Abstract
The role of the glucocorticoid-regulated protein annexin 1 during the process of phagocytosis has been studied using annexin 1 null peritoneal macrophages. Wild type and annexin 1 null macrophages were incubated with several distinct phagocytic targets. No differences were observed in rate or the maximal response with respect to IgG complexes or opsonised zymosan phagocytosis, as assessed by monitoring the production of reactive oxygen species.
When annexin 1 null macrophages were incubated with non-opsonised zymosan particles, they exhibited impaired generation of reactive oxygen species, which was linked to a defect in binding of cells to the particles, as determined with fluorescent zymosan. This phenomenon was further confirmed by electron microscopy analysis, where annexin 1 null macrophages internalised fewer non-opsonised zymosan particles.
Specific alterations in macrophage plasma membrane markers were observed in the annexin 1 null cells. Whereas no differences in dectin-1 and FcγR II/III expression were measured between the two genotypes, decreased membrane CD11b and F4/80 levels were measured selectively in macrophages lacking annexin 1.
These cells also responded with an enhanced release of PGE2 and COX-2 protein expression following addition of the soluble stimulants, LPS and heat-activated IgG.
In conclusion, these results suggest that participation of endogenous annexin 1 during zymosan phagocytosis is critical and that this protein plays a tonic inhibitory role during macrophage activation.
Keywords: Zymosan, CD11b, inflammation
Introduction
In evolutionary terms, phagocytosis of invading pathogens is probably the most ancient inflammatory function of blood leukocytes. Resident macrophages utilise this mechanism to fulfil their role as ‘sentinel' cells as well as orchestrate the host inflammatory response by promoting the recruitment of highly specialised white blood cells, such as the granulocytes, to the site of infection/inflammation (Gordon, 2003). But macrophages are also central to resolution of inflammation and the maintenance of homeostasis. Invading pathogens neutralised by granulocytes, as well as apoptotic granulocytes themselves, are removed by the phagocytosing macrophage (Ward et al., 1999). This process is therefore central to both the genesis and resolution of inflammation and hence to host survival.
Annexin 1 is a 37-kDa protein with anti-inflammatory properties. Originally identified as a mediator of specific glucocorticoid actions (Flower, 1988), this protein inhibits local autacoid generation and leukocyte recruitment, and modulates the organism's hormonal response to inflammation through effects on the anterior pituitary gland (Flower & Rothwell, 1994; Buckingham & Flower, 1997; Perretti, 1998). The inflammatory reaction in animals passively immunised against annexin 1, or in transgenic animals lacking the protein, is exacerbated and prolonged (Perretti et al., 1996; Hannon et al., 2003). These effects have been explained as the failure of a ‘braking mechanism,' which normally acts to limit leukocyte–endothelium interaction and reduces the fraction of leukocytes migrating into the subendothelial matrix tissue (Lim et al., 1998; Gavins et al., 2003).
However, we have not addressed in detail the role of annexin 1 in macrophage function. Human and rodent macrophages contain high levels of annexin 1 (Ambrose et al., 1992; Peers et al., 1993), which is externalised following glucocorticoid treatment or cell activation (De Caterina et al., 1993). Treatment of primary cells (Getting et al., 1997; Goulding et al., 1998) and macrophage-like immortalized cells with human recombinant annexin 1 or annexin 1-derived mimetics (peptides corresponding to a portion of the N-terminus) inhibited phagocytosis of IgG complexes and the consequent oxidative burst. A phagocytosis-inhibitory protein released from macrophages treated with glucocorticoids was tentatively identified as belonging to the annexin family (Becker & Grasso, 1988; Becker et al., 1988).
The intracellular fate of annexin 1 during phagocytosis has been relatively more studied. In a study performed with Brucella suis, prominent annexin 1 staining was associated along the phagosome (Harricane et al., 1996). This pheno-menon was stimulus specific, such that annexin 1 localisation around the phagosome was not observed following macrophage ingestion of live Escherichia coli (Harricane et al., 1996) In addition, intracellular re-location of annexin 1 in relation to the phenomena of phagocytosis or exocytosis has also been reported (Sjolin et al., 1994). Taking advantage of the recent generation of a colony of annexin 1 null mice (Hannon et al., 2003), we undertook to determine the potential role that macrophage annexin 1 could play in the process of phagocytosis itself. Using a panel of different phagocytic stimuli and a series of markers of cell activation, we demonstrated a stimulus-specific defect in cells lacking this protein.
Methods
Animals and peritoneal macrophage (MØ) collection
Annexin 1 null mice and wild-type littermate controls were bred in-house (Hannon et al., 2003). All animals were fed on a standard chow pellet diet with free access to water and maintained on a 12-h light–dark cycle. Animal work was performed in accordance with the U.K. Home Office regulations Animals (Scientific Procedures) Act 1986.
To harvest peritoneal cells (>80% MØ), mice were killed by exposure to CO2, and peritoneal cavities washed with 3 ml of sterile PBS supplemented with 3 mM EDTA and 25 U ml−1 heparin. After gentle massage, the cavity was opened by abdominal incision and lavage fluid collected with a sterile 1-ml plastic pipette. Routinely, samples from 3–4 animals were pooled in 15-ml polypropylene tubes and kept on ice.
Cell surface marker expression
Aliquots (250 μl) of a peritoneal MØ preparation (1 × 106 cells ml−1 in PBC, that is PBS, 1 mM Ca2+ and 0.2 mg ml−1 BSA) were plated into 96-well plates, together with 20 μl blocking human IgG (16 mg ml−1) and 20 μl of either rat anti-mouse CD11b (0.8 μg ml−1) rat anti-mouse F4/80 (5 μg ml−1) rat anti-mouse FcγRIII/II (5 μg ml−1) and rat anti-mouse dectin 1 (10 μg ml−1). After 45 min at 4°C, cells were washed and then stained with 40 μl of FITC-conjugated rabbit anti-rat IgG antibody (1 : 80 dilution) for 30 min at 4°C. For quantification of total cell CD11b expression, macrophages were initially fixed in 2% paraformaldehyde at 4°C for 30 min, prior to immunostaining as described above. A final saponin concentration of 0.02% was maintained throughout the experiment, as previously described (Perretti & Flower, 1996). In all cases, flow cytometry was performed using a FACScan (Becton Dickinson, Cowley, U.K.) with an air-cooled 100-mW argon ion laser tuned to 488 nm connected to an Apple MAC G3 computer running Cell Quest II software. The number of molecules of endogenous antigen per sample was quantified by acquiring 10,000 events in the FL-1 channel (wavelength 548 nm), and calculated as median fluorescence intensity (MFI) units.
