Abstract
The ductus venosus is actively regulated in the fetus, but questions remain on the presence of a functional sphincter at its inlet. Using fetal sheep (0.6–0.7 gestation onwards), we have examined the morphology of the vessel and have also determined whether endothelin-1 (ET-1) qualifies as a natural constrictor being modulated by prostaglandins (PGs).
Masson's staining and α-actin immunohistochemistry showed a muscular, sphincter-like formation at the ductus inlet and a muscle layer within the wall of the vessel proper. This muscle cell component increased with age.
ET-1 contracted dose-dependently isolated sphincter and extrasphincter preparations of the ductus from term fetus. This ET-1 effect also occurred in the premature, but its threshold was higher.
BQ123 (1 μM) caused a rightward shift in the ET-1 dose–response curve, while indomethacin at a threshold concentration (28 nM) tended to have an opposite effect.
Big ET-1 also contracted the ductus sphincter but differed from ET-1 for its lesser potency and inhibition by phosphoramidon (50 μM).
The ductus sphincter (term and preterm) and extrasphincter (term) released 6-keto-PGF1α (hence PGI2) and, to a lesser degree, PGE2 at rest and their release increased dose-dependently upon ET-1 treatment. Both basal and stimulated release was curtailed by endothelium removal.
BQ123 and phosphoramidon reduced slightly the contraction of ductus sphincter to indomethacin (2.8 μM).
We conclude that the ductus contains a contractile mechanism in the sphincter and extrasphincter regions. ET-1 lends itself to a role in the generation of contractile tone and its action may be modulated by prostaglandins.
Keywords: Ductus venosus, endothelin, prostaglandin I2, prostaglandin E2, fetal and neonatal physiology
Introduction
The ductus venosus is a fetal shunt that, in connecting the portal sinus with the inferior vena cava, allows a large proportion of well-oxygenated umbilical vein blood to bypass the liver and reach rapidly the central circulation, specifically heart and brain (Edelstone & Rudolph, 1979; Bellotti et al., 2000; Kiserud et al., 2000b). After birth, the ductus ceases its function and undergoes closure, but the timing of this process varies individually and between species so that the vessel may persist for a certain period as a portocaval shunt (Zink & Van Petten, 1980; Momma et al., 1992; Fugelseth et al., 1997). The issue of whether prenatal patency and postnatal closure are actively or passively determined has been debated through the years, the specific point of contention being the functional significance of a tissue ridge at the junction of the ductus with the portal sinus (Coceani, 1993; Kiserud, 1999; Mavrides et al., 2002; Ailamazyan et al., 2003; Tchirikov et al., 2003). According to some investigators, including us, this structure is a true sphincter with autonomous regulation of its muscle (see Coceani, 1993; Kiserud, 1999). We have shown, in particular, that the vessel wall is endowed with a noradrenergic innervation (Coceani et al., 1984) along with relaxing and contractile non-neural mechanisms that are, respectively, prostaglandin (conceivably PGI2)- and cytochrome P450 (CYP450)-linked (Adeagbo et al., 1982, 1985, 1990). In contrast to our position, however, Rudolph and his associates (Paulick et al., 1990; Rudolph et al., 1991) have argued that patency and closure are passive processes merely denoting an adjustment of the vessel wall to the prevailing hemodynamic state. To compound difficulties, there is evidence suggesting that any active regulation is not limited to the inlet region, but may extend to the whole ductus (Momma et al., 1992; Mavrides et al., 2002).
Recent advances in ultrasound imaging have shed light on this problem (Bellotti et al., 1998, 2000; Kiserud, 1999; Kiserud et al., 2000b). While confirming the importance of extrinsic factors – resistance of the hepatic vascular district, magnitude of the umbilicocaval pressure gradient, blood viscosity – for the control of ductal flow (see Kiserud, 1999), this new line of investigation has yielded direct evidence, both in animals and humans, of an active tone regulation in the putative sphincter region as well as in the remainder of the vessel (Bellotti et al., 1998; Kiserud et al., 2000a). Coincidentally, it has demonstrated that ductus closure is delayed in preterm compared to term infants and, moreover, has ascertained that this sign of prematurity may subside after antenatal treatment with corticosteroids (Fugelseth et al., 1998; Loberant et al., 1999; Kondo et al., 2001). The latter observation is consistent with a gestation-linked maturation of local mechanism(s) that are amenable to upregulation.
The present study originates from this premise and from allied research on the ductus arteriosus where a functional complex, linking a CYP450 with endothelin-1 (ET-1), has been implicated in the generation of contractile tension and the postnatal closure of the vessel (Coceani et al., 1999; Coceani, 2000; see also Taniguchi et al., 2001). Within this complex, CYP450 is regarded as the sensing site for stimuli and ET-1 as the effector (Coceani, 2000). Since a CYP450-based contractile mechanism has also been found in the ductus venosus (Adeagbo et al., 1990), we aimed to determine whether ET-1 lends itself to an equivalent effector role. Any such function for ET-1 would accord with the broader role being assigned to this peptide in the perinatal circulation (Perreault & Coceani, 2003). An additional aim was to ascertain whether ET-1, besides exerting a direct constrictor effect, may stimulate the release of a vasorelaxant prostaglandin with modulator properties. The latter occurrence not only would agree with findings in other cell systems (De Nucci et al., 1988; Suzuki et al., 1992), but could also reconcile an effector function of ET-1 with the observed variability in the timing of ductus venosus closure. In the process, we have also examined the morphology of the vessel, both term and preterm, to resolve the questions raised by recent findings reportedly arguing against the existence of a muscle sphincter in the inlet region (Mavrides et al., 2002; Tchirikov et al., 2003).
Methods
General procedure
Experiments were performed on time-dated preterm (two age groups: 119–125 days, 0.85 gestation; 104–108 days, 0.7 gestation) and near-term (133–142 days gestation; term 145 days) pregnant sheep of mixed breed. A single animal at 98 days gestation (0.67 gestation) was also used for the morphological analysis (see below). At all ages, surgical procedure and procedure for isolating the ductus venosus followed a published protocol (Adeagbo et al., 1982). Rings of the ductus (width ∼3 mm), consisting of the inlet region (henceforth named sphincter preparation) or a section outside the sphincter (extrasphincter preparation), were then prepared taking care not to touch the luminal surface with sharp objects or damage the muscle in removing the adhering liver parenchyma. In some sphincter preparations, however, the endothelium was removed by rubbing filter paper (Whatman No. 41) over the intimal surface and its successful removal was confirmed by scanning electron microscopy (Adeagbo et al., 1990). Loss of the endothelium could not be verified by a pharmacological test, because the ductus venosus seemingly lacks an EDRF/NO-based relaxing mechanism (A. Adeagbo, L. Kelsey, F. Coceani, unpublished data). Ductal rings being collected for the morphological analysis (see below) were wider and comprised the sphincter region and a portion of the vessel proper. All preparations were placed in ice-cold Krebs solution gassed with 5% CO2 in N2 before further workup.
