Abstract
Our study was undertaken to investigate whether bacterial endotoxin/lipopolysaccharide (LPS) affects the neurogenic vasopressor response in rats in vivo by presynaptic mechanisms and, if so, to characterize the type of presynaptic receptor(s) operating in the initial phase of septic shock.
In pithed and vagotomized rats treated with pancuronium, electrical stimulation (ES) (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers or intravenous bolus injection of noradrenaline (NA) (1–3 nmol kg−1) increased the diastolic blood pressure (DBP) by about 30 mmHg. Administration of LPS (0.4 and 4 mg kg−1) under continuous infusion of vasopressin inhibited the neurogenic vasopressor response by 25 and 50%, respectively. LPS did not affect the increase in DBP induced by exogenous NA.
The LPS-induced inhibition of the neurogenic vasopressor response was counteracted by the cannabinoid CB1 receptor antagonist SR 141716A (0.1 μmol kg−1), but not by the CB2 receptor antagonist SR 144528 (3 μmol kg−1), the vanilloid VR1 receptor antagonist capsazepine (1 μmol kg−1) or the histamine H3 receptor antagonist clobenpropit (0.1 μmol kg−1). The four antagonists by themselves did not affect the increase in DBP induced by ES or by injection of NA in rats not exposed to LPS.
We conclude that in the initial phase of septic shock, the activation of presynaptic CB1 receptors by endogenously formed cannabinoids contributes to the inhibition of the neurogenic vasopressor response.
Keywords: Presynaptic inhibition, septic shock, LPS, lipopolysaccharide, cannabinoid CB1 receptor, cannabinoid CB2 receptor, SR 141716A, vanilloid VR1 receptor, histamine H3 receptor, pithed rat
Introduction
Sepsis is the most frequent cause of vasodilatory shock. The pathogenic cascade involves the activation by bacterial endotoxin/lipopolysaccharide (LPS) of intracellular signalling pathways in monocytes and macrophages, the release of inflammatory mediators that promote the dysfunction of endothelium and the impairment of myocardial contractility and may, eventually, lead to cardiovascular collapse (for a review, see, Guha & Mackman, 2001; Landry & Oliver, 2001; Cohen, 2002).
In early stages of septic shock, various inflammatory mediators, for example, histamine, prostaglandins, kinins, nitric oxide and cytokines, are produced that cause the acute vasodilation in animals (Vincent, 1998). Recently, endogenous cannabinoids have also been described to contribute to the development of hypotension in the early stages of shock. Thus, anandamide and 2-arachidonoylglycerol are generated by platelets and macrophages during septic (Varga et al., 1998; Wagner et al., 1998), hemorrhagic (Wagner et al., 1997) and cardiogenic shock (Lagneux & Lamontagne, 2001; Wagner et al., 2001) and cause hypotension through the activation of cannabinoid CB1 receptors, which may be located on the endothelium or on the vascular smooth muscle. Additionally, Orliac et al. (2003) demonstrated that anandamide potentiated the vasorelaxation in the isolated mesenteric bed from endotoxemic rats in a manner sensitive to capsazepine, suggesting that vanilloid VR1 receptors at least partially contribute to the hypotension observed in septic shock.
The possibility has also to be considered that presynaptic receptors located on the sympathetic neurones may be involved in the vascular effects of LPS. The sympathetic axon terminals innervating the resistance vessels are endowed with numerous presynaptic receptors (for a review, see, Boehm & Kubista, 2002), but little information is so far available as to which of them are activated during sepsis. Takakura et al. (1994) reported that the neurogenic contraction of isolated endothelium-denuded rat tail artery strips treated with LPS was depressed by L-arginine, suggesting that endogenously formed nitric oxide, via a presynaptic mode of action, may be associated with the reduction of sympathetic tone. Furthermore, in rat mesenteric arteries the overproduction of both nitric oxide and prostanoids by endothelium was shown to reduce the electrically evoked contraction after administration of LPS (Fatehi-Hassanabad et al., 1995). Moreover, Li et al. (1998) and Cheng et al. (2002) demonstrated that the activation of presynaptic histamine H3 receptors during sepsis resulted in the depression of left ventricular contractility in canine heart, hence contributing to the cardiovascular collapse.
The present study was undertaken to examine whether administration of LPS to pithed rats alters the neurogenic vasopressor response. Since LPS indeed decreased the neurogenic vasopressor response, we next studied whether this effect can be overcome by selective antagonists at cannabinoid CB1 and CB2 receptors and at vanilloid VR1 receptors. In addition, we examined the influence of an antagonist at the presynaptic histamine H3 receptor, which, like the CB1 receptor (Ma-linowska et al., 1997; Niederhoffer & Szabo, 1999; Niederhoffer et al., 2003), inhibits the neurogenic vasopressor response in pithed animals (Malinowska & Schlicker, 1991).
Methods
General procedure
Male Wistar rats weighing 170–280 g were anesthetized by intraperitoneal (i.p.) injection of pentobarbitone sodium (300 μmol kg−1). After cannulation of the trachea the animals were pithed and artificially ventilated with air (10 ml kg−1; 60 strokes min−1) using the respiratory system Ugo Basile (Hugo Sachs Elektronik, March-Hugstetten, Germany). Both vagus nerves were cut in their cervical segment. Diastolic blood pressure (DBP) was measured from the right carotid artery via the pressure transducer DTX (Spectramed, Bromma, Sweden). Heart rate was recorded from the ECG by means of subcutaneous electrodes. Body temperature was maintained constant at approximately 36–37°C using a heating pad (Bio-Sys-Tech, Białystok, Poland) and monitored by a rectal probe transducer. The transducers were connected to the monitor Trendscope 8031 (AxMediTec, Białystok, Poland). The left femoral vein was cannulated for intravenous (i.v.) administration of drugs. All substances (with the exception of pentobarbitone, see above) were given via this route in a volume 0.5 ml kg−1. The right femoral vein was prepared for infusion of vasopressin by means of a Graseby 3100 syringe pump (Graseby Medical, Watford, Herts, U.K.). After 15–30 min of equilibration, during which the cardiovascular parameters were allowed to stabilize, experiments were performed.