In selected experiments, cell samples were also visualised by confocal microscopy. After flow cytometry, cytospins of antibody labelled cells were prepared. The nuclear stain propidium iodide (15 μl) and Slowfade™ (15 μl) were added to the slide prior to mounting the coverslip. The cells were viewed on a Zeiss LSM 510 (Carl Zeiss, Jena, Germany) confocal microscope connected to a PC running LSM 510 software.
Prostaglandin E2 release
Peritoneal cells (1 × 106 per well) were added to 24-well plates and cultured for 2 h at 37°C in 5% CO2 and 95% O2 atmosphere. Nonadherent cells were then removed, adherent cells washed and incubated in foetal calf serum (FCS)-free RPMI 1640, prior to stimulation with 200 μg/ml−1 of boiled zymosan, 200 μg ml−1 heat-aggregated IgG or 10 ng ml−1 LPS. Cell-free supernatants were collected 2 h later. These samples were then frozen at −20°C prior to detection of the release of prostaglandin E2 (PGE2), using a commercially available enzyme immunoassay.
Cells from the same experiments were used to monitor the expression of key pro-inflammatory enzymes, cyclooxygenase 2 (COX-2) or inducible nitric oxide synthase (iNOS). Western blotting analysis was used here according to well-established and published protocols (Wu et al., 1995; Croxtall et al., 2003).
Assays of MØ phagocytosis
The phagocytic ability of peritoneal MØ collected from wild type or annexin 1 null mice was compared using three distinct assays. Phagocytic uptake of antigen–antibody complexes was monitored by a real-time flow cytometric assay as described by Getting et al. (1997). This assay relies upon the intracellular oxidation of an X-rhodamine derivative of dihydrodichlorofluorescein (XR-DHDCF). Briefly, peritoneal cells were re-suspended to a concentration of 1 × 106 cells ml−1) in RPMI-1640 medium supplemented with 2% FCS and kept incubated at 37°C for in a shaking water bath. An aliquot of the cell suspension (250 μl) was removed and pulsed with 10 μl of Fc oxyburst RED, and uptake of these complexes by the peritoneal macrophages was monitored in real time by flow cytometry performed using FACScan analyser (Becton Dickinson, Cowley, U.K.). Quantification of the fluorescence was acquired in the FL-3 channel (670 nm) during 204 s of reaction. Cumulative changes in fluorescence at constant time interval were then constructed and the rate and maximum fluorescence measured.
The oxidative burst induced during cell phagocytosis was monitored by flow cytometry with cells loaded with dihydro-rhodamine 123 (DHR 123) (Euzger et al., 1999). Briefly, 50 μl (containing approximately 1.25 × 105 cells) was added to 200 μl saline supplemented with 5 mM HEPES, cells were incubated with 8 μM DHR 123 for 5 min prior to addition of saline (vehicle), opsonised or non-opsonised boiled zymosan (500 μg ml−1). Zymosan A was opsonised by a 60-min incubation with either wild type or annexin 1 null mouse serum (for respective genotypes) at 37°C, after which the particles were washed extensively and added to cells at a final concentration of 200 μg −1 ml. The reaction was halted at particular time-points (0–60 min) with ice-cold PBS supplemented with 0.02% sodium azide. Samples were analysed immediately by flow cytometry.
The extent of zymosan association with cells, as opposed to ingestion by phagocytosis, was investigated using fluorescein-labelled zymosan. Fluorescent zymosan was added to 1 × 106 macrophages at time 0 and incubated at 37°C; the FITC-zymosan amount was calibrated to two particles per macrophage in order to reduce spill over fluorescence. At different times, phagocytosis was halted by washing cells with 500 μl PBS supplemented with 2 mM EDTA and 4 mg ml−1 lidocaine hydrochloride prior to fixation with 4% paraformaldehyde, certain control groups were preincubated (30 min), and maintained throughout the assay with cytochalasin D. Samples were placed in an ice bath and analysed within 1 h by flow cytometry. The assay used above does not distinguish between binding and ingestion. Attempts to adjust the flow cytometric assay protocol to measure ingestion alone by trypan blue quenching and ethidium bromide (Drevets & Campbell, 1991; Giaimis et al., 1994) of extracellular fluorescence proved to be ineffective in our hands (data not shown). Repetitive washing of the cells proved the best method to remove most unbound FITC labelled particles.
Macrophage phagocytosis was assessed by double-blind examination of electron micrographs. To do so, peritoneal macrophages were resuspended at 1 × 106 cells ml−1 in RPMI-1640 supplemented with 10% FCS and 1% penicillin–streptomycin, and a 3-ml aliquot was placed in six-well cell culture plates for 2 h. After a 2-h adherence period at 37°C in 5% CO2 and 95% O2 atmosphere, the medium was aspirated and the plates washed three times with RPMI-1640 medium, prior to addition of zymosan A (200 μg ml−1). Phagocytosis was halted at 180 min, by washing cells with 5 ml 0.1 M sodium cacodylate prior to fixing in 2.5% glutaraldehyde (20 min at room temperature). Cells were post-fixed in 0.1 M sodium cacodylate (0.1 M) supplemented with 1% osmium tetroxide and potassium ferrocyanide for 30 min. Following extensive washing, cells were dehydrated through graded ethanol solutions (50 and 70% for 5 min each, 90% for 10 min, two changes of 100%, 15 min at each concentration). The cells were then transferred into small aluminium dishes, further dehydrated with propylene oxide, and incubated in a mix containing 9.5 g araldite, 10.5 g dodecenyl succinic anhydride and 0.6 g benzyldimethylamine. The dishes were left overnight at room temperature, heated for 2 days in an oven at 60°C, and cooled down to remove the Melinex™ plastic, thus leaving the cells embedded in the araldite. Using a Reichert-Jung Ultracut E microtome, the araldite blocks were trimmed to size and 80-μm thick sections were cut on a diamond knife and collected on 300 mesh copper grids. The grids were stained with 2% aqueous uranyl acetate for 40 min and rinsed in three changes of distilled water, the first change being at 45°C. The sections were viewed and photographed on a JEM-1200 EX electron microscope at an accelerating voltage of 60 kV.
