Abstract
5-Lipoxygenase (5-LO) is a crucial enzyme in the synthesis of the bioactive leukotrienes (LTs) from arachidonic acid (AA), and inhibitors of 5-LO are thought to prevent the untowarded pathophysiological effects of LTs.
In this study, we present the molecular pharmacological profile of the novel nonredox-type 5-LO inhibitor CJ-13,610 that was evaluated in various in vitro assays.
In intact human polymorphonuclear leukocytes (PMNL), challenged with the Ca2+-ionophore A23187, CJ-13,610 potently suppressed 5-LO product formation with an IC50=0.07 μM. Supplementation of exogenous AA impaired the efficacy of CJ-13,610, implying a competitive mode of action.
In analogy to ZM230487 and L-739.010, two closely related nonredox-type 5-LO inhibitors, CJ-13,610 up to 30 μM failed to inhibit 5-LO in cell-free assay systems under nonreducing conditions, but inclusion of peroxidase activity restored the efficacy of CJ-13,610 (IC50=0.3 μM).
In contrast to ZM230487 and L-739.010, the potency of CJ-13,610 does not depend on the cell stimulus or the activation pathway of 5-LO. Thus, 5-LO product formation in PMNL induced by phosphorylation events was equally suppressed by CJ-13,610 as compared to Ca2+-mediated 5-LO activation. In transfected HeLa cells, CJ-13,610 only slightly discriminated between phosphorylatable wild-type 5-LO and a 5-LO mutant that lacks phosphorylation sites.
In summary, CJ-13,610 may possess considerable potential as a potent orally active nonredox-type 5-LO inhibitor that lacks certain disadvantages of former representatives of this class of 5-LO inhibitors.
Keywords: 5-Lipoxygenase; leukotriene; CJ-13,610; polymorphonuclear leukocyte; inflammation
Introduction
Upon stimulation, certain inflammatory cell types, mainly granulocytes and monocytes/macrophages, possess the ability to release leukotrienes (LTs) and related lipid metabolites such as 5(S)-hydro(pero)xyeicosatetraenoic acid (5-H(P)ETE). These bioactive lipids are generated by the initial conversion of arachidonic acid (AA) to 5-HPETE and LTA4 via the enzyme 5-lipoxygenase (5-LO). The subsequent conversion of LTA4 by LTA4 hydrolase gives LTB4, whereas metabolism by LTC4 synthase leads to the cysteinyl-LTs C4, D4, and E4 (Samuelsson et al., 1987; Funk, 2001). LTB4 is a potent chemotactic and chemokinetic mediator that activates granulocytes (Claesson & Dahlen, 1999) and augments phagocytosis of macrophages (Mancuso et al., 1998). The cysteinyl-LTs cause smooth muscle contraction, mucus secretion, plasma extravasation, vasoconstriction, and recruitment of eosinophils (Funk, 2001). Aside of these functions, more recent studies suggest a role of 5-LO products also in carcinogenesis and cell survival (Romano & Claria, 2003), in the metabolism of bone (Chen et al., 1994; Garcia et al., 1996), and finally in atherosclerotic processes (Mehrabian & Allayee, 2003; Dwyer et al., 2004). Accordingly, anti-LT therapy is beneficial in the treatment of asthma and other inflammatory diseases (Steinhilber, 1999), but may have potential also in the treatment of pancreatic and prostate cancer (Ghosh & Myers, 1998; Ding et al., 1999; Romano & Claria, 2003), osteoporosis (Alanko et al., 1998), and atherosclerosis (Mehrabian & Allayee, 2003; Dwyer et al., 2004).
5-LO contains a nonheme iron in the active site. In the resting state of the enzyme the iron is in the ferrous (Fe2+) form, whereas the ferric (Fe3+) form is necessary to enter the catalytic cycle (for a review see Rådmark, 2002). Accordingly, reducing compounds and iron-ligand inhibitors (such as zileuton, which is approved for the therapy of asthma in the U.S.) are direct 5-LO inhibitors acting at the active site iron. Despite their high potency in vitro, these compounds exhibit only poor bioavailability and only modest selectivity for 5-LO (Steinhilber, 1999).
In search of potent and selective 5-LO inhibitors, nonredox-type 5-LO inhibitors such as ZD2138 and its ethyl analogue ZM230487 were identified as highly potent, orally active inhibitors of LT biosynthesis that specifically and enantioselectively interact with 5-LO. Although these compounds significantly reduced a number of acute inflammatory responses (McMillan & Walker, 1992; Smith et al., 1995), they failed to inhibit more chronic inflammatory processes (Nasser et al., 1994; Turner et al., 1996). It was found that an elevated peroxide tone decreases the potency of ZM230487 that may limit the therapeutic value under chronic inflammatory conditions, connected to increased peroxide levels (Werz et al., 1998). Moreover, the potency of ZM230487 depends on the 5-LO activation pathway (Fischer et al., 2003). Thus, the sensitivity of 5-LO for ZM230487 in polymorphonuclear leukocytes (PMNL) was about 10- to 100-fold lower for 5-LO activated by phosphorylation as compared to Ca2+-mediated enzyme activation. Finally, due to poor aqueous solubility and moderate oral absorption, the compounds exhibit unsatisfactory bioavailability.
