Abstract
The functional characterization of hispidulin (4′,5,7-trihydroxy-6-methoxyflavone), a potent benzodiazepine (BZD) receptor ligand, was initiated to determine its potential as a modulator of central nervous system activity.
After chemical synthesis, hispidulin was investigated at recombinant GABAA/BZD receptors expressed by Xenopus laevis oocytes. Concentrations of 50 nM and higher stimulated the GABA-induced chloride currents at tested receptor subtypes (α1−3,5,6β2γ2S) indicating positive allosteric properties. Maximal stimulation at α1β2γ2S was observed with 10 μM hispidulin. In contrast to diazepam, hispidulin modulated the α6β2γ2S-GABAA receptor subtype.
When fed to seizure-prone Mongolian gerbils (Meriones unguiculatus) in a model of epilepsy, hispidulin (10 mg kg−1 body weight (BW) per day) and diazepam (2 mg kg−1 BW per day) markedly reduced the number of animals suffering from seizures after 7 days of treatment (30 and 25% of animals in the respective treatment groups, vs 80% in the vehicle group).
Permeability across the blood–brain barrier for the chemically synthesized, 14C-labelled hispidulin was confirmed by a rat in situ perfusion model. With an uptake rate (Kin) of 1.14 ml min−1 g−1, measurements approached the values obtained with highly penetrating compounds such as diazepam.
Experiments with Caco-2 cells predict that orally administered hispidulin enters circulation in its intact form. At a concentration of 30 μM, the flavone crossed the monolayer without degradation as verified by the absence of glucuronidated metabolites.
Keywords: Hispidulin, GABAA receptor, partial positive allosteric modulator, epilepsy, blood–brain barrier, Caco-2
Introduction
γ-Aminobutyric acid (GABA) and GABAA receptors belong to the major inhibitory system in the central nervous system (CNS). GABA opens a chloride ion selective channel in GABAA receptors, which are equally modulated by a wide range of drugs including barbiturates, steroids and benzodiazepines (BZDs). These receptor modulators interact with distinct allosteric binding sites on the GABAA receptor complex. The BZD binding site, so-called BZD receptor, can be occupied by a variety of substances classified as positive allosteric modulators, antagonists or negative allosteric modulators according to their intrinsic activity (Sigel & Buhr, 1997). BZDs, the most widely prescribed tranquilizers, are considered classical ligands of this modulatory site. They act as positive allosteric modulators by increasing channel-opening frequency and confer anxiolytic, anticonvulsant, sedative-hypnotic, muscle-relaxant effects (Sieghart & Sperk, 2002). An intensive search has been undertaken over the past decade with the aim to separate these diverse actions and to find new, more selective BZD receptor ligands as drug candidates in the treatment of epilepsy, anxiety and sleep disturbances. Natural flavonoids count among these candidates and hold promise as BZD receptor ligands (Medina et al., 1998). They comprise flavones, flavonols, anthocyanins and related ubiquitous constituents of higher plants and represent a significant part of our daily diet. Several biological activities of flavonoids have emerged, for example, antioxidant, antiviral, anticancer and chemopreventive properties (Daniel & Wenzel, 2003). Effects of plant flavonoids on the CNS are known since 1990, when the existence of natural anxiolytic flavonoids was first described (Medina et al., 1998). Flavones like chrysin, apigenin, wogonin and the recently isolated 6-methylapigenin have been shown to possess anxiolytic effects in vivo. In contrast, their sedative, anticonvulsant and myorelaxant effects appeared less prominent indicating a possible more selective action of these substances (Medina et al., 1998; Hui et al., 2002; Marder et al., 2003).
Although the sources of many active flavones are well known, information on their bioavailability and their active forms in vivo is limited. In particular, absorption, metabolism and CNS delivery of most flavonoid agents are poorly understood. Recently, hispidulin (4′,5,7-trihydroxy-6-methoxyflavone) was isolated from sage (Salvia officinalis L.) and identified as a potent ligand of the central human BZD receptor in vitro. With an IC50 value of 1.3 μM, hispidulin showed the strongest binding activity to the BZD receptor in comparison to other flavones isolated (Kavvadias et al., 2003). Hispidulin is a naturally occurring flavone commonly found in several Artemisia and Salvia species. Several in vitro studies have demonstrated its potent antioxidative, antifungal, anti-inflammatory and antimutagenic activities (Chulasiri et al., 1992; Gil et al., 1994; Tan et al., 1999). The lack of pharmacokinetic data and unresolved issues with regard to the crossing of the blood–brain barrier (BBB) prompted us to investigate its synthesis in a radiolabelled form. In this paper, we describe the conventional synthesis of flavones as applied to generating 14C-labelled hispidulin, and present a comprehensive characterization of this natural ligand, including effects on inhibitory hyperpolarization, BBB permeability and anticonvulsive activity in vivo.
Methods
Animals
Seizure-prone, male Mongolian gerbils (Meriones unguiculatus) weighing 70–102 g were kindly provided by the Leibniz-Institute for Neurobiology (Magdeburg, Germany) and partly bred at the Institute for Medical Chemistry (Veterinary University, Vienna, Austria). The in situ brain perfusion study was carried out on three male Wistar rats (Harlan UK Limited, Oxon, U.K.) aged 6 months with a body weight (BW) of 250 g. The animals were housed in single cages under standard laboratory conditions (20°C, 55% humidity, 12 h light/dark cycle) with free access to food and water.
All experiments were performed in accordance with the UK Home Office, Animal Procedures Act 1986 and in strict compliance with the NIH Guide for the care and use of laboratory animals.
Syntheses
General experimental procedures
Column chromatography for compound purification was performed with Merck 60 silica gel (0.040–0.063 mm). 1H-NMR spectra of unlabelled compounds were recorded on Bruker AC-250 MHz instrument. Chemical shifts are reported (δ) in ppm and coupling constants (J) in Hz relative to the internal standard tetramethylsilane (TMS). Chemical and radiochemical purity of 14C-labelled compounds was controlled by high-performance liquid chromatography (HPLC) analysis with the absorbance detector Spectroflow 757 (Kratos, Weiterstadt, Germany) and the flow scintillation analyzer 500 TR (Packard, Groningen, Netherlands) using authentic references from synthesis of unlabelled hispidulin.
