Abstract
Control of inflammatory pain can result from activation of opioid receptors on peripheral sensory nerves by opioid peptides secreted from leukocytes in response to stress (e.g. experimental swim stress or surgery). The extravasation of immunocytes to injured tissues involves rolling, adhesion and transmigration through the vessel wall, orchestrated by various adhesion molecules.
Here we evaluate the relative contribution of selectins, integrins α4 and β2, and platelet–endothelial cell adhesion molecule-1 (PECAM-1) to the opioid-mediated inhibition of inflammatory pain.
We use flow cytometry, double immunofluorescence and nociceptive (paw pressure) testing in rats with unilateral hind paw inflammation induced by complete Freund's adjuvant.
In inflamed tissue, 43–58% of hematopoietic cells (CD45+) expressed opioid peptides. L-selectin and β2 were coexpressed by 7 and 98% of opioid-containing leukocytes, respectively. Alpha4 integrin was expressed in low levels by the majority of leukocytes. Opioid-containing cells, vascular P- and E-selectin and PECAM-1 were simultaneously upregulated.
Swim stress produced potent opioid-mediated antinociception in inflamed tissue, unaffected by blockade of PECAM-1. However, blockade of L- and P-selectins by fucoidin, or of α4 and β2 by monoclonal antibodies completely abolished peripheral stress-induced antinociception. This coincided with a 40% decrease in the migration of opioid-containing leukocytes to inflamed tissue.
These findings establish selectins and integrins α4 and β2, but not PECAM-1, as important molecules involved in stress-induced opioid-mediated antinociception in inflammation. They point to a cautious use of anti-inflammatory treatments applying anti-selectin, anti-α4 and anti-β2 strategies because they may impair intrinsic pain inhibition.
Keywords: Adhesion molecules, selectins, integrins, PECAM-1, opioids, opioid-containing leukocytes, antinociception, inflammation, pain
Introduction
Inflammatory pain (associated with for example surgery, arthritis or cancer) is among the most prevalent forms of acute and chronic pain. This pain can be locally inhibited by leukocyte-derived opioid peptides without eliciting central side effects (Stein, 1995; Machelska & Stein, 2002; Stein et al., 2003). Opioid-containing immune cells migrate to inflamed tissues (Przewlocki et al., 1992; Stein et al., 1996; Cabot et al., 1997) and, concurrently, opioid receptors are upregulated on peripheral terminals of sensory neurons (Mousa et al., 2001). In response to stressful stimulation (e.g. surgery or experimental swim stress) leukocytes can locally liberate opioids (predominantly β-endorphin) that bind to peripheral opioid receptors leading to potent antinociception in animals and humans (Stein et al., 1990a, 1993; Cabot et al., 1997). This is demonstrated by the blockade of swim stress-induced antinociception by antibodies against β-endorphin and opioid receptor antagonists (Stein et al., 1990a; Machelska et al., 2003). Furthermore, immunosuppression abolishes this antinociception, demonstrating the functional relevance of immunocytes (Stein et al., 1990b; Przewlocki et al., 1992).
Leukocytes are directed to injured tissues by various adhesion molecules. Circulating cells initially tether and roll along the vessel wall, mediated primarily by leukocytic L-selectin and by endothelial P- and E-selectins. This is followed by firm adhesion involving leukocyte integrins, in particular α4β1 (CD49d/CD29; very late antigen-4) and β2 (CD18). Thereafter, the cells migrate through the endothelium engaging immunoglobulin superfamily members, predominately platelet–endothelial cell adhesion molecule-1 (PECAM-1/CD31) on leukocytes and endothelium (Butcher & Picker, 1996; von Andrian & Mackay, 2000). All these molecules are constitutively expressed and are upregulated in inflammation, except L-selectin, which is rapidly shed upon activation (Johnson et al., 1993; Schaible et al., 1994; Bennett et al., 1996; Issekutz TB et al., 1996; Mousa et al., 2000). Their blockade has been proposed as a novel anti-inflammatory strategy (Bogen et al., 1994; Issekutz AC et al., 1996, 2001). However, since some of these treatments can be detrimental to pain control (Machelska et al., 1998, 2002), we sought to evaluate comprehensively the relative contribution of selectins, integrins α4 and β2, and PECAM-1 to intrinsic opioid-mediated inhibition of inflammatory pain in order to identify precisely those molecules that promote intrinsic opioid antinociception vs those that do not. We approached this (1) by investigating the coexpression of these adhesion molecules with opioid peptides; (2) by assessing the effects of the selectin blocker fucoidin and monoclonal antibodies (mAbs) against α4, β2 and PECAM-1 on stress-induced antinociception, and (3) by evaluating the migration of opioid-containing leukocytes to inflamed tissue.
Methods
Animals
Experiments were performed in male Wistar rats (140–160 g) (bred at Freie Universität Berlin, Germany) and were approved by the local animal care committee (the Landesamt für Arbeitsschutz, Gesundheitsschutz und Technische Sicherheit, Berlin, Germany) and carried out in accordance with the International Association for the Study of Pain (Zimmermann, 1983). Rats were housed individually in cages and maintained on a 12 h light/dark schedule with food pellets and water ad libitum. Room temperature was maintained at 22±0.5°C and a relative humidity between 60 and 65%.
Induction and evaluation of inflammation
Rats received an intraplantar (i.pl.) injection of 0.15 ml of complete Freund's adjuvant (Calbiochem, La Jolla, CA, U.S.A.) into the right hind paw under brief halothane (Willy Rüsch GmbH, Böblingen, Germany) anesthesia. Experiments were performed 6 h later. Paw volume and dorsal surface temperature were measured with a plethysmometer (Ugo Basile, Comerio, Italy) and a contact thermometer (Cooper Instrument Corporation, Middlefield, CT, U.S.A.), respectively, and were determined by averaging two consecutive trials (Stein et al., 1990a).