Chemicals
The following items were purchased from Sigma-Aldrich (Poole, Dorset, U.K.): bovine serum albumin, calcium chloride, EDTA, human IgG, lidocaine hydrochloride, para-formaldehyde, phosphate buffer saline tablets, saponin (Saponaria species S2149), sodium azide and zymosan A. FCS HEPES buffer and RPMI-1640 were purchased from GIBCO BRL (Paisley, Scotland, U.K.). Rat-anti-mouse CD11b (clone 5C6) was a generous gift from Dr N. Gozzard, Celltech (Slough, U.K.). Rat-anti-mouse Dectin 1 (Clone 2A11) was a generous gift from Dr G. Brown, Sir William Dunn School of Pathology (University of Oxford, U.K.). Rat-anti-mouse F4/80 (clone Cl: A31) and FITC-conjugated rabbit anti-rat IgG antibody were purchased from Serotec (Kidlington, Oxford, U.K.). Rat-anti-mouse FcγRIII/II was purchased from BD PharMingen (Cowley, Oxford, U.K.). DHR 123, Fc oxyburst™ RED, fluorescein-labelled zymosan and Slowfade™ were all purchased from Molecular Probes (Eugene, OR, U.S.A.). PGE2 enzyme immunoassay was purchased from Amersham Pharmacia Biotech (Little Chalfont, U.K.). Glutaraldehyde, sodium cacodylate, osmium tetroxide, potassium ferrocyanide, ethanol, propylene oxide, araldite, dodecenyl succinic, anhydride, benzyldimethylamine and uranyl acetate were all purchased from TABB (Reading, U.K.). COX-2 polyclonal antibody was purchased from Cayman polyclonal antibody (Nottingham, U.K.). iNOS was purchased from Santa Cruz (Wiltshire, U.K.). All other chemicals were of analytical grade.
Statistical analysis
Flow cytometry and PGE2 release experiments were repeated n times with mixed cell populations obtained from three mice per group. Two independent samples of at least 10 cells per treatment were used for electron microscopy. In all cases data are expressed as mean±s.e.m. of n distinct experiments. Statistical differences among groups were analysed by Student's t-test. A probability value <0.05 was accepted to reject the null hypothesis.
Results
Annexin 1 null macrophages exhibit a defect in zymosan phagocytosis
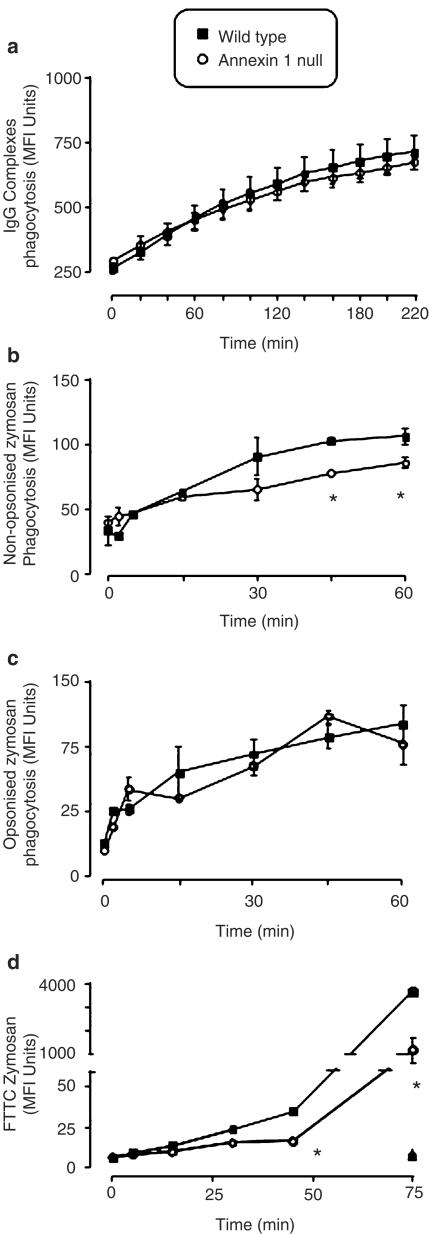
No differences were observed in the rate or maximal response of macrophage phagocytosis, as assessed by generation of reactive oxygen species, when IgG complexes were used as a stimulus (Figure 1a), but when non-opsonised zymosan was used macrophages from annexin 1 null mice exhibited a reduction in phagocytosis compared to wild-type cells (Figure 1b) at 45 and 60 min. However, when opsonised zymosan was used as a stimulus, the difference between wild type and annexin 1 null cells disappeared (Figure 1c). Macrophage incubation with FITC labelled zymosan preparation confirmed a lower degree of particle uptake in the absence of annexin 1 (Figure 1d), which was already significant at 30 min and marked at 45 and 60 min post-zymosan. However, the latter experiment could not differentiate between cell attachment and actual ingestion of zymosan particles per se. This point was addressed further by electron microscopy analysis, which demonstrated that wild-type cells ingested a large number of particles (Figure 2c) and that this was visibly much attenuated in annexin 1 null macrophages (Figure 2d) although no apparent morphological differences were evident between the two types of cells under resting conditions (Figure 2a,b). Analysis of this process demonstrated that 3 h was the optimum sampling point since the large majority (∼85%) of zymosan particles were ingested in wild-type cells, with few particles associated with the external macrophage plasma membrane (Figure 3). In annexin 1 null MØ, particles were not ingested, but remained loosely attached to the cell membrane. Figure 2d inset shows an example of one annexin 1 null macrophage surrounded by zymosan particles that it is apparently unable to ingest relative to wild-type cells.
Figure 1.