Synthesis of [14C]hispidulin
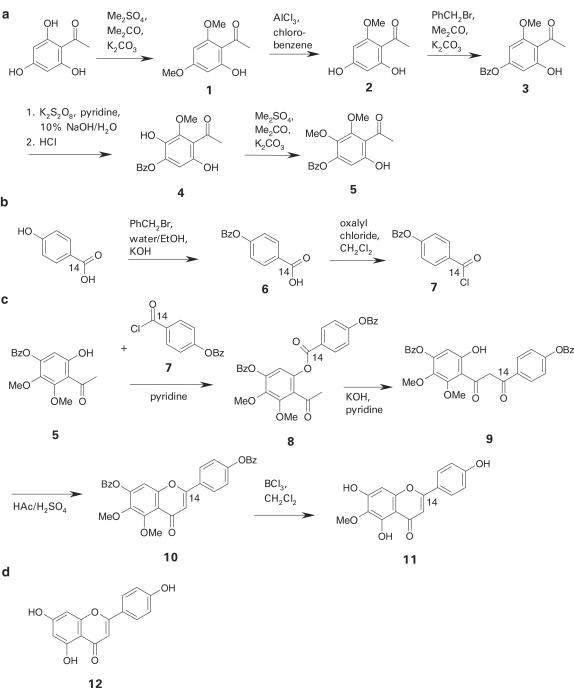
4-Benzyloxy-2,3-dimethoxy-6-hydroxyacetophenone (5)
Potassium carbonate (3 g, 21.7 mmol) was added to a solution of 2,4,6-trihydroxyacetophenone-monohydrate (2 g, 10.8 mmol) in acetone (30 ml). The mixture was heated to 65°C. Three portions of dimethyl sulfate (3 × 0.7 ml, 22.1 mmol) were added to the reaction solution at 3 h intervals. The solution was further heated for 3 h, filtered and the filtrate evaporated to give 2,4-dimethoxy-6-hydroxyacetophenone (1) as a yellow solid (92%): 1H-NMR (250 MHz, DMSO-d6): δ 2.61 (CO-CH3, s, 3H), 3.88 (2-OCH3, s, 3H), 3.93 (4-OCH3, s, 3H), 6.15 (H-3, d, J=2.1 Hz, 1H), 6.18 (H-5, d, J=2.1 Hz, 1H), 13.88 (6-OH, s, 1H). Acetophenone 1 (1.5 g, 7.7 mmol) and dry aluminum chloride (1.5 g, 11.3 mmol) were suspended in chlorobenzene (20 ml) and refluxed for 1 h. The organic solvent was evaporated under reduced pressure and the residue mixed with ice-cold water–HCl (1 : 1) solution (40 ml). The white precipitate was filtered, dissolved in ethyl acetate and extracted with 10% sodium hydroxide solution (3 × 50 ml). After acidifying the aqueous phase, the 2,4-dihydroxy-6-methoxyacetophenone (2) was re-extracted with ethyl acetate (3 × 100 ml) and crystallized from ethyl acetate (45–60%). Alternatively, column chromatography was performed according to Jain et al. (1985) to give 2 as a yellow solid: 1H-NMR (250 MHz, DMSO-d6): δ 2.58 (CO-CH3, s, 3H), 3.88 (-OCH3, s, 3H), 5.94 (H-3, d, 2.1 Hz, 1H), 6.04 (H-5, d, 2.1 Hz, 1H), 13.88 (2-OH, s, 1H). Compound 2 (0.5 g, 2.75 mmol) was dissolved in dry acetone (30 ml) and the solution heated under reflux overnight after adding potassium carbonate (3 g, 21.7 mmol) and benzyl bromide (0.43 ml, 3.6 mmol). After evaporating the solvent, 4-benzyloxy-2-methoxy-6-hydroxyacetophenone (3) was obtained as a brown solid (99%): 1H-NMR (250 MHz, DMSO-d6): δ 2.62 (CO-CH3, s, 3H), 3.93 (-OCH3, s, 3H), 5.24 (-CH2-, s, 2H), 6.25 (H-3, d, 2.5 Hz, 1H), 6.28 (H-5, d, 2.4 Hz, 1H), 7.49 (-Ph, m, 5H). The phenolic compound 3 (0.5 g, 1.8 mmol) and sodium hydroxide (0.4 g) were dissolved in 4 ml water and 0.5 ml pyridine. A solution of potassium persulfate (0.55 g, 2.0 mmol) in water (11 ml) was added over 4 h at a constant temperature of 15°C. After stirring at room temperature for 24 h, the mixture was acidified to pH 5 and filtered. The filtrate was extracted with diethyl ether (2 × 15 ml). The aqueous phase was acidified with HClconc (2 ml) and refluxed for 1 h after addition of diethyl ether (15 ml). The diethyl ether phase was dried over sodium sulfate and evaporated to give 4-benzyloxy-2,5-dihydroxy-6-methoxyacetophenone (4) as brown syrup (29%): 1H-NMR (250 Hz, DMSO-d6): δ 2.66 (CO-CH3, s, 3H), 3.91 (6-OCH3, s, 3H), 5.28 (-CH2-, s, 2H), 6.46 (Har, s, 1H), 7.48 (Ph, m, 5H). For partial methylation of intermediate 4 (0.15 g, 0.5 mmol), 1.5 equivalents of dimethyl sulfate (0.07 ml, 0.7 mmol) and potassium carbonate (0.8 g) were dissolved in dry acetone (8 ml) and refluxed overnight. After filtration and evaporation of the solvent, compound 5 was purified by silica gel column chromatography (2.8 × 42 cm) using chloroform as eluent. The product 5 was obtained in fractions with 500–700 ml elution volume (59%): 1H-NMR (250 Hz, CDCl3): δ 2.66 (CO-CH3, s, 3H), 3.81 (2-OCH3, s, 3H), 4.01 (3-OCH3, s, 3H), 5.14 (-CH2-, s, 2H), 6.30 (Har, s, 1H), 7.42 (Ph, m, 5H), 13.40 (6-OH, s, 1H).
4-Benzyloxy-14COCl-benzoyl chloride (7)
To a solution of radiolabelled 4-hydroxy-14COOH-benzoic acid (55 mCi mmol−1, 250 μCi) in water–ethanol (1 : 3) mixture (450 μl), unlabelled 4-hydroxybenzoic acid (45 mg, 0.33 mmol), aqueous 4 M potassium hydroxide solution (170 μl) and benzyl bromide (45 μl, 0.38 mmol) were added. The reaction mixture was heated under reflux for 6 h. After addition of 4 M potassium hydroxide solution (0.5 ml), the mixture was further heated for 2 h, cooled down to room temperature and centrifuged at 5000 × g. The supernatant was removed and the residue was recrystallized in acetic acid (1 ml) to obtain 4-benzyloxy-14COOH-benzoic acid (6) as white solid (chemical yield: 36%; total 14C activity: 78 μCi). The dried acid 6 (27 mg, 0.12 mmol) was suspended in dichloromethane (1 ml), and oxalyl chloride (28 μl, 0.15 mmol) was added. The reaction mixture was stirred at room temperature for 8 h. 4-Benzyloxy-14COCl-benzoyl chloride (7) was obtained after evaporation of the solvent under reduced pressure (chemical yield: 100%; total 14C activity: 76 μCi).
