Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lipid that affects a variety of cellular processes through both its actions as a second messenger and via activation of a family of G protein-coupled receptors (S1P1–5).
The study of S1P receptor pharmacology, particularly S1P4, has been hindered by the lack of high-affinity radioligands with good specific activity. The studies presented herein characterize [3H]DH-S1P as a stable, high-affinity radioligand for S1P4 pharmacology.
Using a transfected Ba/F3 cell line selected for high hS1P4 surface expression, we compared the consequences of different cellular backgrounds and commercial sources of sphingophospholipids on S1P4 characterization. The development and subsequent use of the assay described has enabled us to extensively and definitively characterize the pharmacology of the human S1P4 receptor.
Keywords: Sphingosine-1-phosphate, dihydrosphingosine-1-phosphate, S1P4
Introduction
Sphingosine-1-phosphate (S1P) is a bioactive lipid that affects a variety of cellular processes (Spiegel et al., 1996) through both its actions as a second messenger (Olivera et al., 1993) and via activation of a family of G protein-coupled receptors (S1P1–5; Pyne et al., 2000). With the exception of S1P4 (previously called edg6; Chun et al., 2002), the S1P receptors are reported to bind S1P with low nanomolar affinity (Mandala et al., 2002). These affinity values were generated by both saturation- and competition-binding analysis using [3H]- and [33P]-labeled sphingophospholipids. Survey of the published literature reveals a variety of S1P binding affinities reported for S1P4, ranging from 10 to 20 nM (Yamazaki et al., 2000; Kohno et al., 2003), 50 to 70 nM (Van Brocklyn et al., 2000), and 100 to 150 nM (Mandala et al., 2002). Dihydrosphingosine-1-phosphate (DH-S1P; also known sphinganine-1-phosphate) and phytosphingosine-1-phosphate (phS1P) are also recognized as S1P4 ligands. DH-S1P is structurally similar to S1P but lacks the trans double bond at the 4 position; again, the reported binding constants for DH-S1P have varied considerably from 10 to 210 nM (Van Brocklyn et al., 1998; 2000). PhS1P, which has the same structure as DH-S1P but contains an additional hydroxyl group at the 4 position, was recently characterized as the highest affinity ligand for S1P4, Kd=0.3–1.0 nM, Ki=2–3 nM (Candelore et al., 2002).
The study of S1P receptor pharmacology has been complicated by a number of issues. First, there is a dearth of high-affinity radioligands for S1P receptors which have high specific activity and are commercially available. This is especially problematic in S1P4 due to its low affinity for S1P relative to the other S1P receptors (see above). Secondly, though recombinant expression of S1P4 has been reported in hemopoeitic L1.2, Jurkat, and HEL cells (An et al., 1999; Candelore et al., 2002; Graler et al., 2003; Kohno et al., 2003) and fibroblastic cell lines, CHO-K1, CHO-dhfr-, HEK293, and COS (Okamoto et al., 1998; Van Brocklyn et al., 2000; Yamazaki et al., 2000), in most instances receptor expression has been modest (Kohno et al., 2003). Finally, the radioligand and its sphingophospholipid competitors (which are available from several commercial sources) are vulnerable to degradation by endogenous sphingophospholipid lyases/phosphatases (Van Veldhoven et al., 2000), which could prohibit steady-state binding analysis.
The studies presented herein describe the development of a robust radioligand-binding assay using the commercially available radioligand [3H]DH-S1P. Using a transfected Ba/F3 cell line selected for high hS1P4 surface expression, we compared the ramifications of different cellular backgrounds and commercial sources of sphingophospholipids on S1P4 characterization. The development of this assay has allowed us to extensively and definitively characterize the affinity of lipids for the human S1P4 receptor.
Methods
Cells and cell culture
Murine IL-3-dependent pro-B cells Ba/F3, mouse L1-2 cells, and human Jurkat T lymphoma cells (clone E6-1) were all cultured in RPMI 1640 medium with 4. 5 g l−1 glucose, 15 mM HEPES, 2 mM L-glutamine, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin, and 50 μM β-mercaptoethanol. Ba/F3 culture medium also contained 1 ng ml−1 of recombinant mouse IL-3 (Biosource International, Camarillo, CA, U.S.A.). Chinese hamster ovary-K1 (CHO-K1) cells were maintained in Dulbecco's modified Eagle's-F12 medium (DMEM-F12) supplemented with 10 mM HEPES, 1 mM L-glutamine, and 10% FBS, pH 7.4. HEK293 and HEL cells were cultured in Eagle's minimum essential medium (MEM) with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5 g l−1 sodium bicarbonate, 0.1 mM non-essential amino acids, and 1.0 mM sodium pyruvate, 10% FBS. L1-2 cells were cultured in RPMI 1640 medium with 4.5 g l−1 glucose,15 mM HEPES, 2 mM L-glutamine, 0.01 mM nonessential amino acids and 0.05 mM 2-mercaptoethanol, and 10% FBS. HL-60 cells were maintained in DMEM medium supplemented with 2 mM L-glutamine, 100 μg ml−1 streptomycin and 100 μg ml−1 penicillin, and 10% FBS. HUT78 cells were cultured in Iscove's modified Dulbecco's medium supplemented with 1% penicillin/streptomycin/L-glutamine bicarbonate, 20% FBS. All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cloning and stable expression of hS1P4
The full-length human hS1P4 cDNA was PCR-amplified from peripheral blood mononuclear cell cDNA using forward primer AAGGCTAGCAACGCCACGGGGACCCCGGTGGCC and reverse primer TTCCGCGGCCGCTCAGATGCTCCGCACGCTGGAGAT. The product was cut with (Nhe1) (5′) and (Not1) (3′) and ligated into pME18neo-CD8-Flag, a mammalian expression vector derived from the SRα expression vector (Takebe et al., 1988), which contains a CD8 leader and a C-terminal Flag epitope. Ba/F3 and CHO/K1 cells were transfected by electroporation and Lipofectamine 2000 (Life Technologies, Gaithersburg, MD, U.S.A.), respectively and a stable population selected by resistance to G418 (1 mg ml−1, Life Technologies, Gaithersburg, MD, U.S.A.). Clonal cell lines expressing hS1P4 were then established by limiting dilution of stable transfectants and surface expression of the Flag epitope.
