Abstract
Nicotine has been reported to normalize deficits in auditory sensory gating in the cases of schizophrenia, suggesting an involvement of nicotinic acetylcholine receptors in attentional abnormalities. However, the mechanism remains unclear. The present study investigated the effects of nicotine on the disruption of prepulse inhibition (PPI) of the acoustic startle response induced by apomorphine or phencyclidine in rats.
Over the dose range tested, nicotine (0.05–1 mg kg−1, s.c.) did not disrupt PPI. Neither methyllycaconitine (0.5–5 mg kg−1, s.c.), an α7 nicotinic receptor antagonist, nor dihydro-β-erythroidine (0.5–2 mg kg−1, s.c.), an α4β2 nicotinic receptor antagonist, had any effect on PPI.
Nicotine (0.01–0.2 mg kg−1, s.c.) dose-dependently reversed the disruption of PPI induced by apomorphine (1 mg kg−1, s.c.), but had no effect on the disruption of PPI induced by phencyclidine (2 mg kg−1, s.c.).
The reversal of apomorphine-induced PPI disruption by nicotine (0.2 mg kg−1) was eliminated by mecamylamine (1 mg kg−1, i.p.), but not by hexamethonium (10 mg kg−1, i.p.), indicating the involvement of central nicotinic receptors.
The antagonistic action of nicotine on apomorphine-induced PPI disruption was dose-dependently blocked by methyllycaconitine (1 and 2 mg kg−1, s.c.). However, dihydro-β-erythroidine (1 and 2 mg kg−1, s.c.) had no effect.
These results suggest that nicotine reverses the disruption of apomorphine-induced PPI through central α7 nicotinic receptors.
Keywords: Nicotine, apomorphine, phencyclidine, prepulse inhibition
Introduction
Prepulse inhibition (PPI) is the phenomenon in which the amplitude of the acoustic startle response is reduced by a preceding weak stimulation, and PPI is thought to reflect the operation of a sensorimotor gating system in the brain. There are a number of studies showing that PPI of the acoustic startle is deficient in the cases of schizophrenia (Braff & Geyer, 1990; Ludewig & Vollenweider, 2002). It has been proposed that the dopaminergic and glutamatergic systems may contribute to the gating deficits observed in patients with schizophrenia (Braff & Geyer, 1990). Animal studies have shown that dopamine agonists or N-methyl-D-aspartate (NMDA) antagonists disrupt PPI (Mansbach et al., 1988; Davis et al., 1990; Peng et al., 1990; Keith et al., 1991; Johansson et al., 1995). The disruption of PPI induced by dopaminergic stimulation such as apomorphine is reversed by dopamine D2 receptor antagonists with potency correlating well with clinically effective dosages of each drug (Swerdlow et al., 1994). Phencyclidine, a noncompetitive NMDA receptor antagonist, causes psychotomimetic symptoms in human subjects and also disrupts PPI in animal subjects. Atypical antipsychotics such as clozapine have variously been reported to reverse (Bakshi et al., 1994; Bakshi & Geyer, 1995) or not reverse (Johansson et al., 1994) the disruption of PPI induced by phencyclidine. Thus, PPI of acoustic startle in animals is a model for the same aspects of schizophrenia, and disruption of PPI induced by apomorphine or phencyclidine in animals has been used as a tool to screen antipsychotic drugs (Swerdlow et al., 1994).
Nicotine has been found to enhance PPI in both human and animal subjects. Nicotine administration via cigarette smoking to a group of overnight smoking-deprived smokers increases PPI (Kumari et al., 1996), and nicotine administered subcutaneously to a group of male nonsmokers also enhances PPI as compared to saline administration (Kumari et al., 1997). In rats, acute administration of nicotine increases PPI, particularly at lower doses (Acri et al., 1994). However, it has been reported that high doses of nicotine and α4β2 nicotinic receptor agonist, but not α7 nicotinic receptor agonist, disrupt PPI (Schreiber et al., 2002), suggesting an involvement of α4β2 nicotinic receptors in the mechanisms of PPI.
A total of 70–90% of schizophrenic patients are cigarette smokers. This rate is markedly higher than the 25–30% of the general population's smoking rate (Glassman, 1993; Ziedonis & George, 1997). Nicotine has been reported to normalize deficient auditory sensory gating in both schizophrenics and their family members (Adler et al., 1992, 1993). Moreover, post-mortem studies of schizophrenic patients have shown a reduced number of [125I]-α-bungarotoxin-sensitive nicotinic acetylcholine hippocampal receptors (Freedman et al., 1995). It has been reported that smoking decreases when schizophrenic patients switch treatments from typical antidopaminergic neuroleptic drugs to clozapine (George et al., 1995, McEvoy et al., 1995), and that smokers show greater therapeutic responses to clozapine than nonsmokers (McEvoy et al., 1999). These studies have led researchers to hypothesize that nicotine has ‘therapeutic' effects on the attentional abnormalities in schizophrenia (Adler et al., 1992, 1993). However, the precise role of nicotinic acetylcholinergic systems underlying deficits in auditory sensory gating in schizophrenics remains unclear. In the present study, we investigated whether or not nicotine reverses the disruption of PPI induced by apomorphine or phencyclidine in rats, and also examined the involvement of nicotinic receptor subtypes by testing several nicotinic receptor antagonists.