2-14C-hispidulin (2-14C-4′,5,7-trihydroxy-6-methoxyflavone) (11)
Acetophenone derivative 5 (36 mg, 0.12 mmol) was added to a solution of 14C-labelled 4-benzyloxybenzoyl chloride (7) (29 mg, 0.12 mmol, 76 μCi) in dry pyridine (0.7 ml) and the mixture was stirred at room temperature for 3 h. The reaction solution was poured on ice-cold 3% HCl (11 ml) and extracted with ethyl acetate (25 ml). The organic phase was washed twice with aqueous saturated sodium carbonate, once with water and then evaporated under reduced pressure to give ester 8 (chemical yield: 81%; total 14C activity: 55 μCi). Ester 8 was dried in vacuum and redissolved in dry pyridine (0.5 ml). After addition of freshly powdered potassium hydroxide (175 mg), the mixture was stirred at 60°C for 4 h. The reaction was stopped by pouring on ice-cold 3% HCl (11 ml). The diketone compound 9 was extracted with ethyl acetate (25 ml), washed with aqueous saturated sodium carbonate and then evaporated under reduced pressure to give the diketone 9 (chemical yield: 55%; total 14C activity: 26 μCi). Diketone 9 was dissolved in acetic acid (1.7 ml), and sulfuric acid (42 μl) was carefully added. The solution was stirred at 60°C for 90 min and then poured on ice (10 g). The resulting flavone 10 was extracted with ethyl acetate (25 ml) and dried under reduced pressure after solvent evaporation (chemical yield: 60%; total 14C activity: 14 μCi). For selective demethylation and deprotection of the hydroxy groups, flavone 10 was dissolved in dichloromethane (1 ml) and cooled down to about −65°C. Boron trichloride solution (1 M in dichloromethane, 0.25 ml) was then added and the mixture was stirred at –65°C for 90 min. The reaction was stopped by addition of saturated sodium bicarbonate (1 ml), and the aqueous solution was extracted with diethyl ether (3 × 5 ml). The crude 2-14C-hispidulin (11) was purified by silica gel column chromatography (1.9 × 18 cm) with methanol : chloroform (3 : 97) as eluent. The major activity of pure flavone 11 (3 mg, 0.01 mmol) was found in fractions eluted with 55–70 ml of the MeOH/CHCl3 mixture (chemical yield: 44%; total 14C activity: 9 μCi). Identity was confirmed by HPLC analysis using unlabelled hispidulin as reference.
Synthesis of hispidulin
Unlabelled hispidulin was synthesized from 4-benzyloxy-2,3-dimethoxy-6-hydroxyacetophenone (5) and unlabelled 4-benzyloxybenzoic acid chloride (7), both prepared in separate routes from 2,4,6-trihydroxyacetophenone and 4-hydroxybenzoic acid, respectively. Synthesis steps were carried out on a large gram-scale as described above for 11. The product was purified by silica gel column chromatography (diameter 2.2 cm, length 42 cm) and was eluted by methanol : chloroform (3 : 97) with an elution volume of 250–370 ml. Identity was confirmed on the basis of published spectral data using isolated natural hispidulin as reference (UV, NMR, MS) (Kavvadias et al., 2003).
Electrophysiological studies
Xenopus laevis oocytes were prepared, injected and defolliculated and currents were recorded as previously described (Sigel, 1987; Sigel et al., 1990). Briefly, oocytes were injected with 50 nl of capped, polyadenylated cRNA dissolved in 5 mM K-HEPES (pH 6.8). For the triple subunit combinations, 10 nM of the cRNA coding for the different α subunits (α1, α2, α3, α5 and α6) and the β2 subunit was used, whereas 50 nM of the γ2S subunit cRNA was used (Boileau et al., 2002). RNA transcripts were synthesized from linearized plasmids encoding the desired protein using the mMESSAGE mMACHINE® kit according to the manufacturers' recommendations. A poly(A) tail of about 300 residues was added to the transcripts by using yeast poly(A) polymerase. The cRNA combinations were co-precipitated in EtOH and stored at −20°C. Transcripts were quantified on agarose gels after staining with Radiant Red Fluorescent RNA Stain by comparing staining intensities with various amounts of molecular weight markers. Electrophysiological experiments were performed by the two-electrode voltage clamp method at a holding potential of −80 mV. The medium (pH 7.4) contained 90 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2 and 5 mM HEPES–NaOH (pH 7.4). GABA, diazepam and hispidulin were applied for 20 s and a washout period of 4–15 min was allowed to ensure full recovery from desensitization. Hispidulin was dissolved every day freshly in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. The stock solution of Apigenin was 10 mM in DMSO. The final concentration of DMSO in the medium was always adjusted to 0.5%, except when hispidulin was assayed at 100 μM. In this case, final DMSO concentration in the medium was 1%. These concentrations of DMSO did not by themselves affect significantly GABA-elicited currents. Both flavone compounds were tested for their effect on GABA concentrations that elicited 2–5% of the maximal current amplitude.
Gerbil model
Only seizure-sensitive animals were used in the experiments. They were assigned to treatment groups of 10 animals each (with the exception of eight animals in the diazepam group). Drugs (hispidulin and diazepam) were dissolved in ethanol, diluted with water and added to a pastry manufactured with ground chow (Altromin® Pellets). Pellets were formed to give final concentrations of 1.08 mg hispidulin or 0.21 mg diazepam and max. 1.4% ethanol per 5 g (daily portion). For the control group, pellets from ground chow, ethanol and water were manufactured in the same manner and used as vehicle. Test substances were administered orally for 7 days at a dosage of 10 mg kg−1 hispidulin and 2 mg kg−1 diazepam per day. During this period, the animals were left undisturbed to avoid any unintended provocation of seizures. Epileptic seizures were induced by a standardized handling procedure before and after 7 days of treatment. For this purpose, each animal was stroked on the back and was separately placed outside its home cage while its seizure activity was monitored and graded in terms of severity as follows: no visible seizure or only minimal seizure correlates (no noticeable changes of gross behavior, possibly twitching of vibrissae and pinnae for a few seconds) and full seizure (clonic–tonic seizure, loss of righting reflexes and body rollover). Only seizures occurring within 3 min of provocation tests were counted.
In situ rat brain perfusion model
Hemiperfusions were carried out using a modified short-duration technique previously described by Takasato et al. (1984). Briefly, [14C]hispidulin (1 μCi, specific activity 67.5 μCi mmol−1) was dissolved in ethanol (1 ml), diluted with perfusion buffer (according to Egleton et al., 1998) to give a final ethanol concentration of 5% and subsequently perfused via the right carotid artery of rats for 30, 45 and 60 s (n=3 animals for each time point). The right hemisphere of the brain was dissected and the 14C content was determined with a scintillation counter. Results were expressed as volume of distribution Vd (μl g−1) and uptake rate Kin (ml min−1 g−1). To ensure that ethanol concentration did not disrupt the BBB and affect permeability data, a control experiment was performed perfusing [14C]sucrose (as a vascular marker) in perfusate containing ethanol (5% final concentration) for 60 s.