Cell membrane preparation
Cell membranes were prepared as previously described (Hipkin et al., 1997). Cells were pelleted by centrifugation, incubated in homogenization buffer (10 mM Tris – HCl, 5 mM EDTA, 3 mM EGTA, pH 7.6) and 1 mM PMSF for 30 min on ice. The cells were then lysed with a Dounce homogenizer using stirrer type RZR3 polytron homogenizer (Caframo, Wiarton, Ont., Canada) with 12 strokes at 900 r.p.m. The intact cells and nuclei were removed by centrifugation at 500 × g for 5 min. The cell membranes in the supernatant were then pelleted by centrifugation at 100,000 × g for 30 min. The membranes were then resuspended in GlyGly buffer (20 mM glycine : glycine, 1 mM MgCl2, 250 mM sucrose, pH 7.2), aliquoted, quick frozen, and stored at −80°C. Protein concentration in membrane preparations was determined using the method of Bradford (1976).
Preparation of lipids
All lipids were purchased either as concentrated stocks in CHCl3 or in powdered form. Powdered lipids were solubilized in 10 mM NaOH with sonication (1 mM stock), aliquoted, and stored at −20°C. All lipid dilutions for assay were carried out in the appropriate buffer containing 0.8% fatty acid-free BSA.
Radioligand-binding assay
D-erythro-[4,5-3H]-dihydrosphingosine-1-phosphate (specific activity=60 Ci mmol−1), D-erythro-[3-3H]-sphingosine-1-phosphate (15 Ci mmol−1), and D-erythro-[33P]-sphingosine-1-phosphate (3000 Ci mmol−1) were purchased from American Radiolabeled Chemicals (St Louis, MO, U.S.A.). Radioligand competition- and saturation-binding assays were measured using a scintillation proximity assay (SPA) as previously described (Cox et al., 2001). Membranes (2–4 μg per assay point) in binding buffer (50 mM Tris pH 7.5, 150 mM NaCl, 0.8% fatty acid-free BSA) (Sigma-Aldrich Chemicals, St Louis, MO, U.S.A.) were preincubated for 30 min at room temperature with 200–320 μg WGA-SPA beads, transferred to a 96-well Isoplate (Wallac, Gaithersburg, MD, U.S.A.) and further incubated at room temperature with the designated concentrations of radioligand and unlabeled lipids for 15 min–16 h. Ligand affinities from competition bindings were calculated from binding IC50 using the Cheng–Prusoff equation (Cheng et al., 1973).
Biophysical analysis of sphingophospholipids
Samples for measurement of NMR spectra were dissolved in 1 : 1 deuteromethanol : deuterated acetic acid (Sigma-Aldrich, St Louis, MO, U.S.A.). NMR spectra were measured on a Varian 400 MHz NMR spectrometer (Varian Instrument Corp, Palo Alto, CA, U.S.A.) using either 64 or 96 scans to obtain adequate signal-to-noise ratios. The phospholipid mass spectrometry samples were prepared in a mixture of ethanol (Pharmco, 200 proof USP, Brookfield, CT)/DMSO (Fisher). The samples were analyzed by Fast Atom Bombardment mass spectrometry (FABMS) on a JEOL M Station JMS 700 double-focusing magnetic sector instrument (JEOL USA, Inc; Peabody, MA, U.S.A.) at 6 KeV acceleration energy. Thioglycerol was used as the FAB matrix.
Flow cytometric analysis of surface hS1P4 expression
Expression of surface receptor expression was measured as previously described (Bober et al., 1995). Cells were collected and centrifuged at 400 × g for 5 min at 4°C. The cell pellet was resuspended in 20 μl of normal mouse serum (Sigma, St Louis, MO, U.S.A.) containing 0.02% NaN3 to block nonspecific binding of antibodies to the cell surface and receptor internalization, respectively, and incubated at room temperature (RT) for 20 min. Cells were then incubated with 10 μl (1 μg ml−1) of Bio M2 anti-Flag antibody (Sigma, St Louis, MO, U.S.A.) at 4°C (dark) for 30–45 min. Unbound antibody was removed by dilution with 4 ml of cold FACS buffer (Dulbeccos phosphate buffered saline, 1% bovine serum albumin, 5 mM EDTA, 0.01% NaN3) and centrifuged. The cell pellet was resuspended in 10 μl of streptavidin-PE (BD Pharmingen, San Diego, CA, U.S.A.) and incubated at 4oC for 30 min. Cells were then washed twice and analyzed on the BD-FACScan (Becton-Dickerson Immunocytometry Systems, Mountain View, CA, U.S.A.).
Materials
The lipids 1-acyl-2-hydroxy-sn-glycerol-3-phosphate (18 : 1 LPA), sphingosine-1-phosphate (S1P), D-erythro-sphingosine (SH), sphinganine-1-phosphate (dihydro-S1P; DH-S1P), sphingosylphosphorylcholine (SPC), 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine(18 : 1 LPC),1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (18 : 1 LPE), D-ribo-phytosphingosine-1-phosphate (phS1P), phytosphingosine 4-hydroxysphinganine (PSph), and n-octanoyl (8 : 0) ceramide-1-phosphate (C1P) were purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). DH-S1P and S1P were also purchased from BioMol (Plymouth Meeting, PA, U.S.A.) and Sigma-Aldrich Chemicals (St Louis, MO, U.S.A.). Nonlinear regression analysis of the data and calculation of EC50 and Ki was performed using Prism 2.0c (GraphPad Software, San Diego, CA, U.S.A.). All other reagents were of the best grade available and purchased from common suppliers.