Nociceptive thresholds
The nociceptive thresholds were assessed using the paw pressure algesiometer (modified Randall-Selitto test; Ugo Basile). Rats were held under paper wadding and incremental pressure was applied via a wedge-shaped, blunt piston onto the dorsal surface of the hind paw by means of an automated gauge. The pressure required to elicit paw withdrawal, the paw pressure thresholds (PPT), was determined. A cutoff of 250 g was employed to avoid tissue damage. Three consecutive trials, separated by 10 s intervals, were conducted and the average determined. The contralateral paw was tested in the same way. The sequence of paws was alternated between animals to avoid ‘order' effects. Testing was performed according to Stein et al. (1990a).
Antibodies
All Abs used for flow cytometry are monoclonal and were purchased from BD Biosciences (Heidelberg, Germany), unless otherwise stated. These were as follows: mouse anti-rat: CD3-phytoerythrin (PE)-conjugated (4 μg ml−1; T-cell marker), CD4-PE (4 μg ml−1), CD11b-fluorescein isothiocyanate (FITC; 10 μg ml−1), CD45-CyChrome (CyC; 4 μg ml−1; marker of all hematopoietic cells), RP-1-PE (12 μg ml−1; granulocyte marker), ED1-FITC (2 μg ml−1; monocyte/macrophage marker; Serotec, Oxford, England), anti-L-selectin-FITC (clone HRL1, subtype hamster IgG; 20 μg ml−1), anti-α4 (clone TA-2, subtype IgG1; 20 μg ml−1; Endogen, Woburn, MA, U.S.A.), anti-β2-FITC (clone WT.3, subtype IgG1; 20 μg ml−1) and mouse 3E7 (subtype IgG2a; 20 μg ml−1; Gramsch Laboratories, Schwabhausen, Germany) recognizing the pan-opioid sequence Tyr-Gly-Gly-Phe at the N-terminus of opioid peptides (Gramsch et al., 1983). Isotype-matched control Abs were as follows: hamster IgG, mouse IgG1, mouse IgG1-FITC and mouse IgG2a (all at 20 μg ml−1). Secondary Abs were as follows: rat anti-mouse IgG1-FITC (1 μg ml−1) and rat anti-mouse IgG2a+b-PE (1.5 μg ml−1).
For double immunofluorescence, the following Abs were used: polyclonal rabbit anti-rat β-endorphin (Peninsula Laboratories, Belmont, CA, U.S.A.), monoclonal mouse-anti-rabbit E-selectin crossreacting with rat E-selectin (generously provided by B. Wolitzky, Hoffman-La Roche, Nutley, NJ, U.S.A.), polyclonal rabbit anti-human P-selectin crossreacting with rat P-selectin (BD Biosciences) and monoclonal mouse anti-rat PECAM-1 (clone TLD-3A12; NatuTec GmbH, Frankfurt/Main, Germany). Secondary Abs were as follows: goat anti-rabbit-Texas red and horse anti-mouse-FITC (Vector Laboratories, Burlingame, CA, U.S.A.).
For behavioral experiments, the following mouse anti-rat mAbs were used: anti-α4 (clone TA-2; Endogen), anti-β2 (clone WT.3), anti-PECAM-1 (clone TLD-3A12) (both from BD Biosciences) and mouse IgG (Sigma, Deisenhofen, Germany). The TA-2, WT.3 and TLD-3A12 mAbs have been shown to inhibit leukocyte migration to arthritic joints and dermal inflammation or to decrease T-cell proliferation (Issekutz & Issekutz, 1995; Issekutz AC et al., 1996; Issekutz TB et al., 1996; Williams et al., 1996). The TA-2 mAb is directed against the α4 subunit and therefore recognizes two integrins sharing this subunit, α4/β1 and α4/β7. Because α4/β7 primarily mediates lymphocyte migration to gut and associated lymphoid tissues (Hamann et al., 1994) the TA-2 mAb most probably targets α4/β1 in our model, as documented by others (e.g. Issekutz AC et al., 1996; Issekutz TB et al., 1996). The WT.3 mAb recognizes the β2 subunit (Tamatani et al., 1991), which is shared by the three integrins CD11a (lymphocyte function-associated antigen-1; LFA-1), CD11b (Mac-1) and CD11c (p150,95) (Sanchez-Madrid et al., 1983).
Flow cytometry
First we evaluated the surface expression of adhesion molecules on leukocytes from blood and inflamed paws. Because no such cells were found in contralateral noninflamed paws (Rittner et al., 2001), these were not examined. At 6 h after induction of inflammation, rats were killed with halothane and heparinized blood was obtained by cardiac puncture, and treated by hypotonic lysis. Subcutaneous paw tissue was dissected, cut, enzymatically digested and filtered (Rittner et al., 2001; Machelska et al., 2002). Single cell suspensions were stained with anti-L-selectin, anti-α4, anti-β2 or control Abs. Gating was performed for blood granulocytes by forward/sideward scatter characteristics, and for CD45+ cells in paws to identify hematopoietic cells and to exclude nonviable cells. Next, we examined the coexpression of L-selectin and β2 with opioids by CD45+ cells in paws. Because of technical difficulties related to low expression of α4 by granulocytes (Issekutz TB et al., 1996; van den Berg et al., 2001), we did not perform co-staining of α4 with opioids in the paw tissue. For triple staining of adhesion molecules, opioids and CD45, the DAKO Intrastain kit (DAKO Diagnostika, Hamburg, Germany) was used. Cells were incubated with anti-L-selectin or anti-β2, fixed, permeabilized and stained with 3E7, rat anti-mouse IgG2a+b and anti-CD45. This strategy was used since surface lectin staining was not preserved after fixation with paraformaldehyde and saponin permeabilization (Verdier et al., 2000). Incubation periods were 15 min at room temperature, except 3E7 (30 min). As demonstrated by previous competition experiments (Rittner et al., 2001), 3E7 produces opioid-specific staining. Data consist of three independent experiments.
