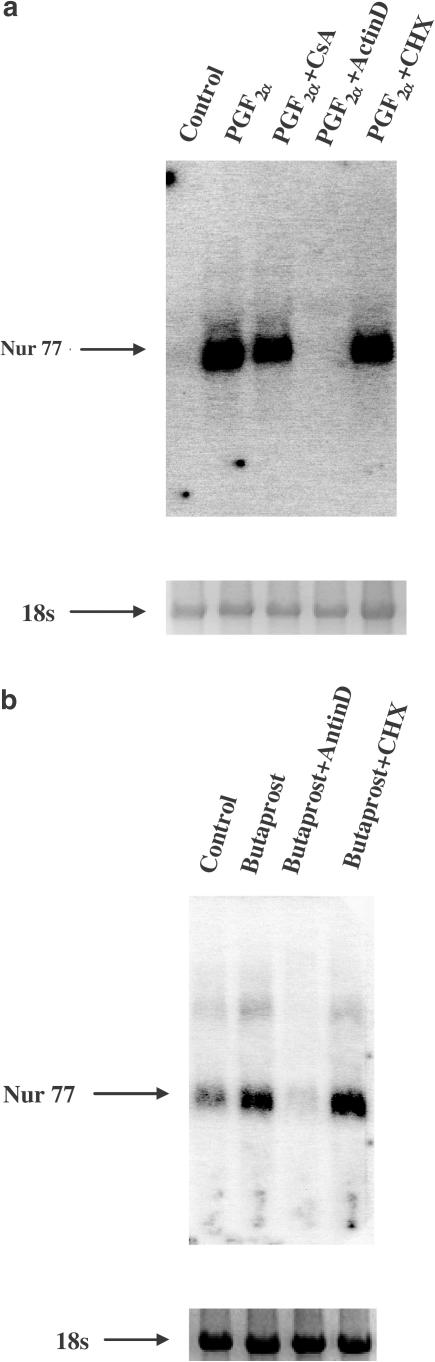
Upregulation of Nur77 mRNA in PGF2α- and Butaprost-treated hFP- and hEP2-HEK 293/EBNA cells may be due to an increase in the rate of synthesis, a decrease in the rate of degradation, or a combination of both. To test these possibilities, a transcription inhibitor, actinomyocin D (10 μg ml−1, actinD), was used to pretreat the cells for 30 min with continued incubation with 10−7 M PGF2α or Butaprost for 6 h. ActinD, at a concentration of 10 μg ml−1, completely prevented PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression (Figure 4a, b). Thus, it is most likely that Nur77 mRNA transcription rates are increased by PGF2α and Butaprost.
Figure 4.
Transcriptional regulation is involved in PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression in hFP- and hEP2-HEK293/EBNA cells. hFP-HEK293/EBNA cells or hEP2-HEK293/EBNA cells were pretreated with 10 μg ml−1 actinomycin D (actinD) or 10 μg ml−1 cycloheximide (CHX) for 30 min, with continued 10−7 M PGF2α (a) or 10−7 M Butaprost (b) for an additional 6 h. Arrows indicate mRNA levels of Nur77 and 18s rRNA. The intensity of 18s rRNA was used as an internal control to normalize the RNA-loading differences.
To determine if PGF2α- and Butaprost-induced upregulation of Nur77 mRNA requires de novo protein synthesis, a protein synthesis inhibitor, cycloheximide (10 μg ml−1, CHX), was used to pretreat the cells for 30 min with continued incubation in the presence of 10−7 M PGF2α or Butaprost for 6 h. CHX did not block PGF2α- or Butaprost-induced upregulation of mRNA expression, suggesting that PGF2α- and Butaprost-induced upregulation of Nur77 mRNA expression did not require de novo protein synthesis (Figure 4a, b).