Methods
Animals
Male Wistar strain rats (at 8–10 weeks of age) were obtained from Charles River Laboratories (Atsugi, Japan). All animals were housed in an animal room maintained at 22±2°C under a 12/12-h light/dark cycle with lights on from 0700. Food and water were available ad libitum. The experimental protocol was conducted according to the Guidelines of the Ethics Review Committee for Animal Experimentation of Okayama University Medical School.
Drugs
The following drugs were used: (−)-nicotine tartrate (Sigma, St Louis, MO, U.S.A.), phencyclidine (synthesized at Fukuyama University), hexamethonium bromide (Sigma), mecamylamine hydrochloride (RBI, Natick, MA, U.S.A.), dihydro-β-erythroidine hydrobromide (DHβE, RBI), methyllycaconitine (MLA, RBI) and haloperidol injection (Dainippon Pharmaceutical Co., Osaka, Japan). All drugs were dissolved in physiological saline (0.9% sodium chloride). The pH of the nicotine solution was neutralized with sodium hydroxide. Apomorphine hydrochloride (Sigma) was dissolved in saline containing 0.1% ascorbic acid and the solution was kept on ice in the dark to protect against oxidative degradation. Clozapine (RBI) was suspended in 1% Tween 80. All drugs were expressed as the free base. Each drug was administered in a volume of 0.2 ml 100 g−1 body weight. Haloperidol and clozapine were administered intraperitoneally, and the other drugs were administered subcutaneously.
Measurement of startle response and prepulse inhibition
One startle chamber (SR-LAB, San Diego Instruments, San Diego, CA, U.S.A.) was used. The chamber consisted of a Plexiglas cylinder (8.9 cm in diameter × 20 cm long) resting on a Plexiglas frame in a sound-attenuated, lighted, ventilated enclosure. Background noise and acoustic noise bursts were presented via a loudspeaker mounted 12 cm above the cylinder. Startle responses, reflecting the motion of animals in the cylinder following the acoustic stimulus, were detected by a piezoelectric transducer mounted below the frame. Stabilimeter readings were rectified and recorded by a microcomputer and interface ensemble (San Diego Instruments).
After a 5 min acclimation period, the PPI test was started. Throughout both the preliminary period and the test session, the chamber light was on, and background noise was set at 65 dB; all sounds were given as white noise. The test session included five initial startle stimuli (120 dB of 20 ms duration), to accustom the rats to the startle stimuli, followed by three different types of 10 trials (total 30 trials) presented in random order: a pulse (P) trial (120 dB of 20 ms duration), and two prepulse and pulse (PP-P) trials, which involved a prepulse (70 or 80 dB of 20 ms duration) followed by the same pulse as in the P trial 100 ms later. The average intertrial interval was 40 s (range 20–60 s), and this interval was randomized. The startle responses were measured every 100 ms from the onset of the pulse presentation, and the maximum value was defined as the startle amplitude. The animals' startle amplitudes in response to repetitions of each trial type were averaged across the session. The experimental schedule was controlled by a microcomputer.
Apomorphine was administered 15 min before the measurement of startle amplitude. Nicotine, phencyclidine, haloperidol and clozapine were administered 30 min before the measurement. Nicotinic receptor antagonists (mecamylamine, hexamethonium, MLA and DHβE) were administered 45 min before the measurement.
Statistical analysis
Values were represented as a group mean and as standard errors of mean. PPI in PP70-P or PP80-P trial was calculated as a percent inhibition of the startle amplitude in the P trial according to the following formula: (startle amplitude (P trial) − startle amplitude (PP-P trial))/startle amplitude (P trial) × 100. The five initial startle stimuli were excluded from the statistical analysis. Repeated measures analysis of variance (two-way ANOVA) with drug treatment as a between-subjects factor and pulse intensity as a within-subject factor was used. Whenever the drug treatment factor or the drug treatment factor × pulse intensity interaction was significant, post hoc comparison was carried out. The post hoc individual comparisons were performed with Dunnett's test or Student's t-test. The significance level was set at P<0.05.
Results
Figure 1 shows the effects of nicotine or nicotinic receptor antagonists on PPI in rats. Nicotine (0.05–1 mg kg−1, s.c.) had no effect on PPI in both prepulses of 70 and 80 dB by itself. Neither MLA (0.5–5 mg kg−1, s.c.) nor DHβE (0.5–2 mg kg−1, s.c.) had any significant effect on PPI. Table 1 shows the effects on startle amplitude. DHβE (0.5–2 mg kg−1, s.c.) dose-dependently increased startle amplitude. A repeated measures ANOVA revealed a significant dose effect of DHβE (F(3,28)=3.132, P<0.05) and no significant dose × pulse intensity interaction (F(6,56)=0.876, NS). The post hoc comparisons using Dunnett's test revealed significant differences of startle amplitude at a dose of 2 mg kg−1 in the PP70-P, PP80-P and P trials. However, neither nicotine (0.05–1 mg kg−1, s.c.) nor MLA (0.5–5 mg kg−1, s.c.) had any effect on startle amplitude.
