Abstract
Signaling networks involving different receptor systems allow extracellular signals to be integrated and transformed into various biological activities. In this report, we studied the activity of the c-Jun N-terminal kinase (JNK) subgroup of mitogen-activated protein kinases (MAPKs), in response to stimulation by G protein-coupled receptors (GPCRs) and co-activation with epithermal growth factor receptor (EGFR).
Stimulation of exogenous GPCRs in Cos-7 cells induced JNK activation of different magnitudes depending on their G-protein coupling specificities (Gq>Gi>Gs), and a moderate JNK activation was linked to stimulation of endogenous EGFR by EGF.
Co-stimulation with GPCR agonists and EGF resulted in differential augmentation of JNK activities, with Gi-coupled receptors associated with a synergistic JNK activation upon co-stimulation with EGF, while Gq- and Gs-coupled receptors were incapable of triggering this effect.
This Gi/EGF-induced synergistic JNK activation was inhibited by pertussis toxin and AG1478, and may involve Src family tyrosine kinases, PI3 K, Ca2+/calmodulin and small GTPases as important intermediates, while Ca2+ mobilization was triggered by the stimulation of Gq-coupled receptor or EGF treatment, but not by the Gi- or Gs-coupled receptors.
Transient expression of Gβγ subunits with EGF treatment, or co-activation of exogenous Gi-coupled receptor with thapsigargin also resulted in a synergistic JNK activation. Activation of Gi-coupled receptor accompanied with EGF treatment enhanced the expression level and activity of MAPK phosphatase type I, which occurred after the maximal synergistic JNK activation.
Our results support a mechanistic model where EGF signaling may differentially regulate the JNK activities triggered by GPCRs of different coupling specificities.
Keywords: GPCR, EGF, Src, PI3 K, Ca2+/CaM, GTPases, JNK, MKP
Introduction
In mammalian cells, cross-communication between different transmembrane receptor signaling systems enables multiple extracellular signals to be received and then integrated into different biological responses. Receptors with tyrosine kinase activity (RTKs) and G protein-coupled receptors (GPCRs) are two major groups of signal transduction systems where, upon binding of extracellular ligands, both are capable of stimulating the regulatory pathways of the mitogen-activated protein kinases (MAPKs) (reviewed by Lowes et al., 2002). The basic assembly of MAPK pathways is a three-component module conserved from yeast to human (i.e. MAPK kinase kinase → MAPK kinase → MAPK). There are at least three subtypes of MAPK. The extracellular signal-regulated kinase (ERK) is mainly stimulated by growth factors, while c-Jun N-terminal kinase (JNK) and p38 MAPK are more responsive to cellular stress and cytokines. MAPKs modulate the activities of various proteins including other protein kinases and transcription factors. As one of the major subgroups of MAPKs, JNKs phosphorylate and activate the transcriptional activities of c-Jun, ATF-2 and Elk-1. JNK activities are important for cell proliferation, differentiation, survival and apoptosis, and these differential biological responses may arise as the consequences of cell type specificities, the magnitude and duration of JNK activation (Kobayashi & Tsukamoto, 2001) and the co-operative effects with other subgroups of MAPK.
Different receptor systems transmit stimulatory signals to the MAPK pathways with similar principles, but involve both common and different intermediates. For example, activation of the epidermal growth factor receptor (EGFR) by EGF induces JNK activation in a Rac-dependent manner (Fanger et al., 1997). Phosphoinositide-3-kinase (PI3 K), a lipid kinase which is activated by EGFR activation, may be responsible for delivering activation signals to Rac (Akasaki et al., 1999). Moreover, EGF treatment is linked to the activation of the γ isoform of phospholipase C (PLCγ), which hydrolyzes phosphatidylinositol (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-triphosphatase (IP3). IP3 then releases Ca2+ from intracellular stores, which then modulates the activities of various effectors including MAPKs. Different G protein families (Gs, Gi, Gq and G12) are also linked to activation of JNK, in most cases, the stimulated kinase activities are dependent on Rac-related GTPases (Chan & Wong, 2000). Receptors coupled to the Gq family induce Ca2+ transients by activating the β-isoforms of phospholipase C (PLCβ) (Piiper et al., 1997), while Gs- and Gi-coupled receptors are linked to stimulatory and inhibitory effects, respectively, on the adenylyl cyclase-mediated cAMP formation (Balmforth et al., 1986; Mollereau et al., 1994). Both Ca2+ and cAMP serve as mediators for MAPK activation in various cell types (Eguchi et al., 2001; Yamauchi et al., 2001); however, the former is likely to be a more potent activator than the latter for activating the JNK pathway (Li et al., 1997). On the other hand, GPCRs which are solely coupled to G12 have not been identified so far, although overexpression of the activated α-subunit mutant of G12 enhances JNK activity (Voyno-Yasenetskaya et al., 1996). Interestingly, the Gβγ subunits released from different G protein families following GPCR activation, especially for the Gi-coupled receptors, seem to be critically important for MAPK regulation, probably due to their stimulatory effects on Src and PI3K two kinase families involved in JNK activation (Luttrell et al., 1996; Lopez-Ilasaca et al., 1998).
In addition to the EGFR autophosphorylation induced by EGF, the GPCR-mediated Src activity has also been linked to the transactivation of EGFR (Biscardi et al., 1999; Prenzel et al., 1999; Pierce et al., 2001), but these two modes of mechanism are unlikely to be identical stimulatory events (Aviezer & Yayon, 1994; Luttrell et al., 1996). Moreover, the JNK activation in response to Gq-coupled angiotensin II receptor was not effectively inhibited by functional blockade of EGFR (Eguchi et al., 2001), and the GPCR-mediated phosphorylation of focal adhesion kinase is independent of the transactivation of EGFR (Salazar et al., 2003). GPCR signaling appears to potentiate EGFR-induced DNA synthesis, and a synergy between these two receptor systems has been demonstrated in terms of cell proliferation (Krymskaya et al., 2000). Due to the similarities and differences of the signaling mechanisms mediated by EGFR and GPCRs, co-stimulation of these two receptor types may generate different biological responses, such as JNK activities, as compared to individual stimulation. In this report, we used transfected Cos-7 cells as a model to examine these issues. We demonstrated that differential activation of JNK occurred upon co-stimulation of EGFR by EGF and GPCRs of different coupling specificities, with EGF signaling co-operating with Gi-coupled receptor activation to induce a synergistic JNK activation, while co-stimulations of EGFR with Gs- or Gq-coupled receptors were incapable of triggering this response.
Methods
Reagents
The cDNAs encoding the dominant-negative mutants RasS17N and RacT17N were generous gifts from Dr Eric J. Stanbridge (University of California, Irvine). cDNAs of other dominant-negative mutants including RhoT19N and Cdc42T17N were provided by Dr Marc Symons (Picower Institute for Medical Research, NY, U.S.A.). The cDNA encoding the HA-tagged JNK was donated by Dr T. Voyno-Yasenetskaya (University of Illinois, Chicago, IL, U.S.A.). Plasmids of BK2R, H1R and SecR were provided by Dr J. Fred Hess (Merck Research Laboratories, Rahway), Dr Marianne D. De Backer (Janssen Research Foundation, Beerse, Belgium) and Dr Shigekazu Nagata (Osaka University, Japan), respectively. [γ-32P]ATP was purchased from DuPont NEN (Boston, MA, U.S.A.). PTX and 12CA5 (Anti-HA) antibody were purchased from List Biological Laboratories (Campbell, CA, U.S.A.) and Roche Molecular Biochemicals (Indianapolis, IN, U.S.A.), respectively. Protein A-agarose and cell culture reagents (including Lipofectamine PLUS™) were obtained from Invitrogen (Carlsbad, CA, U.S.A.). The protein tyrosine phosphatase (PTP) assay system, phospho-Akt (Ser308) antibody and the MAPK phosphatase-1 antibody (MKP-1 M-18) were obtained from New England Biolabs (Beverly, MA, U.S.A.), Cell Signaling Technology (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), respectively. Agonists for various GPCRs (e.g. nociceptin/orphanin FQ, dopamine, somatostatin, melatonin, human chorionic gonadotropin, secretin, vasopressin, bombesin, bradykinin, carbachol and histamine), thapsigargin, BAPTA-AM, W-7 and Na3VO4 were purchased from Sigma (St Louis, MO, U.S.A.). Epidermal growth factor (EGF), Sp-cAMPS, AG1478, radicicol, PP2, wortmannin and LY294002 were obtained from Calbiochem (San Diego, CA, U.S.A.), and the reagents for FLIPR® (Fluorometric Imaging Plate Reader) calcium assay were purchased from Molecular Devices (Sunnyvale, CA, U.S.A.).
