Abstract
Nitric oxide (NO) and α2-adrenoceptor and imidazoline agonists such as moxonidine may act centrally to inhibit sympathetic activity and decrease arterial pressure.
In the present study, we investigated the effects of pretreatment with L-NAME (NO synthesis inhibitor), injected into the 4th ventricle (4th V) or intravenously (i.v.), on the hypotension, bradycardia and vasodilatation induced by moxonidine injected into the 4th V in normotensive rats.
Male Wistar rats with a stainless steel cannula implanted into the 4th V and anaesthetized with urethane were used. Blood flows were recorded by use of miniature pulsed Doppler flow probes implanted around the renal, superior mesenteric and low abdominal aorta.
Moxonidine (20 nmol), injected into the 4th V, reduced the mean arterial pressure (−42±3 mmHg), heart rate (−22±7 bpm) and renal (−62±15%), mesenteric (−41±8%) and hindquarter (−50±8%) vascular resistances.
Pretreatment with L-NAME (10 nmol into the 4th V) almost abolished central moxonidine-induced hypotension (−10±3 mmHg) and renal (−10±4%), mesenteric (−11±4%) and hindquarter (−13±6%) vascular resistance reduction, but did not affect the bradycardia (−18±8 bpm).
The results indicate that central NO mechanisms are involved in the vasodilatation and hypotension, but not in the bradycardia, induced by central moxonidine in normotensive rats.
Keywords: α2-Adrenoceptors, imidazoline receptors, hypertension, nitric oxide, blood flow, vascular resistance, blood pressure
Introduction
Nitric oxide (NO) is an important mediator that modulates cardiovascular function by a peripheral or central mechanism of action (Gardiner et al., 1990; Zanzinger et al., 1995; Colombari et al., 1998; Kadekaro & Summy-Long, 2000; Patel et al., 2001; Sy et al., 2001). The presence of the enzyme nitric oxide synthase (NOS) that catalyzes the synthesis of NO from L-arginine has been demonstrated in different areas of the central nervous system involved in cardiovascular control; these include the nucleus tractus solitarii (NTS), rostral ventrolateral medulla (RVLM), caudal ventrolateral medulla (CVLM) and paraventricular nucleus of the hypothalamus (PVN) (Vincent & Kimura, 1992; Patel et al., 2001). NO is known to act centrally to decrease sympathetic activity and consequently mean arterial pressure (MAP). Inhibition of NO synthesis by NOS inhibitors injected intracerebroventricularly or into specific central sites involved in cardiovascular regulation, such as the NTS, RVLM and PVN, increases sympathetic activity and MAP (Zanzinger et al., 1995; Kadekaro & Summy-Long, 2000; Patel et al., 2001). It has been suggested that the central NO system is involved in the development of hypertension (Patel et al., 2001); inhibition of central NOS reduces the hypotension induced by centrally acting drugs (Colombari et al., 1998; Dobrucki et al., 2001; Sy et al., 2001).
Moxonidine, an α2-adrenoceptor/imidazoline receptor agonist, is a centrally acting anti-hypertensive drug that reduces arterial pressure by inhibiting sympathetic activity (Ernsberger et al., 1993; 1994; 1997). The RVLM that contains the sympathetic pre-motor neurons involved in cardiovascular regulation (Barman & Gebber, 1989; Guyenet et al., 1989) has been implicated as one of the most important central sites for the anti-hypertensive action of moxonidine. α2-Adrenoceptors and imidazoline receptors have been shown to exist in the RVLM (Feldman et al., 1998) and microinjections of α2-adrenoceptor/imidazoline agonists into the RVLM produce hypotension and bradycardia (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger et al., 1997; Tolentino-Silva et al., 2000). Although α2-adrenoceptors have been shown to be involved in the hypotension produced by clonidine (Guyenet 1997), the anti-hypertensive responses induced by moxonidine have been found to depend on the activation of imidazoline receptors located in the RVLM (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger et al., 1997; Tolentino-Silva et al., 2000). The importance of brain stem mechanisms for the hypotensive responses to moxonidine is also suggested by a reduction in splanchnic sympathetic nerve activity induced by moxonidine injected into the 4th brain ventricle (4th V) in spontaneously hypertensive rats (SHR) (Nurminen et al., 1998). In normotensive rats, moxonidine injected into the lateral cerebral ventricle (LV) has been found to have no effect on MAP or HR (Moreira et al., 2003).