Caco-2 system model
Caco-2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 1% MEM non-essential amino-acid solution, GLUTAMAX-1 (2 mM) and penicillin–streptomycin (100 U ml−1; 100 μg ml−1), and maintained at 37°C in a humidified atmosphere (5% CO2). For transport studies, Caco-2 cells (passage 30–50) were seeded on Transwell inserts (polycarbonate membrane, 12 mm diameter and 0.4 μm pore size, Corning Costar Co.) at a density of 1 × 105 cm−2. The transendothelial electrical resistance (TEER) across the Caco-2 monolayers was measured daily using an epithelial volt-ohmmeter (EVOM, World Precision Instruments Inc., Hertfordshire, U.K.) with a planar electrode chamber (Endohm-12, World Precision Instruments Inc.). Monolayers used in transport studies (20–25 days postseeding on Transwells) exhibited TEER >500 Ω cm−2. Inserts were washed with Hanks' balanced salt solution (HBSS) pH 7.4 and were incubated at 37°C for 30 min. Hispidulin and apigenin (30 mM in DMSO) were diluted to 30 μM in transport buffer (HBSS containing 25 mM HEPES, 0.1% BSA (w v−1)). BSA was included to stabilize membrane and tight junctions. 3H-Mannitol (0.1 μCi ml−1, specific activity 20 Ci mmol−1) was used as a marker for the paracellular (tight junctional) pathway to ensure that integrity was maintained during the permeability studies. Aliquots (0.5 ml) of the transport buffer containing 30 μM hispidulin or apigenin were added to the donor (apical) side of the inserts. The receiver (basolateral) chamber contained 1 ml of transport buffer (HBSS, 25 mM HEPES and 0.1% BSA). Plates were then incubated at 37°C in a Wesbart shaking plate set at 200 rpm (Wesbart Ltd, U.K.). After 30 min, the inserts were transferred into new wells containing fresh transport buffer (1 ml). After 60 min, 50 μl aliquots from receiver (t=30 and 60 min) and donor wells (t=60 min) were collected, added to scintillation cocktail and the [3H] radioactivity was measured using a Beckman scintillation counter. Flavonoid content in donor and receiver wells was analyzed by HPLC with photodiode array detection. To identify glucuronide conjugates, samples (200 μl) were incubated with β-glucuronidase (20 μl, 10,000 U ml−1) for 2 h at 37°C and assessed for a decrease in the suspected glucuronide peak and concomitant increase in the respective aglycone peak. Stability of both flavonoids was verified by measurements following incubation of samples in transport buffer at 37°C for 60 min.
HPLC analysis
HPLC was performed on a Waters HPLC system using a Nova-Pak C18 column (4.6 × 250 mm, 4 μm) and a guard column (4.6 × 15 mm, 4 μm) as previously described (Youdim et al., 2003). Flavonoids were identified by comparing retention times (RTs), photodiode array spectra and sample spiking with reference substances.
Chemicals
The following chemicals and reagents of analytical purity were used for the synthesis and experiments: acetic acid, acetone, aluminum chloride, apigenin, benzyl bromide, boron trichloride solution (1 M in dichloromethane), chlorobenzene, chloroform, diazepam, dichloromethane, diethyl ether, dimethyl sulfate, DMSO, DMSO-d6, ethanol, ethyl acetate, GABA, 4-hydroxybenzoic acid, methanol, potassium carbonate, potassium hydroxide, potassium persulfate, pyridine, flumazenil (Ro15-1788), sodium hydroxide, sodium sulfate, sulfuric acid, TMS and 2,4,6-trihydroxyacetophenone-monohydrate from Sigma-Aldrich (Deisenhofen, Germany). 4-Hydroxy-14COOH-benzoic acid was obtained from American Radiolabelled Chemicals Inc. (St Louis, MO, U.S.A.). Ultima-FloTM AF LSC-Cocktail (Packard Bioscience Company, Groningen, Germany) was used for scintillation counting.
The following were used for electrophysiological studies: mMESSAGE mMACHINE® kit (Ambion Inc.), yeast poly(A) polymerase (Amersham Biosciences, U.K.), Radiant Red Fluorescent RNA Stain (Bio-Rad, U.S.A.) and RNA Ladder (Gibco BRL, Crewe-Cheshire, U.K.). Caco-2 cells were obtained from the European Collection of Animal Cell Cultures (ECACC, Wiltshire, U.K.). Further on, fetal bovine serum, DMEM, MEM solution, GLUTAMAX-1, penicillin–streptomycin and HBSS were from Invitrogen (U.K.), and HEPES, BSA, β-glucuronidase (type L-II from Limpets) and 3H-Mannitol were from Sigma (Dorset, U.K.).
Data analysis
Electrophysiological studies
Currents were measured using a modified OC-725 amplifier (Warner Instruments Corp.) in combination with an XY-recorder or digitized using a MacLab200 (AD Instruments). Current responses have been fitted to the Hill equation [I=Imax(1+(IC50[A]−1)n)−1], where I is the peak current at a given concentration of GABA, Imax the maximum current, IC50 the concentration of agonist eliciting half-maximal current and n the Hill coefficient. The resulting data are shown as mean±s.e.m.
Gerbil model
Paired proportion tests (http://www.ubmail.ubalt.edu/~harsham/Business-stat/otherapplets/PairedProp.htm) were used to examine the null hypothesis that 7 days' pharmacological treatment had no modulatory effect on seizure propensity. P-value of less than 0.05 was considered as significant.
In situ rat brain perfusion model
Results were expressed as volume of distribution Vd (μl g−1) using the following equation: Vd=(dpm g−1)tissue (μl dpm−1)perfusate, where dpm expresses disintegrations per minute of the sample. The uptake rate Kin (ml min−1 g−1) was obtained as slope of the plot of Vd (ml g−1) against time (min). Log Poct was calculated using the KowWin (LogKow) software at http://www.esc.syrres.com/interkow/logkow.htm.