Figure 1.
Lack of effects of nicotine, MLA and DHβE on PPI in rats. Nicotine was administered 30 min before the measurement of PPI. MLA or DHβE was administered 45 min before the measurement. Each point represents the mean PPI of eight rats in the PP70-P (70 dB prepulse followed by 120 dB pulse) and PP80-P (80 dB prepulse followed by 120 dB pulse) trials. The vertical lines represent±s.e.m.
Table 1.
Effects of co-administration of apomorphine and nicotine or nicotinic receptor antagonist on startle amplitude in rats
| Drug (dose (mg kg−1)) | Startle amplitude | ||
| P trial | PP70-P trial | PP80-P trial | |
| Saline | 1239±246 | 630±113 | 403±81 |
| Ni (0.05) | 629±85 | 382±35 | 239±36 |
| Ni (0.2) | 1066±255 | 538±133 | 331±62 |
| Ni (0.5) | 933±151 | 522±55 | 279±40 |
| Ni (1) | 1182±370 | 527±88 | 274±43 |
| Saline | 837±95 | 489±60 | 332±34 |
| DHβE (0.5) | 902±110 | 598±111 | 386±78 |
| DHβE (1) | 1046±220 | 745±181 | 453±122 |
| DHβE (2) | 1596±373* | 1266±293** | 863±235* |
| Saline | 945±187 | 613±105 | 326±60 |
| MLA (0.5) | 1000±157 | 687±96 | 380±79 |
| MLA (1) | 1158±235 | 874±195 | 473±91 |
| MLA (2) | 738±118 | 572±66 | 333±36 |
| MLA (5) | 800±119 | 681±136 | 386±70 |
| Naive | 1350±242 | 770±148 | 434±74 |
| Apo (1) | 1426±219 | 1455±214* | 1064±126** |
| Apo (1)+Ni (0.01) | 1077±152 | 801±119 | 570±87 |
| Apo (1)+Ni (0.05) | 1296±260 | 963±171 | 559±112 |
| Apo (1)+Ni (0.02) | 1344±424 | 1349±497 | 895±281 |
| Naive | 1011±133 | 652±73 | 352±51 |
| Apo (1) | 1301±339 | 1333±336 | 998±339 |
| Apo (1)+Hal (0.1) | 1074±314 | 1150±398 | 633±293 |
| Apo (1)+Hal (0.3) | 1068±353 | 848±311 | 526±210 |
| Apo (1)+Hal (1) | 549±126 | 384±61# | 160±26# |
| Naive | 811±88 | 530±59 | 317±46 |
| Apo (1) | 884±204 | 924±214 | 765±228 |
| Apo (1)+Ni (0.2) | 1505±303 | 1436±351 | 841±243 |
| Apo (1)+Ni (0.2)+Mec (1) | 1867±222 | 1945±226 | 1243±131 |
| Apo (1)+Ni (0.2)+Hex (10) | 1039±269 | 877±202 | 486±124 |
| Naive | 980±252 | 571±107 | 512±139 |
| Apo (1) | 1371±291 | 1377±205** | 935±153 |
| Apo (1)+Ni (0.2) | 1758±463 | 1224±261 | 887±201 |
| Apo (1)+Ni (0.2)+MLA (1) | 1919±428 | 1955±460 | 1262±361 |
| Apo (1)+Ni (0.2)+MLA (2) | 1563±258 | 1560±234 | 1307±265 |
| Naive | 931±96 | 666±76 | 399±38 |
| Apo (1) | 1209±280 | 1336±301 | 971±262 |
| Apo (1)+Ni (0.2) | 1499±264 | 1112±198 | 751±101 |
| Apo (1)+Ni (0.2)+DHβE (1) | 1958±326 | 1697±295 | 1042±170 |
| Apo (1)+Ni (0.2)+DHβE (2) | 1475±129 | 1306±103 | 867±80 |
Apomorphine (Apo), nicotine (Ni) and nicotinic receptor antagonists (MLA, DHβE, mecamylamine (Mec), hexamethonium (Hex)) were administered 15, 30 and 45 min before the measurement of startle amplitude, respectively. Haloperidol (Hal) was administered 15 min before the apomorphine injection. The three measures of startle amplitude are P trial (120 dB pulse alone), PP70-P trial (70 dB prepulse followed by 120 dB pulse) and PP80-P trial (80 dB prepulse followed by 120 dB pulse). Each value represents mean±s.e.m. of eight animals.
P<0.05,
P<0.01 compared with the naive (two-way ANOVA followed by Student's t-test).
P<0.05 compared with the apomorphine alone (two-way ANOVA followed by Dunnett's test).