Recently, novel ionizable imidazolylphenyl analogues of ZD2138 with improved solubility and better oral absorption were developed, with an in vitro activity comparable to that of ZD2138 (Mano et al., 2003). Structural modification led to improved pharmacokinetic and toxicological characteristics, exemplified by CJ-13,454, a practical lead for orally active nonredox-type 5-LO inhibitors (Mano et al., 2004). In this study, we evaluated the molecular pharmacology of CJ-13,610, a recently developed analogue of these imidazolylphenyl compounds, using various in vitro test systems with relevance for the in vivo pharmacology.
Methods
Materials
CJ-13,610 was provided by Glaxo Smith Kline (Stevenage, U.K.). The materials and sources were: Dulbecco's modified Eagle's medium (DMEM), GibcoBRL, Life Technologies (Rockville, MD, U.S.A.); fetal calf serum, bovine insulin, Ca2+-ionophore A23187, arachidonic acid, dithiothreitol (DTT), glutathione peroxidase (GPx), sodium arsenite (SA), Sigma (Deisenhofen, Germany); HPLC solvents, Merck (Darmstadt, Germany); 13(S)-hydroperoxy-9Z,11E-octadecadienoic acid (13(S)-HPODE), Cayman; oligonucleotides, Cyber Gene (Huddinge, Sweden).
Cell culture, plasmids, and transient transfections
Human PMNL were freshly isolated from leukocyte concentrates obtained at St Markus Hospital (Frankfurt, Germany). In brief, venous blood was taken from healthy adult donors and subjected to centrifugation at 4000 × g for 20 min at 20°C for preparation of leukocyte concentrates. PMNL were promptly isolated by dextran sedimentation, centrifugation on Nycoprep cushions (PAA Laboratories, Linz, Austria), and hypotonic lysis of erythrocytes as described previously (Werz et al., 2002a). PMNL (7.5 × 106 cells ml−1; purity >96–97%) were finally resuspended in phosphate-buffered saline pH 7.4 (PBS) plus 1 mg ml−1 glucose (PG buffer), or alternatively in PBS plus 1 mg ml−1 glucose and 1 mM CaCl2 (PGC buffer) as indicated.
HeLa cells were maintained in DMEM, supplemented with 10% fetal calf serum and 100 μg ml−1 streptomycin and 100 U ml−1 penicillin at 37°C in a 5% CO2 incubator. Plasmid DNA (pcDNA3.1-5LO (Provost et al., 2001) or pcDNA3.1-5LO-S271A-S663A (Werz et al., 2002b), 10 μg each) was transiently transfected into HeLa cells using the calcium phosphate method, cultured for 48 h, and assayed for 5-LO product formation as described elsewhere (Fischer et al., 2003).
Expression and purification of 5-LO from Escherichia coli
Expression of 5-LO was performed in E. coli JM 109 cells, transfected with pT3-5LO, and purification of 5-LO was performed as described previously (Fischer et al., 2003). In brief, cells were lysed by incubation in 50 mM triethanolamine/HCl pH 8.0, 5 mM EDTA, soybean trypsin inhibitor (60 μg ml−1), 1 mM phenylmethylsulfonyl fluoride (PMSF), and lysozyme (500 μg ml−1), homogenized by sonication (3 × 15 s) and centrifuged at 19,000 × g for 15 min. Proteins including 5-LO were precipitated with 50% saturated ammonium sulfate during stirring on ice for 60 min. The precipitate was collected by centrifugation at 16,000 × g for 25 min and the pellet was resuspended in 20 ml PBS containing 1 mM EDTA and 1 mM PMSF. After centrifugation at 100,000 × g for 70 min at 4°C, the 100,000 × g supernatant was applied to an ATP-agarose column (Sigma A2767), and the column was eluted as described previously (Brungs et al., 1995). Partially purified 5-LO was immediately used for in vitro activity assays.
Determination of 5-LO product formation in intact cells
For assays of intact cells, 7.5 × 106. freshly isolated PMNL or 2 × 106 HeLa cells were finally resuspended in 1 ml PGC buffer. After preincubation with the indicated compounds at 37°C, 5-LO product formation was started by the addition of the indicated stimuli plus exogenous AA as indicated. After 10 min at 37°C, the reaction was stopped with 1 ml of methanol and 30 μl of 1 N HCl, 200 ng prostaglandin B1, and 500 μl of PBS were added. Formed 5-LO metabolites were extracted and analyzed by HPLC as described (Werz & Steinhilber, 1996). 5-LO product formation is expressed as ng of 5-LO products per 106 cells that includes LTB4 and its all-trans isomers, 5(S),12(S)-di-hydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid (5(S),12(S)-DiHETE), and 5(S)-hydro(pero)xy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-H(p)ETE). Cysteinyl LTs (LTC4, D4, and E4) were not detected and oxidation products of LTB4 were not determined.
Determination of 5-LO product formation in cell-free systems
For the determination of 5-LO activity in cell homogenates, 7.5 × 106 freshly isolated PMNL were resuspended in PBS containing 1 mM EDTA, sonicated (3 × 10 s) at 4°C, and 1 mM ATP was added. For determination of the activity of recombinant isolated 5-LO, partially purified 5-LO (0.5 μg in 5 μl) was added to 1 ml of a 5-LO reaction mix (PBS, pH 7.4, 1 mM EDTA, 25 μg ml−1 phosphatidylcholine, 1 mM ATP, and 20 μg ml−1 γ-globulin). Samples of either cell homogenates or partially purified 5-LO were supplemented with DTT (1 mM), GSH (1 mM), GPx-1 (30 mU), and CJ-13,610 as indicated. After 5–10 min at 4°C, samples were prewarmed for 30 s at 37°C and 2 mM CaCl2 and AA at the indicated concentrations were added to start 5-LO product formation. The reaction was stopped after 10 min at 37°C by the addition of 1 ml ice-cold methanol and the formed metabolites were analyzed by HPLC as described for intact cells.