Experimental protocol
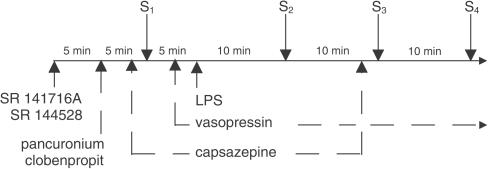
The experimental protocol is shown in Figure 1. Each animal received an injection of pancuronium (0.8 μmol kg−1) to avoid twitches associated with electrical stimulation (ES). Pancu-ronium was also administered to those rats in which an increase in blood pressure was induced by the injection of noradrenaline (NA) (see below) to obtain identical experimental conditions. After 5 min, the first stimulus (S1) was applied. An increase in blood pressure was induced by bolus injection of NA (1–3 nmol kg−1) or by ES (1 Hz, 1 ms, 50 V for 10 s) of preganglionic sympathetic nerve fibers (electrical field generated between the pithing rod in the vertebral column and an indifferent electrode placed ventrally) delivered from Stimulator T (Hugo Sachs Elektronik, March-Hugstetten, Germany). The dose of NA was adjusted in each experiment to obtain an initial increase in DBP of comparable magnitude to that induced by ES. A single dose of LPS (0.4 or 4 mg kg−1) or an equivalent volume of its vehicle (saline) was slowly injected (i.e. over a time period of 1 min) 5 min after S1. In order to prevent the LPS-mediated fall in basal DBP, an infusion of vasopressin was started 2 min earlier at a rate of 0.04–0.4 IU kg−1 h−1. The rate of vasopressin infusion was increased to 0.6–6 IU kg−1 h−1 immediately after the administration of LPS (4 mg kg−1). Two or three subsequent stimuli (S2, S3 and S4) were administered at 10 min intervals following the injection of LPS or its vehicle. The effect of LPS or its vehicle on the neurogenic rise in basal DBP was also examined in the presence of the histamine H3 receptor antagonist clobenpropit (0.1 μmol kg−1), the cannabinoid CB1 receptor antagonist SR 141716A (0.03 or 0.1 μmol kg−1) or the cannabinoid CB2 receptor antagonist SR 144528 (3 μmol kg−1), which were administered as indicated in Figure 1. Since the effect of the vanilloid VR1 receptor antagonist capsazepine (1 μmol kg−1) was short-lived, the drug was given twice, that is, 2 min before S1 and again 2 min before S3. A different time schedule was used for those experiments in which the influence of antagonists on the NA-induced increase in blood pressure was examined (in the absence of LPS). NA was injected twice (S1 and S2); a single dose of antagonist under study or its vehicle (control groups) was administered i.v. 5 min after S1. The S2 was induced 10 min after the administration of SR 141716A (0.1 μmol kg−1) or SR 144528 (3 μmol kg−1), 5 min after clobenpropit (0.1 μmol kg−1) or 2 min after capsazepine (1 μmol kg−1).
Figure 1.
Experimental protocol used to examine the influence of LPS on the electrically- or noradrenaline-induced increase in DBP in pithed and vagotomized rats. Each animal received an injection of pancuronium (0.8 μmol kg−1). After 5 min, the first stimulus (S1) was applied. DBP was increased by bolus injection of noradrenaline (1–3 nmol kg−1) or by ES (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers. A single dose of LPS (0.4 or 4 mg kg−1) or vehicle was slowly injected (over a time period of 1 min) 5 min after S1. An infusion of vasopressin started 2 min before the administration of LPS. Stimulations (S2–S4) were repeated at intervals of 10 min following the administration of LPS or its vehicle. The effects of LPS or its vehicle were also examined after administration of clobenpropit (0.1 μmol kg−1, given along with pancuronium), SR 141716A (0.03 or 0.1 μmol kg−1) or SR 144528 (3 μmol kg−1; each compound was given 5 min prior to pancuronium). Due to its short-lived effects, capsazepine (1 μmol kg−1) was injected 2 min prior to S1 and S3.
All experiments were approved by the Local Animal Ethics Committee in Białystok (Poland).
Calculations and statistics
Results are given as means±s.e.m. (n=number of animals). To quantify the effect of LPS on the electrically or NA-induced increase in DBP, the ratios S2/S1, S3/S1 and S4/S1 were determined. These ratios were expressed as percentages of the corresponding ratios obtained from the vehicle-treated animals. To define the receptor(s) involved in the inhibitory action of LPS, similar ratios were determined in the presence of the respective receptor antagonists. For comparison of mean values, Student's t-test for paired or unpaired data was used. When two or more treatment groups were compared to the same control, the one-way analysis of variance (ANOVA) followed by Dunnett's test was used. Differences were considered significant when P<0.05.
Drugs
Capsazepine, clobenpropit dihydrobromide (Tocris Cookson Ltd, Bristol, U.K.); N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride (SR 141716A), N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR 144528) (Sanofi Recherche, Montpellier, France); (−)-NA bitartrate, pancuronium dibromide, [Lys8]-vasopressin, LPS from Escherichia coli serotype 0127:B8 (LPS) (Sigma, München, Germany); pentobarbitone sodium (pentobarbital) (Biowet, Puławy, Poland). Pancuronium, clobenpropit and LPS were dissolved in isotonic saline. Vasopressin was provided by the manufacturer as an aqueous solution (18.2 IU ml−1), which was diluted (1 : 13.5) in isotonic saline before the experiment. Noradrenaline was dissolved in saline containing ascorbic acid 6 mmol l−1. A stock solution of SR 141716A was prepared in a mixture of ethanol and Cremophor El (1 : 1) and further diluted (1 : 50) in isotonic saline immediately before the experiment. A stock solution of SR 144528 was prepared in a mixture of DMSO, ethanol and Cremophor El (2 : 1 : 1) and further diluted (1 : 10) in isotonic saline immediately before the experiment. Capsazepine was first dissolved in DMSO and further diluted (1 : 10) in isotonic saline containing 10% Tween 80 and 10% ethanol. Isotonic saline and the solvents for SR 141716A and SR 144528 did not affect basal DBP and the heart rate (HR).