In a preliminary study, apomorphine (0.1–3 mg kg−1, s.c.) and phencyclidine (1–4 mg kg−1, s.c.) produced a dose-dependent decrease in PPI in both prepulses of 70 and 80 dB. Based on the results, submaximal doses of apomorphine (1 mg kg−1, s.c.) or phencyclidine (2 mg kg−1, s.c.) were chosen for the subsequent tests. Figure 2 shows the effects of nicotine or haloperidol on apomorphine-induced disruption of PPI in rats. A repeated measures ANOVA for naive and apomorphine (1 mg kg−1, s.c.) revealed a significant effect of apomorphine treatment (F(1,14)=62.397, P<0.001) and no significant dose × prepulse intensity interaction (F(1,14)=0.007, NS). Student's t-test showed significant differences (P<0.01) in both prepulses of 70 and 80 dB. The decrease in PPI induced by apomorphine was reversed by haloperidol (0.1–1 mg kg−1, i.p.) in a dose-dependent manner. To evaluate the effect of haloperidol treatment, a repeated measures ANOVA was applied to all apomorphine-treated groups (four groups). A significant effect of haloperidol treatment (F(3,28)=5.185, P<0.01) and no significant dose × pulse intensity interaction (F(3,28)=1.147, NS) were revealed. The post hoc comparisons using Dunnett's test showed significant differences between the apomorphine-alone group and the apomorphine+haloperidol (0.3 and 1 mg kg−1, i.p.) group in prepulse of 80 dB. On the other hand, nicotine (0.01–0.2 mg kg−1, s.c.) produced a significant dose-dependent reversal of apomorphine-induced disruption of PPI (F(3,28)=12.362, P<0.001) and no significant dose × prepulse intensity interaction (F(3,28)=0.456, NS). Dunnett's test showed that the apomorphine-induced decrease of PPI was significantly reversed by nicotine at doses of 0.05 and 0.2 mg kg−1 in both prepulses of 70 and 80 dB.
Figure 2.
Effects of nicotine and haloperidol on apomorphine-induced disruption of PPI in rats. Apomorphine was injected 15 min before the measurement of PPI. Nicotine or haloperidol was administered 15 min before the apomorphine injection. Each column represents the mean PPI with s.e.m. in the PP70-P (70 dB prepulse followed by 120 dB pulse) and PP80-P (80 dB prepulse followed by 120 dB pulse) trials (n=8). **P<0.01 compared with the naive group (two-way ANOVA followed Student's t-test). #P<0.05, ##P<0.01 compared with the apomorphine-alone group (two-way ANOVA followed by Dunnett's test).
Table 1 shows the effect on startle amplitude in apomorphine-treated rats. A repeated measures ANOVA for naive and apomorphine (1 mg kg−1, s.c.) in the apomorphine+nicotine (0.01–0.2 mg kg−1)-treated group revealed no significant effect of apomorphine treatment (F(1,14)=4.100, NS) and a significant dose × pulse intensity (F(2,28)=5.920, P<0.01). Haloperidol decreased the startle amplitude in apomorphine-treated rats. To evaluate the effect of haloperidol (0.1–1 mg kg−1, i.p.) treatment, a repeated measures ANOVA was applied to all apomorphine-treated groups (four groups). No significant effect of haloperidol treatment (F(3,28)=1.621, NS) and a significant dose × prepulse intensity interaction (F(6,56)=2.274, P<0.05) were revealed. Dunnett's test showed that 1 mg kg−1 of haloperidol significantly (P<0.05) reduced startle amplitude in both prepulses of 70 and 80 dB.
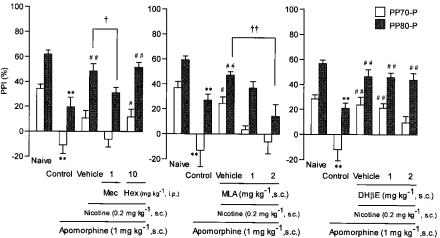
Figure 3 shows the effects of nicotinic receptor antagonists on the reversal of apomorphine-induced PPI disruption by nicotine (0.2 mg kg−1, s.c.). Mecamylamine (1 mg kg−1, i.p.), which freely crosses the blood–brain barrier, blocked the antagonistic action of nicotine. This was confirmed by a main effect for mecamylamine treatment (F(1,14)=7.527, P<0.05) and no significant dose × prepulse intensity interaction (F(1,14)=0.006, NS). The post hoc Student's t-test showed a significant difference (P<0.05) between the nicotine+apomorphine group and the mecamylamine+nicotine+apomorphine group in a prepulse of 80 dB. In contrast, hexamethonium (10 mg kg−1, i.p.), which crosses the blood–brain barrier poorly, had no effect on PPI. MLA (1 and 2 mg kg−1, s.c.) dose-dependently blocked the reversal of apomorphine-induced PPI disruption by nicotine. A repeated measures ANOVA revealed a significant dose effect of MLA (F(2,21)=7.669, P<0.01) and no significant dose × prepulse intensity interaction (F(2,21)=1.361, NS). Dunnett's test showed a significant difference (P<0.01) at a dose of 2 mg kg−1 MLA compared with the vehicle-treated group in the PP80-P trial. DHβE (1 and 2 mg kg−1, s.c.) had no effect on the antagonistic action of nicotine. Table 1 shows the effect on startle amplitude. A repeated measures ANOVA for naive and apomorphine (1 mg kg−1, s.c.) revealed a significant effect of apomorphine treatment and/or a significant treatment × pulse intensity in all experimental groups. However, co-administration of nicotine (0.2 mg kg−1, s.c.) and mecamylamine (1 mg kg−1, i.p.), hexamethonium (10 mg kg−1, i.p.), MLA (1 and 2 mg kg−1, s.c.), DHβE (1 and 2 mg kg−1, s.c.) had no significant effect on the apomorphine-induced change in startle amplitude.