Morphological analysis
Freshly dissected specimens of the ductus venosus were obtained from near-term (134 days gestation; n=1) and preterm (104–106 and 98 days gestation; n=3 and 1) fetuses. Preparations were cut open, gently stretched and pinned to a wax board for fixation in buffered formalin and subsequent embedding in paraffin. Serial sections were prepared along the main axis of the vessel, with the direction of the cut being perpendicular to the exposed surface, and were stained with hematoxylin–eosin and Masson trichrome stains. Certain sections from each specimen were also treated with an antiserum against α-actin (dilution, 1 : 600; Amersham, Piscataway, U.S.A.) and a positive reaction was ascertained with the Dako visualization system (Dimension Laboratories, Mississauga, Canada).
Pharmacological analysis
As previously reported (Adeagbo et al., 1982, 1985), ductal rings, intact (sphincter and extrasphincter) or endothelium-denuded (sphincter), were suspended between platinum/iridium hooks in a 20-ml organ bath, and their tension was recorded isometrically via a transducer (Grass FT 0.03C) coupled to a Grass polygraph. A smaller bath (capacity, 2.2 ml) was used when collecting fluid for the measurement of prostaglandins (see below). Both baths were made of glass and had a water jacket to keep the temperature constant at 37°C. The initial load on the preparation was adjusted gradually to 200–300 mg (100 mg weight=0.98 mN) with both preterm and term animals. This value had been extrapolated from the transmural tension in vivo (Adeagbo et al., 1982) and was kept constant regardless of age knowing that intraluminal pressure does not change appreciably during the last third of gestation (Nicolini et al., 1989; Weiner et al., 1989). Each bath was supplied from several reservoirs, and a system of three-way valves allowed the perfusion fluid to be changed rapidly. Both reservoir and organ bath were continuously bubbled with the required gas mixture, and perfusion rate was about 2 ml min−1. A gas mixture of 5% CO2 in N2 was used when mounting the preparation inside the bath, while a mixture of 95% O2 : 5% CO2 was used in the actual experiment to duplicate the neonatal state and, hence, ensure optimal conditions for the postulated constrictor function of ET-1. This oxygen concentration, exceeding the physiological range, was selected bearing in mind the thickness of the ductus, specifically in its sphincter part, and the attendant steep gradient of the gas across the vessel wall. Illumination was kept constant in all, but few, experiments in which the room had been darkened. However, the contractile effect of ET-1 showed no obvious change between light and dark.
The study comprised several protocols. In protocol 1, ET-1 was added to the bath in sequential doses (0.001–100 nM) and its effect was assessed on the basal tone of sphincter preparations, both term and preterm, and on extrasphincter term preparations. Sphincter preparations at term were also studied after their endothelium had been removed and during treatment with BQ123 (1 μM) or indomethacin (28 nM). A relatively low concentration of indomethacin was used with the aim to inhibit enough of the prostaglandin synthesis for the detection of any modulation of the ET-1 action without eliciting the major rise in basal tone typical of a conventional dose (2.8 μM, see Adeagbo et al., 1982). All tests with ET-1 on intact sphincter (term and preterm) and extrasphincter rings as well as on part (three out of nine experiments) of the endothelium-denuded, term sphincter rings were carried out while measuring the prostaglandin release (see protocol 3). An equivalent experiment was performed in protocol 2, with big ET-1 (0.001–100 nM) being tested on intact and endothelium-denuded, term sphincter preparations. Big ET-1 action was also assessed in the presence of phosphoramidon (50 μM), and ET-1 (1–3.5 nM) was used as a reference to confirm the specificity of any inhibitory effect. In protocol 3, release of prostaglandin E2 (PGE2) and 6-keto-PGF1α (the stable byproduct of PGI2) was determined in sphincter (term and preterm) and extrasphincter (term) preparations before and during exposure to increasing concentrations of ET-1 (0.001–100 nM). The effect of the peptide on prostaglandin release was also ascertained in term sphincter rings lacking the endothelium. The two prostaglandins were studied in view of their potency as ductus relaxant (Adeagbo et al., 1982), notwithstanding the fact that only PGI2 may be a natural agent (Adeagbo et al., 1985). The thromboxane A2 (TXA2) analogue, ONO-11113 (1–100 nM), and excess potassium (55 mM) were used as reference spasmogens on the intact term sphincter. In all cases, to measure prostaglandin release from the vessel, the flow of fluid through the bath was interrupted and incubation was continued for 30 min. An aliquot of the bathing fluid (about 1 ml) was then collected, taking care not to uncover the tissue, and was quickly transferred to an ice-cold vial. Only in the case of ET-1 tests, with the inevitable frothing being caused by albumin in the vehicle, was a portion of the specimen momentarily exposed to air during sample collection. Samples were frozen in an acetone-solid CO2 bath and were subsequently stored at −20°C until analysis. Four to 10 collections were made in each experiment depending on the protocol, and the interval between them was 15 min with controls and subthreshold concentrations of ET-1. With higher concentrations, it varied instead depending on the time required for tissues to regain a stable tension between tests (about 0.5–1.5 h). Regardless of the protocol, the first sample was not taken into consideration since it showed relatively higher variability in the yield of prostaglandins. At the end of the experiment, specimens were dried by draining them on metal foil and were weighed. Wet weight was slightly different between term (sphincter, 6.49±0.3 mg; extrasphincter, 5.51±0.9 mg) and preterm (sphincter, 3.7±0.35 and 3.81±0.41 mg, respectively at 119–125 and 104–108 days gestation) preparations. Values of prostaglandin release (pg 100 mg−1 min−1) apply to this weight. In protocol 4, the contractile response of the term sphincter to indomethacin (2.8 μM) was recorded before, during, and after treatment with BQ123 (1 μM; intact preparation) or phosphoramidon (50 μM; intact and endothelium-denuded preparation). Both ET-1-suppressing agents were introduced into the bath 10 min prior to the start of the indomethacin treatment and remained in contact with the tissue throughout the development of the indomethacin effect. The idea in studying the BQ123 (or phosphoramidon)-indomethacin interaction was to verify whether, in keeping with results in the ductus arteriosus (Coceani et al., 1992; Taniguchi and Muramatsu, 2003), the contractile tone of the ductus venosus is sustained by ET-1.
Measurement of prostaglandins
PGE2 and 6-keto-PGF1α were measured directly in the ductus perfusate using radioimmunoassy kits (Dupont, Mississauga, Canada) with 125I-labelled ligands (Coceani et al., 1995). For this purpose, samples were lyophilized and the dry residue was resuspended in 250 μl of assay buffer. Both prostaglandins were measured in every sample. The limit of detection of the assay, defined as the lowest concentration on the linear portion of the standard curve, was 0.25–0.5 and 2–5 pg, respectively for PGE2 and 6-keto-PGF1α. Compounds being assayed showed no cross-reactivity among themselves and against ONO-11113 (0.1 μM). Control tests also confirmed the lack of any interference from Krebs medium alone.
Solutions and drugs
The Krebs medium had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1, MgSO4 0.9, dextrose 11.1 and NaHCO3 25. Potassium-Krebs solution (55 mM) was prepared by substituting NaCl with an equimolar amount of KCl. Depending on the stage of the experiment (see above), the solution was bubbled with gas mixtures containing either no O2 or 95% O2 plus 5% CO2 and, when necessary, balance N2. PO2 was measured with a gas analyzer (mod. 1301, Instrumentation Laboratories, Lexington, U.S.A.) and was, respectively, 1.59±0.02 and 85±0.5 kPa (pH 7.4).