Cell culture and transfection
Cos-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v v−1) heat-inactivated fetal calf serum (HIFCS), 50 U ml−1 penicillin and 50 μg ml−1 streptomycin, and grown at 37°C in an environment of 5% CO2. The cells were transferred to six-well plates (for JNK assay) and 12-well plates (for IP and cAMP assays) at 5 × 105 cells per well and 1.5 × 105 cells per well, respectively. Transfection was performed by means of Lipofectamine PLUS™ Reagents following the supplier's instructions. Approximately 60–75 % of the cell population will take up the cDNAs, as indicated by co-transfecting a plasmid DNA encoding β-galactosidase as a reporter. For MKP-1 assay, Cos-7 cells (at 60% confluency) in 100 mm dish were used for transfection. The receptor expression levels in transfected Cos-7 cells are usually within the range of pmol mg−1 of protein (Pang et al., 1998).
Assay for inositol phosphate (IP) formation
At 1 day after transfection, Cos-7 cells were labeled for 18 h with 0.75 ml of inositol-free DMEM containing [3H]-myo-inositol (5 μCi ml−1) and 10% HIFCS, followed by serum starvation for 18 h. The cells were then pre-treated in assay medium (20 mM HEPES-buffered DMEM with 20 mM LiCl) for 10 min, and subsequently stimulated in the presence or absence of the indicated drugs for 30 min at 37°C. The reactions were terminated by aspiration of drug-containing medium, followed by the addition of ice-cold 20 mM formic acid solution. After 1 h incubation at 4°C, cell extracts were subjected to ion exchange chromatography as described previously (Tsu et al., 1995).
cAMP assay
Transfected Cos-7 cells were labeled with 2 μCi ml−1 of [3H]adenine in DMEM (10% HIFCS, v v−1) for 18 h. After the serum starvation for 18 h, cells were treated with the assay medium (DMEM containing 20 mM of HEPES and 1 mM of 1-methyl-3-isobutylxanthine) in the presence or absence of the indicated drugs for 30 min at 37°C. The reactions were terminated by aspiration of drug-containing medium, followed by the addition of ice-cold 5% trichloroacetic acid (TCA) solution with 1 mM ATP (1 ml per well) and kept at 4°C for 1 h. Intracellular levels of [3H]-cAMP were determined by sequential chromatography as described previously (Chan et al., 2002).
In vitro JNK assay
Transfected Cos-7 cells were serum-starved for 18 h and then treated with various inhibitors as indicated. After that, the cells were stimulated with the appropriated drugs for indicated duration, it was then terminated by washing the cells with phosphate-buffered saline, followed by addition of 500 μl of ice-cold detergent-containing lysis buffer (50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 40 mM NaP2O7, 1% Triton X-100, 1 mM DTT, 200 μM Na3VO4, 100 μM PMSF, 2 μg ml−1 leupeptin, 4 μg ml−1 aprotinin and 0.7 μg ml−1 pepstatin). Lysates obtained were subjected to JNK assay as described previously (Chan et al., 2002; Kam et al., 2003).
PI3K/Akt assay
Transfected Cos-7 cells were serum-starved overnight and then treated individually or simultaneously with GPCR agonists and EGF for 30 min. Stimulated cells were lysed as described in the JNK assay, and the lysate of each sample (30 μg) was resolved in 12% SDS–PAGE. The presence of stimulatory phosphorylation of Akt was detected by the phospho-Akt (Ser308) antibody obtained from Cell Signaling Technology (Beverly, MA, U.S.A.).
Preparation of labeled substrate for phosphatase reaction
32P-labeled myelin basic protein (MBP) was prepared as described by the supplier (New England Biolabs). Briefly, 40 μl of a Abl (1250 U ml−1) and MBP (18.5 mg ml−1) mixture was mixed with 20 μl of 10 × reaction buffer (500 mM Tris–HCl, pH 7.5, 100 mM MgCl2, 10 mM EGTA, 20 mM DTT and 0.1% Brij 35), 20 μl of ATP buffer (10 mM, containing 50 μCi of [γ-32P]ATP) and H2O to make up a final volume of 200 μl. Incubation was performed overnight at 30°C to allow complete phosphorylation of MBP. The reaction was terminated by adding 1/9 volume of 100% TCA, left on ice for 30 min, then centrifuged at 12,000 × g for 10 min at 4°C. The pellet was washed five times with 1 ml of 20% TCA to remove excess [γ-32P]ATP, and then dissolved in 1 ml of solubilization buffer (50 mM Tris–HCl, pH 8.5, 0.1 mM Na2EDTA, 2 mM DTT and 0.01% Brij 35) to obtain a substrate solution of 20 μM MBP (with 4 mol of 32P per mole of MBP). This substrate solution was diluted with phosphatase assay buffer (50 mM Tris–HCl, pH 7.0, 0.1 mM Na2EDTA, 5 mM DTT and 0.01% Brij 35) to 50 μM with respect to the incorporated 32P, stored at 4°C and used within two half-lives of 32P.
In vitro MKP-1 assay
Cos-7 cells transiently expressing the ORL1 receptor in 100 mM dishes were serum-starved for 18 h and then stimulated by nociceptin/orphanin FQ (OFQ; 100 nM) and EGF (100 ng ml−1) of different durations. The stimulation was terminated by the lysis buffer (1 ml) used in JNK assay without the tyrosine phosphatase inhibitor Na3VO4. The supernatant was collected for each sample by centrifugation at 16,000 × g for 5 min. In all, 50 μl of each supernatant was used for the detection of MKP-1 expression in Western blot, and the remaining was incubated overnight at 4°C with MKP-1 antibody (5 μg per sample), followed by incubation with 50 μl of protein A-agarose (50% slurry) at 4°C for 1 h. The resulting immunoprecipitates were washed twice with the lysis buffer and then twice with the phosphatase assay buffer. Washed immunoprecipitates were resuspended in 40 μl of phosphatase assay buffer with or without 200 μM of Na3VO4, and the phosphatase reactions were initiated by addition of 10 μl of 32P-labeled MBP (as described above), and a blank reaction (without cell extract) was set up as a control. The reaction mixtures were incubated at 30°C for 15 min and then terminated by adding 200 μl of ice-cold 20% TCA, mixed and placed on ice for 10 min. After centrifugation at 16,000 × g for 5 min, 200 μl of TCA supernatant was transferred to 2 ml of aqueous-compatible scintillation fluid for counting of released phosphate. The MKP-1 activity was interpreted as the ratio of the phosphate counts between agonist-treated samples and the untreated sample (the basal), while the phosphate count of the blank reaction was subtracted from each sample count before.
Measurement of intracellular Ca2+ transient by FLIPR®
Cos-7 cells were seeded in 96-well plates (clear bottom and black wall) at 2.5 × 104 cells per well and transfected with various receptor cDNAs as indicated. Transfected cells were subjected to 18 h of serum-starvation, and then washed with HBSS, aspirated, followed by addition of 100 μl of HBSS per well. In all, 100 μl of FLIPR fluorescent dye (with 2.5 mM probenecid) was added to each well before incubation at 37°C for 1 h. Finally, the 96-well plate containing the labeled cells was transferred to the Fluorometric Imaging Plate Reader (FLIPR), and 50 μl of HBSS (with or without indicated agonists) was added to each well. The fluorescent signals that reflect the intracellular Ca2+ transients were monitored by an excitation wavelength of 488 nM and detection with the emission wavelength from 510 to 570 nM.
Results
Regulation of phospholipase C and adenylyl cyclase activities by GPCRs of different coupling specificities
Growing evidences have suggested that certain GPCRs are functionally coupled to more than one family of G proteins (Eason & Liggett, 1995). In order to study how different G protein signals cross-talk with other transmembrane receptor systems, one has to clearly establish the G protein-coupling specificities of the GPCRs in question. This can be demonstrated by their stimulatory or inhibitory effects on adenylyl cyclase and PLC. In total, 12 GPCRs were examined in Cos-7 cells (Table 1). Activation of Gs-coupled dopamine D1 receptor (D1R), lutropin hormone receptor (LHR), secretin receptor (SecR) and vasopressin V2 receptor (V2R) in transfected Cos-7 cells was associated with significant increase of cAMP formation (Table 1), while Gi-coupled opioid receptor-like receptor (ORL1R), dopamine D2 receptor (D2R), somatostatin type I receptor (SSTR1) and melatonin MT1 receptor (MT1R) were capable of suppressing forskolin-stimulated cAMP accumulation (Table 1). However, increased IP formation was observed upon activation of Gs-coupled SecR and V2R, but not for the D1R and LHR, and none of the Gi-coupled receptors examined were able to stimulate IP formation significantly (Table 1). These results showed that ORL1R, D2R, SSTR1 and MT1R were suitable candidates for Gi-coupled receptors, while D1R and LHR were better choices for Gs-coupled receptors in term of coupling specificities. Gq-coupled gastrin-releasing peptide preferring bombesin receptor (GRPR), bradykinin type II receptor (BK2R), muscarinic M1 receptor (M1R) and histamine H1 receptor (H1R) were effective at stimulating intracellular IP formation, but M1R and H1R were linked to increased cAMP level (Table 1); hence GRPR and BK2R seem to be the more suitable representatives for receptors coupled to Gq.