The possible involvement of NO-dependent mechanisms in the responses induced by clonidine and rilmenidine (α2-adrenoceptor/imidazoline receptor agonists), administered centrally or peripherally, has been proposed by several authors (Soares de Moura et al., 2000; 2001; Dobrucki et al., 2001; Figueroa et al., 2001; Sy et al., 2001; 2002). Dobrucki et al. (2001) showed that the α2-adrenoceptor/imidazoline receptor agonist clonidine, injected into the rat LV, decreased MAP and HR and simultaneously increased NO release in the NTS, and that blockade of NOS activity by injection of the NOS inhibitor NG-nitro-L-arginine-methyl ester (L-NAME) into the LV abolished these effects of clonidine. In contrast, intracisternal pretreatment with L-NAME in rabbits reduced the hypotension produced by an injection of α-methyl-noradrenaline (α2-adrenoceptor agonist), but not that induced by either clonidine or rilmenidine injected at the same site (Sy et al., 2001). Also in rabbits, blockade of NOS in the RVLM abolished the hypotension produced by central activation of α2-adrenoceptors, but not that induced by central activation of imidazoline receptors (Sy et al., 2002). Hence, in rats, the central blockade of NOS seems to reduce the hypotension produced by activation of imidazoline receptors, whereas in rabbits NO is involved only in the hypotension dependent on activation of central α2-adrenoceptors.
Although the hypotensive and bradycardic responses produced by α2-adrenoceptor/imidazoline receptor agonists are well established, other specific hemodynamic changes elicited by such drugs have not been investigated. Differences in the effects of central L-NAME on the hypotensive responses induced by α2-adrenoceptor/imidazoline receptor agonists have recently been reported (Dobrucki et al., 2001; Sy et al., 2001; 2002). Only the hypotensive responses induced by clonidine, a drug that binds equally to α2-adrenoceptors and imidazoline receptors, have been tested after L-NAME injections into the LV (Dobrucki et al., 2001). The possible role of central NO in the hypotensive response produced by moxonidine, that is suggested to depend on the activation of central imidazoline receptors located specially in the RVLM (Ernsberger et al., 1993; 1994; 1997), remains unclear. Hence, in the present study we investigated the effects of L-NAME (injected into the 4th V) on the hypotension, bradycardia and vasodilatation induced by intraventricular moxonidine (injected into the 4th V) in normotensive rats.
Methods
Surgical procedures
All experiments were performed in accordance with the Brazilian National Health and Medical Research Council code of practice for the care and use of animals for scientific purposes, and were approved by the Animal Experimentation Ethics Committee of the Federal University of São Paulo-School of Medicine. Experiments were performed on adult male Wistar rats weighing 300–350 g. Animals were anesthetized with urethane (1.2 g kg−1 of body weight intravenously (i.v.)) after induction with halothane (2% in 100% O2). A catheter (PE-10 connected to PE-50) was inserted into the femoral artery for measurement of pulsatile arterial pressure, MAP and HR. A femoral vein catheter was used for the administration of drugs. To record pulsatile arterial pressure, MAP and HR, the arterial catheter was connected to a P23 Db pressure transducer (Statham Gould) coupled to a pre-amplifier (model ETH-200 Bridge Bio Amplifier, CB Sciences) connected to a Powerlab computer recording system (Powerlab 16SP, ADInstruments). The animals were unresponsive to noxious toe pinch and maintained a steady level of arterial pressure. The trachea was cannulated and the animals were artificially ventilated with 100% O2. The colonic temperature was maintained at 37°C with a thermostatically controlled heating table.
Immediately after vein and artery catheterization, a midline laparotomy was performed and miniature pulsed Doppler flow probes were placed around the renal artery, superior mesenteric artery and low abdominal aorta for measurement of renal, mesenteric and hindquarter blood flow, respectively. The probes were fixed to the surrounding tissues with suture thread. Data from animals in which the probes moved during the experiment were not considered for analysis.
The flow probes were connected to a Doppler flowmeter (Dept of Bioengineering, University of Iowa, Iowa City, IA, U.S.A.) coupled to a Powerlab computer recording system (model Powerlab 16SP, ADInstruments) for blood flow recording. Details of the Doppler flow recording technique, including the reliability of the method for estimation of flow velocity, have been described previously by Haywood et al. (1981). Relative renal, mesenteric and hindquarter vascular resistance changes were calculated as the ratio of MAP and Doppler shifts.