ET-1 (human/porcine type from Peptide Institute, Osaka, Japan or from Peninsula, Belmont, U.S.A.) and big ET-1 (human type, Peptide Institute) were dissolved in sterile water containing 0.05% human serum albumin (final concentration, 100 μM). Appropriate aliquots of these stock solutions were stored at −20°C until use. The TXA2 analogue 9, 11-epithio-11, 12-methano-TXA2 (ONO-11113, courtesy of ONO Pharmaceutical, Osaka, Japan) was dissolved in distilled ethanol (5 mg ml−1), and aliquots (stored at −70°C) were diluted with Tris buffer (pH 7.4). Indomethacin (Sigma, St Louis, U.S.A.) was also dissolved in distilled ethanol (10 mg ml−1) before preparation of the final solution in the Krebs medium. Ethanol in the fluid bathing the isolated ductus preparations did not exceed 0.01% (indomethacin) or 0.001% (ONO-11113). Phosphoramidon (Sigma) and BQ123 (courtesy of Merck Frosst, Montreal, Canada) were dissolved directly in the Krebs medium. Doses of all compounds are given in molar concentrations and refer to their final concentration in the bath. Vehicle alone, without or with ethanol (see above), had no effect on either the vessel tone or the release of prostaglandins.
Analysis of data
Effects of contractile agents were measured by the fractional rise in tension over the basal tension. Basal tension, which varied depending on the animal preparation, straddled the applied tension (see Results).
Data are expressed as the mean±s.e.m. Comparisons were made using a Student's t-test or ANOVA, followed by the Duncan's posthoc test. Differences are considered significant for P<0.05.
Results
In agreement with previous reports (Adeagbo et al., 1982, 1985, 1990; Coceani et al., 1984), the ductus venosus sphincter from term lambs adjusted its resting tone at levels equal, or slightly below, the loading tension when first set up in Krebs medium gassed with 5% CO2 in N2. Subsequent exposure to oxygen (95% O2–5% CO2) caused most preparations to contract and this contraction (116±8 mg; n=75) abated, partially or completely, through the equilibration period. Conversely, the contractile response to both excess potassium (55 mM) and the TXA2 analogue ONO-11113 (0.1 μM) was sustained (470±38 and 702±91 mg, respectively; n=36 and 7) and, in the case of ONO-11113, had also phasic discharges superimposed. Equally persistent and often interspersed with spontaneous motility was the contraction to indomethacin at the high concentration (2.8 μM) (264±21 mg; n=31). However, at the low concentration (28 nM), the effect of the inhibitor was not maintained and was also variable in magnitude (max. contraction, 176±70 mg; n=3). No major change was noted in the response of the vessel to either oxygen (116±10 mg; n=37) or other spasmogens (K+, 351±27, n=19; indomethacin, 325±32 and 77±13 mg, respectively at the high and low concentration, n=10 and 5) following the removal of the endothelium. The premature ductus responded marginally to oxygen at 119–125 days of gestation (two of five experiments; mean, 37 mg) and showed no response at all in the youngest fetuses (104–108 days gestation; n=7). Nevertheless, even the least mature preparations were contracted by excess potassium (72±5 mg; n=3) and ONO-11113 (209 mg; n=2). The latter finding confirms and extends a previous report concerning the early appearance of contractile mechanisms in the course of gestation (Adeagbo et al., 1985).
The extrasphincter part of the ductus differed from the sphincter proper in developing little (88±12 mg; n=4) or no tension (four experiments) when being transferred from a low to a high PO2 medium.
Morphology of the vessel
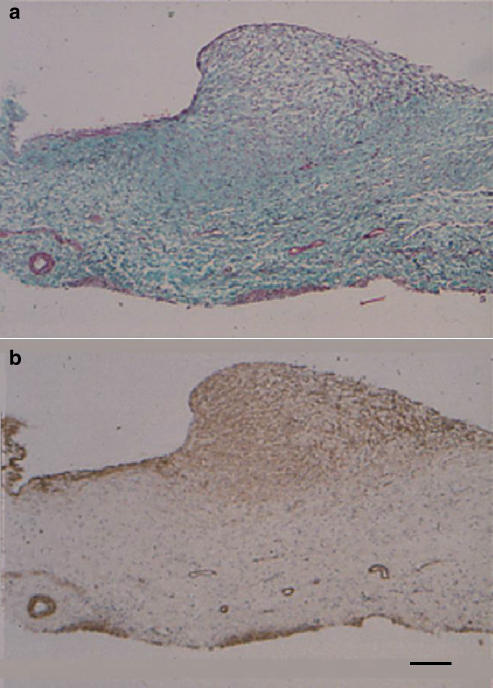
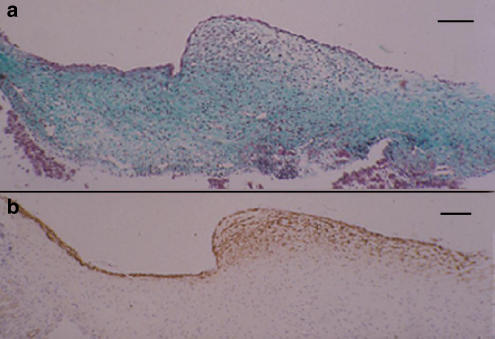
Examination of the near-term fetal ductus by Masson's stain and α-actin immunostaining confirmed an earlier report (Coceani et al., 1984) and showed that muscle cells are arranged in a multilayered, sphincter-like structure at the junction of the vessel with the portal sinus (Figure 1a and b). Equally evident, albeit much thinner, was a muscle formation extending along the ductus proper beneath the endothelium (Figure 1b). Similar features were observed at 0.7 gestation, although the sphincter appeared less prominent and with muscle cells more sparsely arranged (Figure 2a and b). Even at 0.6 gestation, the luminal surface of the ductus had at the site of the inlet a slight protuberance with scattered muscle cells within its texture (data not shown).
Figure 1.
Fetal lamb at term. Longitudinal section of the ductus venosus showing the inlet region, with a prominent sphincter-like formation, and a segment of the vessel proper. (a) Masson's trichrome staining. (b) α-Actin immunostaining. Note the accumulation of muscle at the site of the inlet and the muscle layer beneath the endothelium along the vessel. Bar 100 μm for both panels.
Figure 2.
Fetal lamb at 0.7 gestation. Longitudinal section of the ductus venosus showing the inlet region with a sphincter-like formation and a segment of the vessel. (a) Masson's trichrome staining. (b) α-Actin immunostaining. The muscle component was less developed than in the term preparation but, otherwise, it was similarly arranged. Bar 100 μm.