Table 1.
JNK activation in response to EGF treatment and GPCRs of different coupling specificities
| JNK activity (fold induction)a | cAMP level | IP level | |||||
|---|---|---|---|---|---|---|---|
| GPCR | GPCR agonists | Agonist | EGF | Agonist + EGF | % increaseb | % decreasec | % increased |
| Gs-coupled | |||||||
| D1R | Dopamine (10 μM) | 1.4±0.2 | 1.7±0.3 | 2.1±0.5 | 1156±78** | NA | 10±12 |
| LHR | Chorionic | 1.5±0.1 | 1.8±0.2 | 2.2±0.4 | 367±63** | NA | 14±19 |
| Gonadotropin (1 μg ml−1) | |||||||
| SecR | Secretin (1 μM) | 4.6±0.6 | 1.6±0.1 | 4.9±0.6 | 2897±121** | NA | 712±63** |
| V2R | Vasopressin (100 nM) | 2.3±0.2 | 1.8±0.2 | 2.8±0.4 | 1342±119** | NA | 246±59** |
| Gi-coupled | |||||||
| ORL1R | OFQ (100 nM) | 2.5±0.4 | 2.0±0.3 | 6.3±1.1* | NA | 29±11*** | 14±15 |
| D2R | Dopamine (10 μM) | 2.3±0.3 | 2.0±0.1 | 6.0±1.0* | NA | 26±8*** | 19±22 |
| SSTR1 | Somatostatin (100 nM) | 2.5±0.6 | 1.9±0.2 | 5.9±0.5* | NA | 28±7*** | 16±39 |
| mt1R | Melatonin (100 nM) | 2.2±0.2 | 2.1±0.4 | 5.6±0.6* | NA | 30±8*** | 23±21 |
| Gq-coupled | |||||||
| GRPR | Bombesin (100 nM) | 6.7±0.7 | 1.7±0.1 | 7.1±0.6 | 6±18 | NA | 1209±128** |
| BK2R | Bradykinin (100 nM) | 6.8±0.9 | 2.0±0.4 | 7.7±1.2 | 4±29 | NA | 468±77** |
| M1R | Carbachol (200 μM) | 5.9±0.9 | 1.6±0.1 | 6.6±1.0 | 523±74** | NA | 833±67** |
| H1R | Histamine (100 μM) | 6.2±0.5 | 1.8±0.1 | 7.0±0.7 | 356±52** | NA | 642±87** |
Cos-7 cells were transfected with the cDNAs encoding different GPCRs in the absence (for cAMP and IP assays) or presence (for JNK assay) of JNK-HA. Assays were performed as described in Methods. The JNK activities were determined at 30 min after individual or co-treatment with specific GPCR agonists (as indicated) and EGF (100 ng ml−1). For cAMP assays, transfected cells were stimulated with the corresponding agonists in the absence (for Gs- and Gq-coupled receptors) or presence (for Gi-coupled receptors) of forskolin (10 μM) co-treatment for 30 min. For IP assays, agonists were administered to the transfected cells for 30 min.
Values shown represent the mean±s.e. from at least three separate experiments, with the basal JNK activity (in the absence of GPCR agonists) defined as one-fold induction.
Data represent the mean±s.e. of three separate experiments, with the basal level defined as 100%.
Data represent the mean±s.e. of three separate experiments, with the forskolin-enhanced cAMP level defined as 100%. Data analysis was performed by Bonferroni's corrected t-test.
Co-administration of GPCR agonists and EGF triggered the JNK activations synergistically as compared to their individual responses (two-way ANOVA, P<0.05).
Agonist treatment significantly increased cAMP or IP formation as compared to the basal levels (one-way ANOVA, P<0.05).
Agonist treatment significantly suppressed the forskolin-induced cAMP formation (one-way ANOVA, P<0.05). NA, not applicable.
Receptors specifically coupled to Gi, but not Gs and Gq, induced synergistic JNK activation upon co-stimulation of EGFR by EGF
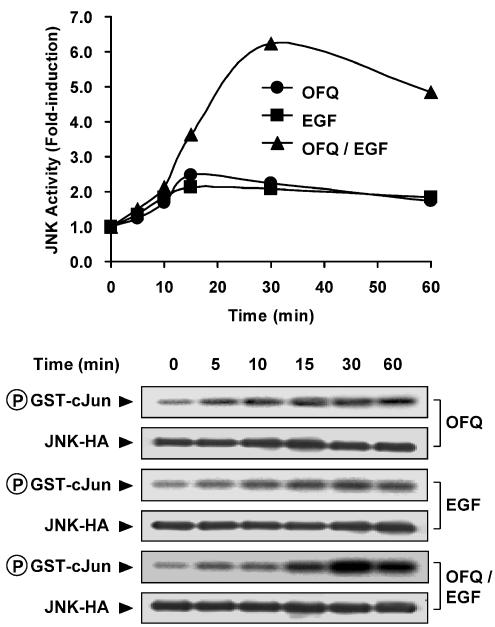
Recent studies demonstrated that both GPCRs and RTKs are linked to activation of JNK (Logan et al., 1997; Chan et al., 2002); in order to examine how these two transmembrane signaling systems co-operate in the regulation of this kinase activity, we transfected Cos-7 cells which endogenously express EGFR, with HA-tagged JNK and GPCRs of different coupling specificities. EGF treatment was associated with a roughly doubled JNK activity, while stimulation of GPCRs induced kinase activation of different magnitudes, with ∼2-fold induction for Gi, ∼1.5-fold for Gs and ∼6-fold for Gq-coupled receptors as compared to their basal responses which were defined as 1.0-fold of the kinase activity (Table 1). Gs-coupled receptors with moderate to weak stimulations of PLC, such as SecR and V2R (Table 1), triggered higher JNK activity than D1R and LHR (Table 1). However, Gq-coupled M1R and H1R, which were associated with weak adenylyl cyclase activation (Table 1), enhanced JNK activity of similar magnitudes as observed for GRPR and BK2R (Table 1). Interestingly, when both GPCR agonists and EGF were co-administered, the resulting JNK activities were stimulated in a differential manner with respect to the coupling specificities of the GPCRs. All of the four Gi-coupled receptors induced a synergistic JNK activation upon co-stimulation with EGF, while stimulation of Gs- and Gq-coupled receptors was not linked to this characteristic upon co-treatment with EGF (Table 1). Using Gi-coupled ORL1R as an example, we revealed that this synergistic activation of JNK was characterized by a slightly delayed time course (maximal at 30 min) as compared to the individual response for ORL1R or EGF stimulation (maximal at around 15–30 min), and the resulting JNK activities remained higher than the basal at 60 min after addition of agonists (Figure 1).
Figure 1.
Time-dependent activation of JNK in response to individual or co-stimulation of EGFR and Gi-coupled ORL1R with EGF and OFQ, respectively. Cos-7 cells co-expressing HA-tagged JNK (JNK-HA) together with ORL1R were stimulated with OFQ (100 nM) and EGF (100 ng ml−1) individually or simultaneously for increasing durations (0–60 min). The expression of JNK-HA for each sample was detected by 12CA5 (Anti-HA) antibody, and the phosphorylation level (32P incorporation) of GST-c-Jun is also illustrated. Data shown represent the averaged values from two separate experiments.
Src family tyrosine kinases, PI3K and Ca2+/calmodulin, (CaM) are important signaling intermediates for the Gi/EGF-induced synergistic JNK activation
To further explore the signaling components involved in the synergistic JNK activation upon co-stimulation of Gi and EGF signaling, we performed pretreatment with target-specific inhibitors before addition of agonists. PTX treatment significantly diminished the ORL1R-induced JNK activation, but had little or no effect on the EGF-induced response (Table 2). AG1478, an EGFR inhibitor, completely suppressed the kinase activation by EGF, however, it had only slight and insignificant inhibitory effect on kinase activity regulated by ORL1R (Table 2). Both PTX and AG1478 significantly inhibited the synergistic JNK activation upon co-activation of ORL1R and EGFR (Table 2). These results suggested that ORL1R was highly dependent on PTX-sensitive Gi proteins instead of functional EGFR to activate the JNK cascade. On the other hand, EGF-induced EGFR activation and the subsequent JNK stimulation were independent on PTX-sensitive Gi proteins. Expression of transducin as a Gβγ scavenger significantly inhibited the JNK activation induced by Gi-coupled ORL1R or by co-stimulation with EGF, while the kinase response triggered by EGF alone was not affected (Table 2). Hence, the Gβγ subunits released upon Gi activation seem to be a major player for the Gi-induced JNK activity, and for the synergistic kinase response towards co-stimulation with EGF.
Table 2.