Subcellular localization of 5-LO
Subcellular localization of 5-LO was investigated as described previously (Werz et al., 2002a). In brief, freshly isolated PMNL (3 × 107) in 1 ml PGC buffer were incubated at 37°C for 10 min with the indicated stimuli and chilled on ice. Nuclear and non-nuclear fractions were obtained after cell lysis by 0.1% NP-40. Aliquots of these fractions were analyzed for 5-LO protein by SDS–PAGE and immunoblotting using anti-5-LO antiserum (AK7, 1551; affinity purified on a 5-LO column). Proteins were visualized by alkaline phosphatase-conjugated IgGs (Sigma) using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma) as substrates.
Statistics
The program ‘GraphPad PRISM 3.0' was used for statistical comparisons. Statistical evaluation of the data was performed using Student's t-test for unpaired observations. A P-value of <0.05 was considered significant.
Results
CJ-13,610 suppresses 5-LO product formation in intact human PMNL
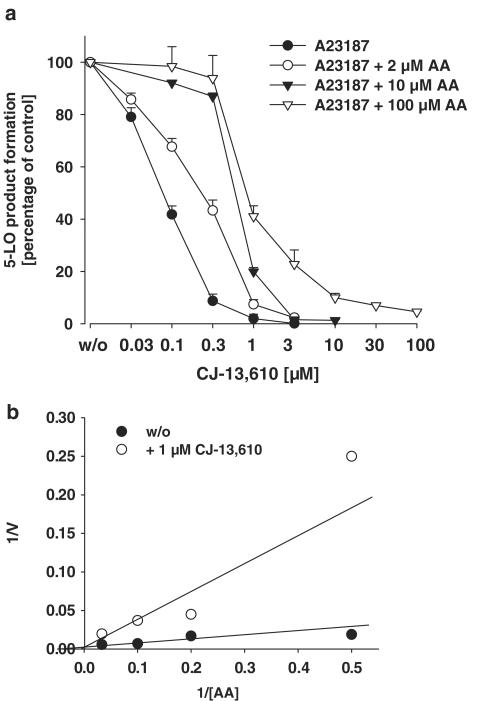
Replacement of the dihydroquinolinone by an imidazolylphenyl of ZM230487 as well as replacement of the ethoxy group of the tetrahydro[2H]pyran by a carboxamide moiety led to a novel series of nonredox-type 5-LO inhibitors (Mano et al., 2003; 2004), exemplified by CJ-13,610 (Figure 1). Freshly isolated human PMNL were preincubated with CJ-13,610 and stimulated with 2.5 μM ionophore A23187 in the absence or presence of exogenous AA. As can be seen in Figure 2a, CJ-13,610 dose dependently suppressed 5-LO product formation in ionophore A23187-stimulated PMNL in the absence of exogenous AA with an IC50 of about 70 nM. Supplementation of AA impaired the efficacy of CJ-13,610 and shifted the IC50 to higher values. Thus, at 2 μM of exogenously added AA, the IC50 was determined as 280 nM and further increase of the substrate concentration (10 or 100 μM) caused further impaired efficacy of CJ-13,610 (IC50 at 100 μM AA was approx. 900 nM). As can be seen from the Lineweaver–Burke plot in Figure 2b, kinetic analysis confirm competitive inhibition of 5-LO by CJ-13,610 in intact PMNL. Moreover, at low AA concentrations, CJ-13,610 completely blocked 5-LO product synthesis, whereas at 100 μM AA, some basal 5-LO activity, even at 100 μM CJ-13,610, still remained. Together, these data indicated that CJ-13,610 is a potent inhibitor of 5-LO product formation in intact cells, apparently acting in a competitive manner.
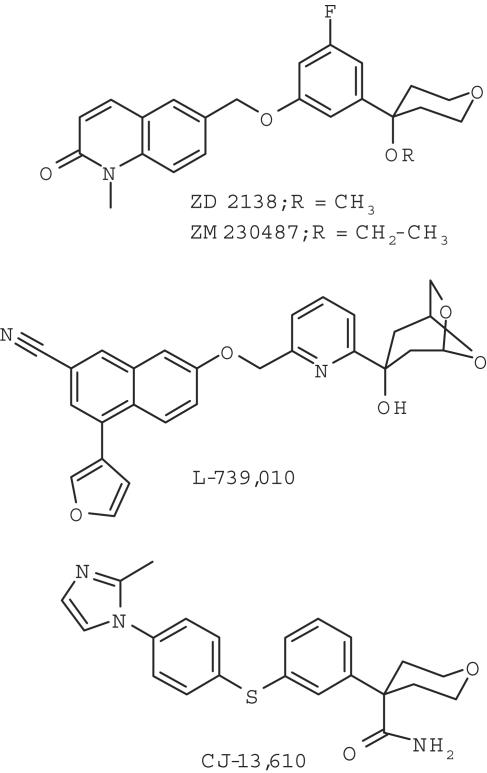
Figure 1.