Figure 3.
Effects of nicotinic receptor antagonists on the reversal of apomorphine-induced PPI disruption by nicotine in rats. Apomorphine, nicotine and nicotinic receptor antagonists were administered 15, 30 and 45 min before the measurement of PPI, respectively. Each column represents the mean PPI with s.e.m. in the PP70-P (70 dB prepulse followed by 120 dB pulse) and PP80-P (80 dB prepulse followed by 120 dB pulse) trials (n=8). **P<0.01 compared with the naive group (two-way ANOVA followed by Student's t-test). #P<0.05, ##P<0.01 compared with the apomorphine-alone group (two-way ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 compared with the apomorphine+nicotine group (two-way ANOVA followed by Dunnett's test or Student's t-test). Mec, mecamylamine; Hex, hexamethonium.
Figure 4 shows the effects of nicotine, haloperidol and clozpine on phencyclidine-induced disruption of PPI in rats. A repeated measures ANOVA for naive and phencyclidine (2 mg kg−1, s.c.) revealed a significant effect of phencyclidine treatment (F(1,14)=63.440, P<0.001) and a significant dose × prepulse intensity interaction (F(1,14)=5.446, P<0.05). Student's t-test indicated significant differences (P<0.01) in both prepulses of 70 and 80 dB. The decrease in PPI induced by phencyclidine (2 mg kg−1, s.c.) was reversed by clozapine (1–10 mg kg−1, i.p.) in a dose-dependent manner. A repeated measures ANOVA revealed a significant main effect for clozapine (F(3,28)=9.954, P<0.001) and a significant dose × prepulse intensity interaction (F(3,28)=4.945, P<0.01). Post hoc Dunnett's test showed a significant difference after a dose of 10 mg kg−1 in the PP80-P trial. However, neither nicotine (0.01–0.2 mg kg−1, s.c) nor haloperidol (0.1–1 mg kg−1, i.p.) had any effect on the phencyclidine-induced disruption of PPI. Table 2 (lower) shows the effect of clozapine on startle amplitude in phencyclidine-treated rats. A repeated measures ANOVA for naive and phencyclidine (2 mg kg−1, s.c.) revealed no significant effect of phencyclidine treatment (F(1,14)=0.357, NS) and a significant dose × pulse intensity interaction (F(2,28)=11.787, P<0.001). Clozapine (1–10 mg kg−1, i.p.) dose-dependently reduced the startle amplitude in phencyclidine-treated rats. To evaluate the effect of clozapine treatment, a repeated measures ANOVA was applied to all phencyclidine-treated groups (four groups). A significant effect of clozapine treatment (F(3,28)=3.454, P<0.05) and a significant dose × pulse intensity interaction (F(6,56)=2.492, P<0.05) were revealed. Dunnett's test showed that 10 mg kg−1 of clozapine significantly (P<0.01) reduced the startle amplitude in the 120 dB pulse-alone group. However, co-administration of nicotine (0.01–0.2 mg kg−1, s.c.) and haloperidol (0.1–1 mg kg−1, i.p.) had no significant effect on the phencyclidine-induced change in startle amplitude.
Figure 4.
Effects of nicotine, haloperidol and clozapine on phencyclidine-induced disruption of PPI in rats. All drugs were administered 30 min before the measurement of PPI. Each column represents the mean PPI with s.e.m. in the PP70-P (70 dB prepulse followed by 120 dB pulse) and PP80-P (80 dB prepulse followed by 120 dB pulse) trials (n=8). **P<0.01 compared with the naive group (two-way ANOVA followed by Student's t-test). ##P<0.01 compared with the phencyclidine-alone group (two-way ANOVA followed by Dunnett's test).
Table 2.
Effects of co-administration of phencyclidine and nicotine or clozapine on startle amplitude in rats
| Drug (dose (mg kg−1)) | Startle amplitude | ||
| P alone | PP70-P | PP80-P | |
| Naïve | 900±162 | 619±109 | 351±52 |
| PCP (2) | 526±82.4 | 523±93 | 469±90 |
| PCP (2)+Ni (0.01) | 767±99 | 723±96 | 693±90 |
| PCP (2)+Ni (0.05) | 796±96 | 768±83 | 685±81 |
| PCP (2)+Ni (0.2) | 717±56 | 669±57 | 631±59 |
| Naive | 1025±116 | 637±57 | 377±42 |
| PCP (2) | 846±202 | 807±170 | 702±180 |
| PCP (2)+Hal (0.1) | 796±249 | 785±214 | 717±272 |
| PCP (2)+Hal (0.3) | 857±94 | 867±99 | 684±103 |
| PCP (2)+Hal (1) | 728±74 | 708±93 | 537±68 |
| Naive | 892±152 | 522±81 | 345±48 |
| PCP (2) | 544±48 | 540±73 | 492±57 |
| PCP (2)+CZP (1) | 688±54 | 639±51 | 535±58 |
| PCP (2)+CZP (3) | 554±113 | 464±90 | 374±59 |
| PCP (2)+CZP (10) | 446±77## | 370±61 | 195±38 |
Phencyclidine (PCP), nicotine (Ni), haloperidol (Hal) and clozapine (CZP) were administered 30 min before the measurement of startle amplitude. The three measures of startle amplitude are P trial (120 dB pulse alone), PP70-P trial (70 dB prepulse followed by 120 dB pulse) and PP80-P trial (80 dB prepulse followed by 120 dB pulse). Each value represents mean±s.e.m. of eight animals.