Results
Recombinant expression of hS1P4 in CHO-K1 and Ba/F3 cells
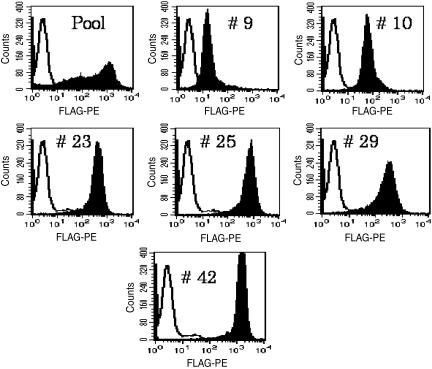
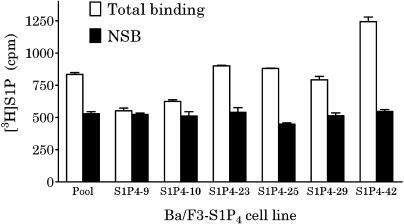
CHO-K1 and Ba/F3 were transfected with the plasmid encoding the human S1P4 receptor with an N-terminal FLAG sequence. A stable pool was selected by antibiotic resistance. Clonal cell lines, isolated by limiting dilution, were then evaluated for surface S1P4 expression by flow cytometric analysis. Clonal transfectants in both Ba/F3 (Figure 1) and CHO-K1 cell lines (data not shown) were isolated, which express S1P4 on the cell surface. S1P4 expression in the various Ba/F3 lines was confirmed by competition-binding analysis with [3H]S1P using scintillation proximity assay (SPA) technology (Figure 2; as described in Methods). [3H]S1P bound in a displaceable manner with specific binding, correlating well with S1P4 expression as measured by flow cytometric analysis (see Figure 1). However, in the highest-expressing clone (#42), the ratio of total to nonspecific binding with [3H]S1P is still only 2.5 : 1. No radioligand binding was detectable in membranes from parental Ba/F3 or CHO-K1 cells (data not shown). Based on high surface expression, two cell lines (CHO-S1P4-10 and Ba/F3-S1P4-42) were then utilized for subsequent S1P4 pharmacology.
Figure 1.
Fluorescence-activated cell sorting for S1P4 expression in Ba/F3-S1P4 cells. Surface S1P4 expression was assessed in Ba/F3-S1P4 cell lines (pool or clones) by fluorescence-activated cell sorting using the N-terminal Flag tag.
Figure 2.
Binding analysis in Ba/F3-S1P4-42 membranes using radiolabeled S1P and DH-S1P. Membranes (2 μg per well) from stable Ba/F3-S1P4 cell lines (pool or clones) were incubated in binding buffer at room temperature (as described in Methods) with [3H]S1P with (solid bars) or without (open bars) 1 μM phS1P.
Binding analysis of S1P4 with different radioligands
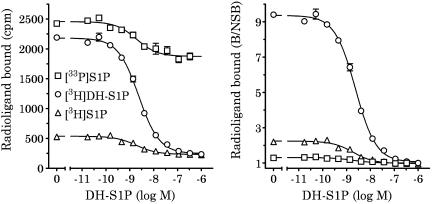
Due to the modest signal : noise in binding assays using [3H]S1P, we assessed the utility of other commercially available radioligands. [3H]DH-S1P and [33P]S1P have approximately four- and 200-fold higher specific activity than tritiated S1P (60, 3000, and 15 Ci mmol−1, respectively). Moreover, DH-S1P may (Van Brocklyn et al., 1998) or may not (Van Veldhoven et al., 2000) bind S1P4 with higher affinity than S1P. In order to test the utility of these radioligands, Ba/F3-S1P4-42 membranes were incubated with the indicated concentration of each trace and the designated concentrations of unlabeled DH-S1P. DH-S1P displaced all the three radioligands (Figure 3) with good apparent affinity (IC50<5 nM). However, the signal : noise with [3H]DH-S1P (≅9 : 1) was significantly better than either [3H]S1P (≅2 : 1) or [33P]S1P (≅1.3 : 1) (Figure 3, right). Based on this finding, we extended our studies of S1P4 pharmacology using [3H]DH-S1P.
Figure 3.
Competition-binding analysis in Ba/F3-S1P4-42 membranes using [3H]S1P, [33P]S1P or [3H]DH-S1P. Membranes (2 μg per well) from stable Ba/F3-S1P4-42 cells were incubated in binding buffer at room temperature (as described in Methods) with 50 nM [3H]S1P, 250 pM [33P]S1P or 4 nM [3H]DH-S1P and the indicated concentrations of DH-S1P (left). The same data are expressed as counts bound/nonspecific binding (B/NSB; right). Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2).
S1P4 pharmacology in Ba/F3-S1P4 membranes
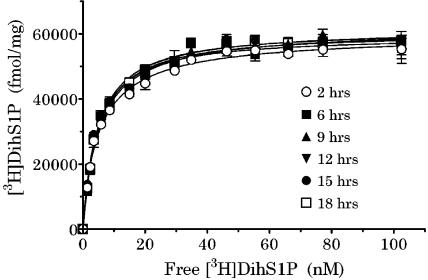
Ba/F3-S1P4-42 membranes were incubated in binding buffer (as described in Methods) containing the indicated concentrations of [3H]DH-S1P in the absence or presence of excess unlabeled phS1P. Saturation analysis (Figure 4) established that S1P4 was highly expressed in Ba/F3-S1P4-42 cells (40±20 pmol mg−1; n=2) and bound [3H]DH-S1P with high affinity (Kd=3.95±0.75 nM, slope=1.0±0.1, n=2). The saturation-binding analysis was counted repeatedly over 18 h at room temperature. The specific binding was very stable, reaching steady state by 2–4 h.
Figure 4.