Results
Chemical synthesis
A route for the chemical synthesis of hispidulin, including its 14C-labelled form, was developed in our laboratory utilizing the Baker Venkataraman rearrangement starting with 4-benzyloxy-2,3-dimethoxy-6-hydroxyacetophenone (5) and 4-benzyloxybenzoic acid chloride (7) (Figure 1c). Both educts were prepared by separate routes from 2,4,6-trihydroxyacetophenone and 4-hydroxybenzoic acid. The acetophenone 5 was synthesized from commercially available 2,4,6-trihydroxyacetophenone in five steps as shown in Figure 1a. Transformation into 2,4-dimethoxy-6-hydroxyacetophenone (1) was achieved by reaction with dimethyl sulfate (Srivastava & Srivastava, 1987). Subsequent selective demethylation with aluminum chloride in chlorobenzene was performed to obtain 2,4-dihydroxy-6-methoxyacetophenone (2) (Jain et al., 1985). After protection of the 4-hydroxy group with a benzyl group, compound 3 was applied to Elbs persulfate oxidation (Baker et al., 1939). In the last step of the acetophenone preparation, the newly introduced hydroxy group at position C-5 was methylated using dimethyl sulfate. The benzoyl chloride 7 was prepared in two steps according to Figure 1b. Starting from 4-hydroxybenzoic acid, we protected the hydroxy group with a benzyl group and prepared the acid chloride by reaction of compound 6 with oxalyl chloride (Cherpeck, 1998; Bracon et al., 1999). For hispidulin synthesis, compounds 5 and 7 (76 μCi) were converted into dry pyridine to the corresponding benzoyl ester (Figure 1c). The crude benzoate was treated with potassium hydroxide to induce an intramolecular Claisen condensation resulting in formation of diketone 9. The diketone 9 cyclized to the corresponding flavone by heating in acetic acid with small amounts of sulfuric acid (Horie et al., 1997). The protecting groups and the labile methoxy group at C-5 were selectively cleaved by reaction with boron trichloride in dichloromethane at about −65°C to obtain hispidulin, which was identified on the basis of published spectral data (UV, NMR, MS) (Kavvadias et al., 2003). For synthesis of 14C-labelled hispidulin (11), radiolabelled 4-hydroxy benzoic acid with a total activity of 250 μCi was used. Radiolabelled flavone 11 (total activity: 9 μCi) was obtained with 12% isotopic incorporation and a specific activity of 67.5 μCi mmol−1 as determined by liquid scintillation counting after silica gel column purification.
Figure 1.
Chemical syntheses of 14C-labelled hispidulin 11 (c) and its building blocks 5 (a) and 7 (b) and the structure of apigenin (d).
Electrophysiological studies
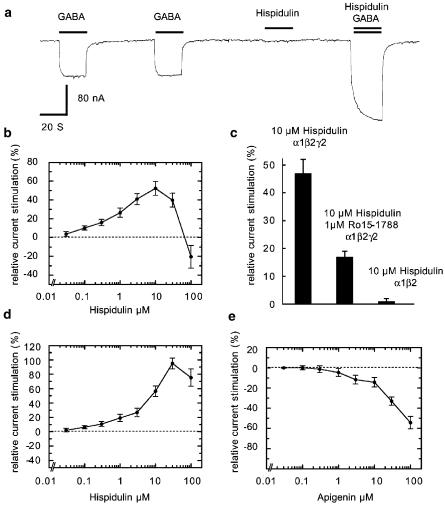
In electrophysiological studies at recombinant GABAA receptors expressed in X. laevis oocytes, hispidulin exhibited a positive allosteric modulatory effect. The current elicited by GABA in α1β2γ2 GABAA receptors was enhanced in a concentration-dependent way (Figure 2b). Maximum relative stimulation at α1β2γ2 was achieved with 10 μM hispidulin. Interestingly, at higher concentrations, a second inhibitory phase was observed. At 10 μM, stimulation by hispidulin amounted to about 24% of the stimulation by 1 μM diazepam. The potentiation of 10 μM hispidulin amounted to 47±5% (n=3, s.e.m.) and was largely blocked by co-application of the BZD receptor antagonist Ro15-1788 with a residual stimulation of 17±2% (n=3, s.e.m.) (Figure 2c).
Figure 2.
Functional properties of hispidulin and apigenin. (a) Hispidulin at 10 μM by itself did not elicit any current but stimulated currents elicited by 4 μM GABA at recombinant α1β2γ2S GABAA receptors expressed by X. leavis oocytes. (b) Concentration dependence of allosteric stimulation by hispidulin. (c) Stimulation by hispidulin (10 μM) at α1β2γ2S GABAA receptors is partially inhibited by co-application of the BZD receptor antagonist Ro15-1788 (1 μM). α1β2 GABAA receptors are not stimulated by 10 μM hispidulin. (d) Allosteric stimulation by hispidulin at α6β2γ2S GABAA receptors. (e) Inhibition by apigenin of GABA-induced currents at recombinant α1β2γ2S GABAA receptors. The points indicate the mean±s.e.m. (n=3–5).
Substance concentrations of about 50 nM and higher stimulated the GABA-induced chloride current at all five receptor subtypes (α1−3,5,6β2γ2) investigated (not shown). In contrast to diazepam, hispidulin also enhanced the GABA-activated current at α6β2γ2 GABAA receptors (Figure 2d).
In order to relate hispidulin's effects to the effects of a familiar flavone, apigenin was equally investigated (Figure 1d). This compound reduced the GABA-induced chloride ion current and therefore acted as negative allosteric modulator of GABA (Figure 2e).
Gerbil model
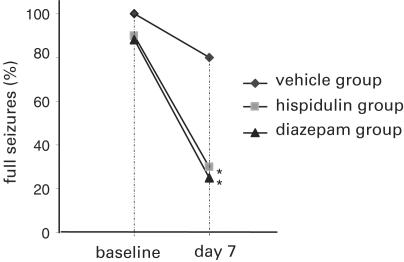
For the investigation of hispidulin effects on ictal events, gerbils were selected for their susceptibility to the triggering of seizures by typical environmental stressors. A visible confirmatory seizure was inducible at baseline in 28 animals immediately prior to treatment. Drug effects of hispidulin and diazepam on seizure incidence are presented in Figure 3. No ictal phenomena were observed during the period of treatment. When re-exposed to the same stimuli on day 7, gerbils experienced milder/fewer seizures in both the hispidulin (10 mg kg−1 BW per day) and the diazepam (2 mg kg−1 BW per day) groups (full seizures in 30% (P<0.04) and 25% (P<0.04) of animals, respectively), when compared to gerbils in the vehicle group (full seizures in 80% of animals).
Figure 3.
Effects of vehicle, hispidulin and diazepam on the occurrence of epileptic seizures in the Mongolian gerbil model (M. unguiculatus) before and after the application of test substances. Paired proportion tests (http://www.ubmail.ubalt.edu/~harsham/Business-stat/otherapplets/PairedProp.htm) were used to examine the null hypothesis that 7 days' pharmacological treatment had no modulatory effect on seizure propensity. *P<0.04.