P<0.01 compared with the phencyclidine alone (two-way ANOVA followed by Dunnett's test).
Discussion
Previous studies have shown that nicotine increases (Acri et al., 1994, 1995) or decreases (Schreiber et al., 2002) sensorimotor gating by disparities in the dose, prepulse intensity, age, species and strain. For example, the enhancing effect of nicotine depends on the dose, with lower doses being more effective (Acri et al., 1994); prepulse intensity, with louder prepulses being more effective (Acri et al., 1994, 1995; Curzon et al., 1994); and age, with older rats being more affected (Acri et al., 1995). Acri et al. (1994) reported that nicotine at lower doses (0.001–0.01 mg kg−1), but not at higher doses (0.1–5.0 mg kg−1), enhances PPI at the prepulse level of 65 dB in rats. In the present study, nicotine at doses of 0.05–1 mg kg−1 had no effect on PPI at prepulse signal levels of 70 or 80 dB. On the other hand, several studies have suggested that the α4β2 nicotinic receptor subtypes are related to the auditory sensory gating deficit in rodents. Schreiber et al. (2002) have reported that α4β2 nicotinic receptor agonist A-85380, but not α7 nicotinic receptor agonists GTS-21 and AR-R-17779, disrupts PPI in Sprague–Dawley rats (Schreiber et al., 2002). Olivier et al. (2001) have reported that GTS-21 and AR-R17779 are inactive in the PPI procedure in DBA/2J mice, a strain with no sensory inhibition under routine experimental conditions. But in the present study, neither MLA, an α7 nicotinic receptor antagonist, nor DHβE, an α4β2 nicotinic receptor antagonist, had any effect on PPI in Wistar rats.
Previous studies have demonstrated that a dopaminergic receptor agonist, apomorphine, causes strong impairment of PPI in rats (Davis et al., 1990; Peng et al., 1990), and the disruption of PPI is reversed by dopamine D2 receptor antagonists such as haloperidol (Swerdlow et al., 1994). In the present study, we not only confirmed these previous findings but also found that nicotine produces a significant dose-dependent reversal of apomorphine-induced disruption of PPI without changing the normal PPI by itself. It has been demonstrated that cigarette smoking normalizes deficient auditory sensory gating in schizophrenics and their family members (Adler et al., 1993). Thus, our findings in the current animal study support the hypothesis that nicotine has ‘therapeutic' effects on attentional abnormalities in schizophrenia (Adler et al., 1992, 1993).
In the present study, the reversal of apomorphine-induced PPI disruption by nicotine (0.2 mg kg−1) was antagonized by mecamylamine, a centrally acting nicotinic receptor antagonist, but not by hexamethonium, a peripherally acting nicotinic receptor antagonist. These results suggest that the central nicotinic receptors are responsible for the improvement of apomorphine-induced PPI disruption by nicotine. This concept is further supported by the finding that the antagonistic action of nicotine was blocked by MLA, an α7 nicotinic receptor antagonist. However, DHβE, an α4β2 nicotinic receptor antagonist, had no effect on the antagonistic action of nicotine. It has been reported that low doses of nicotine (0.08–0.33 mg kg−1) regulate gene expression of dopamine biosynthetic enzymes via α7 nicotinic receptors in the rat brain (Serova & Sabban, 2002). Taken together, these findings and the results of the current study indicate that the central α7 nicotinic receptors mediate sensory inhibition and play an important role in the antagonistic action of nicotine on PPI deficits induced by excessive dopaminergic stimulation. However, it has also been reported that mice lacking α7 nicotinic receptors display normal PPI (Paylor et al., 1998) and that α7 nicotinic receptor agonists, GTS-21 and AR-R-17779, do not disrupt PPI in Sprague–Dawley rats (Schreiber et al., 2002). These findings suggest that α7 nicotinic receptors contribute little to sensory gating responses under normal physiological conditions. The discrepancy of our results may relate to the differences of species and strain. Further studies are necessary to clarify the pharmacological role of the central α7 nicotinic receptors.