To assess the involvement of selectins and integrins in trafficking of opioid-containing leukocytes, rats (n=6 per group) received intravenous (i.v.) fucoidin (10 mg kg−1; Sigma), which blocks L- and P-selectins (Bevilacqua & Nelson, 1993), anti-α4 in combination with anti-β2 (each at 4 mg kg−1), and as controls 0.9% NaCl or mouse IgG (8 mg kg−1) under brief halothane anesthesia immediately before induction of inflammation. In these and in behavioral (see below) experiments, 0.9% NaCl was used to dissolve fucoidin and to dilute Abs, and injections were performed in a volume of 0.6 or 1.2 ml per rat for single or combined injections, respectively. After 6 h, blood samples (1 ml) and subcutaneous paw tissue were obtained. Whole blood (100 μl) was co-stained with anti-CD4 and anti-CD11b for 15 min and treated with fluorescence activated cell staining (FACS) lysing solution according to the manufacturer's instructions (BD Biosciences). Leukocytes were analyzed by expression of CD4 and CD11b as follows: CD4−/CD11b+, granulocytes; CD4+/CD11b+, monocytes/macrophages; CD4+/CD11b−, T cells. Surface and intracellular staining of leukocytes from paws was performed as described (Rittner et al., 2001). Briefly, single cell suspensions were incubated with anti-CD45 and anti-CD3. For intracellular staining, cells were fixed with 1% paraformaldehyde, permeabilized with saponin buffer and stained for 15 min with anti-RP-1, anti-ED1 or 3E7. For 3E7 staining, cell suspensions were stained with the secondary anti-mouse IgG2a+b for 30 min. Samples were finally fixed with 1% paraformaldehyde before analysis. Negative controls included the replacement of the primary Abs with isotype-matched irrelevant Abs.
To calculate absolute numbers of cells per paw and in blood, the stained cell suspensions were analyzed together with a known number of fluorescent beads in a TRUCOUNT tube (BD Biosciences). Numbers of cells per tube were calculated in relation to the known number of fluorescent TRUCOUNT beads and extrapolated for the whole paw. At least 10,000 FACS events were collected in FACScan and analyzed using CellQuest software (BD Biosciences).
Double immunofluorescence
To assess the coexpression of P-selectin, E-selectin and PECAM-1 with opioids, rats (n=5) were deeply anesthetized with halothane and perfused transcardially with 0.1 M phosphate-buffered saline (PBS) and fixative solution (PBS containing 4% paraformaldehyde; pH 7.4). The skin with adjacent subcutaneous tissue was removed from both hind paws, postfixed for 30 min at 4°C in the fixative solution and cryoprotected at 4°C in PBS containing 10% sucrose. The tissue was embedded in tissue-Tek compound (OCT, Miles Inc., Elkhart, IN, U.S.A.), frozen, cut (7 μm) and mounted on slides (Mousa et al., 2000). The sections were incubated with anti-E-selectin (4 μg ml−1) or anti-PECAM-1 (1 : 500) in combination with anti-β-endorphin (1 : 1000) and then with secondary Abs. In the case of P-selectin, staining was detectable after tyramide signal amplification. At 2 h after incubation with anti-P-selectin (1 : 8000), biotin-labeled goat anti-rabbit IgG was applied and visualized by a horse radish peroxidase streptavidin-conjugated Ab followed by deposition of fluorescein-tyramide (NEN™ Life Science Products, Boston, MA, U.S.A.). The sections were then incubated with anti-β-endorphin (1 : 1000) for 2 h, then with a mouse anti-rabbit Texas red-conjugated, washed with PBS, mounted in vectashield (Vector Laboratories) and viewed under a fluorescence microscope (Zeiss, Jena, Germany) with appropriate filters. The control experiments included preabsorption of anti-β-endorphin with β-endorphin (Peninsula Laboratories) and omission of either the primary or the secondary Abs.
The expression of adhesion molecules was quantified by an observer blinded to the experimental protocol, using a Zeiss microscope (objective: × 20; eyepiece: × 10) (Mousa et al., 2000; Machelska et al., 2002). The mean number of P-selectin-, E-selectin- and PECAM-1-expressing blood vessels in three sections per animal and five squares (each of 384 mm2) per section was calculated. The percentage of adhesion molecule-stained vessels was determined according to the following formula: number of P-selectin, E-selectin- or PECAM-1-stained vessels/number of all vessels × 100.
Behavioral experiments
To activate endogenous opioidergic pathways of pain inhibition, rats (6–8 per group) were exposed to cold (2–4°C) water swim stress for 1 min in a metal container. The PPT were evaluated before and 1 min after stress (i.e. at the time of their maximum elevation) (Stein et al., 1990a; Machelska et al., 1998, 2002, 2003). To assess opioid receptor contribution, naloxone hydrochloride (1.125 μg 0.1 ml−1 0.9% NaCl; Sigma) or 0.9% NaCl (0.1 ml) was injected into inflamed paws 5 min before stress, and PPT were evaluated analogously. Next, we evaluated the involvement of selectins, integrins and PECAM-1 in stress-induced antinociception. Immediately before induction of inflammation, rats received i.v. injections of either fucoidin (10 mg kg−1), anti-α4 (4–8 mg kg−1), anti-β2 (2–8 mg kg−1), anti-PECAM-1 (1–10 mg kg−1), anti-α4 in combination with anti-β2 (each at 4 mg kg−1) or, as controls, mouse IgG (1–10 mg kg−1) or 0.9% NaCl. The dose of fucoidin was found to be the most effective in an earlier study (Machelska et al., 1998). After 6 h, paw temperature was measured, baseline PPT were taken and rats were subjected to stress. After 1 min, PPT were re-evaluated and paw volume was measured thereafter. The experimenter was blinded to the respective treatments.
Data analysis
Data are presented as means±s.e.m. and are expressed in raw values or as a percentage of control (100%). Two-sample comparisons were performed using the paired t-test for dependent data, t-test for independent normally distributed data and Mann–Whitney test for independent not normally distributed data. Multiple comparisons were performed using analysis of variance (ANOVA) followed by appropriate post hoc tests. Differences were considered significant if P<0.05.