After the fixation of the flow probes, animals were placed in a stereotaxic apparatus in prone position. Injections into the 4th V were made 13.3 mm caudal to bregma, 0.0 mm lateral to midline and 7.0 mm below the dura mater. Injections into the 4th V were 1 μl and were made with a 10 μl Hamilton syringe connected by polyethylene tubing (PE-10) to the injection cannula.
Histology
At the end of the experiment, a 2% solution of Evans blue was microinjected into the 4th V (1 μl). The animals were killed by an overdose of urethane (1.8 g kg−1, i.v.). Saline followed by 10% buffered formalin was perfused through the heart. The brains were frozen, cut coronally into 50 μm sections and stained with Neutral red. Only animals with injections into the 4th V were considered for statistical analysis.
Experimental protocols
Effects of the combination of L-NAME and moxonidine injected into the 4th V on MAP, HR and regional blood flows
All studies were performed in rats anesthetized with urethane (1.2 g kg−1,. i.v.). Recordings began 10 min after the connection of the arterial line to the pressure transducer. Blood flows, MAP and HR were continuously recorded for 90 min and were analyzed every 10 min. Control (baseline) values were recorded for 10 min and were analyzed immediately before the L-NAME (10 nmol μl−1) or saline injection (first treatment). These values were used as a reference to calculate the changes produced by the treatments. After 20 min, moxonidine (20 nmol μl−1) or acidified saline (vehicle – 1 μl) was injected into the 4th V and the cardiovascular responses were evaluated for the next hour.
Four groups of animals were used to investigate the cardiovascular effects of the combination of L-NAME and moxonidine injected into the 4th V: (1) saline into the 4th V followed by vehicle into the 4th V (control group); (2) saline into the 4th V followed by moxonidine into the 4th V; (3) L-NAME into the 4th V followed by vehicle into the 4th V; (4) L-NAME into the 4th V followed by moxonidine into the 4th V.
Effects of the combination of i.v. L-NAME and intraventricular moxonidine (in the 4th V) on MAP, HR and regional blood flows
The dose of L-NAME used was the same as that injected centrally (10 nmol per rat) and the purpose of this treatment was to show that the effects of a central injection of L-NAME are not due to its diffusion into the periphery.
Four groups of rats were used to test the effects of the combination of the 10 nmol of L-NAME (i.v.) and intraventricular moxonidine (injected into the 4th V). The same protocol as that described above was used, except that L-NAME or saline were injected i.v. instead of into the 4th V.
Statistical analysis
All values are expressed as means±s.e.m. Statistical analysis of baseline MAP, HR and vascular resistances was performed by use of two-way analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc test. The maximum changes in MAP, HR and vascular resistances were analyzed by Student's t-test. A value of P<0.05 was considered significant.
Drugs
Moxonidine hydrochloride (20 nmol μl−1), a gift from Solvay Pharma (Germany), was injected into the 4th V. L-NAME (NG-nitro-L-arginine methyl ester, an NO synthesis inhibitor) from Sigma Chemical Co., U.S.A., was injected into the 4th V at a dose of 10 nmol μl−1 and i.v. at a dose of 10 nmol 0.1 ml−1. Moxonidine was dissolved in isotonic saline acidified with acetic acid (pH adjusted to 4.6). Isotonic acidified saline (vehicle – 1 μl) was used as a control for moxonidine. L-NAME was dissolved in isotonic saline.