Identification of the Nur77 gene promoter regions that confer the regulatory responses to PGF2α and Butaprost treatments
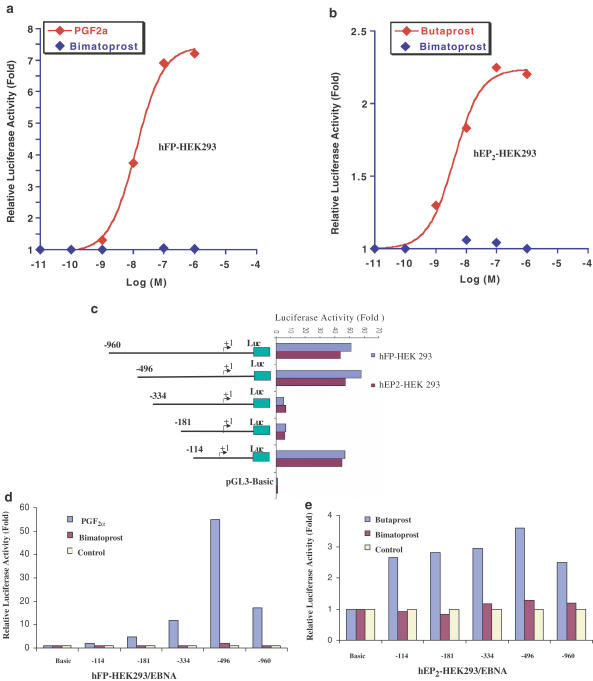
To further study the transcription mechanisms of hFP- and hEP2-mediated Nur77 mRNA expression, Nur77 promoters (−960 to +67 bp; Gene Bank: U17590) were isolated from a human genomic DNA library (Clontech) and subcloned into a luciferase reporter plasmid, pGL3, at the XhoI/HindIII site. Nur77 promoter luciferase reporter plasmids were transfected into both hFP-HEK 293/EBNA cells and hEP2-HEK 293/EBNA cells and then treated with PGF2α vs Bimatoprost or Butaprost vs Bimatoprost at concentrations ranging from 10−11 to 10−6 M. Both PGF2α and Butaprost, but not Bimatoprost, activated the Nur77 promoter in a dose-dependent manner (Figure 5a, b). These results suggested that Bimatoprost did not activate either the prostaglandin FP receptor or EP2 receptor. These data are matched to Northern blot analyses (Figure 6a, b).
Figure 5.
Identification of Nur77 promoter regions that are responsive to PGF2α and Butaprost treatment. (a) pGL3-N-960 plasmids were transfected in HEK 293/EBNA cells (a) or hEP2-HEK 293/EBNA cells (b), and then the transfected cells were treated with PGF2α or Butaprost at concentrations ranging from 10−11 to 10−7 M for 6 h. A variety of deletion constructs for Nur 77 promoter were transfected into hFP-HEK 293/EBNA cells or hEP2-HEK 293/EBNA cells. The basal transcription activities of Nur77 promoter regions were determined (c). Relative luciferase activity was expressed as fold changes of ratio compared with control. The HEK 293/EBNA cells transfected with the deletion constructs were treated with 10−7 M PGF2α vs Bimatoprost (d) or Butaprost vs Bimatoprost (e) for 6 h. Relative luciferase activity was expressed as fold changes of ratio compared with untreated. The luciferase assay results shown in the figures are representative of experiments independently repeated at least three times.
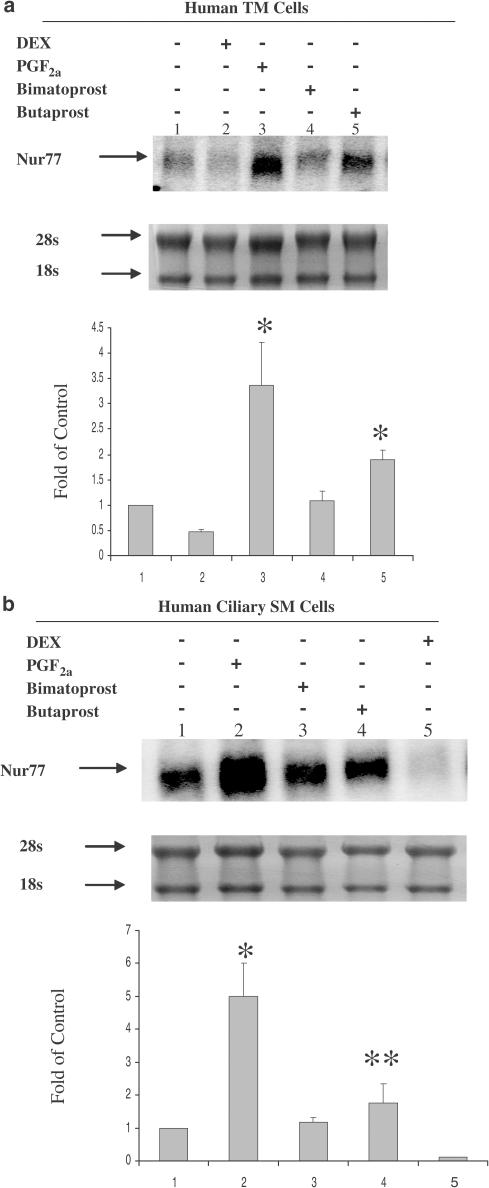
Figure 6.
PGF2α and Butaprost, but not Bimatoprost, upregulated Nur77 mRNA expression in human ciliary SM and TM cells. Human TM cells (a) and ciliary SM cells (b) were treated with 10−7 M of Dexamethasone (DEX), PGF2α, Bimatoprost or Butaprost for 6 h. Arrows indicate mRNA levels of Nur77 and rRNAs (a and b, top panels). The intensity of 28s and 18s rRNA bands was used as an internal control to normalize the RNA-loading differences. The data represent mean±s.d. of three independent experiments (a and b, bottom panels). *P<0.01 vs control, **P<0.05 vs control.