Generally, a drug-induced decrease in PPI concomitant with a pronounced decrease in startle amplitude can potentially be explained by sedation. Clozapine, which is strongly sedative, has been reported variously to reverse (Bakshi et al., 1994; Bakshi & Geyer, 1995) or not reverse (Johansson et al., 1994) the disruption of PPI induced by phencyclidine. In the present study, clozapine showed a significant decrease in startle amplitude and a significant reversal of phencyclidine-induced PPI disruption. On the other hand, consistent with previous reports (Peng et al., 1990), apomorphine enhanced startle amplitude and disrupted PPI in rats in the present study. Several studies have shown that the magnitude of PPI is independent of the startle amplitude (Johansson et al., 1995). Abduljawad et al. (1997) have reported that clonidine, an α2-adorenoceptor agonist, and diazepam, a benzodiazepine receptor agonist, reduced the acoustic startle amplitude but did not alter the degree of PPI in humans. These findings suggest that the two pathways relate to this experimental paradigm: one is a process of sensory information input, and other is a process of the motor output in response to a startling stimulus. Therefore, it is speculated that apomorphine impairs both pathways. The enhancement of startle amplitude and the impairment of PPI by apomorphine were significantly antagonized by haloperidol. In contrast, nicotine only inhibited apomorphine-induced disruption of PPI but did not alter apomorphine-induced enhancement of startle amplitude. These results suggest that nicotine improves or activates the process of the sensory information input involved in PPI.
There are several possible explanations for the ability of nicotine to reverse apomorphine-induced PPI deficits. First, this ability may result from a direct enhancing effect of PPI via nicotinic receptors. Another possible explanation for the antagonistic action of nicotine may involve different direct or indirect interactions with other neurotransmitter systems known to affect PPI. Nicotine facilitates the release of a number of neurotransmitters including dopamine (Imperato et al., 1986; Rowell, 1995) or glutamate (Toth et al., 1993), both of which have been shown to be involved in PPI (Swerdlow et al., 1994). However, since excessive dopaminergic transmission produces a disruption of PPI (Davis et al., 1990), the dopaminergic system appears not to be responsible for the antagonistic action of nicotine. On the other hand, previous studies have shown the involvement of glutamatergic mechanisms in the actions of nicotine. For example, nanomolar concentrations of nicotine enhance glutamatergic synaptic transmission by presynaptic nicotinic receptors, and the increase in glutamate release is mediated through α7 subunit-containing nicotinic receptors (McGehee et al., 1995; Gray et al., 1996). In the present study, an α7 nicotinic receptor agonist, MLA, eliminated the antagonistic action of nicotine on apomorphine-induced PPI deficits, suggesting an involvement of α7 nicotinic receptors in this phenomenon. However, there was insufficient evidence to determine whether or not glutaminergic systems are involved in the antagonistic action of nicotine.
NMDA receptor antagonists such as phencyclidine disrupt PPI (Bakshi et al., 1994; Bakshi & Geyer, 1995), suggesting that the glutaminergic system plays an important role in the operation of the sensorimotor gating system. Previous findings have shown that phencyclidine-induced PPI disruption is reversed by the use of atypical antipsychotics such as clozapine (Bakshi et al., 1994) or olanzapine (Bakshi & Geyer, 1995), but not by high-potency dopamine D2 receptor antagonists such as haloperidol (Keith et al., 1991). In the present study, clozapine reversed the disruption of PPI induced by phencyclidine as was discovered with previous study, but haloperidol did not. On the other hand, we observed that nicotine had no effect on the disruption of PPI. It has been reported that phencyclidine, which blocks the ion channels of NMDA receptors, can also block the ion channel of nicotinic receptors (Paterson & Nordberg, 2000). Therefore, there is a possibility that the nicotinic receptor has already been blocked by phencyclidine prior to nicotine administration. Further studies are needed to determine whether or not nicotine affects the NMDA receptor antagonist-induced disruption of PPI.
In summary, nicotine reversed the apomorphine-induced disruption of PPI without changing the normal PPI by itself in rats, and the antagonistic action of nicotine was blocked by nicotinic receptor antagonists, mecamylamine and MLA. These results indicate an involvement of the central α7 nicotinic receptors in the antagonistic action of nicotine on PPI deficits by excessive dopaminergic stimulation.
Acknowledgments
This study was supported by a grant from the Smoking Research Foundation of Japan. Phencyclidine was synthesized with the assistance of Taisho Pharmaceutical Co., Ltd.