Results
General
In pithed and vagotomized rats treated with pancuronium (0.8 μmol kg−1) basal DBP and HR measured immediately before S1 amounted to 48±1 mmHg and 336±3 beats min−1, respectively (n=97). Capsazepine (1 μmol kg−1) given i.v. 2 min before S1 and S3 caused a transient increase in DBP by about 5 mmHg (its solvent increased DBP by about 2–3 mmHg), but did not affect HR. However, in the presence of capsazepine, no statistical differences were observed in DBP between vehicle- and LPS-treated rats. The other antagonists under study did not affect basal DBP and HR by themselves.
In animals not treated with any receptor antagonist, the ES (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers or injection of NA (1–3 nmol kg−1) increased DBP during S1 by about 30 mmHg (Table 1). The degree of the vasopressor response did not change upon repeated ES or addition of NA (S2–S4); in other words, the ratios S2/S1, S3/S1, S4/S1 were close to unity (Table 1). The cannabinoid CB1 receptor antagonist SR 141716A (0.03 and 0.1 μmol kg−1), the cannabinoid CB2 receptor antagonist SR 144528 (3 μmol kg−1), the vanilloid VR1 receptor antagonist capsazepine (1 μmol kg−1) and the histamine H3 receptor antagonist clobenpropit (0.1 μmol kg−1) did not affect the neurogenic vasopressor response by themselves (see S1 values in Table 1).
Table 1.
Control values for changes in diastolic blood pressure induced electrically or chemically in pithed and vagotomized rats pretreated with pancuronium (0.8 μmol kg−1; i.v.)
| Changes in DBP | |||||
|---|---|---|---|---|---|
| Type of stimulation | Antagonist | Induced by S1 (mmHg) | S2/S1 | S3/S1 | S4/S1 |
| Electrical | No | 28±2 | 0.94±0.06 | 1.14±0.05 | 1.10±0.07 |
| SR 141716A 0.1 | 29±2 | 0.94±0.05 | 1.09±0.07 | 1.10±0.10 | |
| SR 144528 3 | 29±2 | 1.03±0.03 | 1.11±0.13 | 1.08±0.12 | |
| Capsazepine 1 | 33±4 | — | 0.94±0.04 | — | |
| Clobenpropit 0.1 | 29±2 | 1.08±0.03 | 1.09±0.06 | 1.10±0.08 | |
| Noradrenaline | No | 30±2 | 1.05±0.04 | 1.07±0.07 | 1.01±0.10 |
Up to four stimuli (S1–S4) were administered to pithed rats. An increase in blood pressure was induced by electrical stimulation (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers or by bolus injection of noradrenaline (1–3 nmol kg−1, i.v.). SR 141716A or SR 144528 was given 10 min, and pancuronium was administered alone or with clobenpropit 5 min, prior to S1. Capsazepine was injected i.v. 2 min prior to S1 and S3. Doses of the receptor antagonists are given in μmol kg−1. The alterations in DBP are given either as absolute values (for S1) or as ratios (relative to S1) for the three subsequent stimuli (S2, S3 and S4). Means±s.e.m. of 4–21 rats.
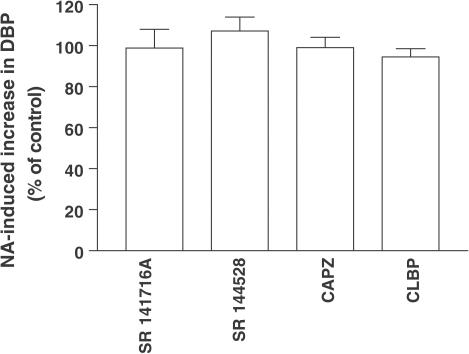
In experiments, in which the influence of antagonists on the NA-induced increase in blood pressure was examined, the corresponding ratios S2/S1 were close to unity (data not shown). As shown in Figure 2, SR 141716A (0.1 μmol kg−1), SR 144528 (3 μmol kg−1), capsazepine (1 μmol kg−1) and clobenpropit (0.1 μmol kg−1) did not affect this parameter.
Figure 2.
Influence of four antagonists on the increase in basal DBP induced by the injection of NA (1–3 nmol kg−1) in pithed and vagotomized rats pretreated with pancuronium (0.8 μmol kg−1). An increase in DBP was induced twice (S1 and S2). Antagonists or their vehicles (control groups) were injected 5 min after S1. The S2 was induced 10 min after the administration of SR 141716A (0.1 μmol kg−1) or SR 144528 (3 μmol kg−1), 5 min after clobenpropit (0.1 μmol kg−1) or 2 min after capsazepine (1 μmol kg−1). The ratios S2/S1 of antagonist-treated animals are expressed as percentages of the corresponding ratios obtained in animals treated with the respective vehicles (% of control). Means±s.e.m. of 3–4 rats.
Influence of LPS on basal DBP
I.v. administration of LPS (0.4 and particularly 4 mg kg−1) produced a profound hypotension within 3 min, which persisted throughout the experiment or even caused death of some of the pithed and vagotomized rats (not shown). To overcome a cardiovascular collapse, vasopressin was infused at an initial dose of 0.04–0.4 IU kg−1 h−1, which elevated basal DBP to about 80 mmHg. When LPS (4 mg kg−1) was administered, the dose of vasopressin was increased to 0.6–6 IU kg−1 h−1. In the presence of vasopressin, the DBP decreased to 45–50 mmHg within 10 min and remained constant throughout the course of experiment, so that DBP measured immediately before S2, S3 and S4 did not differ from the vehicle-treated animals and from the baseline value recorded before LPS injection (see also, the original traces in Figure 3).