Results
Coexpression of selectins, integrins and PECAM-1 with opioids
At 6 h after injection of Freund's adjuvant, rats developed inflammation, confined to inoculated paws and characterized by hyperalgesia (decreased PPT), swelling and elevated paw temperature (for all: P<0.001; paired t-test; data not shown), similar to our previous studies (Machelska et al., 1998, 2002, 2003). To establish optimal conditions of L-selectin and integrin staining, we first examined blood granulocytes. These constituted the predominant (75%) leukocyte subpopulation in inflamed paws at 6 h (controls in Figure 4a and c). Circulating granulocytes expressed high levels of L-selectin and β2, and to a lesser extent α4 (Figure 1a–c), in line with other studies (Tamatani et al., 1991; Issekutz TB et al., 1996; van den Berg et al., 2001).
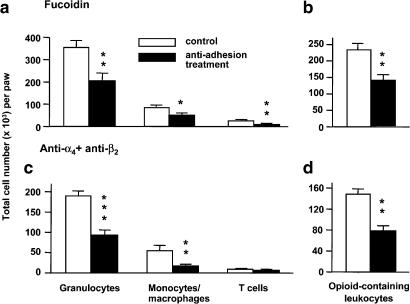
Figure 4.
Effects of concomitant blockade of selectins (a, b) or integrins α4 with β2 (c, d) on leukocyte subpopulation (a, c) and on opioid-containing leukocyte migration (b, d) to inflamed tissue. Fucoidin (10 mg kg−1) or anti-α4 mAb (4 mg kg−1) with anti-β2 mAb (4 mg kg−1) were injected i.v. immediately before induction of unilateral hind paw inflammation. After 6 h, subcutaneous tissue from inflamed paws was obtained and processed for flow cytometry. Data are expressed as means±s.e.m. *P<0.05, **P<0.01, ***P<0.001 (t-test) compared with respective controls.
Figure 1.
Leukocyte expression of α4 and β2 integrins, L-selectin and opioid peptides. Blood and subcutaneous tissue from inflamed paws were obtained 6 h after induction of unilateral hind paw inflammation, and processed for flow cytometry. (a–c) Surface expression of adhesion molecules on blood granulocytes. (d–f) Surface expression of adhesion molecules by CD45+ cells in the paw tissue. In (a–f), thick black lines represent anti-α4, anti-β2 and anti-L-selectin mAbs, respectively, whereas gray areas represent respective isotype-matched control mAbs. (g–l) Expression of adhesion molecules and opioid peptides by CD45+ cells in the paw tissue. (g–i) Controls (staining with isotype-matched mAbs) for double-staining experiments shown in (j–l), respectively. (j) Expression of opioid peptides by CD45+ cells. A total of 43% of CD45+ cells express opioids. (k) Coexpression of β2 with opioid peptides. Of all CD45+ cells, 1% express opioids alone, 51% express β2 alone and 44% coexpress β2 with opioids.. Opioid-expressing cells constitute 45% of all CD45+ cells (both upper quadrants), and 98% of these cells coexpress β2. (l) Coexpression of L-selectin with opioid peptides. Of all CD45+ cells, 54% express opioids alone, 2.3% express L-selectin alone and 4.2% coexpress L-selectin with opioids. Opioid-expressing cells constitute 58% of all CD45+ cells (both upper quadrants), and 7% of these cells coexpress L-selectin.
In inflamed paws, CD45+ cells expressed high levels of β2 and low levels of L-selectin (Figure 1e and f). Although the intensity of α4 staining in paws (Figure 1d) was slightly lower than in blood (Figure 1a), the majority of CD45+ cells expressed α4 (Figure 1d). In all, 43–58% of CD45+ cells expressed opioids (Figure 1j–l). Of these, 7% coexpressed L-selectin (Figure 1l), reflecting the relative lack of single staining for L-selectin (Figure 1f). β2 was expressed by 98% of opioid-containing CD45+ cells (Figure 1k). Specificity was documented by the lack of staining with control Abs (Figure 1g–i). Because of the relatively low expression of α4 by granulocytes (Issekutz TB et al., 1996; van den Berg et al., 2001) and technical difficulties resulting therefrom, we did not examine coexpression of α4 with opioids in paws.
Double immunofluorescence revealed constitutive expression of P-selectin, E-selectin and PECAM-1 by the vascular endothelium in noninflamed paws (Figure 2a, c and e; Table 1). The total number and the percentage of P-selectin-positive vessels was lower compared with PECAM-1, and there were substantially fewer E-selectin- compared with P-selectin- and PECAM-1-positive vessels (P<0.05; ANOVA, Bonferroni test; Table 1). The expression of these molecules in noninflamed paws of Freund's adjuvant-treated rats was similar to those in untreated animals (not shown), consistent with a strictly localized inflammation following i.pl. complete Freund's adjuvant. β-endorphin-positive cells were extremely scarce in noninflamed paws (Figure 2a, c and e).
Figure 2.
Expression of P-selectin (a, b), E-selectin (c, d) and PECAM-1 (e, f) with β-endorphin (a–f) in noninflamed (a, c, e) and inflamed (b, d, f) tissue. Subcutaneous tissue from both hind paws was obtained 6 h after induction of unilateral hind paw inflammation, and processed for double immunofluorescence. Staining of adhesion molecules appears in green, and staining of β-endorphin-containing cells appears in red. Bar=20 μm.
Table 1.
Quantification of P-selectin-, E-selectin- and PECAM-1-expressing blood vessels in the paw
| Staining | Absolute number | Percentage of all vessels | ||
|---|---|---|---|---|
| Noninflamed paw | Inflamed paw | Noninflamed paw | Inflamed paw | |
| P-selectin | 2.8±0.1† | 8.8±0.4* | 25±1.3† | 42±1.8* |
| E-selectin | 0.6±0.07†‡ | 7.9±0.3* | 5±0.7†‡ | 37±1.5* |
| PECAM-1 | 3.4±0.1 | 9.9±0.4* | 31±1.1 | 47±1.8* |
Adhesion molecule expression was assessed by double immunofluorescence. Values are expressed as means±s.e.m.
P<0.001 (paired t-test) compared with noninflamed paws
P<0.05 compared with PECAM-1
P<0.05 compared with P-selectin (ANOVA, Bonferroni test).