Annexin 1 null macrophages display a stimulus-dependent defect in phagocytosis. Peritoneal MØ from WT and annexin-1 null mice were tested for their ability to phagocytose-specific particles. (a) Peritoneal cells (2.5 × 105 per sample) were incubated with IgG complexes and monitored by real-time flow cytometry for phagocytosis-related oxidation and associated fluorescence over a 204-s time frame. (b,c) As above, except that cells were loaded with DHR 123 and stimulated by addition of either (b) non-opsonised zymosan or (c) opsonised zymosan. (d) Analysis of non-opsonised FITC-zymosan association, cytochalasin D control for WT and annexin-1 null Mø shown by  and ▴, respectively. For all panels, data are mean ± s.e.m. of n=4 experiments performed with n=3 mice each. *P<0.05 vs respective WT value.
and ▴, respectively. For all panels, data are mean ± s.e.m. of n=4 experiments performed with n=3 mice each. *P<0.05 vs respective WT value.
Figure 2.
Electron micrographs of peritoneal MØ before and after non-opsonised zymosan incubation. Peritoneal MØ from WT and annexin-1 null mice were incubated with 200 μg ml−1 non-opsonised zymosan for 3 h, prior to fixation and processing as described in the Methods section. (a) Resting WT MØ. (b) Resting annexin-1 null MØ. (c) WT MØ engulfed with several zymosan particles (asterisks highlight some of these phagosomes). (d) Annexin-1 null MØ with fewer zymosan particles. Arrowheads indicate particles associated with the plasma membrane rather than fully internalised. Inset: lower magnification image showing an example of frustrated phagocytosis with several zymosan particles in close vicinity, but not internalised, of the cell. Images are representative of 14 distinct animals. Panels (a–d), bar 1 μm; inset, bar 2 μm.
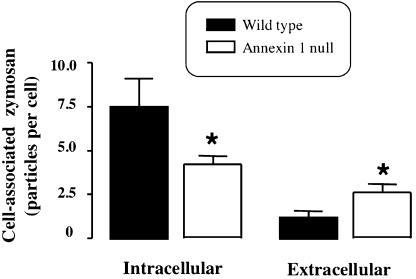
Figure 3.
Quantification of zymosan particle association with WT and annexin-1 null MØs. A ‘blind' analysis of fully internalised or loosely associated (clearly in contact with the plasma membrane) particles was performed on nine distinct electron micrographs prepared as in Figure 2. Data are mean±s.e.m. of n=9 from 14 distinct animals of a genotype. *P<0.05 vs WT value.
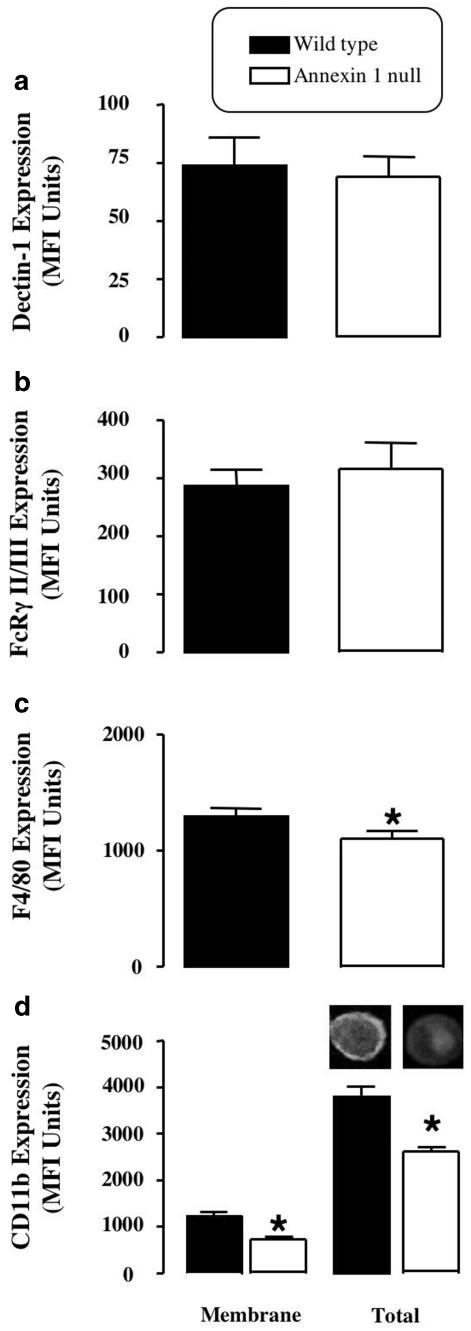
Analysis of macrophage plasma membrane markers
Quantification of selected plasma markers by flow cyto-metry revealed specific alterations in annexin 1 null MØ. Whereas no significant differences were found with respect to dectin 1 (the β glucan receptor) levels (Figure 4a) and FcγRIII/II (Figure 4b), a modest yet significant reduction in the differentiation marker F4/80 was displayed by macrophages lacking annexin 1 (Figure 4c). The most evident difference, though, was observed for CD11b. There was approximately 50% reduction in CD11b plasma membrane expression in annexin 1 null MØ when compared to wild-type cells (Figure 4d). This was also reflected in a reduction in total CD11b levels in annexin 1 null cells, as assessed following cell permeabilisation with saponin (Figure 4d).
Figure 4.
Comparison of membrane marker expression between WT and annexin 1 null peritoneal MØs. Peritoneal MØ from WT and annexin-1 null mice (2.5 × 105 per sample) were stained with specific mAbs to quantify membrane expression of dectin-1 (a), FcRg-II/III (b), F4/80 (c) and CD11b (d). Panel (d) also shows CD11b immunoreactivity in permeabilised MØ to estimate total antigen expression. Inset: immunofluorescent image of total CD11b staining in WT and annexin 1 null MØ. Data are the mean ± s.e.m. of n=4–5 experiments performed with five mice each. *P<0.05 vs respective WT values.