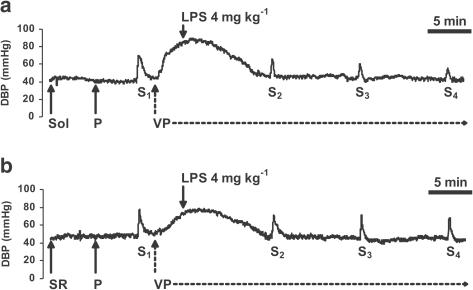
Figure 3.
Traces from representative experiments showing the influence of LPS on the increase in basal DBP induced by ES (S1–S4: 1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers in pithed and vagotomized rats. Experiments were performed in the presence of the cannabinoid CB1 receptor antagonist SR 141716A (SR; 0.1 μmol kg−1; b) or its solvent (Sol; a), injected (solid arrows) 10 min prior to the first period of ES S1 and in the presence of pancuronium (P; 0.8 μmol kg−1), injected (solid arrows) 5 min prior to S1. At 5 min after S1, a single dose of LPS was injected (solid arrows). An infusion of vasopressin (VP; hatched arrows) started 2 min earlier. Three subsequent periods of ES (S2, S3 and S4) were applied at 10 min intervals. For details concerning the experimental protocol, see Figure 1.
Influence of LPS on the electrically and NA-stimulated increase in blood pressure and interaction with receptor ligands
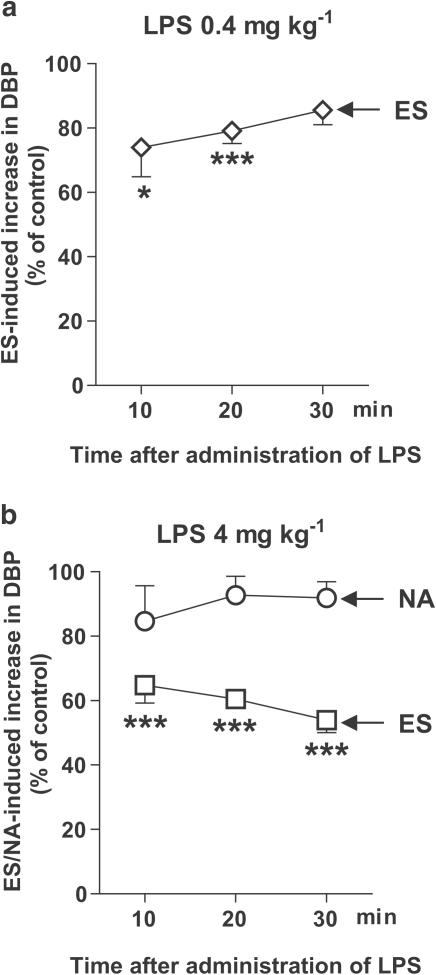
As shown in Figure 4, LPS (0.4 and 4 mg kg−1) attenuated the electrically evoked rise in DBP in pithed rats pretreated with pancuronium and infused with vasopressin. The inhibitory effect of LPS (0.4 mg kg−1) was most pronounced 10 min after its administration (Figure 4a). Higher dose of LPS (4 mg kg−1) produced stronger inhibition, which progressed with time, being more prominent at 30 min (inhibition by about 50%; Figure 4b; see also, the original trace in Figure 3a). By contrast, LPS (4 mg kg−1) did not affect the rise in DBP stimulated by the bolus injection of NA (Figure 4b).
Figure 4.
Influence of LPS on the increase in basal DBP induced by ES (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers or by injection of NA (1–3 nmol kg−1) in pithed and vagotomized rats pretreated with pancuronium (0.8 μmol kg−1). For the experimental protocol, see Figure 1. The ratios S2/S1, S3/S1 and S4/S1 for the increase in DBP in LPS-treated animals were expressed as percentages of the corresponding ratios obtained in vehicle-treated animals (% of control). Means±s.e.m. of 4–9 rats. *P<0.05, ***P<0.001 compared to the corresponding control.