Chemical structures of nonredox-type 5-LO inhibitors. In comparison to ZD2138 or ZM230487, for CJ-13,610 the dihydroquinolinone pharmacophore was replaced by a imidazolylphenyl moiety, the alkoxy group by a carboxamide group, and the methyl ether was replaced by a sulfide group.
Figure 2.
CJ-13,610 suppresses 5-LO product formation in intact human PMNL. (a) Freshly isolated PMNL (7.5 × 106 in 1 ml PGC buffer) were preincubated with CJ-13,610 at the indicated concentrations for 15 min at 37°C. Cells were stimulated with ionophore A23187 (2.5 μM) with or without AA at the indicated concentrations. After 10 min at 37°C, 5-LO products were extracted and determined by HPLC as described in the Methods section. Results are given as mean± s.e., n=4. (b) Kinetic analysis of 5-LO product inhibition by 1 μM CJ-13,610 is given as Lineweaver–Burke plot. The AA concentrations were 2, 5, 10, and 30 μM.
Inhibition of 5-LO in cell-free systems by CJ-13,610 requires GPx activity
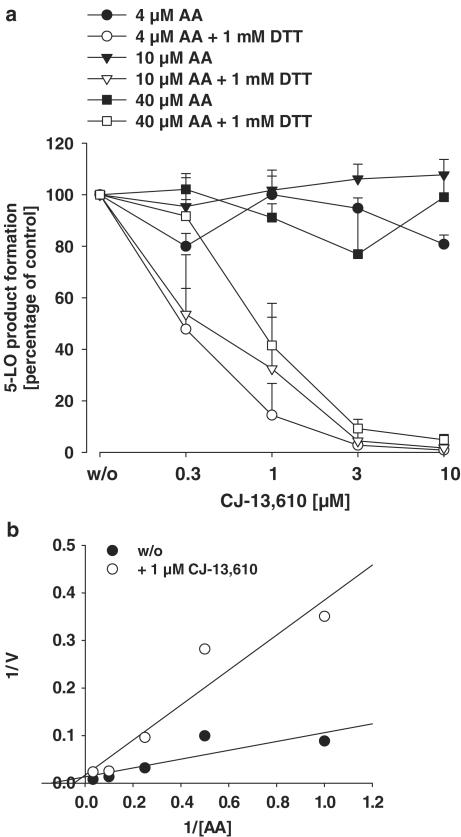
In a previous study, we found that ZM230487 and L739.010 exhibit only low efficacy in cell-free assay systems, but replenishment of peroxidase activity by the addition of GPx-1 plus GSH to purified 5-LO enzyme or by supplementation of thiols (DTT or GSH) to cell homogenates leads to potent 5-LO inhibition (Werz et al., 1997; 1998). CJ-13,610 was assayed for 5-LO inhibition in whole homogenates of human PMNL. As can be see from Figure 3a, CJ-13,610 (up to 30 μM) failed to suppress 5-LO under standard assay conditions, regardless of the AA concentration. However, replenishment of GPx activity by the addition of 1 mM DTT renders CJ-13,610 a potent 5-LO inhibitor. Thus, at 4, 10, and 40 μM AA, similar IC50 values (approx. 280, 320, and 700 nM, respectively) were determined as compared to intact PMNL. Again, as observed in intact cells, elevation of the AA concentration impairs the potency of CJ-13,610 in homogenates, and kinetic analysis (Figure 3b) indicate competitive properties of CJ-13,610.
Figure 3.
Inhibition of 5-LO by CJ-13,610 in whole-cell homogenates of human PMNL. (a) Whole homogenates of human PMNL were prepared as described, preincubated with the indicated concentrations of CJ-13,610 in the presence or absence of 1 mM DTT for 5–10 min on ice. Samples were prewarmed at 37°C for 30 s, then AA (4, 10, or 40 μM) and 2 mM CaCl2 were added. After another 10 min at 37°C, 5-LO activity was determined as described in the Methods section. Results are given as mean± s.e., n=3–4. (b) Kinetic analysis of 5-LO product inhibition by 1 μM CJ-13,610 is given as Lineweaver–Burke plot. The AA concentrations were 1, 2, 4, 10, and 20 μM.
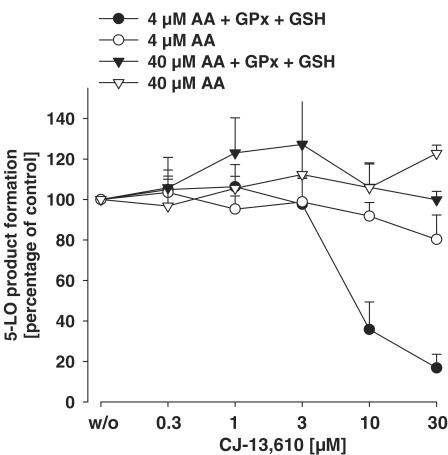
Next, human recombinant 5-LO was expressed in E. coli, partially purified, and CJ-13,610 was tested for enzyme inhibition under reducing (presence of GPx and GSH) and nonreducing conditions. In the absence of GPx, 5-LO activity was not significantly inhibited by CJ-13,610 up to 30 μM (Figure 4). However, inclusion of 30 mU GPx-1 and 1 mM GSH caused 5-LO enzyme inhibition with an IC50=5 μM, when the AA concentration was adjusted to 4 μM. In contrast, at 40 μM AA, addition of GPx-1 and GSH could not confer CJ-13,610 potent 5-LO inhibitory activity. Thus, also under these experimental settings, AA impairs the potency of CJ-13,610 under reducing conditions in cell-free assay systems.