In inflamed paws, the total number and the percentage of P-selectin, E-selectin- and PECAM-1-positive vessels was significantly increased compared with noninflamed paws (P<0.001; paired t-test; Figure 2; Table 1). Numerous cells expressed β-endorphin in the vicinity of P-selectin, E-selectin- and PECAM-1-positive vessels (Figure 2b, d and f). Control experiments revealed no immunoreactivity (not shown).
Blockade of selectins, integrins and PECAM-1 and macroscopic inflammation
Hyperalgesia (i.e. baseline PPT measured 6 h after Freund's adjuvant and antiadhesion treatments but before stress) was not significantly changed by any of the antiadhesion treatments (P>0.05; t-test; Table 2). Paw volume was significantly decreased by fucoidin (1.7±0.06 vs 1.3±0.05 ml, control vs fucoidin; P<0.001; t-test) and by combined treatment with anti-α4 and anti-β2 (1.6±0.03 vs 1.4±0.03 ml, control vs anti-α4 and anti-β2; P<0.01; t-test; Table 2). Paw temperature was slightly decreased by anti-β2 (4 mg kg−1; 36.1±0.2 vs 35.3±0.3°C, control vs anti-β2; P<0.05; t-test; Table 2). Other treatments with anti-α4, anti-PECAM-1 or anti-β2 (2 and 8 mg kg−1) did not significantly change these signs of inflammation (P>0.05; t-test; Table 2). None of the antiadhesion treatments caused significant changes in noninflamed paws (P>0.05; t-test; not shown).
Table 2.
Effects of blockade of selectins, integrins and PECAM-1 on macroscopic inflammation
| Treatment (mg kg−1) | Hyperalgesia (PPT) (g) | Swelling (paw volume; % control) | Hyperthermia (paw temperature; % control) |
|---|---|---|---|
| Control | 26±1.5 | 100% | 100% |
| Fucoidin | |||
| 10 | 27±1.9 | 76±3.0*** | 98±0.5 |
| Anti-α4 | |||
| 4 | 31±7.7 | 97±2.5 | 100±1.1 |
| 8 | 27±5.5 | 106±3.3 | ND |
| Anti-β2 | |||
| 2 | 26±3.7 | 101±1.5 | ND |
| 4 | 25±2.0 | 98±3.7 | 98±0.7* |
| 8 | 29±1.0 | 108±2.7 | 101±3.0 |
| Anti-α4 (4 mg)+anti-β2 (4 mg) | 31±4.1 | 91±2.1** | 100±1.0 |
| Anti-PECAM-1 | |||
| 1 | 32±2.2 | 95±2.8 | 100±0.7 |
| 2.5 | 22±1.3 | 105±2.4 | 102±0.4 |
| 5 | 33±2.4 | 101±1.4 | 101±0.5 |
| 10 | 24±2.8 | 108±2.7 | 101±1.1 |
Values are expressed as means±s.e.m.
P<0.05
P<0.01
P<0.001 (t-test) compared with respective controls. ND, not determined.
Blockade of selectins, integrins and PECAM-1 and stress-induced antinociception
Exposure of rats to swim stress produced potent antinociception (PPT elevations) in inflamed (28±1.8 vs 130±7.9 g) but not in noninflamed paws (63±3.3 vs 64±3.1 g) (PPT before vs after stress; P<0.001 and P>0.05; paired t-test, respectively). Naloxone injected into inflamed paws significantly decreased stress-induced antinociception (129±5.1 vs 61±3.2 g, PPT before vs after injection of naloxone; P<0.001; t-test; Figure 3). No significant changes were observed in noninflamed paws (P>0.05; Mann–Whitney test; not shown).
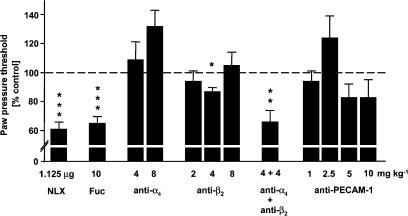
Figure 3.
Effects of blockade of selectins, integrins α4 and β2, and PECAM-1 on stress-induced antinociception. At 6 h after induction of unilateral hind paw inflammation, baseline PPT were measured and naloxone (NLX; 1.125 μg 100 μl−1) was injected into inflamed paws. After 5 min rats were exposed to swim stress and 1 min later PPT were re-evaluated. Fucoidin (Fuc; 10 mg kg−1), anti-α4 (4–8 mg kg−1), anti-β2 (2–8 mg kg−1) and anti-PECAM-1 (1–10 mg kg−1) mAbs were injected i.v. immediately before induction of inflammation. After 6 h PPT were measured, rats were exposed to swim stress and 1 min later PPT were re-evaluated. The dashed line represents controls (100%). Data are expressed as means±s.e.m. *P<0.05, **P<0.01, ***P<0.001 (t-test) compared with controls.
The single blockade by anti-α4, anti-β2 (2 and 8 mg kg−1) or anti-PECAM-1 did not significantly change stress-induced antinociception (P>0.05; t-test; Figure 3). A slight, significant decrease was observed after 4 mg kg−1 of anti-β2 (116±6.1 vs 100±3.2 g; PPT before vs after anti-β2; P<0.05; t-test; Figure 3). This effect was significantly different from that of naloxone (P<0.001; t-test; Figure 3). Fucoidin or combined treatment with anti-α4 and anti-β2 completely abolished peripheral stress-induced antinociception, since their effects were not significantly different from those of naloxone (61±3.2 vs 85±5.9 vs 74±8.7 g, naloxone vs fucoidin vs anti-α4 and anti-β2, respectively; P>0.05; t-test; Figure 3). No significant changes were observed in noninflamed paws after any treatment (P>0.05; t-test; not shown).
Effects of selectin and integrin blockade on migration of opioid-containing leukocytes
Inoculation with Freund's adjuvant caused immigration of leukocytes selectively to injected paws. The majority of these cells were granulocytes, followed by monocytes/macrophages and T cells (controls in Figure 4a and c). In control animals, about 40% of CD45+ cells contained opioids (controls in Figure 4b and d), similar to results presented in Figure 1j–l. In the blood, fucoidin did not significantly change the number of granulocytes and monocytes/macrophages (P>0.05; t-test) but significantly decreased the number of T cells (P<0.01; t-test; Table 3). Combined treatment with anti-α4 and anti-β2 significantly increased the number of all three leukocyte subpopulations in the blood (P<0.05; t-test; Table 3).