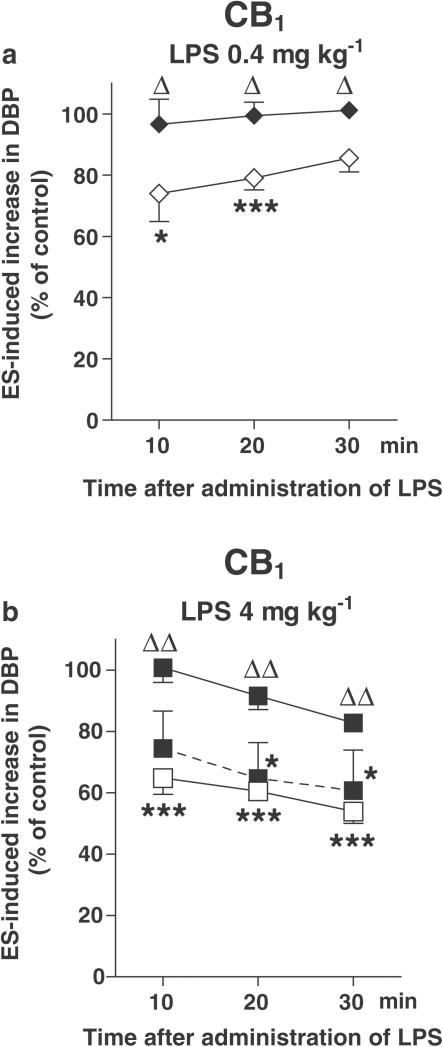
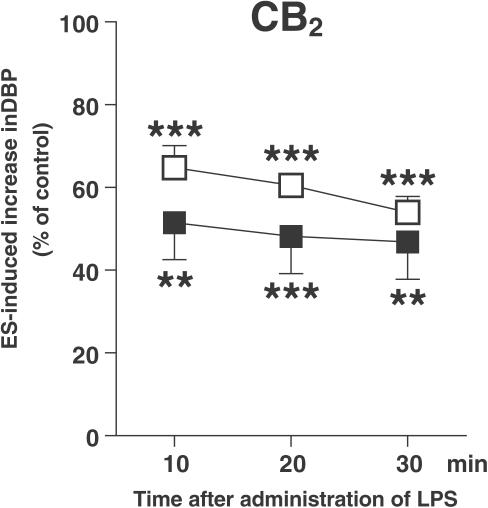
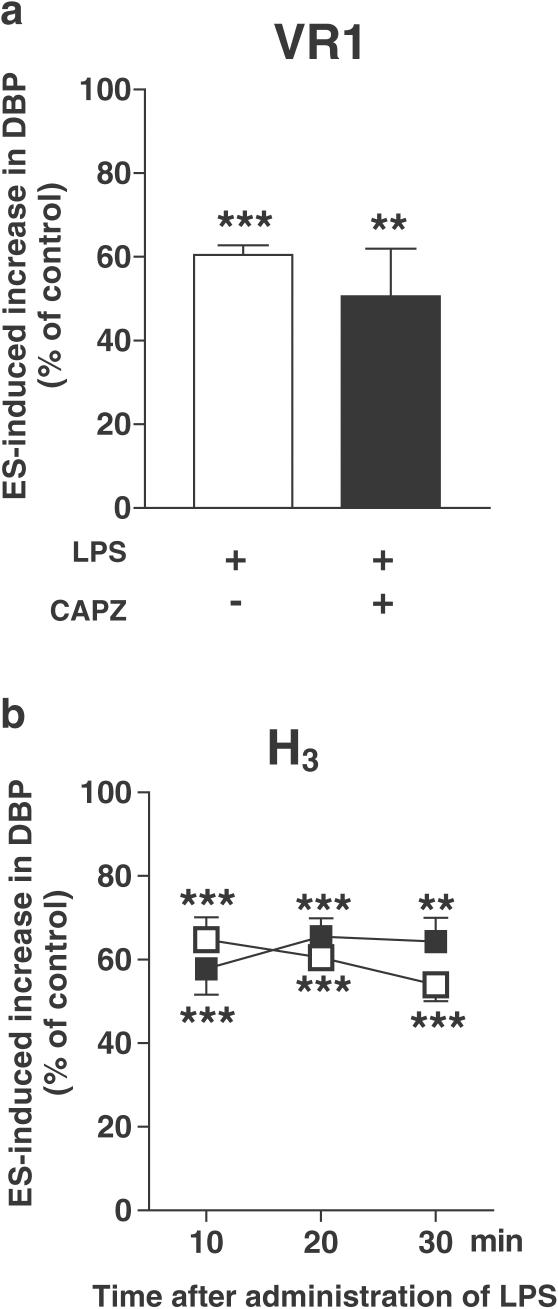
The inhibition of the neurogenic vasopressor response was abolished by the cannabinoid CB1 receptor antagonist SR 141716A (0.1 μmol kg−1) 10, 20 and 30 min after the administration of either dose of LPS (Figures 5a and b; see also, the original trace in Figure 3b). SR 141716A, given at a lower dose (0.03 μmol kg−1), only tended to diminish the response to LPS (4 mg kg−1; Figure 5b). On the other hand, the action of LPS (4 mg kg−1) was not affected by the cannabinoid CB2 receptor antagonist SR 144528 (3 μmol kg−1; Figure 6), the vanilloid VR1 receptor antagonist capsazepine (1 μmol kg−1; Figure 7a) and the histamine H3 receptor antagonist clobenpropit (0.1 μmol kg−1; Figure 7b).
Figure 5.
Influence of the cannabinoid CB1 receptor antagonist SR 141716A on the inhibition of the electrically induced increase in basal DBP by LPS in pithed and vagotomized rats. The preganglionic sympathetic nerve fibers were subjected to four periods (S1–S4) of ES (1 Hz, 1 ms, 50 V for 10 s). SR 141716A 0.03 μmol kg−1 (filled symbols, dashed line) or 0.1 μmol kg−1 (filled symbols, solid line) was administered 10 min before S1. For further details of the experimental protocol, see Figure 1. Both for animals exposed and not exposed (open symbols) to the antagonist, the ratios S2/S1, S3/S1 and S4/S1 for the increase in DBP obtained in the presence of LPS were expressed as percentages of the corresponding ratios obtained in animals treated with the vehicle for LPS (control). Means±s.e.m. of 4–9 rats. *P<0.05, ***P<0.001 compared to the corresponding control. ΔP<0.05, ΔΔP<0.001 compared to the group not receiving SR 141716A.
Figure 6.
Influence of the cannabinoid CB2 receptor antagonist SR 144528 on the inhibition of the electrically induced increase in basal DBP by LPS (4 mg kg−1) in pithed and vagotomized rats. The preganglionic sympathetic nerve fibers were subjected to four periods (S1–S4) of ES (1 Hz, 1 ms, 50 V for 10 s). SR 144528 3 μmol kg−1 (filled squares) was administered 10 min before S1. For further details of the experimental protocol, see Figure 1. Both for animals exposed and not exposed (open squares) to the antagonist, the ratios S2/S1, S3/S1 and S4/S1 for the increase in DBP obtained in the presence of LPS were expressed as percentages of the corresponding ratios obtained in animals treated with the vehicle for LPS (control). Means±s.e.m. of 4–7 rats. **P<0.01, ***P<0.001 compared to the corresponding control.