Saturation-binding analysis with [3H]DH-S1P in Ba/F3-S1P4-42 membranes. Membranes (2 μg per well) from Ba/F3-S1P4-42 cells were incubated in binding buffer at room temperature (as described in Methods) at the indicated times with [3H]DH-S1P in the absence (total binding) or presence of 1 μM ph-S1P (nonspecific binding). Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data (total – nonspecific binding) represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2) (left).
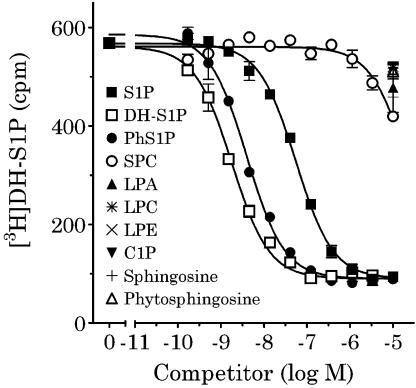
In competition-binding analysis, Ba/F3-S1P4-42 membranes were incubated in binding buffer containing [3H]DH-S1P and the designated concentrations of various lipids including DH-S1P, S1P, SPC, and phS1P. DH-S1P, S1P, SPC, and phS1P inhibited radioligand binding in a concentration-dependent manner (Figure 5), with DH-S1P consistently binding with the highest affinity (DH-S1P>phS1P≫S1P⋙SPC). Results of these competition-binding experiments show that sphingosine (SH), lysophosphatidic acid (LPA), ceramide-1-phosphate (C1P) lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), and phytosphingosine (PS) only minimally displaced [3H]DH-S1P at 10 μM. Cheng-Prusoff conversion of binding IC50 for DH-S1P, S1P, phS1P, and SPC generated binding Ki±s.e.m.=1.6±0.4, 22.8±3.6, 1.8±0.3, and 7533±1639 nM, respectively (n=3–8). The DH-S1P Ki is consistent with (or slightly more potent) the Kd value derived by saturation analysis with [3H]DH-S1P.
Figure 5.
Competition-binding analysis in Ba/F3-S1P4-42 membranes using [3H]DH-S1P. Membranes (2 μg per well) from stable Ba/F3-S1P4-42 cells were incubated in binding buffer at room temperature (as described in Methods) with 4 nM [3H]DH-S1P and the indicated concentrations of unlabeled ligands. Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2).
Functional analysis of S1P4 pharmacology using Ba/F3-S1P4-42 cells was assessed. Ba/F3 cells have elevated cyclase activity which is not efficiently inhibited by activation of any Gi-coupled receptor tested including S1P4 (data not shown). However, in GTPγS exchange assays in membranes from Ba/F3-S1P4-42 cells, the basal levels of binding were dramatically elevated over those measured in membranes from untransfected Ba/F3 cells. Incubation with DH-S1P or S1P stimulated a further 1.3-fold increase in GTPγS binding (but not in parental Ba/F3 membranes) with EC50=4 and 41 nM, respectively (data not shown).
Analysis of S1P4 reagents and expression systems
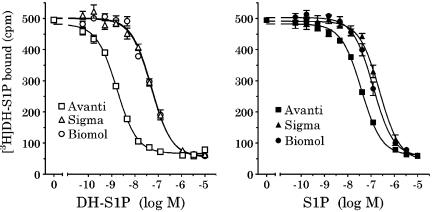
The binding affinities for DH-S1P, S1P, and phS1P that we generated differ from those previously published. However, an examination of the literature indicates that the sphingophospholipids used were obtained from a variety of commercial sources and the assays were performed with an assortment of S1P4-expressing cell lines. To address these disparities, we performed competition-binding assays using ligands purchased from different suppliers and in different recombinant backgrounds. First, Ba/F3-S1P4-42 membranes were incubated in binding buffer containing [3H]DH-S1P and the designated concentrations of DH-S1P, S1P and phS1P purchased from Avanti Polar Lipid, Sigma-Aldrich or Biomol (Figure 6). We found that phS1P bound S1P4 with essentially the same affinity as was reported previously (Candelore et al., 2002) and in both studies the phytosphingolipid was purchased from Avanti Polar Lipid. DH-S1P obtained from Avanti Polar Lipid (Figures 3 and 5) was much more potent in displacing radioligand than that obtained from Biomol (Figure 6, left Ki=36±15 nM, n=2) or from Sigma-Aldrich (Ki=28±8 nM, n=2). S1P (Figure 6, right) obtained from Avanti Polar Lipid (Ki=22±3 nM, n=2) was also more potent in displacing radioligand than that purchased from either Biomol (Ki=112 nM) or Sigma-Aldrich (Ki=153 nM).
Figure 6.
Competition-binding analysis using different commercially available sphingophospholipids. Membranes (2 μg per well) from Ba/F3-S1P4-42 cells were incubated in binding buffer at room temperature (as described in Methods) with 4 nM [3H]DH-S1P and the indicated concentrations of unlabeled DH-S1P (left) or S1P (right). Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2).