Results
Effects of the combination of L-NAME and moxonidine injected into the 4th V on MAP, HR and regional vascular resistances
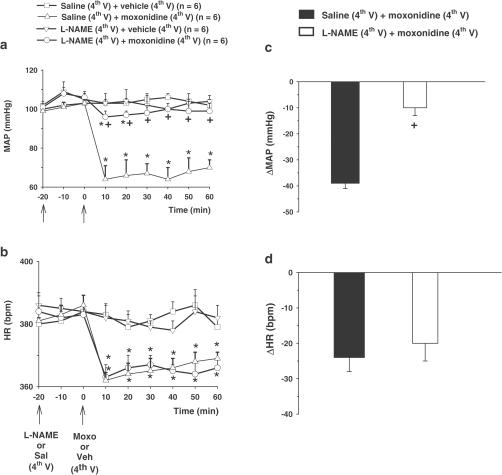
Moxonidine (20 nmol μl−1) injected into the 4th V reduced baseline MAP (66±7 mmHg vs saline: 102±4 mmHg), (F(3, 20)=347. 59, P<0.01) and baseline HR (361±4 bpm vs saline: 384±7 bpm), (F(3, 20)=12.38, P<0.01), (Figure 1a and b). L-NAME (10 nmol μl−1) injected into the 4th V produced no change in the baseline MAP (109±5 mmHg) and baseline HR (383±6 bpm), (Figure 1a and b). Pretreatment with L-NAME (10 nmol μl−1) into the 4th V almost abolished moxonidine-induced hypotension (Figure 1a and c), without affecting the bradycardia response(Figure 1b and d).
Figure 1.
Changes in (a) baseline MAP, and (b) baseline HR, and maximum change in (c) MAP (ΔMAP) and (d) HR (ΔHR) induced by moxonidine (moxo, 20 nmol μl−1) injected into the 4th V following the pre-treatment with L-NAME (10 nmol μl−1) or saline (sal) into the 4th V. The results are represented as means±s.e.m. n=number of rats in each group, veh=vehicle. *different from sal+veh; +different from sal+moxo (P<0.05).
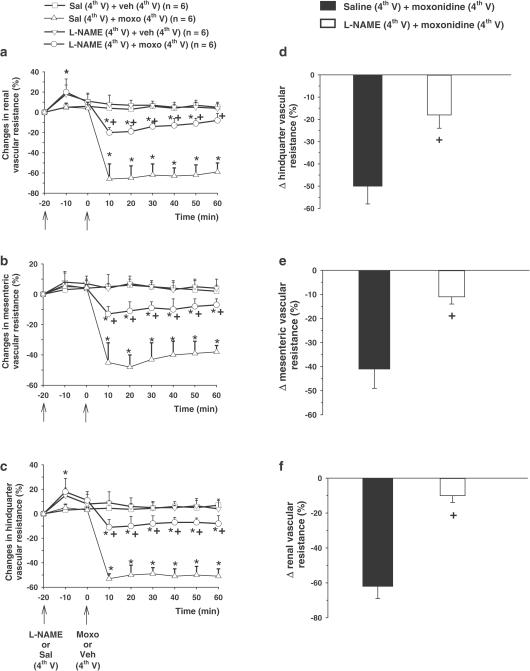
Moxonidine (20 nmol μl−1) injected into the 4th V also markedly reduced renal (−62±15% vs vehicle: 7±5%), (F(3, 20)=1141.81, P<0.01) (Figure 2a), mesenteric (−41±8 % vs vehicle: 6±5%), (F(3, 20)=290.13, P<0.01) (Figure 2b) and hindquarter vascular resistances (−50±8% vs vehicle: 5±6%) (F(3, 20)=641.93, P<0.01), (Figure 2c). Pretreatment with L-NAME, injected into the 4th V, almost abolished moxonidine-induced vasodilatation in the renal (−10±4%) (Figure 2a and d), mesenteric (−11±4%) (Figure 2b and e) and hindquarter vascular beds (−13±6%) (Figure 2c and f). L-NAME alone, injected into the 4th V, produced a transitory small increase (10 min in duration) in the renal (18±9%) (Figure 2a) and hindquarter vascular resistances (15±6%) (Figure 2c).
Figure 2.
Changes in baseline (a), renal (b), mesenteric and (c) hindquarter vascular resistances and maximum change in (d) renal, (e) mesenteric and (f) hindquarter vascular resistances induced by moxonidine (moxo, 20 nmol μl−1) injected into the 4th V following the pre-treatment with L-NAME (10 nmol μl−1) or saline (sal) into the 4th V. The results are represented as means±s.e.m. n=number of rats in each group, veh=vehicle. *Different from sal+veh; +Different from sal+moxo (P<0.05).