Figure 7.
Influence of the vanilloid VR1 receptor antagonist capsazepine (a) and the histamine H3 receptor antagonist clobenpropit (b) on the inhibition of the electrically induced increase in basal DBP by LPS (4 mg kg−1) in pithed and vagotomized rats. The preganglionic sympathetic nerve fibers were subjected to up to four periods (S1–S4) of ES (1 Hz, 1 ms, 50 V for 10 s). Capsazepine 1 μmol kg−1 (CAPZ, filled bar) was administered 2 min before S1 and 2 min before S3; clobenpropit 0.1 μmol kg−1 (filled squares) was injected 5 min before S1. For further details of the experimental protocol, see Figure 1. Both for animals exposed and not exposed (open bar, open squares) to the antagonists, the ratios S2/S1, S3/S1 and S4/S1 for the increase in DBP obtained in the presence of LPS were expressed as percentages of the corresponding ratios obtained in animals treated with the vehicle for LPS (control). With respect to capsazepine, only the S3/S1 ratio was determined. Means±s.e.m. of 4–8 rats. **P<0.01, ***P<0.001 compared to the corresponding control.
Discussion
The aim of the present study was to examine (i) whether endotoxin affects the neurogenic vasopressor response in the pithed rat model and (ii) to characterize the type of presynaptic receptor(s) operating in the initial phase of septic shock.
In the pithed and vagotomized rat, ES (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibers increases DBP. Previous studies from our laboratory showed that the electrically induced increase in basal DBP is solely or predominantly associated with the release of catecholamines (Schlicker et al., 1989), which originate from neuronal endings supplying the resistance vessels but not from the adrenal medulla (Malinowska et al., 1997), and is not related to an increase in cardiac output (Malinowska et al., 1997). LPS inhibited the electrically evoked increase in DBP, but not the response to exogenous NA. Such a pattern of responses clearly indicates the involvement of presynaptic mechanisms in the action of LPS. The fact that LPS did not affect the vasopressor response to exogenously added NA is of interest also for another reason. Thus, LPS inhibited the vasopressor response to other vasoconstrictors, that is, vasopressin and angiotensin II, although this was found in the conscious rat and at a later stage of septic shock (i.e. 2 h after onset) (Tarpey et al., 1998).
The profound decrease in basal DBP caused by LPS (0.4 and 4 mg kg−1) was compensated for by the infusion of vasopressin. The range of doses of vasopressin (0.6–6 IU kg−1 h−1) used in our study was comparable to that applied by Varga et al. (1998) and by Jones et al. (1994) in anesthetized and pithed rats, respectively. Compensation was carried out since the degree of the electrically induced vasopressor response crucially depends on the basal level of the DBP and since the extent of the vasopressor response should be comparable to that in our previous studies, in which presynaptic H3 and CB1 receptors were identified (Malinowska & Schlicker, 1991; Malinowska et al., 1997). Vasopressin was administered, however, also for a second reason. Thus, in the early phase of septic shock a reflex increase in vasopressin is found (Tarpey et al., 1998; Landry & Oliver, 2001; Giusti-Paiva et al., 2002), which, however, cannot occur in pithed animals since reflex loops involving the brain are destroyed.
The possibility that vasopressin acted via a presynaptic site had to be considered. Streefkerk et al. (2002) have demonstrated that the peptide facilitates the sympathetic neurogenic transmission in pithed rats through the activation of presynaptic vasopressin V1 receptors. This mechanism does, however, not play a role under the experimental conditions of our study. First, the facilitatory response to vasopressin became apparent only after the blockade by irbesartan of the angiotensin II AT1 receptors and, second, it occurred at stimulation frequencies higher than 1 Hz only.
Since septic shock is associated with the release of a variety of inflammatory mediators (see Introduction) the question arises which of them might modify the neurogenic vasopressor response. The studies by Takakura et al. (1994) and Fatehi-Hassanabad et al. (1995) suggest that overproduction of nitric oxide reduces the neurogenic vasoconstriction in isolated rat tail and mesenteric arteries exposed to LPS. However, under the experimental conditions of the present study, nitric oxide does not appear to play a role since the impairment of the neurogenic vasopressor response by LPS was apparent within minutes, whereas longer time periods (4–6 h) are required to activate the inducible nitric oxide synthase.
Several lines of evidence implicate the involvement of endogenous cannabinoids, acting via cannabinoid CB1 receptors, in the action of LPS in the anesthetized rat (Varga et al., 1998; Wagner et al., 1998; see also Introduction). The involvement of endogenously formed cannabinoids in the effect of LPS is also suggested by the present study, carried out on pithed and vagotomized rats. Thus, the inhibitory influence of LPS on the neurogenic vasopressor response was abolished by the CB1 receptor antagonist SR 141716A. The latter drug was used at a dose of 100 nmol kg−1 previously shown to counteract the inhibitory action of the cannabinoid receptor agonists WIN 55,212-2 and CP-55,940 on the neurogenic tachycardia in pithed rats (Malinowska et al., 2001b). SR 141716A has frequently been shown to cause effects opposite to those of cannabinoid receptor agonists and the possibility that this drug abolished the inhibitory effect of LPS on the neurogenic vasopressor response due to a facilitatory effect on this parameter had to be considered. This possibility can, however, be ruled out since a facilitatory effect of SR 141716A on the neurogenic vasopressor response was neither found in our previous study (Malinowska et al., 1997) nor in the present one. The drug also does not affect the vasopressor response induced by exogenous NA (present study) or vasopressin (Pfitzer & Szabo, 2004). Finally, the possibility had to be considered that the antagonistic effect of SR 141716A crucially depends on the presence of vasopressin. To address this point a 10-fold lower dose of LPS (0.4 vs 4 mg kg−1) was used in one series of experiments. Under these circumstances, a lower dose of vasopressin could be used to maintain basal DBP. Again, SR 141716A counteracted the LPS-induced inhibition of the neurogenic vasopressor response.