Figure 4.
Inhibition of purified recombinant 5-LO by CJ-13,610. Human recombinant 5-LO was expressed in E. coli and partially purified as described. 5-LO (0.5 μg) was added to a 5-LO reaction mix containing the indicated amounts of CJ-13,610, 1 mM GSH, and 30 mU GPx. After 5–10 min on ice, the samples were prewarmed for 30 s at 37°C and 2 mM CaCl2 and AA (4 or 40 μM) was added. After 10 min at 37°C, formed 5-LO products were extracted and determined by HPLC. Results are given as mean± s.e., n=3.
Exogenous lipid hydroperoxides (LOOH) reduce the efficacy of CJ-13,610 in intact cells
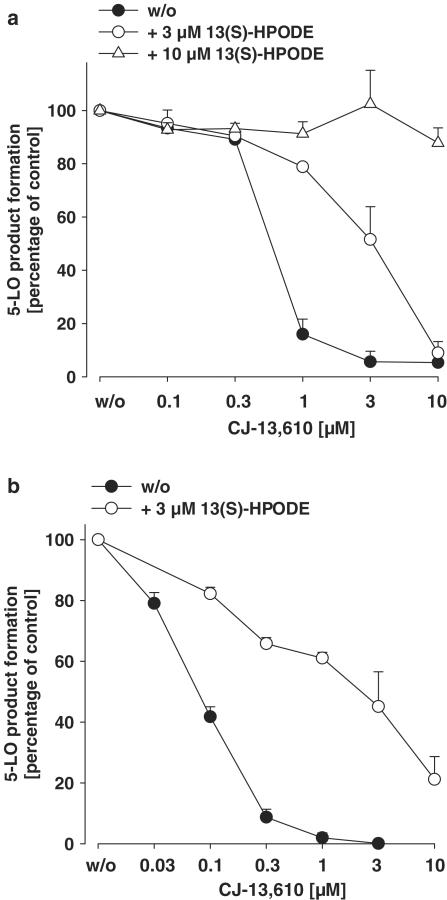
In intact cells, the level of peroxides is controlled by GPx isoenzymes (Ursini et al., 1995). Elevation of the cellular peroxide tone, for example, by exogenous addition of 13(S)-HPODE or diamide, reduced the potency of ZM230487 and L-739.010 (Werz et al., 1998). As shown in Figure 5a, the potency of CJ-13,610 in intact PMNL, stimulated with ionophore A23187 and AA, was considerably impaired when cells had been pretreated with 13(S)-HPODE. This is visualized by a shift of the IC50 value of CJ-13,610 for untreated cells (0.55 μM) to an about six-fold higher value (3.1 μM) in cells exposed to 3 μM 13(S)-HPODE. When the concentration of 13(S)-HPODE was further increased to 10 μM, 5-LO inhibition by CJ-13,610 (up to 10 μM) was completely abolished. Interestingly, in the absence of exogenous AA, 3 μM 13(S)-HPODE led to the same reduced efficacy of CJ-13,610 in PMNL stimulated with ionophore A23187 (IC50=2.8 μM, Figure 5b). This suggests that LOOH are superior to AA in competing with CJ-13,610 and that AA may have no (or at least only modest) additional competitive effects on top of LOOH.
Figure 5.
Elevation of the cellular peroxide tone impairs the potency of CJ-13,610 in intact PMNL. Freshly isolated PMNL (7.5 × 106 in 1 ml PGC buffer) were preincubated with CJ-13,610 at the indicated concentrations at 37°C. After 15 min, 13(S)-HPODE was added as indicated and cells were subsequently stimulated with (a) 2.5 μM ionophore A23187 plus 10 μM AA or with (b) 2.5 μM ionophore A23187 alone. After 10 min at 37°C, 5-LO products were extracted and determined by HPLC. Results are given as mean± s.e., n=3.
Efficacy of CJ-13,610 in intact cells is stimulus independent
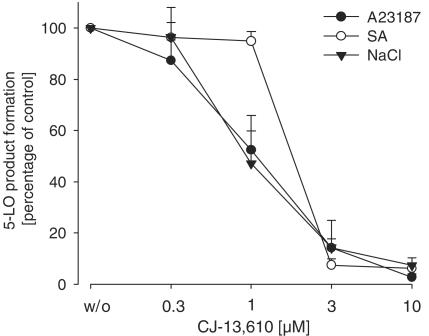
Recently, we could show that the efficacy of ZM230487 and L-739.010 is stimulus dependent and that phosphorylation events of 5-LO may impair the sensitivity of the enzyme against these inhibitors (Fischer et al., 2003). As shown in Figure 6, the potency of CJ-13,610 in PMNL stimulated with 10 μM SA or 300 mM NaCl, which activate 5-LO by phosphorylation events in a Ca2+-independent manner, is comparable to that obtained in cells challenged by ionophore A23187. Cell stress alone (in contrast to ionophore) is not capable of providing sufficient amounts of free AA as 5-LO substrate in intact cells. Therefore, 20 μM AA was included in all experiments in order to ensure equal substrate availability. The results demonstrate that the efficacy of CJ-13,610 is independent of the stimulus used to evoke 5-LO activation and product synthesis, suggesting that 5-LO phosphorylation events have no major impact on the interference of the compound with the enzyme.