Blood–brain barrier study
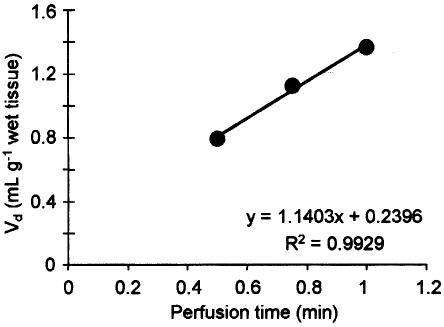
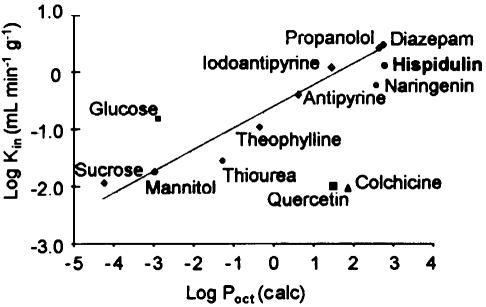
In accordance with our behavioral findings, perfusion of 14C-labelled hispidulin into the right carotid artery of the rat resulted in a linear flux of the flavone to the brain. [14C]hispidulin in artificial saline was perfused into the right carotid artery of the rat for 30, 45 and 60 s. Figure 4 shows the Vd (ml g−1) for hispidulin into the right hemisphere. The rate of uptake (Kin) 1.14 ml min−1 g−1 was obtained from the slope of a plot of Vd (μl g−1) against time (min). Figure 5 shows the relationship between log Kin (experimental) and lipophilicity of hispidulin (log Poct 2.67, calculated octanol–water partition coefficient) compared with the behavior of known passively permeating compounds and efflux transport substrates.
Figure 4.
Volume of distribution (Vd) in the rat brain (right hemisphere) for [14C]hispidulin plotted against time (n=3), as assessed using the Takasato in situ perfusion method. R2=0.9929.
Figure 5.
Relationship between log Kin (experimental) and log Poct (calculated) of passively permeating compounds (diamonds), other flavonoids (naringenin, quercetin), substrates for known uptake (glucose) and efflux (colchicine) transport. Log Kin values with the exception of hispidulin are taken from Qaiser et al. (in preparation; see also Youdim et al., 2004). Log Poct was calculated using the KowWin (LogKow) software at http://www.esc.syrres.com/interkow/logkow.htm. This program uses fragmental analysis of the compound structure for its prediction (values show high correlation with quoted experimental values, P=0.98).
Caco-2 cell layer permeability study
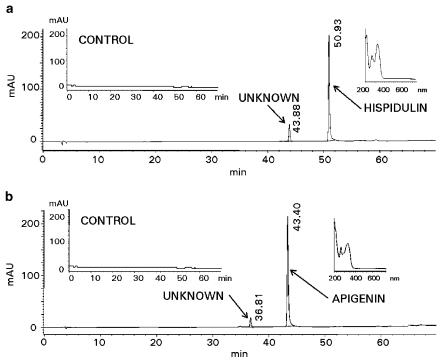
The permeation of hispidulin and apigenin aglycones across the Caco-2 monolayers was investigated in a model of intestinal absorption to test for any compound modification secondary to uptake by epithelial cells. Figure 6a shows the HPLC profile of the receiver medium following hispidulin (30 μM) exposure at the donor side of the Caco-2 cell monolayer for 60 min. The peak with RT at 50.93 min represents the hispidulin aglycone, in parallel with RT and diode array spectra of a standard solution. Apigenin (RT 43.40 min) also crossed the Caco-2 cell monolayer in its intact form as shown in Figure 6b. Following incubation with β-glucuronidase, no change in either peak was noted, suggesting the absence of glucuronidated metabolites of hispidulin or apigenin. Identification of the unknown peaks at RT 43.88 min (Figure 6a) and 36.81 min (Figure 6b) fell outside the scope of the current study.
Figure 6.
HPLC traces at 320 nm obtained from receiver wells of the Caco-2 cells showing permeability of hispidulin (a) and apigenin (b) in their intact aglycone forms.
Discussion
In a previous study on novel natural ligands of the BZD receptor from S. officinalis, we isolated the flavone hispidulin and provided details on its affinity to the BZD binding site in vitro (Kavvadias et al., 2003). The subsequent successful synthesis of hispidulin including its 14C-labelled form allowed us to perform additional in vitro and in vivo investigations.
Electrophysiological studies
Functional effects of hispidulin were investigated in electrophysiological studies at recombinant GABAA receptors expressed in X. laevis oocytes. GABA was always used at concentrations eliciting 2–5% of the maximal current amplitude in the corresponding GABAA receptor. Hispidulin at 10 μM did not by itself elicit current but exhibited a positive allosteric modulatory effect by enhancing the GABA-stimulated current at α1β2γ2S (Figure 2a). This stimulation was concentration dependent (Figure 2b). Maximum relative stimulation at α1β2γ2S was achieved with 10 μM hispidulin. Interestingly, at higher concentrations, a second inhibitory phase was observed. The fact that action of hispidulin is two-phasic, going through a maximum, predicts that an overdose will result in a submaximal effect. At 10 μM, stimulation by hispidulin amounted to about 24% of the stimulation by 1 μM diazepam. Assuming that the optimum stimulation observed corresponds closely to the maximum stimulation, hispidulin is a partial positive allosteric modulator. The potentiation of 10 μM hispidulin at α1β2γ2S amounted to 47±5% (n=3, s.e.m.) and was largely blocked by co-application of the BZD receptor antagonist Ro15-1788 with a residual stimulation of 17±2% (n=3, s.e.m.) (Figure 2c). Potentiation of 10 μM hispidulin at α1β2 amounted to 1±1% (n=3, s.e.m.). We conclude that hispidulin acts predominantly through the BZD binding site.
Substance concentrations of about 50 nM and higher stimulated the GABA-induced chloride current at all five receptor subtypes (α1−3,5,6β2γ2S) investigated (not shown). In this study, we could not detect any receptor subtype selectivity regarding maximal stimulation. The IC50 was 0.8–1.8 μM for α1β2γ2S, α2β2γ2S and α5β2γ2S, and about 5 μM for α3β2γ2S and α6β2γ2S. In contrast to diazepam, hispidulin also enhanced the GABA-activated current at α6β2γ2S GABAA receptors (Figure 2d). At these receptors, the stimulation by 10 μM hispidulin was 65±17% (n=3, s.e.m.) and in the presence of 1 μM Ro15-1788 37±7% (n=3, s.e.m.). Recent studies of Marder et al. (2001) showed absence of binding activity of several flavonoids for GABAA receptors containing α6-subunits. Sterical reasons owing to the small lipophilic pocket L3 at receptors containing α6-unit were hypothesized to explain this phenomenon. In our studies, hispidulin interacted also with this receptor subtype. As with other ligands of the BZD binding site, action at α6β2γ2S receptors seems to depend on ligand structure.