Effects of the combination of i.v. L-NAME and intraventricular moxonidine on MAP, HR and vascular resistance in rats
To show that the effects of a central injection of L-NAME are not due to its diffusion into the periphery, the cardiovascular effects of i.v. L-NAME (10 nmol 0.1 ml−1) combined with intraventricular moxonidine (20 nmol μl−1, into the 4th V) were evaluated. L-NAME (10 nmol 0.1 ml−1, i.v.) alone had no effect on MAP, HR and renal, mesenteric and hindlimb vascular resistances (Table 1). Pretreatment with i.v. L-NAME (10 nmol 0.1 ml−1) also did not affect the central effects of moxonidine (20 nmol μl−1, injected into the 4th V) on MAP (F(3, 20)=345.18, P<0.05), HR (F(3, 20)=35.15, P<0.05) and renal (F(3, 20)=856.83, P<0.05), mesenteric (F(3, 20)=252.50, P<0.05) and hindquarter vascular resistances (F(3, 20)=481.36, P<0.05) (Table 1). No differences in baseline MAP and HR levels were observed between the groups of rats treated with a central injection of moxonidine or vehicle following i.v. L-NAME (10 nmol) or saline (minimum: 100±3 mmHg and 379±9 bpm and maximum: 103±4 mmHg and 382 8 bpm).
Table 1.
Changes in MAP, HR and renal, mesenteric and hindquarter vascular resistances in rats treated with L-NAME (10 nmol.0.1 ml−1) or saline injected i.v., combined with moxonidine (moxo, 20 nmol μl−1) or vehicle into the 4th V
| Treatments | ΔMAP (mmHg) | ΔHR (bpm) | Changes in vascular resistance (%) | ||
|---|---|---|---|---|---|
| Renal | Mesenteric | Hindquarter | |||
| sal+veh | 2±3 | 3±4 | 6±4 | 7±5 | 6±3 |
| L-NAME+veh | 7±4 | 5±2 | 5±5 | 4±6 | 5±4 |
| sal+moxo | −37±9* | −23±13* | −71±23* | −54±18* | −55±14* |
| L-NAME+moxo | −36±5* | −20±5* | −66±10* | −52±9* | −58±17* |
The results are represented as means±s.e.m., n=6 rats in each group. Veh=vehicle, sal=saline, moxo=moxonidine
Different from veh+sal.
Discussion
The results showed that the central blockade of NOS by an injection of L-NAME into the 4th V almost abolishes the hypotension and vasodilatation elicited by a central injection of moxonidine, without changing the bradycardia. These data suggest that NO released centrally is essential for the hypotension and vasodilatation, but not for the bradycardia, induced by moxonidine. The dose of L-NAME (10 nmol), which was effective when injected centrally, had no effect when injected peripherally, which indicates that this dose of L-NAME, injected into the 4th V, only inhibits centrally located NOS.
Intraventricular L-NAME (10 nmol) had no effect itself on MAP, renal, mesenteric and hindquarter vascular resistances. Therefore, its effects on the responses to moxonidine cannot be attributed to changes in baseline MAP or vascular resistance.
The observation that intraventricular moxonidine induced vasodilatation in different vascular beds (renal, mesenteric and hindquarter) suggests that it acts in the brain stem to produce non-selective inhibition of sympathetic activity to various tissues. In contrast to its effects when injected into the 4th V, the same dose of moxonidine injected into the LV was found to have no effect on MAP, HR and mesenteric or hindquarter vascular resistances, but did increase salivary gland vascular resistance (Moreira et al., 2003). Although previous studies have suggested that the hypotensive effects of moxonidine are due to the sympathoinhibition as a result of its action in the brain stem (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger & Haxhiu 1997; Nurminen et al., 1998; Tolentino-Silva et al., 2000), the findings of the present study are the first to show that an α2-adrenoceptor/imidazoline agonist acting centrally can produce vasodilatation in different vascular beds. Although the NTS has been implicated as the site of action of α2-adrenoceptor/imidazoline agonists in the brain stem (Head & Burke, 1998; Sy et al., 2002), evidence has been presented showing that moxonidine reduces sympathetic activity and MAP by an effect in the RVLM (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger & Haxhiu, 1997; Ernsberger et al., 1997; Tolentino-Silva et al., 2000). Hence, it is still unclear whether α2-adrenoceptor/imidazoline agonists activate imidazoline receptors and/or α2-adrenoceptors in the brain stem to inhibit sympathetic activity and reduce arterial pressure, or, as suggested for moxonidine, they activate imidazoline receptors in the RVLM to produce hypotensive responses (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger et al., 1997; Guyenet, 1997; Tolentino-Silva et al., 2000). In the present study, moxonidine was injected into the 4th V, from there it can act in different areas of the brain stem. The RVLM is the most likely site for the action of moxonidine, but, in the present study, it was not possible to exclude any effects induced by moxonidine acting in the NTS. The RVLM is directly involved in the control of the sympathetic fibers to the cardiovascular system and moxonidine acting in this area may nonselectively reduce the activity of sympathetic efferent fibers, resulting in vasodilatation in different vascular beds and bradycardia. The vasodilatation induced by moxonidine was almost totally blocked by central L-NAME, while the bradycardia was not affected; this suggests that different central mechanisms are activated by moxonidine to induce vasodilatation and bradycardia.