Figure 6.
Cell stress does not affect the efficacy of CJ-13,610 in intact cells. Freshly isolated PMNL (7.5 × 106 in 1 ml PGC buffer) were preincubated with CJ-13,610 at the indicated concentrations for 15 min at 37°C. SA (10 μM) and NaCl (300 mM) were added 3 min prior to the addition of 20 μM AA, ionophore A23187 (2.5 μM) was added together with 20 μM AA. After 10 min at 37°C, 5-LO products were extracted and determined by HPLC. Results are given as mean± s.e., n=3.
CJ-13,610 does not inhibit translocation of 5-LO to the nuclear membrane
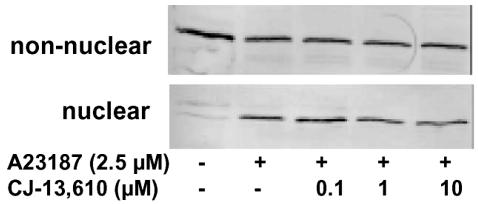
Activation of 5-LO in the cell is accompanied by a rapid translocation of the enzyme to the nuclear membrane, which may stimulate 5-LO product synthesis (Peters-Golden & Brock, 2001). We tested if CJ-13,610 could interfere with the 5-LO translocation process. 5-LO protein was assessed in the nuclear and non-nuclear fraction after subcellular fractionation of PMNL. As shown in Figure 7, in resting PMNL, 5-LO was exclusively in the non-nuclear fraction, whereas the addition of A23187 caused enrichment of 5-LO in the nuclear fraction and a concomitant loss in the non-nuclear fraction. Preincubation of PMNL with CJ-13,610 did not suppress this A23187-induced 5-LO translocation.
Figure 7.
Effects of CJ-13,610 on nuclear 5-LO translocation in intact PMNL. Freshly isolated PMNL (3 × 107 in 1 ml PGC buffer) were preincubated with CJ-13,610 at the indicated concentrations for 15 min at 37°C. Then, 2.5 μM A23187 was added to the samples and incubated for another 5 min at 37°C. 5-LO was detected in nuclear and non-nuclear fractions by immunoblotting after subcellular fractionation. Similar results were obtained in two additional independent experiments.
Effects of CJ-13,610 on 5-LO product synthesis in transfected HeLa cells
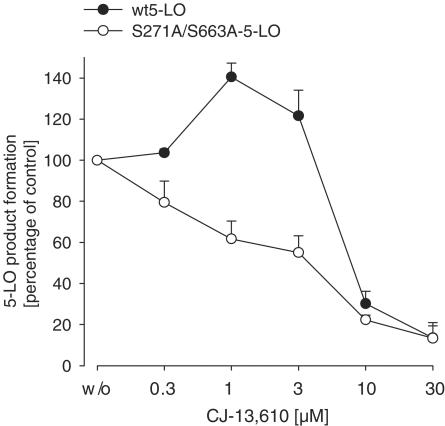
In order to confirm the hypothesis that 5-LO inhibition by CJ-13,610 does not depend on the 5-LO phosphorylation status, HeLa cells were transiently transfected with phosphorylatable wild-type 5-LO (WT-5-LO) or with nonphosphorylatable mutant S271A/S663A-5-LO (lacking the phosphorylation sites for MKs and extracellular signal-regulated kinase (ERKs), respectively), preincubated with CJ-13,610, stimulated with 20 μM AA (5-LO activation conditions that are based on enzyme phosphorylation) and 5-LO product formation was determined. In comparison to intact PMNL, we found significant differences in HeLa cells with respect to 5-LO inhibition by CJ-13,610 (Figure 8). Thus, at low concentrations (1 and 3 μM), CJ-13,610 significantly increased (up to 1.4-fold) product synthesis of WT-5-LO, and the IC50 value of CJ-13,610 was about 10-fold higher in HeLa (approx. 7 μM) as compared to PMNL. Phosphorylation-mediated 5-LO product formation (of WT-5-LO) required about two-fold higher inhibitor concentrations for efficient inhibition as compared to 5-LO product formation by S271A/S663A-5-LO. Notably, the nonphosphorylatable enzyme did not exhibit increased 5-LO product synthesis at low inhibitor concentrations.
Figure 8.
Effects of CJ-13,610 on 5-LO product formation in HeLa cells transformed with WT-5-LO and S271A/S663A-5-LO. HeLa cells were transiently transformed with plasmids pcDNA3.1-5LO or pcDNA3.1-5LO-S271A-S663A (10 μg). Cells (2 × 106) were resuspended in 1 ml PGC buffer and CJ-13,610 was added at the indicated concentrations. After 15 min at 37°C, cells were stimulated with 20 μM AA for another 10 min. 5-LO products were extracted and determined by HPLC. Results are given as mean± s.e., n=4.