Effect of ET-1 and big ET-1
ET-1
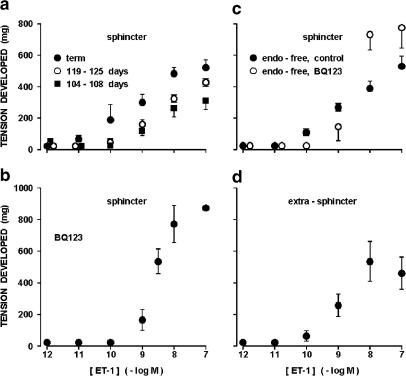
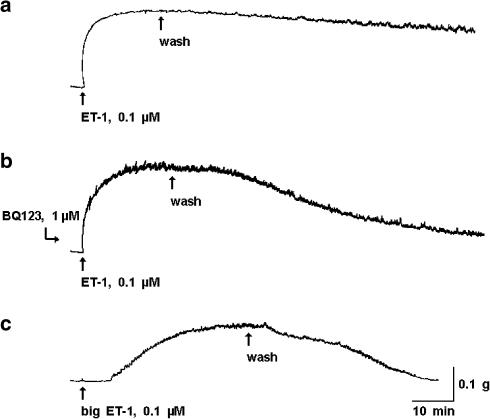
The ductus sphincter from the term lamb contracted in a dose-dependent manner to ET-1 (Figure 3a). As shown in Figure 4a, the contraction began almost instantaneously and developed rapidly to a peak, often with rhythmic discharges or some broad fluctuations superimposed. Reversibility of the response was instead slow, particularly with the high concentrations. Similarly rapid was the ET-1 effect in the younger fetuses, but it had lower magnitude, higher threshold, and little (119–125 days gestation) or no (104–108 days gestation) phasic activity (Figure 3a). No appreciable difference in the ET-1 action was noted at term after removing the endothelium (Figure 3c); however, endothelium-denuded tissues lacked almost completely the phasic activity. Pretreatment of the term sphincter, whether intact or endothelium-free, with BQ123 (1 μM) caused a rightward shift in the ET-1 threshold (Figure 3b and c). However, the peak contraction in the ensuing dose–response curve tended to be greater (Figure 3b and c). Whether enhanced or not over control, the contraction of BQ123–treated tissues to ET-1 showed faster reversal after washing (Figure 4b). Unlike BQ123, indomethacin at the low concentration (28 nM) tended to increase the efficacy of ET-1 (ED50 0.25 and 0.4±0.17 nM, respectively for intact and endothelium-denuded preparations; n=2 and 5).
Figure 3.
Concentration–response curve to ET-1 in the ductus venosus from fetal lamb. (a) Intact ductus sphincter at different gestation ages. The ED50 for the contraction is 0.86±0.49 (n=3), 1.84±0.37 (n=5), and 1.9±0.54 nm (n=5), respectively at term, 119–125 days, and 104–108 days. Dose–response curves for the two preterm groups are significantly different (P<0.001) from the curve at term, but do not reach a significant difference among themselves. (b) Intact ductus sphincter at term; contraction during treatment with BQ123 1 μM (n=3–4, except for the 0.1 μM dose where n=2). (c) Endothelium-denuded ductus sphincter at term; contraction before and during the treatment with BQ123 1 μM (n=9 and 3, respectively). (d) Intact ring preparation from the extrasphincter part of term ductus. The ED50 for the contraction is 1.2±0.3 nM (n=3).
Figure 4.
Ductus sphincter from term fetal lamb. Representative responses to ET-1, (a) before and (b) during treatment with BQ123, and to (c) big ET-1. Note that tracings (a) and (c) are taken from the same experiment. Calibration, 0.1 g weight=0.98 mN.
The extrasphincter part of the ductus behaved as the sphincter in contracting dose-dependently to ET-1, but the threshold concentration was moderately shifted to the right (Figure 3d).
Big ET-1
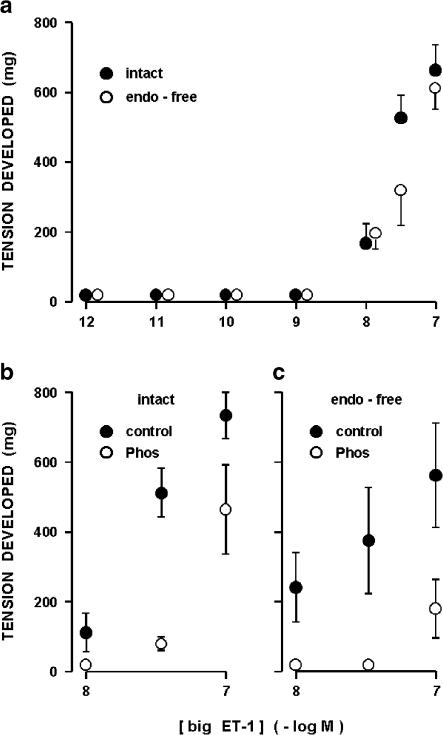
Like ET-1, big ET-1 contracted dose dependently the ductus sphincter from term fetuses (Figure 5a) and, accordingly, the contraction had often phasic discharges interspersed with the tonic component (Figure 4c). However, compared to ET-1 the dose–response curve was shifted to the right and a clear maximum was not attained over the range of concentrations examined (Figure 5a). In addition, even at the highest concentration the response was delayed in onset (4±0.7 min at 0.1 μM) and slow in its progression, and also abated relatively rapidly after washing the preparation (Figure 4c). With one exception, big ET-1 remained effective after the removal of the endothelium (Figure 5a), the only difference from the intact preparation being the absence of phasic activity in most experiments. The single preparation without a response was outwardly normal and, in fact, contracted in the expected manner to ET-1 (506 mg at 0.1 μM). Whether in the presence or the absence of the endothelium, big ET-1 action was curtailed by phosphoramidon (50 μM) (Figure 5b and c). In contrast, treatment with phosphoramidon had no effect on ET-1 (data not shown).
Figure 5.
Concentration–response curve to big ET-1 in the ductus sphincter from term fetal lamb. (a) Intact vs endothelium-denuded vessel (n=3–11). Note that big ET-1 had no effect on one endothelium-denuded preparation (see text) and this result was not included in the computation. (b) and (c) Effect of big ET-1 on intact (n=4–7) and endothelium-denuded (n=3) preparations before and during treatment with phosphoramidon 50 μm (Phos) (P<0.01 for control vs treatment in panels (b) and (c)). The same treatment had no effect on the ET-1 contraction in both the intact (242±47 and 299±57 mg at 1 nM before and during treatment; n=5) and the endothelium-denuded (231 and 237 mg at 3.5 nm before and during treatment; n=1) preparations. Note that different preparations were used in panels (a) vs (b) and (c).