Since cytokines are released in response to LPS (for a review, see, Cohen, 2002) and the second cannabinoid receptor, CB2, has a modulatory role on the immune system including the cytokine network (Klein et al., 2001; Howlett et al., 2002), it was tempting to assume that CB2 receptors might contribute to the hypotensive effect of LPS as well. Our study, however, shows that the inhibition of the neurogenic vasopressor response by LPS was not modified by the selective CB2 receptor antagonist SR 144528, used here at a dose that was previously shown to counteract CB2 receptor-related effects in the rat in vivo (Rinaldi-Carmona et al., 1998).
With respect to the location of the CB1 receptors involved in the LPS-induced hypotensive effect, an entirely different conclusion was reached by Varga et al. (1998) and by ourselves. Our data obtained on pithed rats suggest that the CB1 receptors are located presynaptically on the postganglionic (or perhaps preganglionic) sympathetic nerve fibres innervating the resistance vessels. This observation is generally in agreement with our previous study (Malinowska et al., 1997) and with the studies by Niederhoffer et al. (2003) and Niederhoffer & Szabo (1999), demonstrating that synthetic cannabinoids reduce the neurogenic vasopressor response and plasma NA concentration by activating presynaptic, but not postsynaptic, cannabinoid CB1 receptors in pithed rabbits and rats. On the other hand, Varga et al. (1998) proposed that the hypotension (in the anesthetized rat) is independent of autonomic innervation and occurs solely through the activation of vascular CB1 receptors. Their conclusion is based on the observation that the LPS-induced vasodilatation was preserved when the sympathetic tone was removed by phentolamine. The reason for the discrepancy between both studies is unclear but may be related, for example, to differences in the experimental model (pithed vs anesthetized rat), the dose of LPS (4 vs 15 mg kg−1) or the different time course of the hypotension. We think that it is justified on the basis of our findings to postulate that, in addition to vascular, also presynaptic CB1 receptors (located on the sympathetic nerve fibres of the rat resistance vessels) contribute to the LPS-mediated hypotension. This is the more likely since the dose of SR 141716A used in the present study (100 nmol kg−1) was over 60-fold lower than that in the study by Varga et al. (1998).
The endocannabinoid anandamide relaxes isolated rat vessels via vanilloid VR1 receptors (Zygmunt et al., 1999) and this effect was even more pronounced after pretreatment with LPS (Orliac et al., 2003). In our study, we examined whether vanilloid VR1 receptors might also contribute to the LPS-induced inhibition of the neurogenic vasopressor response. However, the effect of LPS was not modified by the vanilloid VR1 receptor antagonist capsazepine at a dose of 1 μmol kg−1, which was sufficient to antagonize the von Bezold-Jarisch reflex induced by anandamide and methanandamide (via VR1 receptors) in anesthetized rats (Malinowska et al., 2001a).
The possible involvement of presynaptic inhibitory histamine H3 heteroceptors in the regulation of sympathetic activity during septic shock was also considered. The selective and potent histamine H3 receptor antagonist clobenpropit (Hill et al., 1997) did not alter the LPS-mediated inhibition of the neurogenic vasopressor response in pithed rats. Such an effect might have been expected on the basis of our previous report showing the occurrence of H3 receptors on the sympathetic innervation of rat resistance vessels, which were tonically activated by endogenous histamine (Godlewski et al., 1997). Moreover, the activation of presynaptic histamine H3 receptors during sepsis resulted in the depression of left ventricular contractility in canine heart (Li et al., 1998) and contributed to the cardiovascular collapse in dogs (Cheng et al., 2002). As the dose of 100 nmol kg−1 of clobenpropit antagonized the effects of the selective H3 receptor agonists R-α-methylhistamine and imetit in pithed rats (Godlewski et al., 1997), the lack of its activity in the present study may mean that histamine H3 receptors are not active in the initial phase of septic shock, at least not of rats. Another explanation might be that the H3 receptor-dependent mechanism occurs in some vascular beds only or may be restricted to the heart (Levi & Smith, 2000; Cheng et al., 2002). This hypothesis is currently undergoing examination in our laboratory.
In conclusion, our results demonstrate that postganglionic and/or preganglionic sympathetic nerve fibers of rat resistance vessels, in addition to the vascular wall, may serve as targets for mediators released in the initial phase of septic shock. They inhibit the electrically evoked increase in DBP in a manner sensitive to the cannabinoid CB1 receptor antagonist SR 141716A but not to the cannabinoid CB2 receptor antagonist SR 144528, the vanilloid VR1 receptor antagonist capsazepine or the histamine H3 receptor antagonist clobenpropit. Additionally, LPS does not change the vasoconstrictory response to NA. Taken together, our data suggest that endocannabinoids which activate presynaptic inhibitory cannabinoid CB1 receptors play an important role in the initial phase of septic shock.
Acknowledgments
This work was supported by the Medical University of Białystok (No. 3-13461, 3-13466) and by the German Research Foundation (Schl 266/5). We are also indebted to the Alexander von Humboldt-Stiftung (Bonn, Germany) for generously providing some of the equipment. We wish to thank Mrs I. Malinowska for her skilled technical assistance and the pharmaceutical company SANOFI Recherche for the gift of SR 141716A and SR 144528.