Discussion
In the present study, we have evaluated the molecular pharmacology of the novel nonredox-type 5-LO inhibitor CJ-13,610 using various in vitro test systems, relevant for the in vivo pharmacology. In analogy to ZM230487 or L-739.010, two structurally related nonredox-type 5-LO inhibitors, CJ-13,610 potently suppressed 5-LO product formation in intact cells as well as in cell-free assay systems under reducing conditions, whereas an elevated peroxide tone strongly impaired the efficacy of CJ-13,610. However, in contrast to ZM230487 and L-739.010, CJ-13,610 shows competitive kinetics with respect to AA also under reducing conditions, and the efficacy of CJ-13,610 is cell stimulus independent and less affected by the phosphorylation status of 5-LO. Therefore, the pharmacological profile of CJ-13,610 is in part distinct from that of other nonredox 5-LO inhibitors and may possess therapeutic potential as an orally active 5-LO inhibitor, lacking certain disadvantages of former representatives of this inhibitor class. Recently, the efficacy, safety, and tolerability of 6 weeks treatment by oral dosing with CJ-13,610 in adults with chronic obstructive pulmonary disease was evaluated in a phase II multicenter, randomized, double-blind placebo-controlled parallel group study.
Nonredox-type 5-LO inhibitors have been initially designed as active site-directed inhibitors devoid of redox and iron ligand properties that compete with AA at a (hypothetical) substrate binding cleft of 5-LO (McMillan & Walker, 1992). In fact, biochemical characterization of ZM230487 and L-739.010 revealed evidence for competitive kinetics with low affinity under nonreducing conditions, whereas efficient 5-LO inhibition under reducing conditions showed noncompetitive kinetics (Werz et al., 1998). It was found that LOOH or an elevated peroxide tone strongly counteract the high affinity of nonredox 5-LO inhibitors and efficient inhibition of 5-LO product formation required low LOOH levels (Werz et al., 1998). Nevertheless, the precise mode of action and the binding site(s) of nonredox 5-LO inhibitors have not been elucidated yet.
In order to initialize 5-LO catalysis, LOOH are important for the conversion of the active site iron from the ferrous to the ferric state (Rouzer & Samuelsson, 1986; Hammarberg et al., 2001). However, no discrete locale where LOOH bind to 5-LO has been identified. In cell-free systems, CJ-13,610 up to the highest concentration (30 μM) tested was unable to suppress 5-LO, unless peroxide levels were decreased by replenishment of GPx activity. Moreover, in intact PMNL, elevation of the cellular peroxide tone by exogenous addition of 13(S)-HPODE abolished the inhibitory effects of CJ-13,610. Thus, in analogy to ZM230487 or L-739.010 (Werz et al., 1998), also CJ-13,610 requires a low peroxide tone for efficient 5-LO inhibition. Such a pattern is unique for nonredox-type inhibitors since the potencies of iron-ligand 5-LO inhibitors (BWA4C) or 5-lipoxygenase-activating protein (FLAP) inhibitors (MK886) are not affected by the peroxide tone (Werz et al., 1998). Apparently, both CJ-13,160 as well as ZM230487 and L-739.010 compete with activating LOOH at a putative regulatory LOOH-binding site with high affinity, thereby preventing 5-LO catalysis.
Experimental data indicate that 5-LO has two distinct fatty acid-binding sites, one regulatory that may bind LOOH and one at the catalytic site where transformation of AA takes place (Sailer et al., 1998; Burkert et al., 2003). In contrast to ZM230487, the potency of CJ-13,610 under reducing conditions is impaired by AA, suggesting that CJ-13,610 may not only potently interfere with the regulatory LOOH- but also with the arachidonate(substrate)-binding cleft at the active site. Interference of CJ-13,610 at such a substrate-binding cleft could be visualized in this study by different experimental settings: First, in intact PMNL, elevated AA concentrations clearly impaired the efficacy of CJ-13,610; second, under cell-free assay conditions elevated AA concentrations significantly decreased inhibition of crude 5-LO in homogenates supplemented with DTT; and third, inhibition of partially purified 5-LO in the presence of GPx and GSH was apparent at 4 μM AA, but not at 40 μM AA. This pattern is in sharp contrast to ZM230487 and L-739.010, whose high affinities under reducing conditions were not altered by variation of the AA concentration, although competitive kinetics with low affinities were evident under nonreducing conditions (Werz et al., 1997; 1998). Of interest, LOOH are superior to AA in competing with CJ-13,610, and AA may have no additional competitive effects on top of LOOH. Thus, the reduction in the efficacy of CJ-13,610 by 3 μM 13(S)-HPODE was quantitatively almost the same in PMNL stimulated in the absence or in the presence of AA. Together, these findings imply that under certain (more chronic) inflammatory situations, which are associated with elevated cellular levels of LOOH and free AA, a possibly reduced efficacy of CJ-13,610 should be taken into account. In this regard, it was shown that ZD2138 failed to protect against allergen-induced asthmatic responses in asthmatic subjects (Nasser et al., 1994), and did not prevent pulmonary inflammation or the development of airway hyper-responsiveness (Turner et al., 1996).