Src family tyrosine kinases, PI3 K and Ca2+/CaM are important signaling intermediates for the synergistic JNK activation in response to Gi/EGF signaling
| JNK activity (fold induction) | ||||
|---|---|---|---|---|
| Pretreatment | Basal | OFQ | EGF | OFQ+EGF |
| Control | 1.0±0.0 | 2.5±0.4 | 2.0±0.3 | 6.3±1.1 |
| PTX | 1.0±0.1 | 1.3±0.1* | 1.8±0.2 | 1.8±0.2* |
| AG1478 | 1.1±0.2 | 1.9±0.3 | 1.1±0.3* | 2.2±0.1* |
| Transducin | 1.0±0.1 | 1.4±0.2* | 1.9±0.2 | 3.7±0.5* |
| Radicicol | 1.2±0.2 | 1.6±0.2* | 1.8±0.2 | 3.8±0.4* |
| Wortmannin | 1.1±0.1 | 2.6±0.2 | 1.4±0.1* | 3.8±0.3* |
| BAPTA-AM | 0.8±0.1 | 0.9±0.1* | 0.8±0.1* | 0.9±0.1* |
| W-7 | 1.4±0.2 | 1.6±0.4* | 1.6±0.5* | 2.1±1.0* |
Cos-7 cells expressing ORL1R and JNK-HA were pretreated with PTX (100 ng ml−1, overnight), AG1478 (500 nM, 30 min), radicicol (10 μM, 3 h), wortmannin (100 nM, 15 min), BAPTA-AM (50 μM, 30 min) and W-7 (50 μM, 30 min), or co-transfected with transducin. The cells were then stimulated with OFQ (100 nM) and EGF (100 ng ml−1) individually or simultaneously for 30 min before determining the JNK activities. Values shown represent the mean±s.e. from three separate experiments.
Pretreatment of inhibitors or co-expression of transducin significantly suppressed the JNK activation in response to individual or simultaneous administration with OFQ and EGF (Bonferroni's corrected t-test, one-way ANOVA, P<0.05).
The roles of Src family tyrosine kinases and PI3 K isoforms in regulating the receptor-mediated activation of various MAPK had also been suggested by several groups (Luttrell et al., 1996; 1998), and the functional disruption of these molecules by specific inhibitors (radicicol for Src family tyrosine kinases and wortmannin for PI3K) revealed that the ORL1R response was sensitive to inhibition on Src family kinases rather than PI3K, while it was reversed in the case of EGFR (Table 2). The synergistic JNK activation triggered by co-stimulation of these two receptors was also suppressed by radicicol or wortmannin pretreatment (Table 2), or by other inhibitors for Src family kinases and PI3K (PP2 and LY294002, respectively, data not shown). Further experiments demonstrated that both ORL1R- and EGFR-induced JNK activations were highly sensitive to Ca2+ depletion and CaM antagonism by BAPTA-AM and W-7, respectively, and both of them were capable of inhibiting the synergistic kinase response (Table 2).
Co-stimulation with EGF did not significantly affect the GPCR-regulated phospholipase C and adenylyl cyclase activities
Adenylyl cyclase and PLC are the two effectors immediately downstream of G proteins, and their enzymatic products (cAMP for adenylyl cyclase, DAG and IP3 for PLC) have been suggested to have regulatory effects on JNK (Li et al., 1997; Eguchi et al., 2001; Yamauchi et al., 2001). It would be interesting to determine if EGF signaling is capable of modulating GPCR-mediated PLC and adenylyl cyclase activities, and results in modified signal transduction events for JNK. However, EGF treatment itself did not significant affect cAMP or IP accumulation (Table 3), and co-stimulation of Gi-coupled ORL1R and EGFR remained unable to increase the levels of these two second messengers (Table 3). EGF co-treatment neither potentiated the elevation of cAMP level by Gs-coupled D1R, nor facilitated the formation of IP by Gq-coupled GRPR. Moreover, D1R and GRPR remained ineffective in triggering IP and cAMP elevations, respectively, despite co-administration with EGF (Table 3). Hence, it was unlikely that the Gi/EGF-triggered synergistic JNK activation was associated with changes in the adenylyl cyclase and PLC activities.
Table 3.
Co-stimulation with EGF did not significantly affect the adenylyl cyclase and phospholipase C activities induced by agonists of GPCRs
| Drug-induced responses (fold induction) | ||||
|---|---|---|---|---|
| GPCR | Response | Agonist | EGF | Agonist+EGF |
| D1R | cAMP level | 12.8±0.8* | 1.3±0.4 | 13.7±1.5* |
| IP level | 1.0±0.2 | 1.2±0.2 | 1.3±0.2 | |
| ORL1R | cAMP level | 0.8±0.2 | 1.1±0.3 | 1.4±0.6 |
| IP level | 1.0±0.2 | 1.2±0.1 | 1.3±0.2 | |
| GRPR | cAMP level | 1.1±0.4 | 1.1±0.3 | 1.2±0.2 |
| IP level | 9.7±0.5* | 1.2±0.1 | 10.1±0.6* | |
Cos-7 cells were transfected with the cDNAs encoding Gs-coupled D1R, Gi-coupled ORL1R or Gq-coupled GRPR, and then labeled with [3H]adenine for cAMP assay, or [3 H]myo-inositol for IP assay. The cAMP and IP formation, which reflects the activity of adenylyl cyclases and phospholipase C, respectively, was determined at 30 min after individual or co-stimulation with specific agonists (10 μM Dopamine for D1R, 100 nM OFQ for ORL1R, 100 nM bombesin for GRPR and 100 ng ml−1 EGF for EGFR). Data represent the mean±s.e. from three separate experiments, with the corresponding basal activities defined as one-fold of induction.
Agonist treatment significantly increased the cAMP or IP formation over basal levels (Bonferroni's corrected t-test, one-way ANOVA, P<0.05).
Co-stimulation with GPCR agonists and EGF did not co-operate in a synergistic manner on induced Ca2+ transients
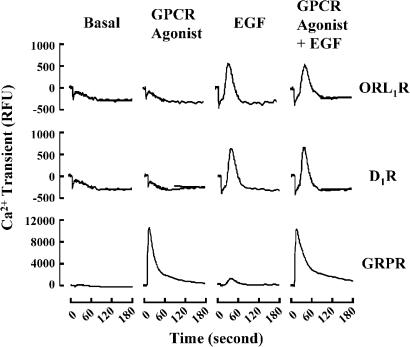
Activation of EGFR by EGF resulted in recruitment of various signaling components including the PLCγ (Tinhofer et al., 1996), while Gq-coupled receptors are linked to the stimulation of PLCβ (Piiper et al., 1997). Both isoforms of PLC are capable of hydrolyzing PIP2 into DAG and IP3, which in turn leads to PKC activation and Ca2+ release. Our recent study of Gq-mediated signaling showed that Ca2+, rather than PKC, served as effective activating signal on JNK pathway (Chan & Wong, 2004). Although EGF induced a weak but insignificant elevation of IP level (Table 3), the more sensitive Ca2+ measurement by FLIPR assay indicated an EGF-induced Ca2+ transient (Figure 2). A greater Ca2+ transient was associated with stimulation of Gq-coupled GRPR, while no enhanced response was observed for Gi-coupled ORL1R and Gs-coupled D1R, and co-stimulation of both GPCRs and EGFR did not further magnify the subsequent Ca2+ release (Figure 2). These results demonstrated that the Gi/EGF-triggered synergistic JNK activation was not due to further elevation of Ca2+ transients upon co-stimulation of the two receptor systems.
Figure 2.
Co-stimulation with GPCR agonists and EGF did not co-operate in a synergistic manner on the induced Ca2+ transients. Cos-7 cells were transfected with Gs-coupled D1R, Gi-coupled ORL1R or Gq-coupled GRPR. Transfected cells were labeled with the FLIPR® fluorescent dye for 1 h, followed by individual or co-stimulation with specific agonists (10 μM dopamine for D1R, 100 nM OFQ for ORL1R, 100 nM bombesin for GRPR and 100 ng ml−1 EGF for EGFR) for the time interval as indicated. Traces represent the averaged values of three separate experiments. The panel for GRPR was rescaled for curve fitting. RFU, relative fluorescent unit.