A 1 kb 5′ flanking fragment of the human Nur77 gene was subcloned in front of a luciferase gene for studying transcriptional regulation of the human Nur77 gene. When pGL3-N-960 plasmid was transfected into the hFP-HEK 293 or hEP2-HEK 293/EBNA cells, a remarkable increase of luciferase activity was observed compared to that of pGL3 basic without promoter (Figure 5c), which indicated that the 1 kb 5′ flanking fragment of human Nur77 gene contains a functional promoter that directs Nur77 gene expression. Deletion analysis of the Nur77 promoter in hFP- and hEP2-HEK 293/EBNA cells (Figure 5c) showed that a deletion from −960 to −496 slightly decreased luciferase reporter activity. The region from −960 to −496 may contain negative regulatory elements for Nur77 expression. Further deletion from −496 to −334 dramatically decreased luciferase activity about 30-fold as compared to pGL3-N-496, suggesting that the region from −496 to −334 may contain strong enhancers. Further deletion from −181 to −114 dramatically increased luciferase activity about 30-fold compared to pGL3-N-181. The region from −181 to −114 may contain strong suppressors. The region from −114 to +1 contains a basic functional promoter with multiple SP-1 and AP-1 elements for transcription activity of the human Nur77 gene in HEK 293/EBNA cells (Uemura et al., 1995).
To further identify which regions in the Nur77 promoter are responsive to PGF2α and Butaprost treatments, a series of Nur 77 promoter deletion constructs (Figure 5c) were transfected into hFP-HEK293/EBNA cells (Figure 5d) or hEP2-HEK293/EBNA 293 cells (Figure 5e), and then treated with 10−7 M PGF2α or Butaprost for 6 h. Relative luciferase activity was calculated as fold changes of ratio compared with untreated. These results suggested that a region from −960 to −496 contains regulatory elements that negatively regulate Nur77 expression in response to both PGF2α and Butaprost treatments. The regions from −496 to −334 contain regulatory elements that positively and dramatically induced Nur77 expression in response to PGF2α and Butaprost treatments. This region (−496 to −334) contains two putative IL-6-RE and a SP-1 elements (Uemura et al., 1995), which may contain novel cis-acting elements that are responsive to PGF2α and Butaprost treatments. The basic Nur77 promoter from −114 to +1 does not contain essential cis-acting elements that regulated Nur77 expression in response to PGF2α and Butaprost treatments. The overall patterns of the Nur77 regulatory regions that responded to PGF2α are similar to those of Butaprost, suggesting that PGF2α- and Butaprost-induced Nur77 expression may share the same regulatory mechanisms at transcriptional levels. Bimatoprost did not activate any of the Nur77 deletion promoters in either hFP- or hEP2-HEK 293/EBNA cells, which further supported the view that Bimatoprost does not interact with either hFP or hEP2 receptors.
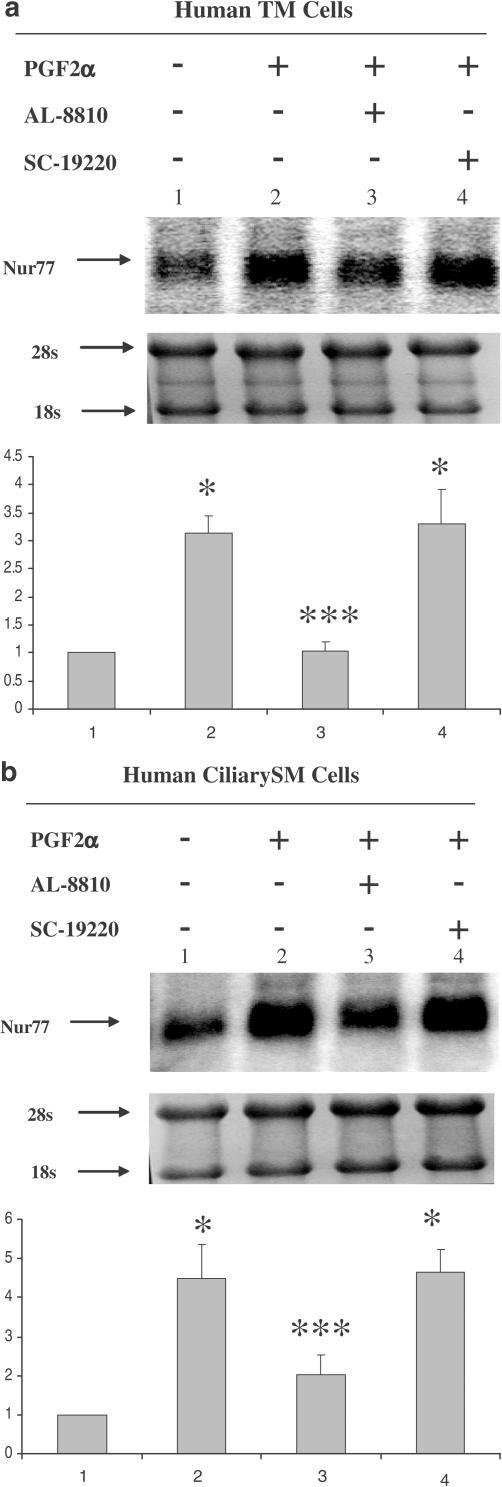
Regulation of Nur77 mRNA expression in human TM and ciliary SM cells following treatment with PGF2α, Butaprost, and Bimatoprost
TM and ciliary SM cells are thought to be the major target cells in the aqueous humor outflow pathway for glaucoma treatments. To further compare the mechanisms of PGF2α, Bimatoprost, and Butaprost in human ocular tissues, each of these compounds at a receptor effective (Abramovitz et al., 2000; Woodward et al., 2003) concentration of 10−7 M was used to treat cultured human TM and ciliary SM cells for 6 h. Northern blot analysis of Nur77 mRNA expression revealed that PGF2α and Butaprost, but not Bimatoprost, induced upregulation of Nur77 mRNA expression in human TM cells (Figure 6a). PGF2α, but not Bimatoprost, dramatically induced upregulation of Nur77mRNA expression in human ciliary SM