Table 3.
Effects of selectin and integrin blockade on the number of circulating leukocytes
| Treatment | Leukocyte counts (cells per μl of whole blood) | ||
|---|---|---|---|
| Granulocytes | Monocytes | T cells | |
| Control | 608±77 | 102±23 | 320±33 |
| Fucoidin (10 mg kg−1) | 446±207 | 164±42 | 117±39** |
| Control | 612±121 | 44±25 | 291±31 |
| Anti-α4 (4 mg kg−1)+anti-β2 (4 mg kg−1) | 1846±521* | 412±124* | 1348±421* |
Leukocyte counts were determined by flow cytometry. Values are expressed as means±s.e.m.
P<0.05
P<0.01 (t-test) compared with respective controls.
In the inflamed paws, fucoidin significantly decreased the number of granulocytes, T cells (P<0.01) and monocytes/macrophages (P<0.05) (t-test; Figure 4a). Combined treatment with anti-α4 and anti-β2 significantly decreased the number of granulocytes (P<0.001) and monocytes/macrophages (P<0.01) but did not change the number of T cells (P>0.05) (t-test; Figure 4c). Fucoidin and combined treatment with anti-α4 and anti-β2 significantly decreased the number of leukocytes containing opioids in the inflamed paws (P<0.01; t-test; Figure 4b and d).
Discussion
The present study shows that endogenous opioid antinociception in inflamed tissue is unaffected by blockade of PECAM-1, but is abolished by blockade of L- and P-selectins, or of α4 and β2 integrins. These data significantly expand our previous studies (Machelska et al., 1998, 2002) in that we have now comprehensively evaluated the relative role of prominent members of each adhesion molecule family. In particular, we now show that (1) in peripheral inflamed tissue, L-selectin and β2 integrins are coexpressed by opioid-containing leukocytes, endothelial P- and E-selectin and PECAM-1 are upregulated simultaneously with an enhanced infiltration of opioid-containing cells; (2) blocking of L- and P-selectins and of integrins α4 and β2, but not PECAM-1, completely abolishes peripheral intrinsic opioid antinociception; and (3) this effect results from the selectin- and integrin-mediated decreased migration of opioid-producing immunocytes to the injured tissue.
We have previously shown that our stress paradigm is an appropriate stimulus to reveal endogenous opioid-mediated inhibition of pain (Stein et al., 1990a, 1990b; Machelska et al., 2003). At 6 h after induction of inflammation, stress-induced antinociception is mediated by both peripheral and central opioid mechanisms (Machelska et al., 2003). Thus, peripherally selective doses of Abs against opioid peptides and of opioid receptor antagonists dose-dependently attenuate (by about 50%), whereas naloxone in centrally acting doses completely blocks this stress-induced antinociception (present study; Machelska et al., 2003). That adhesion molecule blockade abolishes only the peripheral component of stress-induced antinociception (producing a similar effect to that of naloxone in a peripherally selective dose) is consistent with the notion that centrally mediated intrinsic opioid antinociception is independent of leukocyte extravasation. Notwithstanding, peripheral mechanisms of endogenous pain control are potent and of clinical relevance since in patients undergoing surgery for knee injuries, opioid-containing immune cells are detectable in the inflamed synovium and the blockade of intra-articular opioid receptors by naloxone results in significantly increased postoperative pain for up to 4 h (Stein et al., 1993).
The most likely mechanism of anti-selectin and anti-integrin inhibition of intrinsic opioid antinociception is impaired migration of opioid-containing leukocytes. We found a strong upregulation of vascular P- and E-selectins in inflamed tissue. The relatively low expression of L-selectin on opioid-containing leukocytes is most likely due to its shedding required for leukocyte extravasation (Bennett et al., 1996). Although we could not perform co-staining of α4 with opioids, it is reasonable to assume that this integrin is coexpressed by opioid-containing CD45+ cells in paws, because the majority of circulating granulocytes and of CD45+ cells in paws expressed α4 (Figure 1a and d). Furthermore, the vast majority of opioid-producing immunocytes coexpressed β2 integrins. Importantly, fucoidin and anti-α4 combined with anti-β2 substantially decreased the migration of opioid-containing leukocytes to the injured tissue. Consistently, both treatments reduced the immigration of granulocytes and monocytes/macrophages. The number of T cells was reduced by fucoidin but not by anti-α4 combined with anti-β2, which could be due to lower baseline counts of T cells in anti-integrin- compared with fucoidin-treated groups (Figure 4a and c). Blockade of leukocyte extravasation can result in no change or elevated cell numbers in the blood (Teixeira & Hellewell, 1997; Machelska et al., 2002). Consistently, combined treatment with anti-α4 and anti-β2 increased circulating leukocyte counts. Fucoidin produced either no change (granulocytes and monocytes/macrophages) or a drop (T cells) in the number of circulating leukocytes. This was also observed by others (Kuebler et al., 1997; Teixeira & Hellewell, 1997) and has been related to anaphylactoid reactions to sulfated polysaccharides such as fucoidin (Kuebler et al., 1997). Notwithstanding, our functional together with morphological findings indicate that L-and P-selectins and the integrins α4 and β2 permit the extravasation of opioid-producing leukocytes to promote intrinsic inhibition of inflammatory pain.