Gβγ subunits and Ca2+ transient may serve as two major inputs from the Gi and EGF signaling, respectively, to trigger the synergistic JNK activation
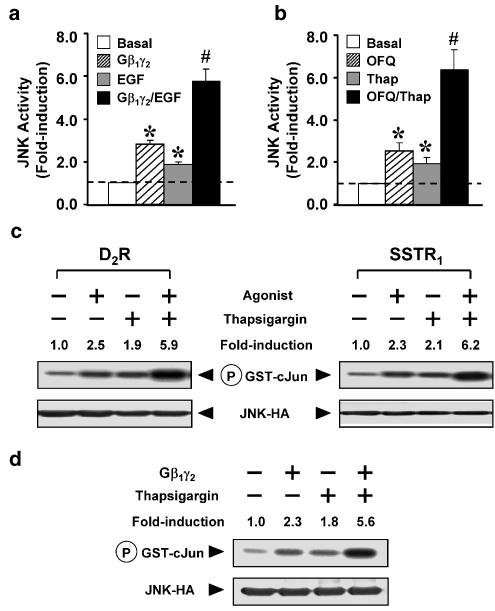
It has been suggested that Gβγ subunits released from G proteins upon GPCR activation play a stimulatory role for JNK activity (Coso et al., 1996). On the other hand, as an important intracellular secondary messenger, Ca2+ can also modulate the JNK pathway (Li et al., 1997). Since the relatively high JNK activation for Gq-mediated signaling could be a co-operative effect between Gβγ and Ca2+ (Chan & Wong, 2004), we suspected that the Gβγ component of Gi, and the Ca2+ transient from EGF signaling, might also co-operate with each other and thus account for the Gi/EGF-induced synergistic JNK activation. Cos-7 cells transiently transfected with Gβ1γ2 were associated with increased JNK activity (Figure 3a), and EGF treatment in the absence of transient Gβ1γ2 expression also stimulated JNK as described previously. Interestingly, a synergistic JNK activation was obtained when both Gβ1γ2 expression and EGF treatment were co-administered (Figure 3a). The same phenomenon can also be observed when different Gi-coupled receptors were co-stimulated with thapsigargin (Figure 3b, c), a chemical agent which is extensively used for elevating the cytoplasmic Ca2+ level. Further investigation showed that co-administration of Gβ1γ2 and thapsigargin also induced this synergistic JNK response (Figure 3d). These results supported the idea that Gβγ and Ca2+ may serve as critical inputs from Gi and EGF signaling, respectively, co-operating with each other to modulate the JNK activation in a synergistic manner.
Figure 3.
Gβγ signaling and Ca2+ transient may serve as two major inputs from the Gi and EGFR, respectively, to trigger the synergistic JNK activation. (a) Cos-7 cells expressing JNK-HA with or without Gβ1 and Gγ2 subunits were stimulated in the absence or presence of EGF (100 ng ml−1) for 30 min. (b) ORL1R and JNK-HA were co-transfected into Cos-7 cells, followed by individual or simultaneous stimulation with OFQ (100 nM) and thapsigargin (Thap, 5 μM) for 30 min before determining the JNK activity. Data shown represent the mean±s.e. from three separate experiments, and dotted lines indicate the corresponding basal activities. (a, b) *Transient expression of Gβ1γ2 subunit and the treatment with EGF, OFQ or thapsigargin significantly increased the JNK activity as compared to the basal (Bonferroni's corrected t-test, one-way ANOVA, P<0.05). #Co-administration of Gβ1γ2 with EGF, or OFQ with thapsigargin induced JNK activations synergistically as compared to their individual responses (Bonferroni's corrected t-test, two-way ANOVA, P<0.05). (c) Cos-7 cells expressing JNK-HA with either Gi-coupled SSTR1 or D2R were also capable of inducing a synergistic JNK activation upon co-treatment with their agonists (100 nM somatostatin for SSTR1 and 10 μM dopamine for D2R) and thapsigargin. (d) Cos-7 cells co-transfected with the cDNAs of JNK-HA with or without Gβ1 and Gγ2 subunits were stimulated in the absence or presence of thapsigargin (Thap, 5 μM, 30 min) before determining the JNK activity. (c, d) Both expression of JNK-HA and the phosphorylation of GST-c-Jun are illustrated. Data of JNK activity (fold-induction) represent the averaged values of two separate experiments.
Gs/cAMP signaling produced an inhibitory effect on Ca2+-induced JNK activation and was incapable of stimulating the PI3K/Akt activity
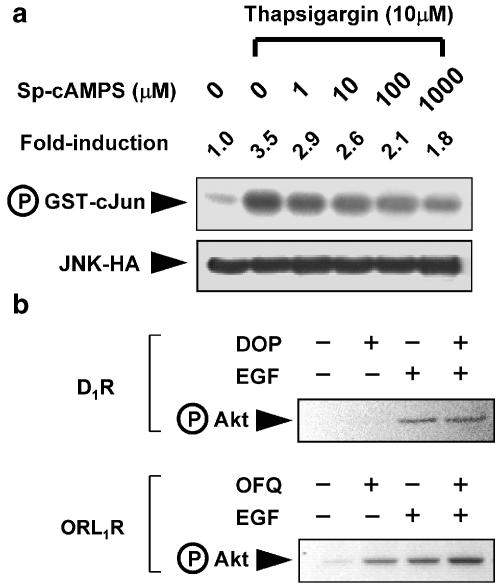
One might argue why Gβγ released from Gs did not synergize with EGF-induced Ca2+ transient to regulate JNK activity. Current knowledge for the functional diversity of different Gβγ isoforms is very limited. However, the opposing regulatory effects of Gi and Gs on adenylyl cyclase-induced cAMP formation could be one of the reasons behind this differential activation of JNK. When Cos-7 cells were treated with thapsigargin to mimic a Ca2+-induced JNK activation, co-administration of Sp-cAMPS (a cell-permeable cAMP analog) effectively suppressed the JNK activity in a dose-dependent manner (Figure 4a), while Sp-cAMPS itself did not significantly activate JNK in the same cells (100 μM for 30 min; 1.2±0.2-fold induction as compared to the basal activity). Moreover, activation of Gs-coupled D1R (Figure 4b) and administration of Sp-cAMPS were not linked to stimulatory phosphorylation of Akt, a major downstream effector of PI3 K, while Gi-linked ORL1R was capable of triggering Akt activation, and potentiated the effect of EGF on the same response (Figure 4b). Since PI3 K is one of the activators for JNK cascade (Logan et al., 1997), and the dependence of functional PI3 K activity for the Gi/EGF-induced synergistic JNK activation (Table 2), the lack of this basic requirement and the possible cAMP-mediated inhibitory effect may account for the inability of Gs-coupled receptors to synergize with the EGF signaling in terms of JNK activation.
Figure 4.
Gs/cAMP signaling suppressed Ca2+-induced activation of JNK, and did not trigger stimulatory phosphorylation of Akt. (a) Cos-7 cells transfected with JNK-HA were treated with thapsigargin (10 μM, 30 min) in the absence or presence of increasing concentration of Sp-cAMPS (0–1000 μM) before determining the JNK activity. The expression of JNK-HA and the phosphorylation of GST-c-Jun are illustrated. Fold induction of JNK activities represent averaged values from two separate experiments. (b) Cos-7 cells expressing Gs-coupled D1R and Gi-coupled ORL1R were stimulated individually or simultaneously with EGF and their agonists (10 μM dopamine (DOP) for D1R, and 100 nM OFQ for ORL1R). The induced stimulatory phosphorylation of Akt was detected by the anti-phospho-Akt (Ser308) antiserum.
The synergism of Gi and EGF signaling may occur at a level upstream of small GTPases
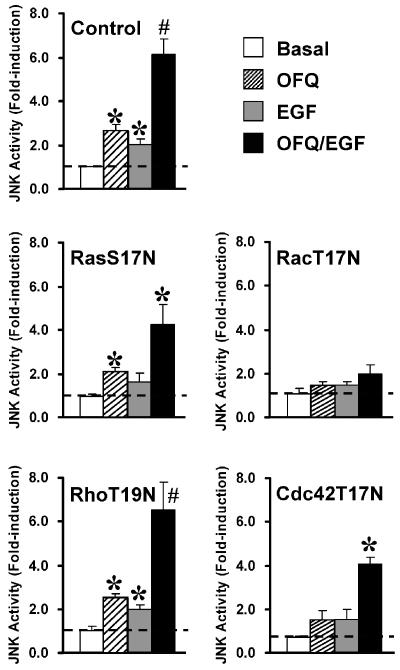
Ras and Rho-related small GTPases have been demonstrated as critical modulators for receptor-mediated JNK activation (Fanger et al., 1997). Functional disruption of small GTPases by expression of their dominant-negative mutants (RasS17N, RacT17N, RhoT19N and Cdc42T17N) is an extensively used method to examine their possible involvement (Chan & Wong, 2000; Chan et al., 2002; Kam et al., 2003). Transient expression of RacT17N significantly diminished the JNK activation upon individual and co-stimulation of Gi-coupled ORL1R and EGFR by OFQ and EGF, respectively (Figure 5). Other mutants such as RasS17N and Cdc42T17N showed weaker inhibitory effects on the induced kinase activation, and no significant changes were associated with RhoT19N (Figure 5). Due to the strong suppressive effect of RacT17N on the JNK activation triggered by individual or co-stimulation of Gi/EGF signaling, the activating signals from Gi-coupled receptor and EGFR may converge at the level of small GTPases, or other signaling intermediates which are positioned upstream of Rac.