It is well accepted that NO either peripherally or centrally has an important role in the maintenance of blood pressure (Calver et al., 1993; Zanzinger et al., 1995; Liu et al., 1996). Inhibition of peripheral NOS results in increased arterial pressure, an indication that NO produced at its basal rate by the vascular endothelium causes vasodilatation (Rees et al., 1989). Although the reduction of continuous NO release by the vascular endothelium is important in normal homeostasis, the mechanisms of the acute pressor effects elicited by inhibition of NO synthesis are not completely clear (Gardiner et al., 1990). Blockade of NO synthesis centrally (with high doses injected peripherally) also influences a peripheral vascular mechanism resulting in pressor responses (Zhang et al., 1997). The present results, showing that a low dose (10 nmol) of L-NAME injected centrally can almost abolish the hypotension and vasodilatation induced by a central injection of moxonidine, without affecting baseline MAP, HR or regional vascular resistances, suggest that a mechanism involving central NO is essential for the hypotension and vasodilatation induced by moxonidine. It has been proposed that the central effects of moxonidine involve the release of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the RVLM (Ernsberger et al., 1997) and the same mechanism has also been suggested to explain the reduced neuronal activity induced by NO in the RVLM (Patel et al., 2001). Therefore, one possible explanation for the present results is that GABA release is reduced after the treatment with L-NAME and that this impairs the ability of centrally acting moxonidine to induce hypotension and vasodilatation.
Recent studies in rats have shown that central L-NAME abolishes clonidine-induced hypotension and bradycardia (Dobrucki et al., 2001). However, in rabbits central L-NAME abolished the hypotension induced by central α-methyl-noradrenaline (a specific α2-adrenoceptor agonist), but did not affect the responses produced by the α2-adrenoceptor/imidazoline receptor agonists clonidine and rilmenidine (Sy et al., 2001), which suggests that in rabbits central NO is not involved in the hypotension produced by the activation of imidazoline receptors. Similar to the previous study with clonidine in rats (Dobrucki et al., 2001), the present results show that central L-NAME inhibits moxonidine-induced hypotension, suggesting that central NO is important for the hypotension induced by the activation of imidazoline receptors in rats. In contrast to the results from this study, central L-NAME also abolished the bradycardia induced by clonidine (Dobrucki et al., 2001), suggesting that central NO is important for the bradycardia produced by clonidine injected into the LV, but not that produced by moxonidine injected into the 4th V. This difference may be due to the activation of different central sites. While the responses to clonidine, injected into the LV, are thought to depend on NO release in the NTS (Dobrucki et al., 2001), the actions of moxonidine have been suggested to depend on an effect in the RVLM (Gomez et al., 1991; Haxhiu et al., 1994; Ernsberger et al., 1997; Tolentino-Silva et al., 2000). As moxonidine-induced vasodilatation was almost totally blocked by central L-NAME, whereas the bradycardia was not affected, different central mechanisms may be activated by moxonidine to induce vasodilatation and bradycardia.
Acknowledgments
We thank Solvay Pharma and Dr P. Ernsberger for the donation of moxonidine. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/PRONEX).