Markers of macrophage activation
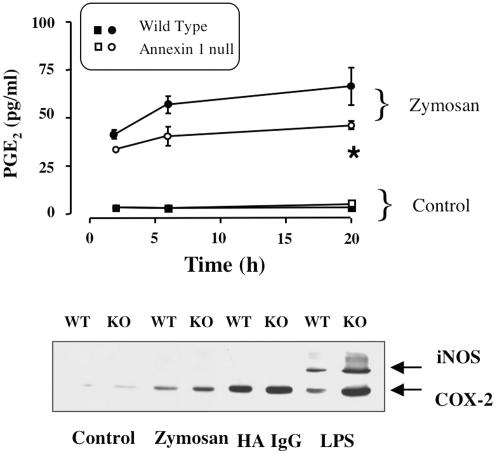
In the last series of experiments, we measured several other parameters of MØ activation to clarify the downstream events that follow zymosan phagocytosis. A marked accumulation of PGE2 was measured in the supernatant of wild type and annexin 1 null MØ during phagocytosis of non-opsonised zymosan, although the response was attenuated in the latter cell type (Figure 5a), presumably in view of the lower extent of particle uptake. This reduction in eicosanoid production was evident at all time points investigated, though significance was reached only at 2 and 6 h post-zymosan. A modest release of PGE2 was detected in the absence of cell stimulation, with no difference apparent between the genotypes (Figure 5a).
Figure 5.
WT and annexin 1 null MØ activation as measured by PGE2 generation and COX-2 or iNOS expression. Peritoneal MØ from WT and annexin-1 null mice (1 × 106 per sample) were seeded in a 24-well plate and activated with zymosan (200 μg ml−1), heat aggregated IgG (HA IgG; 200 μg ml−1) or LPS (10 ng ml−1) for a 0.5–20-h period. (a) Time-course of PGE2 release in response to zymosan. Data are mean±s.e.m. of three experiments performed in triplicate with cells pooled from 7–10 mice. (b) COX-2 and iNOS immunoreactivity in cell extracts as measured following 20-h cell incubation. Gel representative of two experiments.
Modest COX-2 protein expression was detected in both wild type and annexin 1 null MØ after adhesion to plastic; however, this was markedly increased following cell activation with zymosan: annexin 1 null cells exhibited a greater expression of the enzyme (Figure 5b). Cell activation with heat-aggregated IgG also led to a more pronounced COX-2 induction, but with little difference among the genotypes. Finally, LPS produced a substantial COX-2 induction and, alone among the stimuli investigated, also an induction of iNOS (Figure 5b). Table 1 reports the PGE2 values from this set of experiments. In contrast to zymosan, soluble stimulants such as IgG complexes and LPS produced a greater release of PGE2 in annexin 1 null MØ, which was particularly evident after longer incubation (Table 1).
Table 1.
PGE2 release from activated MØ in response to different stimuli
| No stimulus | Heat-Aggregated IgG | LPS | ||||
|---|---|---|---|---|---|---|
| Time (h) | Wild type | Annexin 1 null | Wild type | Annexin 1 null | Wild type | Annexin 1 null |
| 2 | 33±2 | 57±17 | 132±34 | 217±53 | 255±66 | 324±50 |
| 6 | 40±13 | 32±10 | 114±6 | 320±36* | 776±51 | 895±110 |
| 20 | 53±13 | 75±17* | 147±27 | 259±52 | 111±19 | 1800±175* |
Adherent macrophages (1 × 106) prepared from WT or annexin 1 null mice were incubated for the reported times either alone (no stimulus), with heat-aggregated IgG complexes (200 μg ml−1) or LPS (10 ng ml−1). At the end of the incubation period, prostaglandin E2 (PGE2) was assayed in the cell media. Data reported are PGE2 concentrations in pg ml−1, and are expressed as mean±s.e.m. from three experiments performed in duplicate.
P<0.05 vs respective WT values.
Discussion
In the present study, we demonstrate that lack of annexin 1 is functionally associated with a reduced ability of MØ to phagocytose non-opsonised zymosan particles. In contrast, macrophage activation by soluble mediators is either unchanged or exacerbated by this annexin 1 deficiency.
The recent generation of annexin 1 null mice, with the initial characterisation of their inflammatory response (Roviezzo et al., 2002; Hannon et al., 2003), presented an ideal opportunity to test the potential role of glucocorticoid-regulated protein annexin 1 in resident macrophage function. Previous studies demonstrated that glucocorticoids augmented annexin 1 protein expression in human alveolar macrophages as well as in human monocytes and rat peritoneal macrophages (Ambrose et al., 1992; Peers et al., 1993; Comera & Russo-Marie, 1995). In addition, exogenous annexin 1 inhibited activation of monocytes and macrophages, as assessed by superoxide generation, prostanoid release and enzyme or cytokine expression (Maridonneau-Parini et al., 1989; Comera & Russo-Marie, 1995; Wu et al., 1995). More recently, the annexin 1 mimetic peptide Ac2-26 (Perretti, 1998) was shown to inhibit IgG immuno-complex phagocytosis by mouse peritoneal macrophages, as determined by flow cytometry (Getting et al., 1997). In contrast, U937 cells that overexpress annexin 1 show an increase in IgG immuno-complex phagocytosis, whereas cells with reduced annexin 1 expression exhibited a reduction in phagocytosis (Getting, unpublished). Therefore, in the present study we began our analysis by comparing the extent of macrophage phagocytosis and cell activation using a panel of soluble and insoluble stimuli. When zymosan particles were used, a striking difference in the rate of phagocytosis was seen between wild type and annexin 1 null MØ. However, if zymosan was opsonised, this difference disappeared. This is congruent with the absence of any detectable kinetic difference when IgG immunocomplexes were used as phagocytosing stimulus, since opsonisation leads to the formation of IgG complexes around the foreign particle. The defect in zymosan phagocytosis was confirmed by electron microscopy, by which it was demonstrated that zymosan particles were only loosely attached to the external surface of the plasma membrane in the annexin 1 null MØ, rather than being actually ingested.