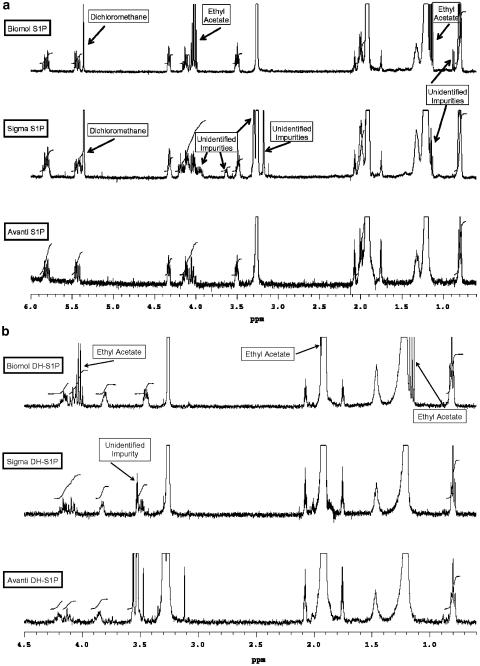
As the ligands from the different manufacturers generated conflicting binding constants, we examined the purity and identity of the S1P and DH-S1P samples by proton NMR spectroscopy and low-resolution mass spectroscopy (Figure 7). All S1P and DH-S1P samples showed the expected molecular ion for the sphingosine-1-phosphates (m z−1=380.4) and the dihydrosphingosine-1-phosphates (m z−1=382.5), respectively. When the NMR spectra were taken, however, there was a significant difference between the Avanti, Sigma, and Biomol samples. The NMR spectrum of the Avanti S1P (Figure 7, top) was in good agreement with that published earlier (Li et al., 1999). The Sigma S1P was contaminated with what appears to be dichloromethane and possibly ethyl acetate, perhaps as a result of insufficient drying after the material had been transferred into the vials. The Sigma S1P also appears to be contaminated with dichloromethane, as well as some unidentified impurities. As was the case with Avanti S1P, the NMR spectrum of Avanti DH-S1P (Figure 7, bottom) was free of obvious contaminants, although the signal-to-noise ratio was not as good as that in the S1P spectra. Once again the Biomol material appeared to contain large amounts of ethyl acetate. NMR and mass spectra of Sigma DH-S1P and phS1P from Avanti appeared to be free of significant impurities (Figure 7, bottom and data not shown). Owing to the presence of solvent or other impurities in the Sigma and Biomol samples, all calculations made using the nominal weight on the bottle would assume a higher concentration for the stock solutions, which could well explain the higher Ki using Biomol or Sigma material. Therefore, we would conclude that the Ki generated in competition-binding assays using Avanti material represents the accurate affinity of these ligands for S1P4.
Figure 7.
1H NMR spectra of Avanti, Sigma, and Biomol S1P and DH-S1P. Samples were dissolved in 1 : 1 deuterated methanol : deuterated acetic acid. Spectra were measured at 400 MHz using either 64 or 96 scans to obtain adequate signal-to-noise ratios. Signals that do not correlate with the spectra reported by Li et al. (1999) are noted.
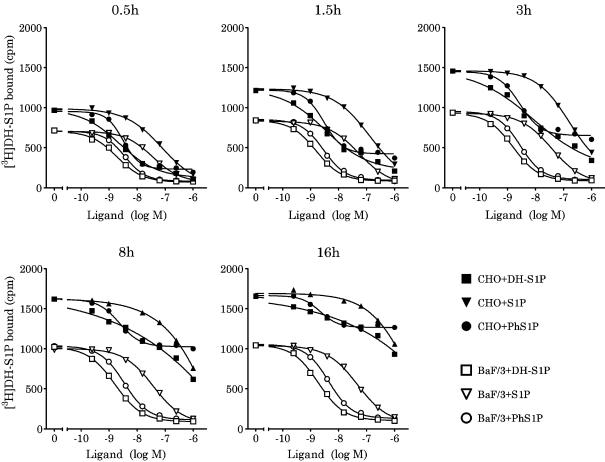
Numerous studies in the S1P receptor field (including S1P4) use cloned receptors expressed in a CHO cell background (Kon et al., 1999; Van Brocklyn et al., 2000; Yamazaki et al., 2000; Candelore et al., 2002; Mandala et al., 2002; Graler et al., 2003). For this reason, we assessed the effect of cell background (Ba/F3 vs CHO-K1) on S1P4 pharmacology. Ba/F3-S1P4-42 and CHO-S1P4-10 membranes were incubated at room temperature in binding buffer containing [3H]DH-S1P and the indicated concentrations of DH-S1P, S1P or phS1P. The bindings were then measured at various times (0.5–16 h). A representative experiment is shown in Figure 8 (summarized in Table 1). The binding IC50's of the three ligands in the Ba/F3 membranes were very stable over the time course of the experiment as were the Hill coefficients (which were at or close to unity). The binding IC50's in CHO-S1P4-10 membranes were similar to those in the Ba/F3 membranes at 30 and 90 min (i.e. binding IC50 DH-S1P<phS1P≪S1P), but altered with longer incubation times. The binding IC50 for DH-S1P and S1P increased dramatically over time in CHO-S1P4-10 membranes while the Hill coefficients were consistently less than unity and gradually decreased with time. Neither the binding IC50 nor the Hill coefficient of phS1P changed appreciably in the CHO membranes. However, the total binding for the trace and nonspecific binding with all the three ligands increased as the experiment proceeded such that the signal to noise decreased over time. Based on these data, we conclude that under these conditions S1P4 binding in CHO membranes will not reach true steady state, such that generation of a binding Ki with these membranes is not possible.
Figure 8.
Competition-binding analysis using Ba/F3-S1P4-42 and CHO-S1P4-10 membranes. Ba/F3-S1P4-42 or CHO-S1P4-10 membranes (2 μg per well) were incubated in binding buffer at room temperature for the indicated times with 4 nM [3H]DH-S1P and the specified concentrations of unlabeled DH-S1P (squares), ph-S1P (circles) or S1P (triangles). Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2).
Table 1.