cells, whereas Butaprost slightly upregulated Nur77 mRNA expression (Figure 6b). The effect of Butaprost is significantly different from control and Bimatoprost in the SM cells with P-value <0.05. Therefore, PGF2α is more efficacious than Butaprost in inducing Nur77 mRNA upregulation in human TM cells and ciliary SM cells. The effects of dexamethasone (DEX) on Nur77 expression will be fully described as a separate research topic elsewhere. To further support that the induction of Nur77 mRNA is through the activation of FP receptors, a selective FP receptor antagonist, AL-8810, and a selective EP1 antagonist, SC-19220, were used in the experiments (Figure 7a and b). AL-8810, but not SC-19220, blocked PGF2α-induced Nur77 mRNA expression in human TM and ciliary SM cells, suggesting that PGF2α-induced Nur77 mRNA expression is via the activation of prostaglandin FP receptors.
Figure 7.
Prostaglandin FP receptor antagonist, AL-8810, blocked PGF2α-induced Nur77 mRNA expression in human ciliary SM and TM cells. Human TM cells (a) and Ciliary SM cells (b) were pretreated with 10−5 M of AL-8810 or SC-19220 for 30 min, and then incubated with 10−7 M PGF2α for 6 h. Arrows indicate mRNA levels of Nur77 and rRNA (a and b, top panels). The intensity of 28s and 18s rRNA bands was used as an internal control to normalize the RNA-loading differences. The data represent mean±s.d. of three independent experiments (a and b, bottom panels). *P<0.01 vs control, ***P<0.01 vs PGF2α alone.
Discussion
A diverse variety of prostaglandins and prostaglandin ethanolamides (prostamides) have been reported to be highly effective in lowering intraocular pressure. Prostanoid FP and EP2 receptor agonists and the prostamide bimatoprost are the most potent ocular hypotensives reported to date (Woodward et al., 1993a, 1993b; 1995; 2001; Dubiner et al., 2001; Noecker et al., 2003). Their effects on intraocular pressure occur predominantly as an increase in aqueous humor outflow via the uveoscleral outflow pathway. The mechanisms that are involved in re-modeling of ciliary body tissue, with a resultant increase in uveoscleral outflow of aqueous humor from the eye, appear to involve the release of metalloproteinases and their modulation. Morphological studies on the ciliary body of monkey eyes treated for 1 year with the FP agonist prodrug Latanoprost, the EP2 agonist AH13205, or the prostamide Bimatoprost revealed remarkably similar effects with respect to the formation of uveoscleral outflow channels (Richter et al., 2003). This was surprising as Latanoprost and AH 13205 act at distinctly different prostanoid receptors and exert their effects via different second messenger pathways (Pierce et al., 1995; Toh et al., 1995). Bimatoprost appears to be pharmacologically independent of FP and EP2 receptors (Woodward et al., 2001; 2003). This implicates a possible common pathway that mediates the effects of these drugs on ciliary muscle re-modelling. The expression of genes that may coordinate this organized remodeling remains to be investigated. To this end, we have studied Nur77. The immediate-early gene Nur77 (also called NGFI-B) encodes an orphan nuclear receptor, a class of ligand-dependent transcriptional modulator proteins, which controls a program of gene expression that is involved in the regulation of cell differentiation and proliferation (Perlmann & Jansson, 1995; Maruyama et al., 1998). To further elucidate the mechanisms of Nur77 at the gene expression/second messenger levels, we compared the effects of three pharmacologically distinct ocular hypotensive agents PGF2α (FP agonist), Butaprost (EP2 agonist), Bimatoprost (prostamide), which appear to behave as mechanistically identical ocular hypotensives at the gross level, on the Nur77 gene expression.