The macrophage expresses several surface molecules involved in the recognition of microorganisms and other foreign particles. These fall into two main categories: the receptors for immunoglobulin (FcR) and complement (CR3 or CD11b/CD18) utilise opsonins for ingestion (Aderem & Underhill, 1999), while the dectin-1 receptor (Brown & Gordon, 2001) recognises conserved motifs on the β glucan particle in conjunction with, lactosylceramide (Zimmerman et al., 1998) and the Toll receptor-2 (Underhill et al., 1999). FACS analysis demonstrated that there were no differences in dectin-1 levels on wild type and annexin 1 null cells. This is an important finding since dectin-1 is the major receptor for both soluble and particulate β glucans on MØ (Brown et al., 2002); dectin-1 has also been shown to regulate β glucan cellular responses including cytokine production (Brown et al., 2003). In contrast, membrane levels of heterodimeric integrin CD11b were reduced in annexin 1 null cells. CD11b is the alpha subunit of a myeloid member of the β2-integrin family, well known to be involved in the recognition of particles and other phagocytosis stimuli (Brown, 1991; Le Cabec et al., 2000). For instance, the MØ uses CD11b to ingest iC3b opsonised particles; in addition, CD11b also possesses a lectin-binding domain that recognises a variety of β glucans, including zymosan (Ross et al., 1999). In addition, CD11b is a ligand for a number of other soluble factors and compounds known to be internalised in macrophages, ranging from galectin-3 to antisense probes (Avni et al., 1998). The reduction of this lectin-binding domain may account for several of the effects observed during this study. However, zymosan uptake defects were largely observed from 30 min onwards, offering a perplexing situation, for this is also the time point when opsonins are believed to be released by activated MØ (Ezekowitz et al., 1984). The CD11b/CD18 complex plays a crucial role in phagocytosis by maintaining the integrity of membrane protrusions around the loosely attached particles to facilitate their ingestion into the phagosome (Dewitt & Hallett, 2002).
Other members of the annexin superfamily are known to be involved in membrane dynamics including endocytosis and lipid raft stabilisation (Gerke & Moss, 2002). In this context, it is worth remembering that annexin 1, as well as other members of the annexin family, has been found to be associated with the early endosomes and phagosomes of the J774 macrophage cell line during latex bead phagocytosis (Diakonova et al., 1997). In these cells, the protein co-localised with F-actin on the cell protrusions and microvilli tips. Punctuate membrane immuno-gold localisation for annexin 1 has also been reported in human adherent neutrophils (Oliani et al., 2000) and rat pituitary folliculo-stellate cells that belong to the macrophage lineage (Traverso et al., 1999). Initial structural studies using mutated proteins suggest that phosphorylation of Ser27 in the N-terminal region of the protein is crucial for phagosome association (Kusumawati et al., 2001). However, (Kusumawati et al. (2001) also showed that deletion of half or all the N-terminal domain (1–33) did not alter annexin 1 association with phagosomes, as seen in J-774A.1 cells. This is an important indication that the core domain, which contains the repeating canonical annexin motif and the phospholipid binding sites (Gerke & Moss, 2002), might govern annexin 1 localisation to the phagosome. During apoptosis cytosolic annexin A1 translocates to the membrane in discrete patches, where it can bind to phosphatidylserine or other receptors from engulfing cells (Arur et al., 2003). Future analysis of reconstitution experiments, with cells taken from the annexin 1 null mouse, will help clarifying this puzzle.
Our previous study with annexin 1 null mice (Hannon et al., 2003) indicated that some of the anti-inflammatory effects of glucocorticoids were no longer apparent in the absence of this protein. One of such effects was hydrocortisone-mediated inhibition of macrophage phagocytosis of IgG complexes. Several studies have addressed the effect of glucocorticoids on macrophage phagocytosis. Interestingly, Grasso and co-workers demonstrated that steroids inhibited phagocytosis through the secretion of a phagocytosis inhibitory protein (Becker & Grasso, 1988), which was recognised by a monoclonal antibody raised against purified preparations of annexin 1. Thus, there was presumptive evidence that an annexin 1-like protein might mediate the anti-phagocytic effect of glucocorticoids.
In the present study, both membrane and total (membrane+cytosolic) levels of CD11b were markedly reduced (∼30%) in annexin 1 null cells. Thus, annexin 1 deficiency was not related to an altered process of exportation of this integrin. It is not known how intracellular annexin 1 regulates CD11b gene expression, or whether this is a direct effect or through post-transcriptional events. Interestingly, glucocorticoids are known to reduce CD11b expression on circulating and resident granulocytes and monocytes (Burton et al., 1995; Das et al., 1997; Lim et al., 2000), and this effect is cycloheximide sensitive (Filep et al., 1997).
The flow cytometric analysis performed during this study showed two further results worth discussion. Firstly, wild type and annexin null macrophages displayed similar levels of membrane FcRγ type II/III and this is in agreement with the lack of differences observed during phagocytosis of IgG complexes or opsonised zymosan. Secondly, a slight reduction in the F4/80-sensitive antigen was measured in annexin 1 null MØ. The significance of these findings is yet unclear, as it is the precise function of the ligand recognised by the F4/80 mAb. Nonetheless, it is certainly a marker of macrophage differentiation, with a significantly lower degree of expression in monocytes, and higher levels in differentiated tissue macrophages (Hirsch & Gordon, 1983). Thus, it is possible that absence of annexin 1 might retard the process of macrophage maturation, a fact that recalls the differential expression of the endogenous protein in monocyte/macrophages as demonstrated more than a decade ago (Ambrose et al., 1992).
Finally, some changes were seen in the cellular responses that follow phagocytosis. The reduced non-opsonised zymosan ingestion by annexin 1 null cells was mirrored by the lack of full PGE2 release compared with wild-type cells at any time-point tested. These functional data were only in part corroborated by COX-2 protein expression at 20 h post-zymosan, since a smaller increase was seen in extracts taken from annexin 1 null cells. Studies in fibroblasts have shown a similar dissociation between PGE2 release and COX-2 expression in the absence of annexin 1, and these cells also display changes in cPLA2 activity (Croxtall et al., 2003). The stimulus specificity of the responses that were altered in the absence of MØ annexin 1 was confirmed by testing the effect of soluble stimuli. Whereas heat-aggregated IgG-mediated response was unmodified in the absence of annexin 1, lipopolysaccharide activation led to a marked upregulation of COX-2 and iNOS protein expression, and this was associated with a prolonged and more pronounced release of PGE2, this may be due to overactivity at the NFκB promoter, that regulates COX-2 expression (Yamamoto et al., 1995). Again, these findings are congruent with studies performed with annexin 1-derived peptides or with anti-annexin 1 neutralising sera (often tested in conjunction with a glucocorticoid), in which iNOS and COX-2 expression, as well as soluble mediator release, was monitored (Wu et al., 1995; Ferreira et al., 1997; Minghetti et al., 1999).