Time course of binding IC50 and Hill coefficients in competition-binding assays with [3H]DH-S1P in CHO-S1P4-10 and Ba/F3-S1P4-42 membranes
| CHO-S1P4-10 | Ba/F3-S1P4-42 | ||||||
|---|---|---|---|---|---|---|---|
| Value | Time (h) | DH-S1P | S1P | PhS1P | DH-S1P | S1P | PhS1P |
| IC50 | 0.5 | 2.2 | 51 | 3.1 | 1.9 | 34 | 3.5 |
| 1.5 | 2.7 | 77 | 3.0 | 1.6 | 45 | 2.9 | |
| 3 | 8.5 | 159 | 2.7 | 1.6 | 37 | 2.8 | |
| 6 | 42 | 1620 | 2.7 | 1.6 | 40 | 3.2 | |
| 8 | NC | NC | 2.3 | 1.6 | 37 | 3.4 | |
| 16 | NC | NC | 2.2 | 1.9 | 47 | 4.4 | |
| Hill | 0.5 | −0.64 | −0.67 | −1.48 | −0.93 | −0.87 | −1.16 |
| 1.5 | −0.57 | −0.67 | −1.28 | −0.96 | −0.78 | −1.09 | |
| 3 | −0.41 | −0.74 | −1.15 | −0.95 | −0.79 | −1.10 | |
| 6 | −0.35 | −0.56 | −1.46 | −0.89 | −0.81 | −1.02 | |
| 8 | −0.19 | −0.43 | −1.16 | −0.90 | −0.82 | −1.01 | |
| 16 | −0.18 | −0.58 | −1.34 | −0.94 | −0.80 | −1.06 | |
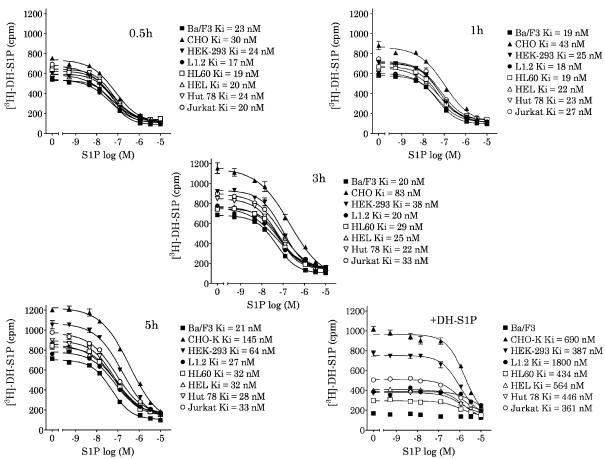
We next assessed assay stability using membranes from a panel of cell lines. Ba/F3-S1P4-42 membranes (2 μg/300 μg WGA-SPA beads) were co-incubated with 2 μg membranes from untransfected cells (Ba/F3, CHO-K1, HEK293, L1.2, HL-60, HEL, HUT78 or Jurkat), 6 nM [3H]DH-S1P and the designated concentrations of S1P for the indicated times. Note that these untransfected cell lines do not have detectable endogenous S1P receptors as measured by radioligand binding (data not shown). We know from previous work that 300 μg WGA-SPA beads have sufficient capacity to bind 4 μg membrane protein (Gonsiorek & Hipkin, unpublished observations). At 30 min incubation, the presence of non-Ba/F3 membranes did not significantly alter the binding curves relative to those measured in the Ba/F3-S1P4-42+Ba/F3 membranes (Figure 9, top left), although the Ki in the presence of CHO-K1 membranes was slightly right-shifted. By 60 min, the increase total-binding and S1P-binding IC50 in assays containing CHO-K1, HEK293 or Jurkat membranes was more evident (Ki=43, 25, and 27 nM, respectively; Figure 9, top right) relative to that in Ba/F3 membranes (Ki=19 nM). The increase in total binding and binding Ki continued through 5 h (Figure 9, bottom left) in the co-incubations with CHO-K1, HEK293 or Jurkat membranes (Ki=145, 64, and 33 nM, respectively). Indeed, by 5 h, the total binding and calculated Ki increased to some degree in all co-incubations relative to the Ba/F3 membranes. At 5 h, 1 μM DH-S1P was added to all incubations in order to assess the reversibility of [3H]DH-S1P binding (Figure 9, bottom right). Bound [3H]DH-S1P was completely displaced from the Ba/F3-S1P4-42 membranes (2 μg per point) co-incubated with wild-type Ba/F3 membranes consistent with a receptor-bound radioligand. However, in all other co-incubations (especially with CHO-K1, HEK 293 and Jurkat membranes), measurable [3H]DH-S1P binding was still evident after the addition of excess cold ligand. The calculated binding Ki did increase although not appropriately in the face of 1 μM DH-S1P. These binding data are not consistent with the competitive binding of ligands at S1P4.
Figure 9.
Effect of co-incubation with different membranes on ligand-binding constants and reversibility in Ba/F3-S1P4-42 membranes. Ba/F3-S1P4-42 membranes (2 μg per well) were co-incubated at room temperature with membranes (2 μg per well) from untransfected Ba/F3, CHO-K1, HEK293, L1.2, HL-60, HEL cells, HUT78 or Jurkat cells, 4 nM [3H]DH-S1P and the specified concentrations of unlabeled S1P from 30 min (top, left) to 5 h (bottom, left). Following the addition of 1 μM DH-S1P (reversibility), the incubation continued for 8 h (bottom, right). Radioligand binding to the membranes was measured by WGA-SPA scintillation. Data represent the mean±s.e.m. of triplicate determinations from a representative experiment (n=2).
Discussion
Analysis of S1P4 mRNA expression indicates that S1P4 is relatively highly expressed in lymphoid and hematopoietic tissue (Graler et al., 1998) and has a more restricted tissue distribution than the other S1P receptor family members (Spiegel et al., 2003). Regrettably, characterization of its expression and pharmacology has been hindered by the lack of S1P4-reactive antibodies or radioligands with high affinity and/or specific activity. The studies described herein establish the value of [3H]DH-S1P as a radioligand for S1P4 pharmacology. [3H]DH-S1P binds S1P4 with 10–20-fold higher affinity than does S1P and has a four-fold higher specific activity than does [3H]S1P. Moreover, we demonstrate that generation of valid binding constants for S1P4: (1) depends on the quality of the sphingophospholipid competitors and (2) dictates the use of certain cell lines for recombinant S1P4 expression based on their ability to degrade sphingolipids.