Figure 5.
The synergism of Gi and EGF signaling on JNK activity was inhibited by functional disruption of small GTPases. Cos-7 cells were co-transfected with the cDNAs of JNK-HA, ORL1R, and dominant-negative mutants of small GTPases (RasS17N, RacT17N, RhoT19N or Cdc42T17N) as indicated. The JNK activity was determined at 30 min after individual or simultaneous stimulation with OFQ (100 nM) and EGF (100 ng ml−1). Data shown represent the mean±s.e. from at least three separate experiments, and dotted lines indicate the corresponding basal activities. *Individual or simultaneous treatment with OFQ and EGF significantly increased the JNK activity as compared to the basal (Bonferroni's corrected t-test, one-way ANOVA, P<0.05). #Simultaneous treatment with OFQ and EGF induced JNK activations synergistically as compared to their individual responses (Bonferroni's corrected t-test, two-way ANOVA, P<0.05).
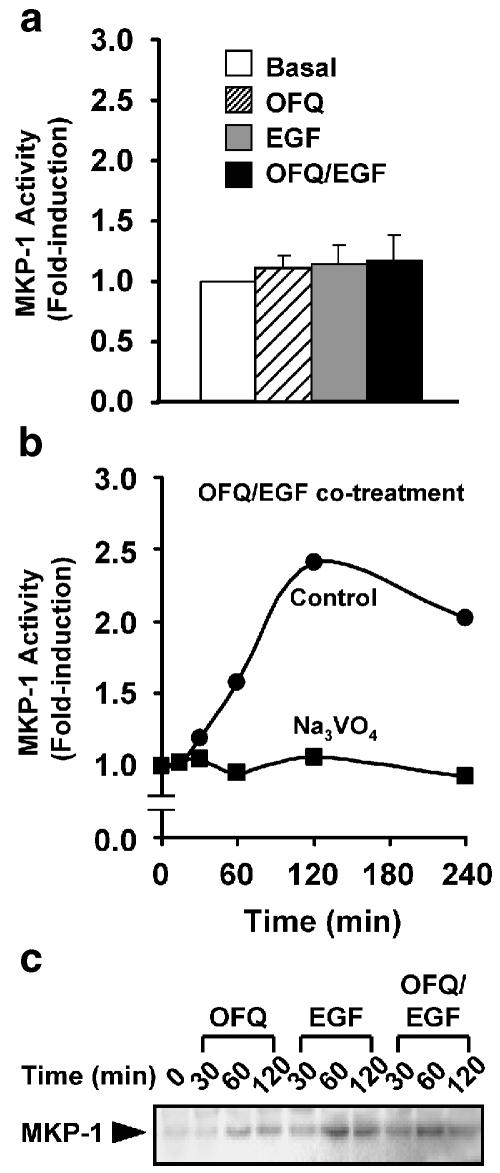
Gi and EGF signaling did not suppress the activity of MKP-1
In addition to an upstream signaling input which positively regulates JNK, an increased activity of the kinase could also be a consequence of decreased negative feedback. Phosphatases of the MKP family dephosphorylate the activation loops of different MAPK subtypes and return them into inactive conformation. The MKP-1 isoform shows a high preference on JNK (Sanchez-Perez et al., 2000), and is expressed in a board range of tissues (Misra-Press et al., 1995). Hence, we examined whether the Gi/EGF-induced synergistic JNK activation was associated with decreased phosphatase activity of MKP-1. The maximal activity of the synergistic JNK activation occurred at 30 min after co-stimulation with EGF and Gi-coupled ORL1R (Figure 1); however, no matter these two stimulatory signals were administered individually or simultaneously, there were no significant changes for the MKP-1 activities up to 30 min of agonist treatment (Figure 6a). Co-stimulation of ORL1R and EGFR gradually increased the MKP-1 activity from 30 to 60 min, reaching the peak at 120 min, and decreased slowly afterwards (Figure 6b). The induced MKP-1 activity was completely inhibited by Na3VO4, an extensively used phosphatase inhibitor for various MAPK and MKP studies (Figure 6b). The expression level of MKP-1 increased gradually for the first 60 min and then decreased with respect to individual stimulation with OFQ or EGF, co-stimulation was not associated with further enhancement of MKP-1 induction as compared to the EGF-induced level (Figure 6c).
Figure 6.
Co-activation of Gi and EGF signaling increased the activity and expression level of MKP-1. (a) Cos-7 cells expressing ORL1R were stimulated individually or simultaneously with OFQ (100 nM) and EGF (100 ng ml−1) for 30 min, or (b) co-stimulated with the two agonists for increasing durations before determining the associated MKP-1 activities in the absence or presence of Na3VO4 (200 μM). Data shown represent the mean±s.e. from three separate experiments (a) or the averaged values of two separate experiments (b). (c) The time-dependent expression pattern of MKP-1 in response to agonist treatments was detected by anti-MKP-1 M-18 antiserum, which recognizes the C-terminus of MKP-1.
Discussion
It is important to clearly define what effectors are linked to the GPCRs under investigation; otherwise, the observed activities will be the integrated responses brought about by multiple effectors, if the examined GPCRs are efficiently coupled to various G protein families. It can be verified by the observation that GPCRs with strong coupling toward Gs (e.g. D1R and LHR), Gi (e.g. ORL1R, D2R, SSTR1 and MT1R) and Gq (e.g. GRPR and BK2R) were linked to increasing capabilities of JNK stimulation (i.e Gs<Gi<Gq). Receptors showing strong coupling with Gq but weak coupling with Gs (e.g. M1R and H1R) stimulated JNK to similar magnitudes as those of Gq-linked GRPR and BK2R. If the situation was reversed as for SecR and V2R, the integration of strong Gs and weak Gq signaling resulted in JNK activities in between the Gs (e.g. D1R) and the Gq (e.g. GRPR)-mediated responses. Among the 12 GPCRs investigated in this report, only Gi-coupled ORL1R, D2R, SSTR1 and MT1R were able to induce a synergistic activation of JNK upon co-stimulation with EGF, while Gs-coupled D1R, LHR, Gq-coupled GRPR, BK2R and receptors showing both Gs and Gq coupling (SecR, V2R, M1R and H1R) were incapable of augmenting the EGF response. Although previous studies suggested that ORL1R might be coupled to the G12 protein, this effect is negligible as compared to its major Gi signaling (Chan & Wong, 2000). ORL1R appears to predominantly utilize a Gβγ/Src family kinase-dependent pathway to stimulate JNK, as in the cases for other Gi-coupled receptors (Chan et al., 2002).
For a synergistic JNK activity to occur, stimulatory signals from the participating systems have to co-operate in a particular manner. Our results suggested that Gβγ subunits released upon Gi activation and the Ca2+ transient induced by EGF signaling may be two critical inputs to induce the synergistic JNK activation. It is consistent with our finding that Src family tyrosine kinases, PI3 K and CaM, were involved in JNK activation, since they have been identified as immediate effectors (Src family tyrosine kinases and PI3 K) and modulator (CaM) for Gβγ and Ca2+, respectively (Schulman & Greengard, 1978; Luttrell et al., 1996). Our previous studies demonstrated that Src family tyrosine kinases, rather than PI3K, serve as important intermediates towards Gi-induced JNK activation (Kam et al., 2003). However, the reason for differential preferences between these two intermediates remains unclear. In contrast, EGF-mediated stimulation of JNK showed a higher dependency on PI3 K (Table 2). In this report, we have shown that activation of Akt, an event mediated by the PI3K-produced phospholipids, was further increased upon co-stimulation of Gi/EGF signaling, while additional enhancement for the activities of PLC, adenylyl cyclase and the induced Ca2+ transient were not observed. Hence, in addition to the Gβγ/Src family tyrosine kinase signaling from Gi and the induced Ca2+ transient by EGF treatment, the enhanced PI3 K signals induced by co-activation of the two receptor systems may also positively regulate the synergistic JNK activation. In fact, the β-isoform of PI3 K has been suggested as an integration point for signals received from Gβγ and receptor tyrosine kinases (Murga et al., 2000). Our recent study showed that activation of Gq-mediated receptors contributes the critical signals (i.e. Src family kinases, PI3 K and Ca2+) required for this synergistic JNK activation (Chan & Wong, 2004), thus resulting in a robust stimulation of JNK that cannot be further enhanced by EGF (Table 1).