In conclusion, this is the first study that takes advantage of the recently generated annexin 1 null mice to clarify a role for this endogenous protein in the process of macrophage phagocytosis. The data obtained indicated that annexin 1 deficiency is associated with subtle changes in the expression of key membrane receptors and this, either directly or indirectly, leads to inadequate phagocytosis of insoluble particles with consequent alterations in downstream markers of cell activation. It is likely that the experiments illustrated here will prompt further investigations into the process of macrophage maturation and differentiation, as well as future molecular analysis more focused on particle ingestion and degradation.
Acknowledgments
We thank Dr B. Ward for her assistance with the electron microscopy. This work was funded by a PhD studentship of the Nuffield Foundation U.K. (Oliver Bird Fund, project RHE/00057/G). M.P. is a Senior Research Fellow of the Arthritis Research Campaign U.K., whereas R.J.F. is a Principal Research Fellow of the Wellcome Trust U.K.
Abbreviations
- COX-2
cyclooxygenase 2
- DHR 123
dihydrorhodamine 123
- FCS
foetal calf serum
- iNOS
inducible nitric oxide synthase
- MØ
macrophage
- MFI
median fluorescence intensity
- PGE2
prostaglandin E2
References
- ADEREM A., UNDERHILL D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- AMBROSE M.P., BAHNS C.L., HUNNINGHAKE G.W. Lipocortin I production by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1992;6:17–21. doi: 10.1165/ajrcmb/6.1.17. [DOI] [PubMed] [Google Scholar]
- ARUR S., UCHE U.E., REZAUL K., FONG M., SCRANTON V., COWAN A.E., MOHLER W., HAN D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- AVNI O., PUR Z., YEFENOF E., BANIYASH M. Complement receptor 3 of macrophages is associated with galectin-1-like protein. J. Immunol. 1998;160:6151–6158. [PubMed] [Google Scholar]
- BECKER J., GRASSO R.J. Suppression of yeast ingestion by dexamethasone in macrophage cultures: evidence for a steroid-induced phagocytosis inhibitory protein. Int. J. Immunopharmacol. 1988;10:325–338. doi: 10.1016/0192-0561(88)90118-x. [DOI] [PubMed] [Google Scholar]
- BECKER J.L., GRASSO R.J., DAVIS J.S. Dexamethasone action inhibits the release of arachidonic acid from phosphatidylcholine during the suppression of yeast phagocytosis in macrophage cultures. Biochem. Biophys. Res. Commun. 1988;153:583–590. doi: 10.1016/s0006-291x(88)81135-5. [DOI] [PubMed] [Google Scholar]
- BROWN E.J. Complement receptors and phagocytosis. Curr. Opin. Immunol. 1991;3:76–82. doi: 10.1016/0952-7915(91)90081-b. [DOI] [PubMed] [Google Scholar]
- BROWN G.D., GORDON S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- BROWN G.D., HERRE J., WILLIAMS D.L., WILLMENT J.A., MARSHALL A.S., GORDON S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G.D., TAYLOR P.R., REID D.M., WILLMENT J.A., WILLIAMS D.L., MARTINEZ-POMARES L., WONG S.Y., GORDON S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKINGHAM J.C., FLOWER R.J. Lipocortin 1: a second messenger of glucocorticoid action in the hypothalamo–pituitary–adrenocortical axis. Mol. Med. Today. 1997;3:296–302. doi: 10.1016/S1357-4310(97)88908-3. [DOI] [PubMed] [Google Scholar]
- BURTON J.L., KEHRLI M.E., JR, KAPIL S., HORST R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995;57:317–325. doi: 10.1002/jlb.57.2.317. [DOI] [PubMed] [Google Scholar]
- COMERA C., RUSSO-MARIE F. Glucocorticoid-induced annexin 1 secretion by monocytes and peritoneal leukocytes. Br. J. Pharmacol. 1995;115:1043–1047. doi: 10.1111/j.1476-5381.1995.tb15916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROXTALL J.D., GILROY D.W., SOLITO E., CHOUDHURY Q., WARD B.J., BUCKINGHAM J.C., FLOWER R.J. Attenuation of glucocorticoid functions in an Anx-A1−/− cell line. Biochem. J. 2003;371:927–935. doi: 10.1042/BJ20021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAS A.M., FLOWER R.J., HELLEWELL P.G., TEIXEIRA M.M., PERRETTI M. A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment. Br. J. Pharmacol. 1997;121:97–104. doi: 10.1038/sj.bjp.0701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CATERINA R., SICARI R., GIANNESSI D., PAGGIARO P.L., PAOLETTI P., LAZZERINI G., BERNINI W., SOLITO E., PARENTE L. Macrophage-specific eicosanoid synthesis inhibition and lipocortin-1 induction by glucocorticoids. J. Appl. Physiol. 1993;75:2368–2375. doi: 10.1152/jappl.1993.75.6.2368. [DOI] [PubMed] [Google Scholar]
- DEWITT S., HALLETT M.B. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J. Cell Biol. 2002;159:181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAKONOVA M., GERKE V., ERNST J., LIAUTARD J.P., VAN DER VUSSE G., GRIFFITHS G. Localization of five annexins in J774 macrophages and on isolated phagosomes. J. Cell Sci. 1997;110:1199–1213. doi: 10.1242/jcs.110.10.1199. [DOI] [PubMed] [Google Scholar]
- DREVETS D.A., CAMPBELL P.A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods. 1991;142:31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- EUZGER H.S., FLOWER R.J., GOULDING N.J., PERRETTI M. Differential modulation of annexin I binding sites on monocytes and neutrophils. Mediators Inflamm. 1999;8:53–62. doi: 10.1080/09629359990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZEKOWITZ R.A., SIM R.B., HILL M., GORDON S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J. Exp. Med. 1984;159:244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA S.H., CUNHA F.Q., LORENZETTI B.B., MICHELIN M.A., PERRETTI M., FLOWER R.J., POOLE S. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br. J. Pharmacol. 1997;121:883–888. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILEP J.G., DELALANDRE A., PAYETTE Y., FOLDES-FILEP E. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation. 1997;96:295–301. doi: 10.1161/01.cir.96.1.295. [DOI] [PubMed] [Google Scholar]