Recently, Candelore et al. (2002) described the enzymatic phosphorylation of phytosphingosine through incubation with [γ-33P]ATP and Saccharomyces cerevisiae membranes. The authors described the resultant [33P]phS1P as a novel radioligand for S1P4 pharmacology using transfected CHO-dhfr- and L1.2 cells. In saturation-binding analysis, it was reported to bind with a Kd=0.3–1 nM although in competition binding it was reported with a Ki=2–3 nM. However, we believe that [3H]DH-S1P may offer some advantages relative to other S1P4 radioligands including [33P]phS1P. Unlike [3H]DH-S1P, [33P]phS1P is not yet commercially available and, as a result, we did not test it in our studies. Tritiated DH-S1P has a much longer radioactive half-life relative to 33P- or 32P-labeled ligands and has a higher specific activity than does [3H]S1P. Lastly, we found that DH-S1P bound with slightly higher affinity than did phS1P and much higher affinity than did S1P. These data differ from the results obtained by Van Brocklyn et al. (2000), who reported that DH-S1P bound S1P4 with lower affinity (Ki=210 nM) than did S1P (Ki=46 nM), and the findings of Candelore et al. (2002), that DH-S1P bound with ≅five-fold lower affinity than did phS1P. However, we established that the pharmacology within this ligand class varied significantly depending on the commercial supplier. This may be the case in the Van Brocklyn study which used lipids obtained from Biomol. We found that competition-binding assays using sphingophospholipids purchased from Biomol and Sigma-Aldrich consistently generated lower affinity values than that purchased from Avanti.
When NMR spectral analysis of Biomol and Sigma lipids were performed, both lipids have evidence of significant solvent contamination. However, the Sigma DH-S1P appeared to be free of significant impurities. The reason(s) for the lower binding affinities with this material is unknown. Regarding phS1P and DH-S1P, our phS1P Ki is in agreement with that reported by Candelore et al. (2002), although we found that DH-S1P bound with higher affinity than noted in that study. As both studies used lipids purchased from Avanti, a more likely explanation may pertain to differences in S1P4 expression systems. In Ba/F3-S1P4 cells, there was little or no degradation of either radioligands or its competitors, as evidenced by the stability of binding IC50 and specific binding over time. However, steady-state binding was not attained in a CHO-K1 expression system nor in co-incubations with membranes from various cell lines previously used for S1P4 expression, such as CHO-dhfr-, CHO-K1 (Okamoto et al., 1998; Van Brocklyn et al., 2000), HEK293 (Yamazaki et al., 2000), human erythroleukemia HEL cells (Okamoto et al., 1998), the murine B cell line L1.2 (Candelore et al., 2002), T-lymphoid Jurkat cells (An et al., 1999; Graler et al., 2003; Kohno et al., 2003), and myeloid leukemia HL-60 cells (Sato et al., 1998). Interestingly, in the co-incubation studies, membranes prepared from various hemopoietic cell lines (L1.2, HL-60, HEL, and Hut78) had relatively little effect on the S1P4 pharmacology when co-incubated with Ba/F3-S1P4-42 membranes and then only with prolonged incubation (8 h). In the Candelore study, the reported affinity for S1P and phosphorylated FTY720 was approximately three-fold lower when measured in a CHO expression line (≅160 and ≅ 45 nM, respectively) than that measured in the same study using membranes from L1.2 cells transfected to express S1P4 (≅50 and ≅15 nM, respectively). We found that co-incubation with HEK293 and Jurkat membranes brought about some time-dependent changes in S1P4 pharmacology, though the effect was not as dramatic as that seen in CHO-K1 co-incubation or in CHO-S1P4-10 membranes (Figure 9). Previous studies showed that HEK 293 cells endogenously express low levels of S1P phosphatase activity (Van Veldhoven et al., 1994; Mandala et al., 2000; Alderton et al., 2001). This activity was quantitated (Le Stunff et al., 2002) as approximately 5 nmol min−1 mg−1. Indeed, it is also worth noting that endogenous sphingophospholipid lyases/phosphatases have been implicated in extensive sphingolipid degradation in fibroblasts (Van Veldhoven et al., 1994).
We found that whereas the potency of S1P to displace [3H]DH-S1P decreased with time, [3H]DH-S1P binding upon co-incubation with CHO-K1, HEK293, and Jurkat membranes increased over the same time period. However, these bound counts were not displaced with excess unlabeled DH-S1P. We made the identical observation in [3H]DH-S1P bindings with CHO-S1P4-10 membranes (data not shown). This lack of reversibility strongly implies that tritiated sphingophospholipid is no longer interacting with a receptor and may be intercolated into the cell membrane. The rate at which a binding assay may destabilize will depend on both assay temperature and the concentration of the reactants. Our assay is incubated at room temperature (vs 37 or 4°C) and uses low concentrations of membranes (2 μg/100 μl) relative to other studies. Again, as an example, Candelore et al. (2002) incubated 66 μg of membranes for L1.2 and 10–24 μg for CHO-S1P4 membranes with radioligand (S1P or phS1P) and competitor for 45–60 min at room temperature. Other studies used more or less membranes but incubated the binding reaction at 4°C for relatively short times (i.e. 15–30 min) (Van Brocklyn et al., 2000; Yamazaki et al., 2000), conditions in which ligand degradation may be minimal. However, based on our binding time course at room temperature, it is unclear if the binding reaction has reached steady state under these conditions.
In conclusion, we have described a novel radioligand [3H]DH-S1P for the study of S1P4. Moreover, we characterized the utility of various cell lines as hosts for recombinant S1P4 expression and commercial sources of sphingophospholipids. Using [3H]DH-S1P, in concert with a Ba/F3 expression system, we have extensively and definitively characterized the pharmacology of the human S1P4 receptor.