Abbreviations
- CVLM
caudal ventrolateral medulla
- GABA
γ-aminobutyric acid
- HR
heart rate
- i.v.
intravenously
- LV
lateral brain ventricle
- L-NAME
NG-nitro-L-arginine-methyl ester
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- NTS
nucleus tractus solitarii
- PVN
paraventricular nucleus of the hypothalamus
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rats
- 4th V
4th brain ventricle
References
- BARMAN S.M., GEBBER G.L. Basis for the naturally occurring activity of rostral ventrolateral medullary sympathoexcitatory neurons. Prog. Brain Res. 1989;81:117–129. doi: 10.1016/s0079-6123(08)62003-8. [DOI] [PubMed] [Google Scholar]
- CALVER A., COLLIER J., VALLANCE J. Nitric oxide and cardiovascular control. Exp. Physiol. 1993;78:303–326. doi: 10.1113/expphysiol.1993.sp003687. [DOI] [PubMed] [Google Scholar]
- COLOMBARI E., DAVISSON R.L., SHAFFER R.A., TALMAN W.T., LEWIS S.J.Hemodynamic effects of L-glutamate in NTS of conscious rats: a possible role of vascular nitrosyl factors Am. J. Physiol. 1998274H1066–H1074.(Part 2) [DOI] [PubMed] [Google Scholar]
- DOBRUCKI L.W., CABRERA C.L., BOHR D.F., MALINSKI T. Central hypotensive action of clonidine requires nitric oxide. Circulation. 2001;104:1884–1886. doi: 10.1161/hc4101.098281. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., ELLIOT H.L., WEIMAN H.J., RAAP A., HAXHIU M.A., HOFFERBER E., LOW-KROGER A., REID J.L., MEST H.J. Moxonidine: a second-generation central antihypertensive agent. Cardiovasc. Drug. Rev. 1993;11:411–431. [Google Scholar]
- ERNSBERGER P., FRIEDMAN J.E., KOLETSKI R.J. The I1-imidazoline receptor: from binding site to therapeutic target in cardiovascular disease. J. Hypertens. 1997;15 suppl. 1:S9–S23. doi: 10.1097/00004872-199715011-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSBERGER P., HAXHIU M.A.The I1-imidazoline-binding site is a functional receptor mediating vasodepression via the ventral medulla Am. J. Physiol. 1997273R1572–R1579.(Part 2) [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., HAXHIU M.A., GRAFF L.M., COLLINS L.A., DRESHAJ I., GROVE D.L., GRAVES M.E., SCHAFER S.G., CHRISTEN M.O. A novel mechanism of action for hypertension control: moxonidine as a selective I1-imidazoline agonist. Cardiovasc. Drugs Ther. 1994;8 Suppl 1:27–41. doi: 10.1007/BF00877082. [DOI] [PubMed] [Google Scholar]
- FELDMAN J., GRENEY H., MONASSIER L., VONTHRON C., BRUBAN V., DONTENWILL M., BOUSQUET P. Does a second generation of centrally acting antihypertensive drugs really exist. J. Auton. Nerv. Syst. 1998;72:94–97. doi: 10.1016/s0165-1838(98)00093-9. [DOI] [PubMed] [Google Scholar]
- FIGUEROA X.F., POBLETE M.I., BORIC M.P., MENDIZABAL V.E., ADLER-GRASCHINSKY E., HUIDOBRO-TORO J.P. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br J Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T., PALMER R.M., MONCADA S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990;15:486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- GOMEZ R.E., ERNSBERGER P., FEINLAND G., REIS D.J. Rilmenidine lowers arterial pressure via imidazole receptors in brainstem C1 area. Eur. J. Pharmacol. 1991;195:181–191. doi: 10.1016/0014-2999(91)90534-w. [DOI] [PubMed] [Google Scholar]
- GUYENET P.G. Is the hypotensive effect of clonidine and related drugs due to imidazoline binding sites. Am. J. Physiol. 1997;273:R1580–R1584. doi: 10.1152/ajpregu.1997.273.5.R1580. [DOI] [PubMed] [Google Scholar]
- GUYENET P.G., HASELTON J.R., SUN M.K. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog. Brain Res. 1989;81:105–116. doi: 10.1016/s0079-6123(08)62002-6. [DOI] [PubMed] [Google Scholar]