Apigenin was also investigated. Despite its strong structural similarity to hispidulin (Figure 1d), apigenin reduced the GABA-induced chloride ion current and therefore acted as negative allosteric modulator of GABA in α1β2γ2S (Figure 2e) in the present electrophysiological study. Most probably it lacks the stimulatory phase observed at low concentrations of hispidulin and only has the inhibitory properties observed at higher concentrations. A similar behavior was observed in α2β2γ2S, α3β2γ2S and α5β2γ2S. Interestingly, in α6β2γ2S, the transient stimulatory phase was also observed. A negative allosteric effect by apigenin has previously been noted by Avallone et al. (2000) in primary cultures of cerebellar granule cells. Thus, its modulatory activity at the BZD receptor site as suggested by behavioral data (Viola et al., 1995) has been difficult to ascertain both in primary cell cultures and in oocytes expressing recombinant receptors (Avallone et al., 2000; Goutman et al., 2003). Sedative or other BZD-like effects observed in vivo are therefore presumed to result from apigenin affinity to an alternative site.
Epileptic seizures are a correlate of hypersynchronous electrical discharges in the brain resulting from neuronal overexcitation or deficient inhibition in a given brain area. The GABA-mediated inhibition is readily enhanced by a large number of BZD receptor ligands. Consequently, classical BZD ligands such as diazepam act as effective anticonvulsants (Treiman, 2001). We therefore hypothesized that other BZD receptor ligands that possess an agonistic pharmacological profile similar to that of hispidulin could also have these effects. In this study, the diazepam-like activity of hispidulin in vitro led us to hypothesize anticonvulsant effects of the flavone in vivo. The Mongolian gerbil (M. unguiculatus) is well characterized as an animal model for studying epileptiform seizures, in particular, human myoclonic and grand mal seizures (Bertorelli et al., 1995). A variable proportion of Mongolian gerbils are known to suffer from clonic–tonic seizures, which may be elicited by exposure to minor environmental stimuli, for example, by manually stroking the animal or by placing it in a novel environment. Seizure sensitivity is enhanced in certain inbred strains well suited for the study of antiepileptic drugs (Rausch et al., 1988). Owing to their innate vulnerability, affected gerbils need not undergo chemical or electroshock challenges to induce seizures (Rausch et al., 1988). Intravenous or intraperitoneal injections are mostly avoided so as not to cause immediate seizure induction. In the present study, efficacy of hispidulin was tested in comparison to diazepam on the basis of earlier observations (Rausch et al., 1988). As we confirmed, antiepileptic treatment with either hispidulin or the classic tranquilizer diazepam aided in the treatment of spontaneous seizures.
Few studies have addressed BBB permeability for flavone derivatives. It was recently shown in an in vitro model by members of our group that a number of flavonoids, for example, naringenin and hesperetin, are able to enter the brain endothelium and cross the BBB as are several flavonoid metabolites (Youdim et al., 2003). Other related compounds, for example, epicatechin, possess little or no potential for permeation. The permeability of hispidulin across the BBB has not been investigated in cell culture or in vivo. The anticonvulsant effects of this flavone, illustrated in seizure-prone Mongolian gerbils, strongly suggested its delivery to the CNS. To further confirm this important mode of distribution, we investigated the penetration of hispidulin into the brain in an in situ rat model. When compared to other highly BBB-penetrating agents, hispidulin approached both diazepam and propanolol in terms of uptake by the total right cerebral hemisphere. When rate of uptake (Kin) by the total right cerebral hemisphere was plotted against compound lipophilicity (log Poct 2.67), the behavior of hispidulin was similar to that of known highly penetrating agents such as diazepam and propanolol. These findings suggest that hispidulin permeates the BBB by simple passive diffusion. However, a control perfusion with [14C]sucrose indicated that 5% ethanol did not disrupt BBB integrity, so the high penetration of [14C]hispidulin was not due to paracellular (junctional) flux, but likely to be transcellular, via passive diffusion across the endothelial cell membranes. As recently observed by Youdim et al. (2003) in an in vitro BBB model, the potential for permeation by other flavonoids is strongly correlated with lipophilicity. Here we show that the lipophilicity of hispidulin appears to facilitate its permeation through the BBB.
Many flavones and their glucoside derivatives are glucuronidated during the absorption process (Spencer et al., 1999). Glucuronidation during transfer across the jejunum and ileum is possible without the need for gut microflora. The more highly reducing phenolics like quercetin or luteolin are absorbed, more than 90% as glucuronidated metabolites. To establish whether orally administered hispidulin enters the blood circulation in its intact form, permeation across the Caco-2 monolayer was investigated. Hispidulin and apigenin were predominantly absorbed without any structural modification. These studies provide evidence that the active aglycone form of hispidulin is present after absorption.
The present study illustrates the chemical synthesis of the flavone hispidulin. As we point out, this compound probably acts as a partial positive allosteric modulator at GABAA receptors, penetrates the BBB and possesses anticonvulsant activity in the CNS. Electrophysiological data and data on BBB permeation provide important insights into the role of hispidulin as a potent anticonvulsant compound and are supported by our in vivo observations, with the limitations of a single-dose design. Further experiments will establish whether hipidulin displays other effects typical of BZDs, such as sedative, anxiolytic and muscle relaxant activity. Further research is warranted on the bioavailability, distribution parameters and the putative, active metabolites plus the effects in other epileptic phenotypes, to put into perspective the flavone's impact on inhibitory brain activity.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft, Bonn (Schr 211-19-3, Schr 211-21-1-2 and Sa 789-1-3). E.S. was supported by the Swiss National Science Foundation Grant 3100-064789.01-1 and M.Z.Q. by the King's College London BBB Consortium with Industry. We are most grateful to Hans Boehme from Leibniz Institute for Neurobiology (Magdeburg, Germany) for generously providing gerbils. We thank Prof. Joan Abbott for her helpful suggestions on this manuscript.