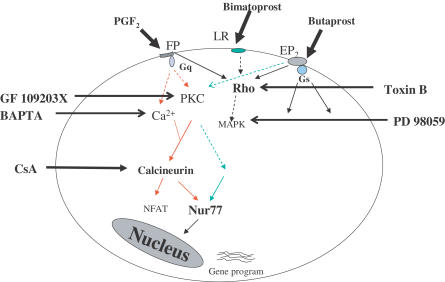
Activation of FP receptors initiated by ligand binding triggers Gαq protein-coupled mechanisms involving activation of phospholipase C, initiation of the second messengers IP3 and diacylglycerol, and a resultant mobilization of intracellular Ca2+ and increased protein kinase C activity (Toh et al., 1995). Butaprost is a synthetic prostaglandin analog that is highly selective for EP2 receptors; it triggers Gαs protein-coupled mechanisms involved in activation of adenylate cyclase, initiation of the cAMP formation with resultant activation of protein kinase A (Pierce et al., 1995). Despite different G protein signaling pathways, activation of both FP and EP2 receptors by their selective agonists induced Nur77 expression via protein kinase C pathways (Figure 8). This is the first observation that showed a link between EP2 receptor stimulation and protein kinase C. Further studies suggested that the downstream PKC signaling initiated by FP receptor activation differs from that of EP2 receptor activation, suggesting that EP2 receptor stimulation may activate a different PKC isoform, linked to a different PKC downstream signaling pathway to trigger Nur77 gene expression. It has been reported that the PKC activator PMA induced only a low level of Nur77 expression, but became highly induced by the addition of calcium ionophore to T-cell hybridoma (Woronicz et al., 1995). This may explain why EP2 receptor stimulation only weakly induced Nur77 expression, whereas FP receptor stimulation strongly induced Nur77 expression. The induction of Nur77 expression by other stimuli has also been reported in other cell types. Nur77 expression was rapidly induced by Nerve Growth Factor (NGF) in PC 12 phenochromocytoma cells via Ca2+ (Katagiri et al., 1997; Milbrandt, 1988), by T-cell receptor activation (TCR) in T-cells via Ca2+ (Liu et al., 1994; Woronicz et al., 1994) and by serum in fibroblasts via AP-1 (Hazel et al., 1988). Thus, the regulation of Nur77 expression may be through diverse intracellular mechanisms that are dependent upon different stimuli.
Figure 8.
Diagrammatic representation of the second messenger pathways involved in the regulation Nur77 expression by PGF2α (FP receptor agonist), Butaprost (EP2 receptor agonist), and Bimatoprost (prostamide). Red and green arrows indicate FP- and EP2-associated pathways, respectively.
Actinomycin D blocked Nur77 mRNA expression in response to PGF2α and Butaprost treatment, which implied that transcription mechanisms were involved in PGF2α- and Butaprost-induced upregulation of Nur77 expression. The 5′-flanking region of Human Nur77 gene has been previously characterized to be a functional promoter for Nur77 transcription (Crabtree, 2001). Activation of Nur77 expression by phorbol esters requires multiple transcription elements between −126 to −72 of the promoter region (William & Lau, 1993). Activation of Nur77 expression by NGF and membrane depolarization involves a region between −60 to −30 of the promoter region (Yoon & Lau, 1993). A region of Nur77 promoter from −322 to −151 is required for T-cell receptor signalling-induced Nur77 expression (Woronicz et al., 1994). In this study, we first identified that a region from −496 to −334 contains positive regulatory elements (enhancers) that are responsive to PGF2α and Butaprost treatment. A region from −960 to −496 contains negative regulatory elements that are responsive to PGF2α and Butaprost treatment. We also identified that a region from −181 to −114 contains negative regulatory elements (suppressors) for Nur77 expression, but these are not responsive to PGF2α and Butaprost treatment. Localization of discrete regions in human Nur77 promoters that respond to PGF2α and Butaprost treatment provides a clue for further studies on the mechanisms of PGF2α- and Butaprost-induced upregulation of Nur77 gene expression at the transcriptional level.
In terms of ocular hypotensive activity resulting from ciliary muscle re-modelling, Nur77 may represent a common modulator associated with both FP and EP2 receptor activation. Bimatoprost represents a novel class of anti-glaucoma compounds where the -COOH typical of prostaglandins is replaced by an amide group (Woodward et al., 2001). Unlike PGF2α and Butaprost, Bimatoprost did not upregulate Nur77 expression in human ciliary SM cells and TM cells and Nur77 would, therefore, be concluded to play no role in its ocular hypotensive effects. In our previous study, we found that Bimatoprost induced upregulation of Cyr61 gene expression in cat iris and human ciliary SM cells (Liang et al., 2003). In contrast to PGF2α, Bimatoprost did not regulate either CTGF or Nur77 upregulation in human ciliary SM and TM cells. These results suggest that Bimatoprost may exert its pharmacological actions through a unique mechanism to lower intraocular pressure, which is independent of Nur77.