- FLOWER R.J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br. J. Pharmacol. 1988;94:987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOWER R.J., ROTHWELL N.J. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- GAVINS F.N., YONA S., KAMAL A.M., FLOWER R.J., PERRETTI M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- GERKE V., MOSS S.E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- GETTING S.J., FLOWER R.J., PERRETTI M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIAIMIS J., LOMBARD Y., POINDRON P., MULLER C.D. Flow cytometry distinction between adherent and phagocytized yeast particles. Cytometry. 1994;17:173–178. doi: 10.1002/cyto.990170210. [DOI] [PubMed] [Google Scholar]
- GORDON S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., EUZGER H.S., BUTT S.K., PERRETTI M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm. Res. 1998;47 Suppl 3:S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- HANNON R., CROXTALL J.D., GETTING S.J., ROVIEZZO F., YONA S., PAUL-CLARK M.J., GAVINS F.N., PERRETTI M., MORRIS J.F., BUCKINGHAM J.C., FLOWER R.J. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB. J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- HARRICANE M.C., CARON E., PORTE F., LIAUTARD J.P. Distribution of annexin I during non-pathogen or pathogen phagocytosis by confocal imaging and immunogold electron microscopy. Cell Biol. Int. 1996;20:193–203. doi: 10.1006/cbir.1996.0024. [DOI] [PubMed] [Google Scholar]
- HIRSCH S., GORDON S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- KUSUMAWATI A., LIAUTARD J.P., SRI WIDADA J. Implication of annexin 1 in phagocytosis: effects of n-terminal domain deletions and point mutations of the phosphorylation site Ser-27. Cell Biol. Int. 2001;25:809–813. doi: 10.1006/cbir.2000.0704. [DOI] [PubMed] [Google Scholar]
- LE CABEC V., COLS C., MARIDONNEAU-PARINI I. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect. Immun. 2000;68:4736–4745. doi: 10.1128/iai.68.8.4736-4745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM L.H., FLOWER R.J., PERRETTI M., DAS A.M. Glucocorticoid receptor activation reduces CD11b and CD49d levels on murine eosinophils: characterization and functional relevance. Am. J. Respir. Cell Mol. Biol. 2000;22:693–701. doi: 10.1165/ajrcmb.22.6.3890. [DOI] [PubMed] [Google Scholar]
- LIM L.H., SOLITO E., RUSSO-MARIE F., FLOWER R.J., PERRETTI M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIDONNEAU-PARINI I., ERRASFA M., RUSSO-MARIE F. Inhibition of O2- generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Invest. 1989;83:1936–1940. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINGHETTI L., NICOLINI A., POLAZZI E., GRECO A., PERRETTI M., PARENTE L., LEVI G. Down-regulation of microglial cyclo-oxygenase-2 and inducible nitric oxide synthase expression by lipocortin 1. Br. J. Pharmacol. 1999;126:1307–1314. doi: 10.1038/sj.bjp.0702423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIANI S.M., CHRISTIAN H.C., MANSTON J., FLOWER R.J., PERRETTI M. An immunocytochemical and in situ hybridization analysis of annexin 1 expression in rat mast cells: modulation by inflammation and dexamethasone. Lab. Invest. 2000;80:1429–1438. doi: 10.1038/labinvest.3780150. [DOI] [PubMed] [Google Scholar]
- PEERS S.H., SMILLIE F., ELDERFIELD A.J., FLOWER R.J. Glucocorticoid-and non-glucocorticoid induction of lipocortins (annexins) 1 and 2 in rat peritoneal leucocytes in vivo. Br. J. Pharmacol. 1993;108:66–72. doi: 10.1111/j.1476-5381.1993.tb13441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRETTI M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen. Pharmacol. 1998;31:545–552. doi: 10.1016/s0306-3623(98)00039-1. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., CROXTALL J.D., WHELLER S.K., GOULDING N.J., HANNON R., FLOWER R.J. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., FLOWER R.J. Measurement of lipocortin 1 levels in murine peripheral blood leukocytes by flow cytometry: modulation by glucocorticoids and inflammation. Br. J. Pharmacol. 1996;118:605–610. doi: 10.1111/j.1476-5381.1996.tb15444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS G.D., VETVICKA V., YAN J., XIA Y., VETVICKOVA J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- ROVIEZZO F., GETTING S.J., PAUL-CLARK M.J., YONA S., GAVINS F.N., PERRETTI M., HANNON R., CROXTALL J.D., BUCKINGHAM J.C., FLOWER R.J. The annexin-1 knockout mouse: what it tells us about the inflammatory response. J. Physiol. Pharmacol. 2002;53:541–553. [PubMed] [Google Scholar]
- SJOLIN C., STENDAHL O., DAHLGREN C.Calcium-induced translocation of annexins to subcellular organelles of human neutrophils Biochem. J. 1994300325–330.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAVERSO V., CHRISTIAN H.C., MORRIS J.F., BUCKINGHAM J.C. Lipocortin 1 (annexin 1): a candidate paracrine agent localized in pituitary folliculo-stellate cells. Endocrinology. 1999;140:4311–4319. doi: 10.1210/endo.140.9.7008. [DOI] [PubMed] [Google Scholar]
- UNDERHILL D.M., OZINSKY A., HAJJAR A.M., STEVENS A., WILSON C.B., BASSETTI M., ADEREM A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- WARD I., DRANSFIELD I., CHILVERS E.R., HASLETT I., ROSSI A.G. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol. Sci. 1999;20:503–509. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- WU C.C., CROXTALL J.D., PERRETTI M., BRYANT C.E., THIEMERMANN C., FLOWER R.J., VANE J.R. Lipocortin 1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3473–3477. doi: 10.1073/pnas.92.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., ARAKAWA T., UEDA N., YAMAMOTO S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN J.W., LINDERMUTH J., FISH P.A., PALACE G.P., STEVENSON T.T., DeMONG D.E. A novel carbohydrate-glycosphingolipid interaction between a beta-(1–3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998;273:22014–22020. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]