Release of prostaglandins: at rest vs ET-1 treatment
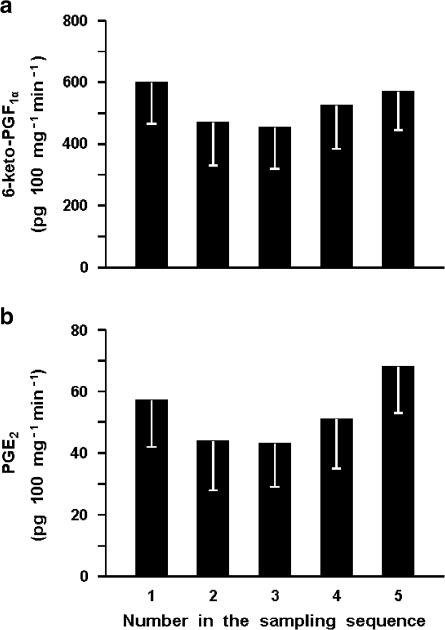
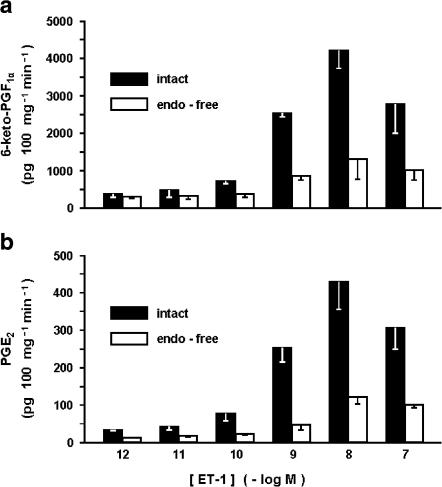
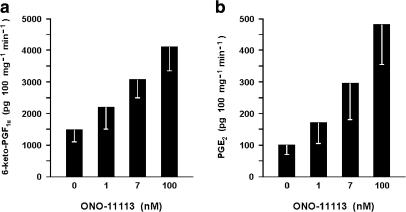
The ductus sphincter from the term lamb released 6-keto-PGF1α and PGE2 under basal conditions, the values for the former compound being severalfold higher and more variable (1232±240 and 78±15 pg 100 mg−1 min−1, respectively for 6-keto-PGF1α and PGE2; n=26 for both). However, in any given experiment, the output of these prostaglandins was fairly stable over time (Figure 6). Removal of the endothelium resulted in lesser yield of both compounds (6-keto-PGF1α, 424±69 pg 100 mg−1 min−1, n=20, P<0.01 vs intact vessel; PGE2, 23±4 pg 100 mg−1 min−1 n=19, P<0.005 vs intact vessel). Likewise, pretreatment with indomethacin curtailed the synthesis of prostaglandins in part or in full depending on the dose used. Specifically, with the low concentration of the inhibitor (28 nM) compounds were still measurable in three of four experiments (235±135 and 35±26 pg 100 mg−1 min−1, respectively for 6-keto-PGF1α and PGE2), while with the high concentration (2.8 μM) 6-keto-PGF1α was consistently below detection (four experiments) and PGE2 straddled the threshold (8 pg 100 mg−1 min−1) in one of the four experiments. ET-1 increased the output of 6-keto-PGF1α and PGE2 in a dose-related fashion, its effectiveness being greater with intact than endothelium-denuded preparations (Figure 7). A similar enhancing action was seen with ONO-11113 over a range of concentrations causing a contraction (Figure 8). However, smooth muscle contraction per se was not an effective stimulus since release of both compounds remained within basal limits during exposure of the tissue to excess potassium (55 mM) (931±132 and 107±20 pg 100 mg−1 min−1, respectively for 6-keto-PGF1α and PGE2; n=6).
Figure 6.
Fetal lamb at term. Prostaglandin release from the ductus venosus sphincter through a period of 4 h. (a) 6-keto-PGF1α. (b) PGE2 (n=4–5 for both compounds). With either prostaglandin, differences between individual samples are not significant. In this and the following figures, the two compounds were measured in the same sample and reference weight applies to the wet weight of the tissue (see Methods).
Figure 7.
Fetal lamb at term. Effect of ET-1 on prostaglandin release from the intact vs endothelium-denuded ductus venosus sphincter. (a) 6-keto-PGF1α. (b) PGE2 (n=3 for both compounds and conditions). P<0.001 intact vs endothelium-free for both panels.
Figure 8.
Fetal lambs at term. Effect of ONO-11113 on prostaglandin release from the ductus venosus sphincter. (a) 6-keto-PGF1α, (b) PGE2 (n=4–5 for both compounds). Contractile responses to ONO-11113 are 387±46, 647±75, and 821±133 mg, respectively with 1, 7, and 100 nM.
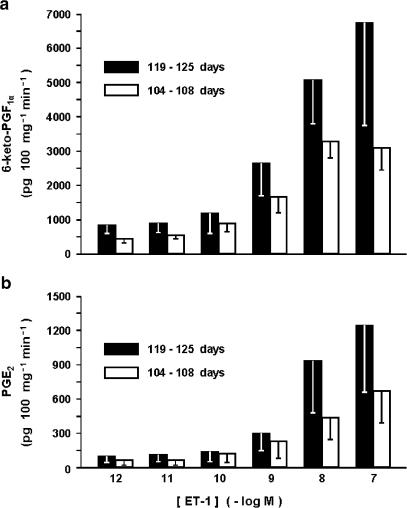
No significant difference in the basal release of either prostaglandin was noted in premature compared to mature preparations and, likewise, ET-1 still exhibited a stimulatory effect prior to term (Figure 9). However, the magnitude of the ET-1 effect tended to be lower in the youngest fetuses (Figure 9).
Figure 9.
Fetal lamb before term. Effect of ET-1 on prostaglandin release from the ductus venosus sphincter at 119–125 days vs 104–108 days gestation. (a) 6-keto-PGF1α (n=4–5). (b) PGE2 (n=3). Differences between the two age groups do not reach significance. Note that release of 6-keto-PGF1α at rest is 1147±383 and 686±229 pg 100 mg−1 min−1, respectively, for the older and the younger age group, while the equivalent values for PGE2 are 63±27 and 64±52 pg 100 mg−1 min−1. With either compound, basal output in the premature does not differ significantly from that seen at term.
The extrasphincter portion of the term ductus was also capable of producing 6-keto-PGF1α and PGE2 at rest and, in fact, its synthetic activity exceeded that of the sphincter region (6-keto-PGF1α, 3415±1555 pg 100 mg−1 min−1; PGE2, 190±55 pg 100 mg−1 min−1; P<0.02 vs sphincter with n=5 for both). Accordingly, at this site too ET-1 enhanced the release of both compounds dose-dependently, the peak being reached at the 10-nM concentration (19,503±9513 and 1528±489 pg 100 mg−1 min−1, respectively for 6-keto-PGF1α and PGE2; n=3 for both).
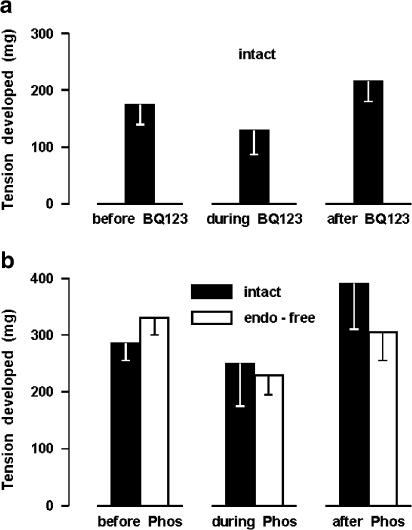
Effect of BQ123 and phosphoramidon on contractile tone
Assuming that ET-1 may be involved in the generation of muscle tone, BQ123 (1 μM) and phosphoramidon (50 μM) were tested on the contractile response of the ductus sphincter to indomethacin (2.8 μM). As shown in Figure 10a, BQ123 reduced slightly, albeit not significantly, the contractile tension elicited by indomethacin. Phosphoramidon, on the other hand, was without effect on the intact preparation, while it curtailed in part the response of the endothelium-denuded preparation (Figure 10b). Whether effective or not against the indo-methacin contraction, both ET-1-suppressing agents produced no obvious change in the basal tone of vessel.