The precise role of early response genes in mediating the ocular effects of the various PGs and Bimatoprost are not likely to be elucidated until an effective means of inhibiting their actions can be employed in living primates. Methods for measuring intraocular pressure in mice have recently been reported (Avila et al., 2001) and disruption of the Nur 77 gene could provide valuable insight into the importance of Nur 77 in mediating drug induced ocular hypotension. It is, however, important to realize that, between the various mammalian species, enormous variations in drug-induced ocular hypotension occur. For example, the rabbit and cat do not predict the effects of FP (Woodward et al., 1989; Stjernschantz, 2001) or DP (Crawford et al., 1992; Woodward et al., 1993b) agonists in primate eyes and are, therefore, unreliable therapeutic indicators. In addition to reducing intraocular pressure, negative consequences of Nur77 upregulation in the eye may possibly occur. Although this is clearly not the case in the anterior segment of the eye, PG-based drugs may achieve the retina in aphakic patients. In this case, Nur77 upregulation may accelerate the neurodegenerative aspects of glaucoma by influencing apoptotic events (Laabich et al., 2001).
In addition to glaucoma, efforts are directed towards prostanoid EP2 and FP agonist therapies for treating osteoporosis (Paralkar et al., 2003). In this case, there is an established link between Nur77 upregulation and bone formation. Thus, PTH induces Nur77 expression in primary mouse osteoblasts and in primary mouse calvariae cultures (Pirih et al., 2003). The existence of a convergent pathway for EP2 and FP agonists via Nur77 in bone remains to be determined.
Abbreviations
- DMEM
Dulbecco's modified eagle's medium
- EBNA
Epstein–Barr nuclear antigen
- EP2
prostaglandin EP2 receptor
- FP
prostaglandin FP receptor
- HEK
human embryonic kidney
- MAP
mitogen-activated protein
- NFAT
nuclear factor of activation T cell
- PGF2α
prostaglandin F2α
- SM
smooth muscle
- TM
trabecular meshwork
References
- ABRAMOVITZ M., ADAM M., BOIE Y., CARRIERE M.-C., DENIS D., GODBOUT C., LAMONTAGNE S., ROCHETTE C., SAWYER N., TREMBLAY N.M., BELLEY M., GALLANT M., DUFRESNE C., GAREAU Y., RUEL R., JUTEAU H., LABELLE M., OUIMET N., METTERS K.M. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- ADAMS J.W., MIGITA D.S., YU M.K., YOUNG R., HELLICKSON M.S., CASTRO-VARGUS F.E., DOMINGO J.D., LEE P.H., BUI J.S., HENDERSON S.A. Prostaglandin F2 alpha stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J. Biol. Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- AVILA M.Y., CARRE D.A., STONE R.A., CIVAN M.M. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest. Ophthalmol. Vis. Sci. 2001;42:1841–1846. [PubMed] [Google Scholar]
- BERGLUND B.A., BORING D.L., HOWLETT A.C. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Research 1999New York: Kluwer Academic/Plenum Publications; 527–533.ed. Honn, K. et al. Vol. 4, pp [Google Scholar]
- CLIPSTONE N.A., CRABTREE G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- CRABTREE G.R. Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- CRAWFORD K.S., KAUFMAN P.L., HUBBARD W.C., WOODWARD D.F. The DP-receptor agonist SQ 27986 raises but does not lower intraocular pressure in ocular normotensive monkeys. J. Glaucoma. 1992;1:94–99. [Google Scholar]
- DAVIS I.J., HAZEL T.G., CHEN R.H., BLENIS J., LAU L.F. Functional domains and phosphorylation of the orphan receptor Nur77. Mol. Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- DAVIS I.J., HAZEL T.G., LAU L.F. Transcriptional activation by Nur77, a growth factor-inducible member of the steroid hormone receptor superfamily. Mol. Endocrinol. 1991;5:854–859. doi: 10.1210/mend-5-6-854. [DOI] [PubMed] [Google Scholar]
- DUBINER H., COOKE D., DIRKS M., STEWART W.C., VANDENBURGH A.M., FELIX C. Efficacy and safety of bimatoprost in patients with elevated intraocular pressure: a 30-day comparison with latanoprost. Surv. Ophthalmol. 2001;45:5353–5360. doi: 10.1016/s0039-6257(01)00212-0. [DOI] [PubMed] [Google Scholar]