We were unable to determine the relative importance of L- vs P-selectin because both are targeted by fucoidin (Bevilacqua & Nelson, 1993). Also, although we observed an upregulation of E-selectin, its functional contribution to intrinsic opioid antinociception awaits further evaluation because selective blockers are not commercially available at present. On the other hand, we found an upregulation of PECAM-1, but a wide dose range of anti-PECAM-1 did not influence antinociception, indicating no involvement of PECAM-1 in this effect. It is conceivable that concomitant blockade of PECAM-1 with other adhesion molecules might be more effective. However, it seems that the simultaneous blockade of several adhesion molecules is not absolutely necessary to interfere with the generation of antinociception because we have previously shown that the individual blockade of another immunoglobulin-like molecule (ICAM-1) nearly abolished stress-induced antinociception (Machelska et al., 2002). In view of this finding, it is interesting to observe that although ICAM-1 is a ligand of β2 integrins their blockade only slightly decreased stress-induced antinociception. It has been shown that integrins interact with each other, which can result in compensatory up- or downregulation (Porter & Hogg, 1997). Thus, it is conceivable that blocking β2 alters α4-mediated functions (and vice versa) in a sense that α4-mediated cell migration is increased. This would explain why we observed no significant changes in stress-induced antinociception following the blockade of either α4 or β2 alone. This is in line with the higher effectiveness of concomitant integrin blockade on leukocyte migration to inflamed joints (Issekutz & Issekutz, 1995).
Effects of anti-adhesion treatments on stress-induced antinociception are apparently independent of their effect on macroscopic inflammation. Both fucoidin and anti-α4 combined with anti-β2 reduced antinociception to similar magnitudes while they decreased paw edema to different degrees and did not change paw temperature. In contrast, the latter parameter was decreased by anti-β2 alone, which had only a minimal effect on stress-induced antinociception and no effect on paw edema. Interestingly, even though blockade of selectins and integrins diminished leukocyte migration and edema of the inflamed paws, hyperalgesia (basal nociceptive thresholds) was not alleviated. It is conceivable that this adhesion blockade does not have a major impact on cells containing hyperalgesic cytokines or hyperalgesic neuropeptides (e.g. substance P, calcitonin gene-related peptide) (Machelska et al., 2001; Cunha & Ferreira, 2003). Furthermore, although this adhesion blockade diminished the immigration of opioid-containing cells, hyperalgesia was not exacerbated. This may be due to a lack of sensitivity of our paw pressure assay. On the other hand, it is likely that the endogenous opioid system is not essential for the tonic control of basal nociceptive thresholds but is mainly recruited under challenging situations. This would be consistent with other studies (Kieffer & Gaveriaux-Ruff, 2002) and with the fact that immune cells need to be stimulated (e.g. by stress or corticotropin-releasing hormone) to release opioids and produce antinociception (Stein et al., 1990a, 1993; Schäfer et al., 1996; Machelska et al., 2003). Our data also suggest that anti-selectin and anti-integrin treatments have no major influence on other immune cell-derived analgesics (e.g. acetylcholine or analgesic cytokines) (Machelska et al., 2001; Cunha & Ferreira, 2003). Thus, it seems that in our model selectins and integrins play an important role in the recruitment of opioid-containing cells to provide endogenous antinociception against inflammatory pain.
In conclusion, our studies indicate that blockade of L- and P-selectins and of α4 and β2 integrins, but not of PECAM-1, can harm intrinsic pain inhibition by way of an impaired migration of opioid-containing leukocytes to the inflamed tissue. Apparently, selectins and integrins play a significant role since peripheral endogenous antinociception is abolished already after single injections of fucoidin or anti-α4 and anti-β2. Although our present studies do not allow conclusions as to the long-term effects of such treatments, these adhesive mechanisms might be of clinical relevance because immune-derived opioids can reduce pain in humans (Stein et al., 1993). Selectins, α4 and β2 integrins as well as PECAM-1 have been proposed as therapeutic targets for novel anti-inflammatory drugs (Bogen et al., 1994; Issekutz AC et al., 1996; Issekutz et al., 2001). Our present data point to a cautious use of anti-selectin, anti-α4 and anti-β2 strategies because they may seriously impair intrinsic inhibition of inflammatory pain. Finally, the recognition that certain adhesion molecules are critical for the recruitment of ‘pain control cells' opens up new ways to approach pathological pain by specific promotion of these adhesive interactions.
Acknowledgments
We thank Prof. A. Hamann (Experimentelle Rheumatologie, Medizinische Klinik, Charité-Universitätsmedizin Berlin, Campus Mitte, Berlin, Germany) for stimulating discussion and helpful suggestions on this manuscript. This study was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 507/B8 and Klinische Forschergruppe 100/1) and by the International Anesthesia Research Society.
Abbreviations
- ICAM-1
intercellular adhesion molecule-1
- i.pl.
intraplantar
- mAb
monoclonal antibody
- PECAM-1
platelet–endothelial cell adhesion molecule-1
- PPT
paw pressure threshold
References
- BENNETT T.A., LYNAM E.B., SKLAR L.A., ROGELJ S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J. Immunol. 1996;156:3093–3097. [PubMed] [Google Scholar]
- BEVILACQUA M.P., NELSON R.M. Selectins. J. Clin. Invest. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGEN S., PAK J., GARIFALLOU M., DENG X., MULLER W.A. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J. Exp. Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER E.C., PICKER L. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- CABOT P.J., CARTER L., GAIDDON C., ZHANG Q., SCHÄFER M., LOEFFLER J.P., STEIN C. Immune cell-derived β-endorphin: production, release and control of inflammatory pain in rats. J. Clin. Invest. 1997;100:142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., FERREIRA S.H. Peripheral hyperalgesic cytokines. Adv. Exp. Med. Biol. 2003;521:22–39. [PubMed] [Google Scholar]
- GRAMSCH C., MEO T., RIETHMULLER G., HERZ A. Binding characteristics of a monoclonal beta-endorphin antibody recognizing the N-terminus of opioid peptides. J. Neurochem. 1983;40:1220–1226. doi: 10.1111/j.1471-4159.1983.tb13560.x. [DOI] [PubMed] [Google Scholar]