Abbreviations
- BBB
blood–brain barrier
- BW
body weight
- BZD
benzodiazepine
- CNS
central nervous system
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- GABA
γ-aminobutyric acid
- GABAA receptors
γ-aminobutyric acid type A receptors
- HBSS
Hanks' balanced salt solution
- HPLC
high-performance liquid chromatography
- Kin
rate of uptake
- Poct
calculated octanol–water partition coefficient
- Ro15-1788
flumazenil
- RT
retention time
- TEER
transendothelial electrical resistance
- TMS
tetramethylsilane
References
- AVALLONE R., ZANOLI P., PUIA G., KLEINSCHNITZ M., SCHREIER P., BARALDI M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59:1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- BAKER W., BROWN N.C., SCOTT J.A. The synthesis of 5-hydroxy-8-methoxyflavone (primetin monomethyl ether) J. Chem. Soc. 1939;2:1922–1927. [Google Scholar]
- BERTORELLI R., ADAMI M., ONGINI E. The Mongolian gerbil in experimental epilepsy. Ital. J. Neurol. Sci. 1995;16:101–106. doi: 10.1007/BF02229081. [DOI] [PubMed] [Google Scholar]
- BOILEAU A.J., BAUR R., SHARKEY L.M., SIGEL E., CZAJKOWSKI C. The relative amount of cRNA coding for γ2 subunits affects stimulation by benzodiazepines in GABAA receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- BRACON F., GUITTARD F., TAFFIN DE GIVENCHY E., CAMBON A. Highly fluorinated monomeres precursors of side-chain liquid cristalline polysiloxanes. J. Polym. Sci. A. 1999;37:4487–4496. [Google Scholar]
- CHERPECK R.E.Preparation and use of aromatic amides of poly(oxyalkylene) carbamates as fuel additives, especially gasoline deposit inhibitors 1998. US Patent 5786499
- CHULASIRI M., BUNYAPRAPHATSARA N., MOONGKARNDI P. Mutagenicity and antimutagenicity of hispidulin and hortensin, the flavonoids from Millingtonia hortensis L. Environ. Mol. Mutagen. 1992;20:307–312. doi: 10.1002/em.2850200409. [DOI] [PubMed] [Google Scholar]
- DANIEL H., WENZEL U.Flavonoids and their possible role in colon cancer prevention and therapy. Exogenous factors in colonic carcinogenesis Falk-Symposium 2003128Dordrecht: Kluwer Academic Publisher; 264–274.ed. Scheppach, W. & Scheurlen, M. Vol [Google Scholar]
- EGLETON R.D., ABBRUSCATO T.J., THOMAS S.A., DAVIS T.P. Transport of opioid peptides into the central nervous system. J. Pharm. Sci. 1998;87:1433–1439. doi: 10.1021/js980062b. [DOI] [PubMed] [Google Scholar]
- GIL B., SANZ M.J., TERENCIO M.C., FERRANDIZ M.L., BUSTOS G., PAYA M., GUNASEGARAN R., ALCARAZ M.J. Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice. Life Sci. 1994;54:333–338. doi: 10.1016/0024-3205(94)90021-3. [DOI] [PubMed] [Google Scholar]
- GOUTMAN J.D., WAXEMBERG M.D., DONATE-OLIVER F., POMATA P.E., CALVO D.J. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur. J. Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- HORIE T., SHIBATA K., YAMASHITA K., KAWAMURA Y., TSUKAYAMA M. Studies of the selective O-alkylation and dealkylation of flavonoids. XXII. A convenient method for synthesizing 3,5,7-trihydroxy-6-methoxyflavones. Chem. Pharm. Bull. 1997;45:446–451. [Google Scholar]
- HUI K.M., HUEN M.S.Y., WANG H.Y., ZHENG H., SIGEL E., BAUR R., REN H., LI Z.W., WONG J.T.-F., XUE H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002;64:1415–1424. doi: 10.1016/s0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- JAIN A.C., GUPTA S.M., BAMNAH P. Aromatic benzhydrylation: part III – synthesis of chromones of triphenylmethane type. Indian J. Chem. 1985;24B:393–397. [Google Scholar]
- KAVVADIAS D., MONSCHEIN V., SAND P., RIEDERER P., SCHREIER P. Constituents of sage (Salvia officinalis L.) with in vitro affinity to human brain benzodiazepine receptor. Planta Med. 2003;69:113–117. doi: 10.1055/s-2003-37712. [DOI] [PubMed] [Google Scholar]
- MARDER M., ESTIU G., BLANCH L.B., VIOLA H., WASOWSKI C., MEDINA J.H., PALADINI A.C. Molecular modeling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABAA receptor complex. Bioorg. Med. Chem. 2001;9:323–335. doi: 10.1016/s0968-0896(00)00250-9. [DOI] [PubMed] [Google Scholar]
- MARDER M., VIOLA H., WASOWSKI C., FERNANDEZ S., MEDINA J.H., PALADINI A.C. 6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharm. Biochem. Behav. 2003;75:537–545. doi: 10.1016/s0091-3057(03)00121-7. [DOI] [PubMed] [Google Scholar]
- MEDINA J.H., VIOLA H., WOLFMAN C., MARDER M., WASOWSKI C., CALVO D., PALADINI A.C. Neuroactive flavonoids: new ligands for the benzodiazepine receptors. Phytomedicine. 1998;5:235–243. doi: 10.1016/S0944-7113(98)80034-2. [DOI] [PubMed] [Google Scholar]
- RAUSCH W.-D., GOLECH S., RIEDERER P., WEISER M. New Trends in Clinical Neuropharmacol. London: John Libbey Co Ltd, London; 1988. Inhibitory activity of tranquilizers, antiepileptics, calcium antagonists, sedatives and other compounds on the epileptiform seizures in the Mongolian gerbil; pp. 55–58. [Google Scholar]
- SIEGHART W., SPERK G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- SIGEL E. Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J. Physiol. (Lond.) 1987;386:73–90. doi: 10.1113/jphysiol.1987.sp016523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGEL E., BUHR A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- SIGEL E., BAUR R., TRUBE G., MÖHLER H., MALHERBE P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- SPENCER J.P.E., CHOWRIMOOTOO G., CHOUDHURY R., DEBNAM E.S., SREI S.K., RICE-EVANS C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458:224–230. doi: 10.1016/s0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA S.D., SRIVASTAVA S.K. Synthesis of a new flavone. Indian J. Chem. 1987;26B:57–58. [Google Scholar]
- TAKASATO Y., RAPOPORT S.I., SMITH Q.R. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 1984;247:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- TAN R.X., LU H., WOLFENDER J.L., YU T.T., ZHENG W.F., YANG L., GAFNER S., HOSTETTMANN K. Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med. 1999;65:64–67. doi: 10.1055/s-1999-13965. [DOI] [PubMed] [Google Scholar]
- TREIMAN D.M. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42 Suppl. 3:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- VIOLA H., WASOWSKI C., LEVI DE STEIN M., WOLFMAN C., SILVEIRA R., DAJAS F., MEDINA J.H., PALADINI A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- YOUDIM K.A., DOBBIE M.S., KUHNLE G., PROTEGGENTE A., ABBOTT N.J., RICE-EVANS C. Interaction between flavonoids and the blood–brain barrier: in vitro studies. J. Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- YOUDIM K.A., QAISER M.Z., BEGLEY D.J., RICE-EVANS C.A., ABBOTT N.J. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic. Biol. Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]