- HAXHIU M.A., DRESHAJ I., SCHAFER S.G., ERNSBERGER P. Selective antihypertensive action of moxonidine is mediated mainly by I1-imidazoline receptors in the rostral ventrolateral medulla. J. Cardiovasc. Pharmacol. 1994;24 Suppl 1:1–8. doi: 10.1097/00005344-199424001-00002. [DOI] [PubMed] [Google Scholar]
- HAYWOOD J.R., SHAFFER R.A., FASTENOW C., FINK G.D., BRODY M.J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am. J. Physiol. 1981;241:H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- HEAD G.A., BURKE S.L. Relative importance of medullary brain nuclei for the sympatho-inhibitory actions of rilmenidine in the anaesthetized rabbit. J Hypertens. 1998;16:503–517. doi: 10.1097/00004872-199816040-00012. [DOI] [PubMed] [Google Scholar]
- KADEKARO M., SUMMY-LONG J.Y. Centrally produced nitric oxide and the regulation of body fluid and blood pressure homeostases. Clin. Exp. Pharmacol. Physiol. 2000;27:450–459. doi: 10.1046/j.1440-1681.2000.03264.x. [DOI] [PubMed] [Google Scholar]
- LIU H., TERRELL M.L., SUMMY-LONG J.Y., KADEKARO M. Drinking and blood pressure responses to central injection of L-NAME in conscious rats. Physiol. Behav. 1996;59:1137–1145. doi: 10.1016/0031-9384(95)02180-9. [DOI] [PubMed] [Google Scholar]
- MOREIRA T.S., TAKAKURA A.C., COLOMBARI E., DE LUCA L.A., JR, RENZI A., MENANI J.V. Central moxonidine on salivary gland blood flow and cardiovascular responses to pilocarpine. Brain Res. 2003;987:155–163. doi: 10.1016/s0006-8993(03)03322-5. [DOI] [PubMed] [Google Scholar]
- NURMINEN M.L., CULMAN J., HAASS M., CHUNG O., UNGER T. Effect of moxonidine on blood pressure and sympathetic tone in spontaneously hypertensive rats. Eur. J. Pharmacol. 1998;362:61–67. doi: 10.1016/s0014-2999(98)00726-2. [DOI] [PubMed] [Google Scholar]
- PATEL K.P., LI Y.F., HIROOKA Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med. 2001;226:814–824. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M., MONCADA S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SY G.Y., BRUBAN V., BOUSQUET P., FELDMAN J. Nitric oxide and central antihypertensive drugs: one more difference between catecholamines and imidazolines. Hypertension. 2001;37:246–249. doi: 10.1161/01.hyp.37.2.246. [DOI] [PubMed] [Google Scholar]
- SY G.Y., BRUBAN V., BOUSQUET P., FELDMAN J. Nitric oxide discriminates the sites and mechanisms of action of centrally acting anti-hypertensive drugs in rabbits. Neuropharmacology. 2002;43:1330–1338. doi: 10.1016/s0028-3908(02)00307-6. [DOI] [PubMed] [Google Scholar]
- SOARES DE MOURA R.S., LEAO M.C., CASTRO RESENDE A.C., MOREIRA C.F., SENA K.M., SILVEIRA S.S., LIMA A.F., NUNES F.R., MESQUITA FERREIRA A.G. Actions of L-NAME and methylene blue on the hypotensive effects of clonidine and rilmenidine in the anesthetized rat. J. Cardiovasc. Pharmacol. 2000;35:791–795. doi: 10.1097/00005344-200005000-00017. [DOI] [PubMed] [Google Scholar]
- SOARES DE MOURA R., RIOS A.A., DE OLIVEIRA L.F., RESENDE A.C., DE LEMOS NETO M., SANTOS E.J., CORREIA M.L., TANO T. The effects of nitric oxide synthase inhibitors on the sedative effect of clonidine. Anesth. Analg. 2001;93:1217–1221. doi: 10.1097/00000539-200111000-00035. [DOI] [PubMed] [Google Scholar]
- TOLENTINO-SILVA F.P., HAXHIU M.A., WALDBAUM S., DRESHAJ I., ERNSBERGER P. Alpha(2)-adrenergic receptors are not required for central anti-hypertensive action of moxonidine in mice. Brain Res. 2000;862:26–35. doi: 10.1016/s0006-8993(00)02089-8. [DOI] [PubMed] [Google Scholar]
- VINCENT S.R., KIMURA H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- ZANZINGER J., CZACHURSKI J., SELLER H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am. J. Physiol. 1995;268:R958–R962. doi: 10.1152/ajpregu.1995.268.4.R958. [DOI] [PubMed] [Google Scholar]
- ZHANG K., MAYHAN W.G., PATEL K.P. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am. J. Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]