Figure 10.
Ductus sphincter from term lamb. Effect of (a) BQ123 1 μM (n=7) and (b) phosphoramidon 50 μM (Phos; n=4 and 9, respectively, for the intact and endothelium-denuded vessel) on the contractile response to indomethacin 2.8 μM. Indomethacin was tested sequentially and normal Krebs was passed through the bath between applications until the contraction had fully subsided (see Methods). Where indicated, BQ123 or phosphoramidon was added to the perfusion fluid 10 min prior to starting the indomethacin test and the treatment was continued throughout the period of exposure to indomethacin. In each panel, the same tissues were studied in the absence or presence of BQ123 (or phosphoramidon). Note that in panel (b) the difference between control and treated endothelium-denuded preparations reaches significance (P<0.05) by paired t-test.
Discussion
Contrary to recent reports (Mavrides et al., 2002; Tchirikov et al., 2003), our study reasserts the existence in the ductus venosus inlet of a muscle structure with the features of a sphincter (see Coceani et al., 1984). At the same time, it shows that the muscle extends along the vessel proper as a distinct layer within the wall. This muscular component, within and without the inlet region, is already evident at 0.6–0.7 gestation, and the finding is compatible with the early development of a local vasoregulatory mechanism. ET-1 is a potent constrictor of both sphincter and extrasphincter sections of the ductus and, in keeping with the morphological data, its action becomes evident prior to term. Coincidentally, the peptide promotes the release of 6-keto-PGF1α (hence, PGI2) and PGE2 from endothelial and extraendothelial, conceivably muscular, sources within the vessel, the former site being more active in this response. Based on this premise, our discussion will address the following issues: the question of a competent sphincter operating in the ductus inlet with the added possibility of muscle tone being actively regulated in the whole vessel; the functional organization of the ET-1 system with the attendant prospect of an involvement of the peptide in the generation of contractile tension and the closure of the ductus at birth; and the operation of an intramural vasodilator mechanism in the possible dual role of direct effector and modulator of ET-1 action.
Against the unequivocal evidence of a sphincter formation being provided here, recent reports negating the existence of any such structure (Mavrides et al., 2002; Tchirikov et al., 2003) are perplexing. In the study of Tchirikov et al. (2003), carried out as ours in fetal sheep, not only is an ordered arrangement of muscle cells missing, but also the tissue ridge itself, which is typical of the inlet region, is seemingly not detected. A methodological reason for the inconsistency pertaining to the stock of sheep is unlikely since, through the present and the early (Coceani et al., 1984) investigations, we had access to a varied assortment of purely bred and crossbred animals and did not detect any difference among them. Tchirikov et al. (2003), however, provide no details on the procedure for sectioning the ductus and a question may be raised on whether the ridge structure has been missed in this process. The negative result of Mavrides et al. (2002), on the other hand, can find an explanation in the fetal age being considered. Human fetuses were studied, in fact, at less than 0.5 gestation, that is a stage in development at which, according to our data, the sphincter structure may be barely visible, if not absent altogether. Whatever the cause for such discrepancy, the evidence from our work is compelling enough to indicate the existence of a muscle sphincter, conceivably functional, at the inlet of the ductus and, by extension, the operation of a vasomotor control beyond the site of the sphincter. Consistent with our conclusion is a report showing that hypoxemia in utero causes a widening of both inlet and midportion sections of the ductus (Kiserud et al., 2000a). Furthermore, after birth the ductus closes uniformly over its entire length (Momma et al., 1992), implying that the contractile drive is similarly expressed in sphincter and extrasphincter regions. Extending further the extrapolation from morphological data, one may also surmise that the postulated vasoregulatory mechanism is not as effective in the preterm as in the term ductus. No data are available in the living fetus to confirm this point. However, in the newborn it has been shown that prematurity is linked to delayed closure of the vessel (Fugelseth et al., 1998; Loberant et al., 1999; Kondo et al., 2001).
The demonstration of the potent constrictor effect of ET-1, with its implicit functional connotation, accords with the broad role being assigned to the compound in the control of the perinatal circulation (Perreault & Coceani, 2003). In addition, it complements previous data on the importance of a CYP450-based mechanism in the generation of contractile tone (Adeagbo et al., 1990) and suggests that an operational scheme, linking CYP450 with ET-1, originally developed in the ductus arteriosus (Coceani, 2000) may also apply to the ductus venosus. Still, what is the actual evidence for a normal function of the ET-1 in this vessel? The potency of the compound is definitely important, especially if one considers that there is no other suitable candidate, at least known at this time, for the role of intramural constrictor. In fact, the involvement of α-adrenergic innervation, which was supported by findings in vitro (Coceani et al., 1984), could not be confirmed in vivo (Kiserud et al., 2000a). TXA2, on the other hand, is as potent as ET-1 in contracting the ductus (Adeagbo et al., 1985; see Results), but in exerting any effect it may only originate from extramural sources (Adeagbo et al., 1982, 1985). Significant in the present context is also the observation that corticosteroids, while enhancing the susceptibility of fetal blood vessels to ET-1 (Docherty et al., 2001), are also capable of promoting the closure of the ductus in the prematurely born infant (Kondo et al., 2001). In addition, tests with big ET-1, specifically the demonstration of the susceptibility of the agent to the interfering action of phosphoramidon, point to the occurrence of a functional converting enzyme in the tissue. Indeed, the ductus may release ET-1 itself (A. Adeagbo, L. Kelsey, F. Coceani, unpublished data), hence it is endowed with a full-fledged synthetic system. In brief, there are several data that qualify ET-1 as an effector in the generation of contractile tone. Their significance, however, is weakened by the finding that ET-1-suppressing agents are little effective in curtailing the elevated tone from treatment with indomethacin. Further work is required to reach a firm conclusion on this issue. In particular, experiments are needed in vivo to verify the efficacy of ET-1 inhibitors in interfering with ductus closure at birth.
While the role of ET-1 needs to be defined further, our study strengthens the concept of the ductus being normally relaxed by a prostaglandin. Not only was it found that prostaglandins are a natural constituent of the vessel, but also that interference with their release through indomethacin treatment causes a constriction which is correlated in magnitude to the degree of inhibition. In theory, both prostaglandins present in the tissue perfusate could serve this function since they are virtually equipotent as ductus relaxant (Adeagbo et al., 1982). However, there are already data identifying the active agent with PGI2 (Adeagbo et al., 1985), and our present observation on its rate of release exceeding severalfold that of PGE2 accords with such concept. As expected from an activation of the ETB receptor subtype by ET-1, PGI2 synthesis is greater in the endothelial than the subendothelial tissue. Peculiarly, however, the indomethacin-induced constriction is virtually the same with intact and endothelium-denuded preparations, thus implying that, despite the existence of an over-riding endothelial source for PGI2, the fraction of the compound causing relaxation is formed primarily, if not exclusively, in the proximity or within the muscle layer.