- HAMANN A., ANDREW D.P., JABLONSKI-WESTRICH D., HOLZMANN B., BUTCHER E.C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- ISSEKUTZ A.C., AYER L., MIYASAKA M., ISSEKUTZ T.B. Treatment of established adjuvant arthritis in rats with monoclonal antibody to CD18 and very late activation antigen-4 integrins suppresses neutrophil and T-lymphocyte migration to the joints and improves clinical disease. Immunology. 1996;88:569–576. doi: 10.1046/j.1365-2567.1996.d01-695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSEKUTZ A.C., ISSEKUTZ T.B. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late activation antigen 4 integrin mechanisms. J. Exp. Med. 1995;181:1197–1203. doi: 10.1084/jem.181.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSEKUTZ A.C., MU J.Y., LIU G., MELROSE J., BERG E.L. E-selectin, but not P-selectin, is required for development of adjuvant-induced arthritis in the rat. Arthritis Rheum. 2001;44:1428–1437. doi: 10.1002/1529-0131(200106)44:6<1428::AID-ART238>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ T.B., MIYASAKA M., ISSEKUTZ A.C. Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J. Exp. Med. 1996;183:2175–2184. doi: 10.1084/jem.183.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON B.A., HAINES G.K., HARLOW L.A., KOCH A.E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993;36:137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- KIEFFER B.L., GAVERIAUX-RUFF C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- KUEBLER W.M., KUHNLE G.E., GROH J., GOETZ A.E. Contribution of selectins to leucocyte sequestration in pulmonary microvessels by intravital microscopy in rabbits. J. Physiol. 1997;501:375–386. doi: 10.1111/j.1469-7793.1997.375bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHELSKA H., CABOT P.J., MOUSA S.A., ZHANG Q., STEIN C. Pain control in inflammation governed by selectins. Nat. Med. 1998;4:1425–1428. doi: 10.1038/4017. [DOI] [PubMed] [Google Scholar]
- MACHELSKA H., MOUSA S.A., BRACK A., SCHOPOHL J.K., RITTNER H.L., SCHÄFER M., STEIN C. Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J. Neurosci. 2002;22:5588–5596. doi: 10.1523/JNEUROSCI.22-13-05588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHELSKA H., MOUSA S.A., STEIN C.Pain and immune function Psychoneuroimmunology 2001San Diego, CA: Academic Press; 111–121.ed. Ader, R., Felten, D. & Cohnen, N. Vol. 2, pp [Google Scholar]
- MACHELSKA H., SCHOPOHL J.K., MOUSA S.A., LABUZ D., SCHÄFER M., STEIN C. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J. Neuroimmunol. 2003;141:30–39. doi: 10.1016/s0165-5728(03)00213-3. [DOI] [PubMed] [Google Scholar]
- MACHELSKA H., STEIN C. Immune mechanisms in pain control. Anesth. Analg. 2002;95:1002–1008. doi: 10.1097/00000539-200210000-00039. [DOI] [PubMed] [Google Scholar]
- MOUSA S.A., MACHELSKA H., SCHÄFER M., STEIN C. Co-expression of β-endorphin with adhesion molecules in a rat model of inflammatory pain. J. Neuroimmunol. 2000;108:160–170. doi: 10.1016/s0165-5728(00)00284-8. [DOI] [PubMed] [Google Scholar]
- MOUSA S.A., ZHANG Q., SITTE N., JI R., STEIN C. Beta-endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissue. J. Neuroimmunol. 2001;115:71–78. doi: 10.1016/s0165-5728(01)00271-5. [DOI] [PubMed] [Google Scholar]
- PORTER J.C., HOGG N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters alpha4beta1- and alpha5beta1-mediated function. J. Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRZEWLOCKI R., HASSAN A.H.S., LASON W., EPPLEN C., HERZ A., STEIN C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- RITTNER H.L., BRACK A., MACHELSKA H., MOUSA S.A., BAUER M., SCHÄFER M., STEIN C. Opioid peptide expressing leukocytes – identification, recruitment and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-MADRID F., NAGY J.A., ROBBINS E., SIMON P., SPRINGER T.A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J. Exp. Med. 1983;158:1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHÄFER M., MOUSA S.A., ZHANG Q., CARTER L., STEIN C. Expression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6096–6100. doi: 10.1073/pnas.93.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAIBLE U.E., VESTWEBER D., BUTCHER E.G., STEHLE T., SIMON M.M. Expression of endothelial cell adhesion molecules in joints and heart during Borrelia burgdorferi infection of mice. Cell Adhes. Commun. 1994;2:465–479. doi: 10.3109/15419069409014211. [DOI] [PubMed] [Google Scholar]
- STEIN C. Mechanisms of disease: the control of pain in peripheral tissue by opioids. N. Engl. J. Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- STEIN C., GRAMSCH C., HERZ A. Intrinsic mechanisms of antinociception in inflammation. Local opioid receptors and beta-endorphin. J. Neurosci. 1990a;10:1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN C., HASSAN A.H.S., LEHRBERGER K., GIEFING J., YASSOURIDIS A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342:321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- STEIN C., HASSAN A.H.S., PRZEWLOCKI R., GRAMSCH C., PETER K., HERZ A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1990b;87:5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN C., PFLUGER M., YASSOURIDIS A., HOELZL J., LEHRBERGER K., WELTE C., HASSAN A.H.S. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J. Clin. Invest. 1996;98:793–799. doi: 10.1172/JCI118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN C., SCHAFER M., MACHELSKA H. Attacking pain at its source: new perspectives on opioids. Nat. Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- TAMATANI T., KOTANI M., MIYASAKA M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur. J. Immunol. 1991;21:627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN BERG J.M., MUL F.P., SCHIPPERS E., WEENING J.J., ROOS D., KUIJPERS T.W. Beta1 integrin activation on human neutrophils promotes beta2 integrin-mediated adhesion to fibronectin. Eur. J. Immunol. 2001;31:276–284. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- VERDIER M., JAYAT C., RATINAUD M.H., TROUTAUD D. Optimization of cell permeabilization for multiparametric flow cytometric analysis with lectin staining. Cytometry. 2000;41:55–61. doi: 10.1002/1097-0320(20000901)41:1<55::aid-cyto8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- VON ANDRIAN U.H., MACKAY C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- WILLIAMS K.C., ZHAO R.W., UENO K., HICKEY W.F. PECAM-1 (CD31) expression in the central nervous system and its role in experimental allergic encephalomyelitis in the rat. J. Neurosci. Res. 1996;45:747–757. doi: 10.1002/(SICI)1097-4547(19960915)45:6<747::AID-JNR11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]