Previous studies suggested that G protein-induced MAPK activation requires transactivation of EGFR, wherein a Src-dependent metalloprotease activity converts proheparin-binding EGF-like growth factor (proHB-EGF) into HB-EGF, which acts as a ligand for EGFR activation (Prenzel et al., 1999). A more recent report demonstrated that Gi-induced ERK activation in Cos-7 cells is only partially dependent on the EGFR transactivation (Pierce et al., 2001). This may also be applicable to the Gi-induced JNK activation, since we only observed a weak but insignificant inhibition on the ORL1R-mediated JNK stimulation in the same cells after pretreatment of the EGFR inhibitor, AG1478. All these findings, together with the differential dependencies on Src family tyrosine kinases and PI3 K for the Gi-coupled receptor and EGF-mediated JNK activation, implied that EGF-induced receptor autophosphorylation and G protein-mediated EGFR transactivation may not be totally equivalent stimulatory events. In fact, binding of HB-EGF to EGFR is dependent on the local concentration of heparin-like molecules expressed on cell surface, while such requirement is not applicable for the interaction between EGF and EGFR (Aviezer & Yayon, 1994). Moreover, among the four RTKs in the EGFR family (ErbB1, ErbB2, ErbB3 and ErbB4), EGF only shows significant binding with ErbB1, while HB-EGF is capable of interacting with ErbB1 and ErbB4 (Paria et al., 1999). Hence, activation of EGFR family through different ligands (e.g. EGF and HB-EGF) may result in differential dimerization, which is probably linked to similar, but not identical signaling events (Schlessinger, 2000).
In agreement with our observation that Sp-cAMPS suppresses the Ca2+-induced activation of JNK, others have shown that administration of other cAMP analogues in μM range is also linked to this inhibitory effect in GN4 cells (Li et al., 1997). However, the identities of proteins involved in this inhibitory mechanism remain unclear. In addition to this cAMP effect, the signals contributed by Gβγ subunits released from Gs could be different from those of Gi. This assumption is supported by the findings that dually coupled β-adrenergic receptors (with Gs and Gi) primarily require Gβγ subunits from Gi to trigger the activation of PI3K/Akt pathway (Jo et al., 2002). In contrast, similar Gβγ subunits may accompany the α-subunits of Gi and Gq (Quitterer & Lohse, 1999). Although it is difficult to determine the precise amount of Gβγ released upon Gi activation in cells transiently transfected with cDNAs encoding Gβγ (0.2 μg per well cDNA for both Gβ1 and Gγ2), our results clearly demonstrated that induction of synergistic JNK activation by Gβγ and EGF is experimentally feasible (Figure 3a). Moreover, the amount of thapsigargin (5 μM) administrated to synergize with Gi signaling (Figure 3b, c) was similar to those applied in various studies on G proteins and MAPKs (Li et al., 1997). On the other hand, activation of Gi-coupled ORL1R did not trigger any Ca2+ transient as in the case of EGF-stimulated EGFR. However, the JNK activities mediated by both receptors were inhibited by disruption of Ca2+/CaM function (Table 2). This result implied that, although Ca2+ elevation is an effective means to stimulate the JNK pathway, a basal physiological Ca2+ level is necessary to maintain the normal function of certain intermediates in the JNK pathway, for stimulatory mechanisms which involve Ca2+ elevation or not.
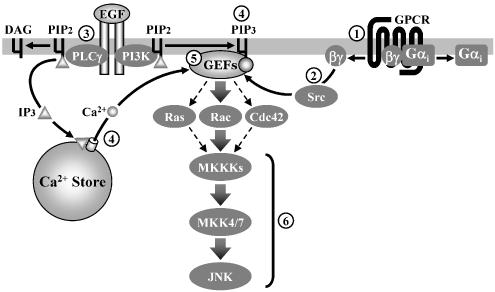
Components which are capable of receiving inputs from multiple signaling intermediates are highly important for the integration of intracellular signals. Members of the guanine nucleotide exchange factors (GEFs) family are capable of regulating the small GTPase activities, which in turn activate the downstream JNK pathway (Fan et al., 1998). GEF proteins such as Ras-GRF1, Ras-GRF2 and Vav isoforms can be stimulated through multiple mechanisms, including phosphorylation by Src family tyrosine kinases (Kiyono et al., 2000), binding of Ca2+ to their CaM-like regions (Fan et al., 1998) and conformational changes induced by interaction with PI3K-phosphorylated lipids (Das et al., 2000). Hence, members of the GEF family may serve as modulators to integrate various inputs (such as Gβγ/Src family tyrosine kinases, PI3K and Ca2+/CaM) from Gi and EGF signaling, and transmit stimulatory signals directly to small GTPases-mediated JNK pathway (Figure 7). This idea is supported by the strong suppressing effect on the synergistic JNK activation when RacT17N was expressed. It is also consistent with the previous reports that Racs serves as an important small GTPase for EGF- and GPCR-mediated JNK activation (Chan et al., 2002; Kam et al., 2003).
Figure 7.
A schematic diagram for the synergistic JNK activation triggered by cross-communication between Gi-coupled receptors and EGF signaling. (1) Activation of Gi-coupled receptors triggers the dissociation of Gi proteins into Gαi and Gβγ subunits, with the former having negligible contribution towards the stimulation of JNK as compared to the latter; (2) Gβγ subunits released from Gi mainly utilize a Src family kinase-dependent pathway to stimulate the JNK cascade; (3) On the other hand, stimulation of EGFR with EGF enables recruitment of PLCγ and PI3K; (4) Stimulated PLCγ and PI3 K induce elevation of intracellular Ca2+ level and phospholipid products (PIP3), respectively; (5) Different signals from activated EGFR and Gi-coupled receptors, such as Src family kinases, Ca2+ and PI3 K may converge at a common locus (e.g. GEFs), which is capable of modulating the activities of small GTPases, and hence the subsequent stimulatory signal through the three-kinase module (6) of JNK cascade (MKKKs → MKK4/7 → JNK).
MAPKs are activated by upstream kinases (i.e. MAPK kinases) which phosphorylate their activation loops, and this stimulatory phosphorylation is reversed by MKPs. MKP-1 has a high substrate preference towards JNK (Sanchez-Perez et al., 2000), and our results showed that its activity was gradually increased, rather than suppressed upon Gi/EGF co-stimulation. Moreover, the induction of MKP-1 and other MKPs is also triggered by MAPK-mediated mechanisms (Zhang et al., 2001). Hence, the Gi/EGF-induced synergistic JNK activation was unlikely to result from decreased inhibitory effects from MKPs.
The biological significance of this differential JNK activation upon co-stimulation of EGFR and GPCRs of different coupling specificities remains unclear. Extensive studies are being performing in our laboratory to investigate the biological consequences of such differential responses in cells endogenously expressing these receptors. Recent studies demonstrated that the magnitude and the duration of MAPK activities could be the critical factors which determine cell fates (Kobayashi & Tsukamoto, 2001). In this report, the Gi/EGF-induced synergistic JNK activity is also associated with a higher magnitude for a given period of time as compared to individual stimulation. This is the first study which demonstrates the possible differential regulation of JNK activity upon co-stimulation with EGF and different GPCRs in a single cell type, with Gs-, Gi- and Gq-coupled receptors showing increasing capability of JNK activation, while EGF acts as a modulating signal to accompany or even synergize with the subsequent kinase response.
Acknowledgments
We thank Eric C.H. Yip and Angel Y.F. Kam for technical support in cell culture, Maggie M.K. Lee for assistance in FLIPR assay and Dr Maurice K.C. Ho for helpful discussion. This work was supported in parts by grants from the University Grants Committee of Hong Kong (AoE/B-15/01), the Research Grants Council of Hong Kong (HKUST 6115/00 M and 2/99C) and the Hong Kong Jockey Club to YHW. YHW was a recipient of the Croucher Senior Research Fellowship.