The question of a prostaglandin-based modulation of ET-1 action remains a moot point. Intuitively, one may think that the marked stimulation of PGI2 and PGE2 synthesis by ET-1 serves a useful purpose as a self-regulatory process. Consistent with this idea is the fact that TXA2, a second potential constrictor formed extramurally (Adeagbo et al., 1982, 1985), is also able to promote prostaglandin release, while no such effect is seen with excess potassium. However, despite this apparent selectivity in spasmogen/prostaglandin interaction, removal of the endothelium does not cause any obvious change in the response to ET-1. Against this negative evidence is the observation that indomethacin, at a threshold concentration, tends to enhance the ET-1 contraction. Perhaps, as in the case of the direct action of the prostaglandins (see above), any modulatory action is sustained by an extraendothelial site of synthesis.
A final point deserves a comment and relates to the peculiar change induced by BQ123 on the dose–response curve to ET-1. With both intact and endothelium-denuded preparations, the ETA receptor antagonist inhibited ET-1 concentrations in the low range, while it had no effect, or even caused some enhancement, with those in the high range. A possible reason for this finding is that muscle cells are endowed with ETA and ETB (i.e. ETB2) receptors, both mediating constriction but differing in their susceptibility to ET-1 (ETA>ETB2). However, while accounting for the lack of inhibition, such arrangement could not explain any enhancement of ET-1 action seen in the BQ123-treated preparations. For the latter, one could surmise the existence of an ETB-mediated upregulation of ET-1 system being triggered by the exogenous peptide. Evidence of this positive feedback mechanism has been reported elsewhere (see Saito et al., 1995). Alternatively, the decreasing effectiveness of BQ123 with increasing ET-1 concentrations may be linked to the marked difference in kinetics of ETA receptors binding between the two compounds (ET-1 slowly reversible/BQ123 rapidly reversible) (Henry & King, 1999). The possibility, on the other hand, of the observed pattern deriving from the occurrence in the tissue of a subtype of BQ123-insensitive ETA receptors seems far-fetched on the basis of available data (see Henry & King, 1999). Equally unfeasible would appear to be the operation of an ETA-based negative feedback mechanism on ET-1 action (see Zhang et al., 1998). Clearly, additional work is required to settle this point. However, if the existence of ETB receptors with constrictor properties were confirmed, then this particular feature will have to be considered in any pharmacological manipulation of the ET-1 system in vivo.
In conclusion, this study demonstrates that ET-1 is a potent constrictor of the ductus venosus, lending itself to a role in the generation of contractile tension and the process of closure of the vessel at birth. Prostaglandins are formed in the tissue and, besides exerting a direct relaxing effect, may also modulate ET-1 action. The concept of ductal tone being actively regulated is reasserted with implications that are both conceptual and practical.
Acknowledgments
This work was supported by the Heart and Stroke foundation of Ontario (Grant T-3329). We gratefully acknowledge the assistance of Dr. E Cutz for the morphological analysis and of Dr. I Bishai for the radioimmunoassay of prostaglandins.
Abbreviations
- CYP450
cytochrome P450
- ET
endothelin
- PG
prostaglandin
- Phos
phosphoramidon
- TX
thromboxane
References
- ADEAGBO A.S.O., BISHAI I., LEES J., OLLEY P.M., COCEANI F. Evidence for a role of prostaglandin I2 and thromboxane A2 in the ductus venosus of the lamb. Can. J. Physiol. Pharmacol. 1985;63:1101–1105. doi: 10.1139/y85-181. [DOI] [PubMed] [Google Scholar]
- ADEAGBO A.S.O., BREEN C.A., CUTZ E., LEES J.G., OLLEY P.M., COCEANI F. Lamb ductus venosus: evidence of a cytochrome P-450 mechanism in its contractile tension. J. Pharmacol. Exp. Ther. 1990;252:875–879. [PubMed] [Google Scholar]
- ADEAGBO A.S.O., COCEANI F., OLLEY P.M. The response of the lamb ductus venosus to prostaglandin and inhibitors of prostaglandin and thromboxane synthesis. Circ. Res. 1982;51:580–586. doi: 10.1161/01.res.51.5.580. [DOI] [PubMed] [Google Scholar]
- AILAMAZYAN E.K., KIRILLOVA O.V., POLYANIN A.A., KOGAN I.Y. Functional morphology of ductus venosus in human fetus. Neuroendocrinol. Lett. 2003;24:28–32. [PubMed] [Google Scholar]
- BELLOTTI M., PENNATI G., DE GASPERI C., BATTAGLIA F.C., FERRAZZI E. Role of ductus venosus in distribution of umbilical blood flow in human fetuses during second half of pregnancy. Am. J. Physiol. 2000;279:H1256–H1263. doi: 10.1152/ajpheart.2000.279.3.H1256. [DOI] [PubMed] [Google Scholar]
- BELLOTTI M., PENNATI G., PARDI G., FUMERO R. Dilatation of the ductus venosus in human fetuses: ultrasonographic evidence and mathematical modeling. Am. J. Physiol. 1998;275:H1759–H1767. doi: 10.1152/ajpheart.1998.275.5.H1759. [DOI] [PubMed] [Google Scholar]
- COCEANI F. The control of the ductus venosus: an update. Eur. J. Pediatr. 1993;152:976–977. doi: 10.1007/BF01957218. [DOI] [PubMed] [Google Scholar]
- COCEANI F.Cytochrome P450 in the contractile tone of the ductus arteriosus: regulatory and effector mechanisms The Fetal and Neonatal Pulmonary Circulations 2000Armonk: Futura Publishing Co; 331–341.ed. Weir, E. K., Archer, S. L. & Reeves, J. T. pp [Google Scholar]
- COCEANI F., ADEAGBO A.S.O., CUTZ E., OLLEY P.M. Autonomic mechanisms in the ductus venosus of the lamb. Am. J. Physiol. 1984;247:H17–H24. doi: 10.1152/ajpheart.1984.247.1.H17. [DOI] [PubMed] [Google Scholar]
- COCEANI F., BISHAI I., ENGELBERTS D., HOUSE R.V., ADAMSON S.L. Response of newborn and adult sheep to pyrogens: relation between fever and brain eicosanoid changes. Brain Res. 1995;700:191–204. doi: 10.1016/0006-8993(95)00946-n. [DOI] [PubMed] [Google Scholar]
- COCEANI F., KELSEY L., SEIDLITZ E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can. J. Physiol. Pharmacol. 1992;70:1061–1064. doi: 10.1139/y92-146. [DOI] [PubMed] [Google Scholar]