- FAHRNER T.J., CARROLL S.L., MILBRANDT J. The NGFI-B protein, an inducible member of the thyroid/steroid receptor family, is rapidly modified posttranslationally. Mol. Cell. Biol. 1990;10:6454–6459. doi: 10.1128/mcb.10.12.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJINO H., PIERCE K.L, SRINIVASAN D., PROTZMAN C.E., KRAUSS A.H.-P., WOODWARD D.F., REGAN J.W. Delayed reversal of shape change in cells expressing FP(B) prostanoid receptors. Possible role of receptor resensitization. J. Biol. Chem. 2000;275:29907–29914. doi: 10.1074/jbc.M003467200. [DOI] [PubMed] [Google Scholar]
- FUJINO H., WEST K.A., REGAN J.W. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- GATON D.D., SAGARA T., LINDSEY J.D., GABELT B.A.T., KAUFMAN P.L., WEINREB R.N. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch. Ophthalmol. 2001;119:1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- HAZEL T.G., NATHANS D., LAU L.F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 1988;85:8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTON E.W., POYSER N.L. Uterine luteolytic hormone: a physiological role for prostaglandin F2alpha. Physiol. Rev. 1976;56:595–651. doi: 10.1152/physrev.1976.56.4.595. [DOI] [PubMed] [Google Scholar]
- KATAGIRI Y., HIRATA Y., MILBRANDT J., GUROFF G. Differential regulation of the transcriptional activity of the orphan nuclear receptor NGFI-B by membrane depolarization and nerve growth factor. J. Biol. Chem. 1997;272:31278–31284. doi: 10.1074/jbc.272.50.31278. [DOI] [PubMed] [Google Scholar]
- LAABICH A., GUANGYU L., COOPER N.G.F. Visual-mediated regulation of retinal CaMKII and its GluR1 substrate is age-dependent. Mol. Brain Res. 2001;91:34–42. doi: 10.1016/s0169-328x(01)00168-1. [DOI] [PubMed] [Google Scholar]
- LIANG Y., LI C., GUZMAN V.M., EVINGER A.J., III, PROTZMAN C.E, KRAUSS A.H.-P., WOODWARD D.F. Comparison of prostaglandin F2alpha, bimatoprost (prostamide), and butaprost (EP2 agonist) on Cyr61 and connective tissue growth factor gene expression. J. Biol. Chem. 2003;278:27267–27277. doi: 10.1074/jbc.M301009200. [DOI] [PubMed] [Google Scholar]
- LIU Z.G., SMITH S.W., MCLAUGHLIN K.A., SCHWARTZ L.M., OSBORNE B.A. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- MANGELSDORF D.J., THUMMEL C., BEATO M., HERRLICH P., SCHUTZ G., UMESONO K., BLUMBERG B., KASTNER P., MARK M., CHAMBON P., EVANS R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA K., TSUKADA T., OHKURA N., BANDOH S., HOSONO T., YAMAGUCHI K. The NGFI-B subfamily of the nuclear receptor superfamily. Int. J. Oncol. 1998;12:1237–1243. doi: 10.3892/ijo.12.6.1237. [DOI] [PubMed] [Google Scholar]
- MATIAS I., CHEN J., PETROCELLIS L.D., BISOGNO T., LIGRESTI A., FEZZA F., KRAUSS A., SHI L., PROTZMAN C.E., LI C., LIANG Y., NIEVES A.L., KEDZIE K.M., BURK R.M., DIMARZO V., WOODWARD D.F.Prostaglandin-ethanolamide (prostamides): in vitro pharmacology and metabolism J. Pharmacol. Exp. Ther. 2004. in press [DOI] [PubMed]
- MILBRANDT J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- NOECKER R.S., DIRKS M.S., CHOPLIN N.T., BERNSTEIN P., BATOOSINGH A.L., WHITCUP S.M. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am. J. Ophthalmol. 2003;135:55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- PARALKAR V.M., BOROVECKI F., KE H.Z., CAMERON K.O., LEFKER B., GRASSER W.A., OWEN T.A., LI M., DASILVA JARDINE P., ZHOU M., DUNN R.L., DUMONT F., KORSMEYER R., KRASNEY P., BROWN T.A., PLOWCHALK D., VUKICEVIC S., THOMPSON D.D. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULSEN R.E., WEAVER C.A., FAHRNER T.J., MILBRANDT J. Domains regulating transcriptional activity of the inducible orphan receptor NGFI-B. J. Biol. Chem. 1992;267:16491–16496. [PubMed] [Google Scholar]
- PERLMANN T., JANSSON L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., GIL D.W., WOODWARD D.F., REGAN J.W. Molecular cloning of human prostanoid receptors. Trends Pharmacol. Sci. 1995;16:253–256. doi: 10.1016/s0165-6147(00)89035-5. [DOI] [PubMed] [Google Scholar]
- PIRIH F.Q., NERVINA J.M., PHAM L., AGHALOO T., TETRADIS S. Parathyroid hormone induces the nuclear orphan receptor NOR-1 in osteoblasts. Biochem. Biophys. Res. Commun. 2003;306:144–150. doi: 10.1016/s0006-291x(03)00931-8. [DOI] [PubMed] [Google Scholar]