Abbreviations
- ANOVA
analysis of variance
- DHβE
dihydro-β-erythroidine
- MLA
methyllycaconitine
- NMDA
N-methyl-D-aspartate
- P
pulse
- PP-P
prepulse and pulse
- PPI
prepulse inhibition
References
- ABDULJAWAD K.A., LANGLEY R.W., BRADSHAW C.M., SZABADI E. Effects of clonidine and diazepam on the acoustic startle response and on its inhibition by ‘prepulse' in man. J. Psychopharmacol. 1997;11:29–34. doi: 10.1177/026988119701100110. [DOI] [PubMed] [Google Scholar]
- ACRI J.B., MORSE D.E., POPKE E.J., GRUNBERG N.E. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology. 1994;114:369–374. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- ACRI J.B., BROWN K.J., SAAH M.I., GRUNBERG N.E. Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacol. Biochem. Behav. 1995;50:191–198. doi: 10.1016/0091-3057(94)00285-q. [DOI] [PubMed] [Google Scholar]
- ADLER L.E., HOFFER L.J., GRIFFITH J., WALDO M.C., FREEDMAN R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- ADLER L.E., HOFFER L.D., WISER A., FREEDMAN R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- BAKSHI V.P., GEYER M.A. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. J. Pharm. Exp. Ther. 1995;271:787–794. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- BAKSHI V.P., SWERDLOW N.R., GEYER M.A. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. Psychopharmacology. 1994;122:198–201. [PubMed] [Google Scholar]
- BRAFF D.L., GEYER M.A. Sensorimotor gating and schizophrenia: human and animal model studies. Arch. Gen. Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- CURZON P., KIM D.J., DECKER M.W. Effect of nicotine, lobeline, and mecamylamine on sensory gating in rats. Pharmacol. Biochem. Behav. 1994;49:877–882. doi: 10.1016/0091-3057(94)90237-2. [DOI] [PubMed] [Google Scholar]
- DAVIS M., MANSBACH R.S., SWERDLOW N.R., CAMPEAU S., BRAFF D.L., GEYER M.A. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology. 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- FREEDMAN R., HALL M., ADLER L.E., LEONARD S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- GEORGE T.P., SERNYAK M.J., ZIEDONIS D.M., WOODS S.W. Effects of clozapine on smoking in chronic schizophrenic outpatients. J. Clin. Psychiatry. 1995;56:344–346. [PubMed] [Google Scholar]
- GLASSMAN A.H. Cigarette smoking: implications for psychiatric illness. Am. J. Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- GRAY R., RAJAN A.S., RADCLIFFE K.A., YAKEHIRO M., DANI J.A. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- IMPERATO A., MULAS A., DI CHIARA G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- JOHANSSON C., JACKSON D.M., SVENSSON L. The atypical antipsychotic, remoxipride, blocks phencyclidine-induced disruption of prepulse inhibition in the rat. Psychopharmacology. 1994;116:437–442. doi: 10.1007/BF02247475. [DOI] [PubMed] [Google Scholar]
- JOHANSSON C., JACKSON D.M., ZHANG J., SVENSSON L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Phramacol. Biochem. Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- KEITH V.A., MANSBACH R.S., GEYER M.A. Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle. Biol. Psychiatry. 1991;30:557–566. doi: 10.1016/0006-3223(91)90025-h. [DOI] [PubMed] [Google Scholar]
- KUMARI V., CHECKLEY S.A., GRAY J.A. Effects of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- KUMARI V., COTTER P.A., CHECKLEY S.A., GRAY J.A. Effects of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle reflex in healthy male non-smokers. Psychopharmacology. 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- LUDEWIG K., VOLLENWEIDER F.X. Impaired sensorimotor gating in schizophrenia with deficit and with nondeficit syndrome. Swiss Med. Wkly. 2002;132:159–165. doi: 10.4414/smw.2002.09873. [DOI] [PubMed] [Google Scholar]
- MANSBACH R.S., GEYER M.A., BRAFF D.L. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- McEVOY J.P., FREUDENREICH O., McGEE M., VANDERZWAAG C., LEVIN E., ROSE J. Clozapine decreases smoking in patients with chronic schizophrenia. Biol. Psychiatry. 1995;37:550–552. doi: 10.1016/0006-3223(94)00365-A. [DOI] [PubMed] [Google Scholar]
- McEVOY J.P., FREUDENREICH O., WILSON W.H. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol. Psychiatry. 1999;46:125–129. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- McGEHEE D.S., HEATH M.J., GELBER S., DEVAY P, ROLE L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- OLIVIER B., LEAHY C., MULLEN T., PAYLOR R., GROPPI V.E., SARNYAI Z., BRUNNER D. The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics. Psychopharmacology. 2001;156:284–290. doi: 10.1007/s002130100828. [DOI] [PubMed] [Google Scholar]
- PATERSON D., NORDBERG A. Neuronal nicotinic receptor in the human brain. Prog. Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- PAYLOR R., NGUYEN M., CRAWLEY J.N., PATRICK J., BEAUDET A., ORR-URTREGER A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn. Memory. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- PENG R.Y., MANSBACH R.S., BRAFF D.L., GEYER M.A. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats: implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- ROWELL P.P. Nanomolar concentrations of nicotine increase the release of [3H]dopamine from rat striatal synaptosomes. Neurosci. Lett. 1995;189:171–175. doi: 10.1016/0304-3940(95)11471-8. [DOI] [PubMed] [Google Scholar]
- SCHREIBER R., DALMUS M., DE VRY J. Effects of α4/β2- and α7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology. 2002;159:248–257. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- SEROVA L., SABBAN E.L. Involvement of alpha 7 nicotinic acetylcholine receptors in gene expression of dopamine biosynthetic enzymes in rat brain. J. Pharmacol. Exp. Ther. 2002;303:896–903. doi: 10.1124/jpet.102.039198. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., BRAFF D.L., TAAID N., GEYER M.A. Assessing the validity of an animal model of deficit sensorimotor gating in schizophrenic patients. Arch. Gen. Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- TOTH E., VIZI E.S., LAJTHA A. Effect of nicotine on levels of extracellular amino acids in regions of the rat brain in vivo. Neuropharmacology. 1993;32:827–832. doi: 10.1016/0028-3908(93)90192-6. [DOI] [PubMed] [Google Scholar]
- ZIEDONIS D.M., GEORGE T.P. Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr. Bull. 1997;23:247–254. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]