Abbreviations
- C1P
ceramide-1-phosphate
- DH-S1P
D-erythro-dihydrosphingosine-1-phosphate
- LPA
18 : 1 lysophosphatidic acid
- LPC
18 : 1 lysophosphorylcholine
- LPE
18 : 1 lysophosphoethanolamine
- phS1P
phytosphingosine-1-phosphate
- PS
phytosphingosine
- S1P
D-erythro-sphingosine-1-phosphate
- SH
D-erythro-sphingosine
- SPC
D-erythro-sphingosinephosphatidylcholine
- WGA-SPA
wheat germ agglutinin bead-scintillation proximity assay
References
- ALDERTON F., DARROCH P., SAMBI B., MCKIE A., AHMED I.S., PYNE N., PYNE S. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 2001;276:13452–13460. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- AN S., BLEU T., ZHENG Y. Transduction of intracellular calcium signals through G protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol. Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- BOBER L.A., GRACE M.J., PUGLIESE-SIVO C., ROJAS-TRIANA A., WATERS T., SULLIVAN L.M., NARULA S.K. The effect of GM-CSF and G-CSF on human neutrophil function. Immunopharmacology. 1995;29:111–119. doi: 10.1016/0162-3109(94)00050-p. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CANDELORE M.R., WRIGHT M.J., TOTA L.M., MILLIGAN J., SHEI G.J., BERGSTROM J.D., MANDALA S.M. Phytosphingosine 1-phosphate: high affinity ligand for the S1P(4)/Edg-6 receptor. Biochem. Biophys. Res. Commun. 2002;297:600–606. doi: 10.1016/s0006-291x(02)02237-4. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHUN J., GOETZL E.J., HLA T., IGARASHI Y., LYNCH K.R., MOOLENAAR W., PYNE S., TIGYI G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- COX M.A., JENH C.H., GONSIOREK W., FINE J., NARULA S.K., ZAVODNY P.J., HIPKIN R.W. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol. Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- GRALER M.H., BERNHARDT G., LIPP M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- GRALER M.H., GROSSE R., KUSCH A., KREMMER E., GUDERMANN T., LIPP M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 2003;89:507–519. doi: 10.1002/jcb.10537. [DOI] [PubMed] [Google Scholar]
- HIPKIN R.W., FRIEDMAN J., CLARK R.B., EPPLER C.M., SCHONBRUNN A. Agonist-induced desensitization, internalization, and phosphorylation of the sst2A somatostatin receptor. J. Biol. Chem. 1997;272:13869–13876. doi: 10.1074/jbc.272.21.13869. [DOI] [PubMed] [Google Scholar]
- KOHNO T., MATSUYUKI H., INAGAKI Y., IGARASHI Y. Sphingosine 1-phosphate promotes cell migration through the activation of Cdc42 in Edg-6/S1P4-expressing cells. Genes Cells. 2003;8:685–697. doi: 10.1046/j.1365-2443.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- KON J., SATO K., WATANABE T., TOMURA H., KUWABARA A., KIMURA T., TAMAMA K., ISHIZUKA T., MURATA N., KANDA T., KOBAYASHI I., OHTA H., UI M., OKAJIMA F. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J. Biol. Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- LE STUNFF H., GALVE-ROPERH I., PETERSON C., MILSTIEN S., SPIEGEL S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell. Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI S., WILSON W.K., SCHROEPFER G.J., JR New methods for determining the enantiomeric purity of erythro-sphingosine. J. Lipids Res. 1999;40:764–772. [PubMed] [Google Scholar]
- MANDALA S., HAJDU R., BERGSTROM J., QUACKENBUSH E., XIE J., MILLIGAN J., THORNTON R., SHEI G.J., CARD D., KEOHANE C., ROSENBACH M., HALE J., LYNCH C.L., RUPPRECHT K., PARSONS W., ROSEN H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- MANDALA S.M., THORNTON R., GALVE-ROPERH I., POULTON S., PETERSON C., OLIVERA A., BERGSTROM J., KURTZ M.B., SPIEGEL S. Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1-phosphate and induces cell death. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7859–7864. doi: 10.1073/pnas.120146897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO H., TAKUWA N., GONDA K., OKAZAKI H., CHANG K., YATOMI Y., SHIGEMATSU H., TAKUWA Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J. Biol. Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- OLIVERA A., SPIEGEL S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- PYNE S., PYNE N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. Ther. 2000;88:115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- SATO K., MURATA N., KON J., TOMURA H., NOCHI H., TAMOTO K., OSADA M., OHTA H., TOKUMITSU Y., UI M., OKAJIMA F. Downregulation of mRNA expression of Edg-3, a putative sphingosine 1-phosphate receptor coupled to Ca2+ signaling, during differentiation of HL-60 leukemia cells. Biochem. Biophys. Res. Commun. 1998;253:253–256. doi: 10.1006/bbrc.1998.9745. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MERRILL A.H., JR Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- TAKEBE Y., SEIKI M., FUJISAWA J., HOY P., YOKOTA K., ARAI K., YOSHIDA M., ARAI N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., GRALER M.H., BERNHARDT G., HOBSON J.P., LIPP M., SPIEGEL S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., LEE M.J., MENZELEEV R., OLIVERA A., EDSALL L., CUVILLIER O., THOMAS D.M., COOPMAN P.J., THANGADA S., LIU C.H., HLA T., SPIEGEL S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell. Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN VELDHOVEN P.P., GIJSBERS S., MANNAERTS G.P., VERMEESCH J.R., BRYS V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1) Biochim. Biophys. Acta. 2000;1487:128–134. doi: 10.1016/s1388-1981(00)00079-2. [DOI] [PubMed] [Google Scholar]
- VAN VELDHOVEN P.P., MANNAERTS G.P. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: evidence for the existence of a sphingosine phosphatase. J. Biochem. 1994;299:597–601. doi: 10.1042/bj2990597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI Y., KON J., SATO K., TOMURA H., SATO M., YONEYA T., OKAZAKI H., OKAJIMA F., OHTA H. Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca(2+) signaling pathway. Biochem. Biophys. Res. Commun. 2000;268:583–589. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]