- RICHTER M., KRAUSS A.H.-P., WOODWARD D.F., LUTJEN DRECOLL E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest. Ophthalmol. Vis. Sci. 2003;44:4419–4426. doi: 10.1167/iovs.02-1281. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., CRAIB S.J., STEVENSON L.A., PERTWEE R.G., HENDERSON A., TOOLE J., ELLINGTON H.C. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J. Pharmacol. Exp. Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- SMITH W.L., MARNETT L.J., DEWITT D.L. Prostaglandin and thromboxane biosynthesis. Pharmacol. Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-p. [DOI] [PubMed] [Google Scholar]
- STJERNSCHANTZ J.W. From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 2001;42:1134–1145. [PubMed] [Google Scholar]
- TOH H., ICHIKAWA A., NARUMIYA S. Molecular evolution of receptors for eicosanoids. FEBS Lett. 1995;361:17–21. doi: 10.1016/0014-5793(95)00129-w. [DOI] [PubMed] [Google Scholar]
- UEMURA H., MIZOKAMI A., CHANG C.S. Identification of a new enhancer in the promoter region of human TR3 orphan receptor gene. A member of steroid receptor superfamily. J. Biol. Chem. 1995;270:5427–5433. doi: 10.1074/jbc.270.10.5427. [DOI] [PubMed] [Google Scholar]
- WEINREB R.N., LINDSEY J.D. Metalloproteinase gene transcription in human ciliary muscle cells with latanoprost. Invest. Ophthalmol. Vis. Sci. 2002;43:716–722. [PubMed] [Google Scholar]
- WILLIAM G.T., LAU L.F. Involvement of JunD in transcriptional activation of the orphan receptor gene nur77 by nerve growth factor and membrane depolarization in PC12 cells. Mol. Cell. Biol. 1993;13:6124–6136. doi: 10.1128/mcb.14.12.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T.E., FAHRNER T.J., JOHNSTON M., MILBRANDT J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- WOLDEMUSSIE E., FELDMANN B.J., CHEN J. Electrophysiological and histological measures of retinal injury in chronic ocular hypertensive monkeys. Exp. Eye Res. 1993;56:385–392. doi: 10.1177/112067219900901S11. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., BOGARDUS A.M., DONELLO J.E., FAIRBAIRN C.E., GIL D.W., KEDZIE K.M., BURKE J.A., KHARLAMBh A., RUNDE E., ANDREWS S.W., PIERCE K.L., REGAN J.W. Molecular characterization and ocular hypotensive properties of the prostanoid EP2 receptor. J. Ocular Pharmacol. 1995;11:447–454. doi: 10.1089/jop.1995.11.447. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., BURKE J.A., WILLIAMS L.S., PALMER B.P., WHEELER L.A., WOLDEMUSSIE E., RUIZ G., CHEN J. Prostaglandin F2 alpha effects on intraocular pressure negatively correlate with FP-receptor stimulation. Invest. Ophthalmol. Vis. Sci. 1989;30:1838–1842. [PubMed] [Google Scholar]
- WOODWARD D.F., KRAUSS A.H.-P., CHEN J., LAI R.K., SPADA C.S., BURK R.M., ANDREWS S.W., SHI L., LIANG Y., KEDZIE K.M., CHEN R., GIL D.W., KHARLAMB A., ACHEAMPONG A., LING J., MADHU C., NI J., RIX P., USANSKY J., USANSKY H., WEBER A., WELTY D., YANG W., TANG-LIU D.D.-S., GARST M.E., BRAR B., WHEELER L.A., KAPLAN L.J. The pharmacology of bimatoprost (Lumigan) Surv. Ophthalmol. 2001;45:5337–5345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., KRAUSS A.H.-P., CHEN J., LIANG Y., LI C., PROTZMAN E., BOGARDUS A., CHEN R., KEDZIE K.M., KRAUSS H.A., GIL D.W., KHARLAMB A., WHEELER L.A., BABUSIS D., WELTY D., TANG-LIU D.D.-S., CHERUKURY M., ANDREWS S.W., BURK R.M., GARST M.E. Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024) J. Pharmacol. Exp. Ther. 2003;305:772–785. doi: 10.1124/jpet.102.047837. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., LAWRENCE R.A., FAIRBAIRN C.E., SHAN T., WILLIAMS L.S. Intraocular pressure effects of selective prostanoid receptor agonists involve different receptor subtypes according to radioligand binding studies. J. Lipid Med. 1993a;6:545–553. [PubMed] [Google Scholar]
- WOODWARD D.F., SPADA C.S., HAWLEY S.B., WILLIAMS L.S., PROTZMAN C.E., NIEVES A.L. Further studies on ocular responses to DP receptor stimulation. Eur. J. Pharmacol. 1993b;230:327–333. doi: 10.1016/0014-2999(93)90569-4. [DOI] [PubMed] [Google Scholar]
- WORONICZ J.D., CALNAN B., NGO V., WINOTO A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- WORONICZ J.D., LINA A., CALNAN B.J., SZYCHOWSKI S., CHENG L., WINOTO A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol. Cell. Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOON J.K., LAU L.F. Transcriptional activation of the inducible nuclear receptor gene nur77 by nerve growth factor and membrane depolarization in PC12 cells. J. Biol. Chem. 1993;268:9148–9155. [PubMed] [Google Scholar]
- YU M., IVES D., RAMESH C.S. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]