Abbreviations
- CAPZ
capsazepine
- CLBP
clobenpropit
- DBP
diastolic blood pressure
- ES
electrical stimulation
- HR
heart rate
- LPS
lipopolysaccharide from Escherichia coli serotype 0127:B8
- NA
noradrenaline
- SR, 141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- SR 144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
References
- BOEHM S., KUBISTA H. Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol. Rev. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- CHENG Z.Q., BOSE D., JACOBS H., LIGHT R.B., MINK S.N. Sepsis causes presynaptic histamine H3 and α2-adrenergic dysfunction in canine myocardium. Cardiovasc. Res. 2002;56:225–234. doi: 10.1016/s0008-6363(02)00543-6. [DOI] [PubMed] [Google Scholar]
- COHEN J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- FATEHI-HASSANABAD Z., FURMAN B.L., PARRATT J.R. The effect of endotoxin on sympathetic responses in the rat isolated perfused mesenteric bed; involvement of nitric oxide and cyclo-oxygenase products. Br. J. Pharmacol. 1995;116:3316–3322. doi: 10.1111/j.1476-5381.1995.tb15141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIUSTI-PAIVA A., DE CASTRO M., ANTUNES-RODRIGUES J., CARNIO E.C. Inducible nitric oxide synthase pathway in the central nervous system and vasopressin release during experimental septic shock. Crit. Care Med. 2002;30:1306–1310. doi: 10.1097/00003246-200206000-00025. [DOI] [PubMed] [Google Scholar]
- GODLEWSKI G., MALINOWSKA B., BUCZKO W., SCHLICKER E. Inhibitory H3 receptors on sympathetic nerves of the pithed rat: activation by endogenous histamine and operation in spontaneously hypertensive rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:261–266. doi: 10.1007/pl00004941. [DOI] [PubMed] [Google Scholar]
- GUHA M., MACKMAN N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- JONES S.B., KOTSONIS P., MAJEWSKI H. Endotoxin enhances noradrenaline release in the rat by peripheral mechanisms. Shock. 1994;2:370–375. doi: 10.1097/00024382-199411000-00012. [DOI] [PubMed] [Google Scholar]
- KLEIN T.W., NEWTON C.A., FRIEDMAN H. Cannabinoids and the immune system. Pain Res. Manage. 2001;6:95–101. doi: 10.1155/2001/326867. [DOI] [PubMed] [Google Scholar]
- LAGNEUX C., LAMONTAGNE D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br. J. Pharmacol. 2001;132:793–796. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDRY D.W., OLIVER J.A. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- LEVI R., SMITH N.C.E. Histamine H3-receptors: a new frontier in myocardial ischemia. J. Pharmacol. Exp. Ther. 2000;292:825–830. [PubMed] [Google Scholar]
- LI X., ESCHUN G., BOSE D., JACOBS H., YANG J.J., LIGHT R.B., MINK S.N. Histamine H3 activation depresses cardiac function in experimental sepsis. J. Appl. Physiol. 1998;85:1693–1701. doi: 10.1152/jappl.1998.85.5.1693. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. H3 receptor-mediated inhibition of the neurogenic vasopressor response in pithed rats. Eur. J. Pharmacol. 1991;205:307–310. doi: 10.1016/0014-2999(91)90915-d. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., GODLEWSKI G., BUCHER B., SCHLICKER E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., KWOLEK G., GÖTHERT M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart and blood pressure in anaesthetized rats. Naunyn- Schmiedeberg's Arch. Pharmacol. 2001a;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., PISZCZ J., KONECZNY B., HRYNIEWICZ A., SCHLICKER E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic OP4 and cannabinoid CB1 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001b;264:233–241. doi: 10.1007/s002100100450. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SCHMID K., SZABO B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- ORLIAC M.L., PERONI R., CELUCH S.M., ADLER-GRASCHINSKY E. Potentiation of anandamide effects in mesenteric beds isolated from endotoxemic rats. J. Pharmacol. Exp. Ther. 2003;304:179–184. doi: 10.1124/jpet.102.041095. [DOI] [PubMed] [Google Scholar]
- PFITZER T., SZABO B. Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369 Suppl 1:R25. doi: 10.1007/s00210-004-1003-9. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIÈRE J.C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SCHLICKER E., URBANEK E., GÖTHERT M. ATP, α,β-methylene ATP and suramin as tools for the characterization of vascular P2x receptors in the pithed rat. J. Autonom. Pharmacol. 1989;9:357–366. doi: 10.1111/j.1474-8673.1989.tb00072.x. [DOI] [PubMed] [Google Scholar]
- STREEFKERK J.O., MATHY M.J., PFAFFENDORF M., VAN ZWIETEN P.A. Vasopressin-induced presynaptic facilitation of sympathetic neurotransmission on the pithed rat. J. Hypertens. 2002;20:1175–1180. doi: 10.1097/00004872-200206000-00030. [DOI] [PubMed] [Google Scholar]
- TAKAKURA K., GOTO Y., KIGOSHI S., MURAMATSU I. Selective inhibition of sympathetic nerve-mediated contraction by L-arginine in lipopolysaccharide-treated tail artery in rats. Eur. J. Pharmacol. 1994;271:367–370. doi: 10.1016/0014-2999(94)90795-1. [DOI] [PubMed] [Google Scholar]
- TARPEY S.B., BENNETT T., RANDALL M.D., GARDINER S.M. Differential effects of endotoxemia on pressor and vasoconstrictor actions of angiotensin and arginine vasopressin in conscious rats. Br. J. Pharmacol. 1998;123:1367–1374. doi: 10.1038/sj.bjp.0701751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA K., WAGNER J.A., BRIDGEN D.T., KUNOS G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- VINCENT J.L. Cardiovascular alterations in septic shock. J. Antimicrob. Chemother. 1998;41 Suppl A:9–15. doi: 10.1093/jac/41.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., HU K., BAUERSACHS J., KARCHER J., WIESLER M., GOPARAJU S.K., KUNOS G., ERTL G. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J. Am. Coll. Cardiol. 2001;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., KUNOS G. Cardiovascular actions of cannabinoids and their generation during shock. J. Mol. Med. 1998;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., ELLIS E.F., RZIGALINSKI B.A., MARTIN B.R., KUNOS G. Activation of peripheral CB1 canna-binoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]