Abbreviations
- CaM
Ca2+/calmodulin
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MKP
mitogen-activated protein kinase phosphatase
- PI3 K
phosphoinositide-3-kinase
- PTX
pertussis toxin
References
- AKASAKI T., KOGA H., SUMIMOTO H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J. Biol. Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- AVIEZER D., YAYON A. Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc Natl. Acad. Sci. U.S.A. 1994;91:12173–12177. doi: 10.1073/pnas.91.25.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALMFORTH A.J., BALL S.G., FRESHNEY R.I., GRAHAM D.I., MCNAMEE H.B., VAUGHAN P.F. D-1 dopaminergic and beta-adrenergic stimulation of adenylate cyclase in a clone derived from the human astrocytoma cell line G-CCM. J. Neurochem. 1986;47:715–719. doi: 10.1111/j.1471-4159.1986.tb00670.x. [DOI] [PubMed] [Google Scholar]
- BISCARDI J.S., MAA M.C., TICE D.A., COX M.E., LEU T.H., PARSONS S.J. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- CHAN A.S.L., LAI F.P.L., LO R.K.H., VOYNO-YASENETSKAYA T.A., STANBRIDGE E.J., WONG Y.H. Melatonin MT1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and –insensitive G proteins. Cell Signal. 2002;14:249–257. doi: 10.1016/s0898-6568(01)00240-6. [DOI] [PubMed] [Google Scholar]
- CHAN A.S.L., WONG Y.H. Regulation of c-Jun N-terminal kinase by the ORL1 receptor through multiple G proteins. J. Pharmacol. Exp. Ther. 2000;295:1094–1100. [PubMed] [Google Scholar]
- CHAN A.S.L., WONG Y.H. Gβγ signaling and Ca2+ mobilization co-operate synergistically in a Sos and Rac-dependent manner in the activation of JNK by Gq-coupled receptors. Cell Signal. 2004;16:823–836. doi: 10.1016/j.cellsig.2003.12.007. [DOI] [PubMed] [Google Scholar]
- COSO O.A., TERAMOTO H., SIMONDS W.F., GUTKIND J.S. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J. Biol. Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- DAS B., SHU X., DAY G.J., HAN J., KRISHNA U.M., FALCK J.R., BROEK D. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J. Biol. Chem. 2000;275:15074–15081. doi: 10.1074/jbc.M907269199. [DOI] [PubMed] [Google Scholar]
- EASON M.G., LIGGETT S.B. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the α2A-adrenergic receptor: evidence for distinct structural determinants that confer Gsversus Gi coupling. J. Biol. Chem. 1995;270:24753–24760. doi: 10.1074/jbc.270.42.24753. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., DEMPSEY P.J., FRANK G.D., MOTLEY E.D., INAGAMI T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p 38 MAPK but not for JNK. J. Biol. Chem. 2001;276:7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- FAN W.T., KOCH C.A., DE HOOG C.L, FAM N.P., MORAN M.F. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr. Biol. 1998;8:935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- FANGER G.R., JOHNSON N.L., JOHNSON G.L. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JO S.H., LEBLAIS V., WANG P.H., CROW M.T., XIAO R.P. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent G(s) signaling during β2-adrenergic stimulation. Circ. Res. 2002;91:46–53. doi: 10.1161/01.res.0000024115.67561.54. [DOI] [PubMed] [Google Scholar]
- KAM A.Y., CHAN A.S.L., WONG Y.H. Rac and Cdc42-dependent regulation of c-Jun N-terminal kinases by the δ-opioid receptor. J. Neurochem. 2003;84:503–513. doi: 10.1046/j.1471-4159.2003.01535.x. [DOI] [PubMed] [Google Scholar]
- KIYONO M., KAZIRO Y., SATOH T. Induction of rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm) following phosphorylation by the nonreceptor tyrosine kinase Src. J. Biol. Chem. 2000;275:5441–5446. doi: 10.1074/jbc.275.8.5441. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI K., TSUKAMOTO I. Prolonged Jun N-terminal kinase (JNK) activation and the upregulation of p53 and p21(WAF1/CIP1) preceded apoptosis in hepatocytes after partial hepatectomy and cisplatin. Biochim. Biophys. Acta. 2001;1537:79–88. doi: 10.1016/s0925-4439(01)00059-x. [DOI] [PubMed] [Google Scholar]
- KRYMSKAYA V.P., ORSINI M.J., ESZTERHAS A.J., BRODBECK K.C., BENOVIC J.L., PANETTIERI R.A., Jr, PENN R.B. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2000;23:546–554. doi: 10.1165/ajrcmb.23.4.4115. [DOI] [PubMed] [Google Scholar]
- LI X., YU H., GRAVES L.M., EARP H.S. Protein kinase C and protein kinase A inhibit calcium-dependent but not stress-dependent c-Jun N-terminal kinase activation in rat liver epithelial cells. J. Biol. Chem. 1997;272:14996–15002. doi: 10.1074/jbc.272.23.14996. [DOI] [PubMed] [Google Scholar]
- LOGAN S.K., FALASCA M., HU P., SCHLESSINGER J. Phosphatidylinositol-3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol. Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ-ILASACA M., GUTKIND J.S., WETZKER R. Phosphoinositide 3-kinase γ is a mediator of Gβγ-dependent Jun Kinase activation. J. Biol. Chem. 1998;273:2505–2508. doi: 10.1074/jbc.273.5.2505. [DOI] [PubMed] [Google Scholar]
- LOWES V.L., IP N.Y., WONG Y.H. Integration of signals from receptor tyrosine kinases and G protein-coupled receptors. Neurosignals. 2002;11:5–19. doi: 10.1159/000057317. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., HAWES B.E., VAN BIESEN T., LUTTRELL D.K., LANSING T.J., LEFKOWITZ R.J. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- LUTTRELL M., GUTKIND J.S., WETZKER R. Phosphoinositide 3-kinase gamma is a mediator of Gβγ-dependent Jun kinase activation. J. Biol. Chem. 1998;273:2505–2508. doi: 10.1074/jbc.273.5.2505. [DOI] [PubMed] [Google Scholar]
- MISRA-PRESS A., RIM C.S., YAO H., ROBERSON M.S., STORK P.J. A novel mitogen-activated protein kinase phosphatase. Structure, expression, and regulation. J. Biol. Chem. 1995;270:14587–14596. doi: 10.1074/jbc.270.24.14587. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.C. ORL1, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MURGA C., FUKUHARA S., GUTKIND J.S. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- PANG L., HASHEMI T., LEE H.J., MAGUIRE M., GRAZIANO M.P., BAYNE M., HAWES B., WONG G., WANG S. The mouse GalR2 galanin receptor: genomic organization, cDNA cloning, and functional characterization. J. Neurochem. 1998;71:2252–2259. doi: 10.1046/j.1471-4159.1998.71062252.x. [DOI] [PubMed] [Google Scholar]
- PARIA B.C., ELENIUS K., KLAGSBRUN M., DEY S.K. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., TOHGO A., AHN S., FIELD M.E., LUTTRELL L.M., LEFKOWITZ R.J. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J. Biol. Chem. 2001;276:23155–23160. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- PIIPER A., STRYJEK-KAMINSKA D., KLENGEL R., ZEUZEM S. CCK, carbachol, and bombesin activate distinct PLCβ isoenzymes via Gq/11 in rat pancreatic acinar membranes. Am. J. Physiol. 1997;272:G135–G140. doi: 10.1152/ajpgi.1997.272.1.G135. [DOI] [PubMed] [Google Scholar]
- PRENZEL N., ZWICK E., DAUB H., LESERER M., ABRAHAM R., WALLASCH C., ULLRICH A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- QUITTERER U., LOHSE M.J. Crosstalk between Gαi- and Gαq-coupled receptors is mediated by Gβγ exchange. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10626–10631. doi: 10.1073/pnas.96.19.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAZAR E.P., HUNGER-GLASER I., ROZENGURT E. Dissociation of focal adhesion kinase and paxillin tyrosine phosphorylation induced by bombesin and lysophosphatidic acid from epidermal growth factor receptor transactivation in Swiss 3T3 cells. J. Cell Physiol. 2003;194:314–324. doi: 10.1002/jcp.10204. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-PEREZ I., MARTINEZ-GOMARIZ M., WILLIAMS D., KEYSE S.M., PERONA R. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene. 2000;19:5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- SCHULMAN H., GREENGARD P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by ‘calcium-dependent regulator'. Proc. Natl. Acad. Sci. U.S.A. 1978;75:5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TINHOFER I., MALY K., DIETL P., HOCHHOLDINGER F., MAYR S., OBERMEIER A., GRUNICKE H.H. Differential Ca2+ signaling induced by activation of the epidermal growth factor and nerve growth factor receptors. J. Biol. Chem. 1996;271:30505–30509. doi: 10.1074/jbc.271.48.30505. [DOI] [PubMed] [Google Scholar]
- TSU R.C., ALLEN R.A., WONG Y.H. Stimulation of type II adenylyl cyclase by chemoattractant formyl peptide and C5a receptors. Mol. Pharmacol. 1995;47:835–841. [PubMed] [Google Scholar]
- VOYNO-YASENETSKAYA T.A., FAURE M.P., AHN N.G., BOURNE H.R. Gα12 and Gβ13 regulate extracellular signal-regulated kinase and c-Jun kinase pathways by different mechanisms in COS-7 cells. J. Biol. Chem. 1996;271:21081–21087. doi: 10.1074/jbc.271.35.21081. [DOI] [PubMed] [Google Scholar]
- YAMAUCHI J., HIRASAWA A., MIYAMOTO Y., ITOH H., TSUJIMOTO G. β2-adrenergic receptor/cyclic adenosine monophosphate (cAMP) leads to JNK activation through Rho family small GTPases. Biochem. Biophys. Res. Commun. 2001;284:1199–1203. doi: 10.1006/bbrc.2001.5103. [DOI] [PubMed] [Google Scholar]
- ZHANG T., WOLFE M.W., ROBERSON M.S. An early growth response protein (Egr) 1 cis-element is required for gonadotropin-releasing hormone-induced mitogen-activated protein kinase phosphatase 2 gene expression. J. Biol. Chem. 2001;276:45604–45613. doi: 10.1074/jbc.M107075200. [DOI] [PubMed] [Google Scholar]