- COCEANI F., LIU Y.-A., SEIDLITZ E., KELSEY L., KUWAKI T., ACKERLEY C., YANAGISAWA M. Endothelin A receptor is necessary for O2 constriction but not closure of ductus arteriosus. Am. J. Physiol. 1999;277:H1521–H1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- DE NUCCI G., THOMAS R., D'ORLEANS-JUSTE P., ANTUNES E., WALDER C., WARNER T., VANE J.R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCHERTY C.C., KALMAR-NAGY J., ENGELEN M., KOENEN C.C., NIJLAND M., KUC R.E., DAVENPORT A.P., NATHANIELSZ P.W. Effect of in vivo infusion of dexamethasone at 0.75 GA on fetal ovine resistance artery responses to ET-1. Am. J. Physiol. 2001;281:R261–R268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- EDELSTONE D.I., RUDOLPH A.M. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am. J. Physiol. 1979;237:H724–H729. doi: 10.1152/ajpheart.1979.237.6.H724. [DOI] [PubMed] [Google Scholar]
- FUGELSETH D., LINDEMAN R., LIESTØL K., KISERUD T., LANGSLET A. Ultrasonographic study of ductus venosus in healthy neonates. Arch. Dis. Child. 1997;77:F131–F134. doi: 10.1136/fn.77.2.f131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUGELSETH D., LINDEMAN R., LIESTØL K., KISERUD T., LANGSLET A. Postnatal closure of ductus venosus in preterm infants ⩽32 weeks. An ultrasonographic study. Early Human Dev. 1998;53:163–169. doi: 10.1016/s0378-3782(98)00051-6. [DOI] [PubMed] [Google Scholar]
- HENRY P.J., KING S. Typical endothelin ETA receptors mediate atypical endothelin-1-induced contractions in sheep isolated tracheal muscle. J. Pharmacol. Exp. Ther. 1999;289:1385–1390. [PubMed] [Google Scholar]
- KISERUD T. Hemodynamics of the ductus venosus. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;84:139–147. doi: 10.1016/s0301-2115(98)00323-6. [DOI] [PubMed] [Google Scholar]
- KISERUD T., OZAKI T., NISHINA H., RODECK C., HANSON M.A. Effect of NO, phenylephrine, and hypoxemia on ductus venosus diameter in fetal sheep. Am. J. Physiol. 2000a;279:H1166–H1171. doi: 10.1152/ajpheart.2000.279.3.H1166. [DOI] [PubMed] [Google Scholar]
- KISERUD T., RASMUSSEN S., SKULSTAD S. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am. J. Obstet. Gynecol. 2000b;182:147–153. doi: 10.1016/s0002-9378(00)70504-7. [DOI] [PubMed] [Google Scholar]
- KONDO M., ITOH S., KUNIKATA T., KUSAKA T., OZAKI T., ISOBE K., ONISHI S. Time of closure of ductus venosus in term and preterm neonates. Arch. Dis. Child. 2001;85:57–59. doi: 10.1136/fn.85.1.F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOBERANT N., HERSKOVITS M., BARAK M., BEN-ELISHA M., HERSCHKOWITZ S., SELA S., ROGUIN N. Closure of the ductus venosus in premature infants: findings on real-time gray-scale, color-flow Doppler, and duplex Doppler sonography. Am. J. Roentgenol. 1999;172:227–229. doi: 10.2214/ajr.172.1.9888772. [DOI] [PubMed] [Google Scholar]
- MAVRIDES E., MOSCOSO G., CARVALHO J.S., CAMPBELL S., THILAGANATHAN B. The human ductus venosus between 13 and 17 weeks of gestation: histological and morphometric studies. Ultrasound J. Obstet. Gynecol. 2002;19:39–46. doi: 10.1046/j.1469-0705.2002.00614.x. [DOI] [PubMed] [Google Scholar]
- MOMMA K., ITO T., ANDO M. In situ morphology of the ductus venosus and related vessels in the fetal and neonatal rat. Pediatr. Res. 1992;32:386–389. doi: 10.1203/00006450-199210000-00003. [DOI] [PubMed] [Google Scholar]
- NICOLINI U., TALBERT D.G., FISK N.M., RODECK C.H. Pathophysiology of pressure changes during intrauterine trans-fusion. Am. J. Obstet. Gynecol. 1989;160:1139–1145. doi: 10.1016/0002-9378(89)90176-2. [DOI] [PubMed] [Google Scholar]
- PAULICK R.P., MEYERS R.L., RUDOLPH C.D., RUDOLPH A.M. Venous and hepatic vascular responses to indomethacin and prostaglandin E1 in the fetal lamb. Am. J. Obstet. Gynecol. 1990;163:1357–1363. doi: 10.1016/0002-9378(90)90719-n. [DOI] [PubMed] [Google Scholar]
- PERREAULT T., COCEANI F. Endothelin in the perinatal circulation. Can. J. Physiol. Pharmacol. 2003;81:644–653. doi: 10.1139/y03-013. [DOI] [PubMed] [Google Scholar]
- RUDOLPH C.D., MEYERS R.L., PAULICK R.P., RUDOLPH A.M. Effect of ductus venosus obstruction on liver and regional blood flows in the fetal lamb. Pediatr. Res. 1991;29:347–352. doi: 10.1203/00006450-199104000-00004. [DOI] [PubMed] [Google Scholar]
- SAITO S., HIRATA Y., IMAI T., MARUMO F. Autocrine regulation of the endothelin-1 gene in rat endothelial cells. J. Cardiovasc. Pharmacol. 1995;26:584–587. [PubMed] [Google Scholar]
- SUZUKI S., SUZUKI A., KAJIKURI J., ITOH T. Endothelin-1-induced prostaglandin E2 production: modulation of contractile response to endothelin-1 in porcine coronary artery. Eur. J. Pharmacol. 1992;217:97–100. doi: 10.1016/0014-2999(92)90517-8. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T., MURAMATSU I. Pharmacological knockout of endothelin ETA receptors. Life Sci. 2003;74:405–409. doi: 10.1016/j.lfs.2003.09.027. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T., AZUMA H., OKADA Y., NAIKI H., HOLLENBERG M.D., MURAMATSU I. Endothelin-1-endothelin receptor type A mediates closure of rat ductus arteriosus at birth. J. Physiol. 2001;537:579–585. doi: 10.1111/j.1469-7793.2001.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCHIRIKOV M., KERTSCHANSKA S., SCHRÖDER H.J. Differential effects of catecholamines on vascular rings from ductus venosus and intrahepatic veins of fetal sheep. J. Physiol. 2003;548:519–526. doi: 10.1113/jphysiol.2002.034470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINER C.P., HEILSKOV J., PELZER G., GRANT S., WENSTROM K., WILLIAMSON R.A. Normal values for human umbilical venous and amniotic fluid pressures and their alteration by fetal disease. Am. J. Obstet. Gynecol. 1989;161:714–717. doi: 10.1016/0002-9378(89)90387-6. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., OLIVER J.R., HOROWITZ J.D. Endothelin B receptor-mediated vasoconstriction induced by endothelin A receptor antagonist. Cardiovasc. Res. 1998;39:665–673. doi: 10.1016/s0008-6363(98)00152-7. [DOI] [PubMed] [Google Scholar]
- ZINK J., VAN PETTEN G.R. Time course of closure of the ductus venosus in the newborn lamb. Pediatr. Res. 1980;14:1–3. doi: 10.1203/00006450-198001000-00001. [DOI] [PubMed] [Google Scholar]