It should be noted that the effects of CJ-13,610 determined in HeLa cells differed from that obtained with PMNL. For example, in HeLa cells, CJ-13,610 significantly increased 5-LO product synthesis at low (<3 μM) concentrations, a phenomenon that cannot be readily explained. Compared to PMNL, the IC50 value was about 10-fold higher in HeLa cells at same substrate concentrations (20 μM AA). The reason for this difference is also not clear. Presumably, the machinery necessary for 5-LO product formation and the existence of regulating cofactors (e.g. FLAP, LOOH and Ca2+ levels, GPx activity) are different in HeLa cells. For example, it is conceivable that CJ-13,610 could interfere with the fatty acid-binding FLAP, necessary for efficient 5-LO product synthesis in intact cells. However, in experiments with HeLa cells (not expressing FLAP (own unpublished observations)), transiently transfected with FLAP, CJ-13,610 was equipotent as compared to MOCK-transfected cells (not shown). Therefore, FLAP should not affect the potency of CJ-13,610, supported by the finding that CJ-13,610 did also not interfere with 5-LO translocation to the nucleus in PMNL, which may be FLAP dependent (Peters-Golden & Brock, 2001). Nevertheless, the potency of CJ-13,610 may depend on the cellular environment, that is, the presence of certain 5-LO regulatory mechanisms or cofactors, which remain to be identified.
Activation of cellular 5-LO in response to external stimuli involves 5-LO translocation from a soluble locale to the nuclear membrane where the enzyme colocalizes with FLAP (Peters-Golden & Brock, 2001), and is mediated by elevation of the intracellular Ca2+ levels and/or phosphorylation of 5-LO by mitogen-activated protein kinase-activated protein kinase (MAPKAP) kinases at Ser-271 and by ERKs 1/2 at Ser-663 (Werz et al., 2000; 2002a, 2002b). It was shown that the activation of 5-LO by stimuli that induce phosphorylation of 5-LO is Ca2+-independent in certain cell types (Werz et al., 2002a; Burkert et al., 2003). The efficacies of the nonredox-type 5-LO inhibitors ZM230487 and L-739.010 depend on the stimulus and activation pathway utilized to induce 5-LO product synthesis (Fischer et al., 2003). Whereas Ca2+-mediated 5-LO activation in PMNL is efficiently suppressed by ZM230487 and L-739.010, 10- to 100-fold higher inhibitor concentrations are required to suppress 5-LO product synthesis induced by phosphorylation (Fischer et al., 2003). In contrast, the efficacy of CJ-13,610 in intact PMNL is not reduced when 5-LO is activated by phosphorylation events. Also, CJ-13,610 exhibited no pronounced difference in the potencies towards phosphorylatable wt- or nonphosphorylatable mutated S217A/S663A-5-LO in HeLa cells. Notably, several diseases related or connected to elevated levels of 5-LO products such as inflammatory reactions, allergic asthma, various types of cancer, and atherosclerosis are associated with an increased phosphorylation status of the cell (Hajjar & Pomerantz, 1992; Johnson & Lapadat, 2002), which determine the susceptibility of 5-LO towards nonredox inhibitors. Owing to the fact that the efficacy of CJ-13,610 does not depend on the 5-LO phosphorylation status, targeting of 5-LO by such a drug could indeed be beneficial for the therapy of these diseases. Although the therapeutic potential of CJ-13,610 may be somewhat limited by reduced efficacy due to an elevated peroxide tone, the compound obviously possesses advantages over former representatives of this class of 5-LO inhibitors that could not attain regular approval due to poor clinical benefits.
Acknowledgments
We thank Astrid Neuß for expert technical assistance and Dr Patrick Provost and David Dishart for providing the plasmid pcDNA3.1-5LO and pcDNA3.1-5LO-S271A-S663A, respectively. This study was supported by grants from the Fonds der Chemischen Industrie, the EU (LEUCHRON, QLRT-2000-01521), and the Deutsche Pharmazeutische Gesellschaft.
Abbreviations
- AA
arachidonic acid
- ERK
extracellular signal-regulated kinase
- FLAP
5-lipoxygenase-activating protein
- GPx
glutathione peroxidase
- GSH
glutathione
- LO
lipoxygenase
- LOOH
lipid hydroperoxides
- LT
leukotriene
- MAPK
mitogen-activated protein kinase
- MK, MAPKAPK
mitogen-activated protein kinase-activated protein kinase
- PBS
phosphate-buffered saline pH 7.4
- PG buffer
PBS containing 1 mg ml−1 glucose and 1 mM CaCl2
- PMNL
polymorphonuclear leukocytes
- SA
sodium arsenite
References
- ALANKO J., SIEVI E., LAHTEENMAKI T., MUCHA I., VAPAATALO H., PARANTAINEN J. Catechol estrogens as inhibitors of leukotriene synthesis. Biochem. Pharmacol. 1998;55:101–104. doi: 10.1016/s0006-2952(97)00398-5. [DOI] [PubMed] [Google Scholar]
- BRUNGS M., RÅDMARK O., SAMUELSSON B., STEINHILBER D. Sequential induction of 5-lipoxygenase gene expression and activity in Mono Mac 6 cells by transforming growth factor-beta and 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U.S.A. 1995;92:107–111. doi: 10.1073/pnas.92.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKERT E., SZELLAS D., RADMARK O., STEINHILBER D., WERZ O. Cell type-dependent activation of 5-lipoxygenase by arachidonic acid. J. Leukocyte Biol. 2003;73:191–200. doi: 10.1189/jlb.0702354. [DOI] [PubMed] [Google Scholar]
- CHEN X.S., SHELLER J.R., JOHNSON E.N., FUNK C.D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- CLAESSON H.E., DAHLEN S.E. Asthma and leukotrienes: antileukotrienes as novel anti-asthmatic drugs. J. Intern. Med. 1999;245:205–227. doi: 10.1046/j.1365-2796.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- DING X.Z., IVERSEN P., CLUCK M.W., KNEZETIC J.A., ADRIAN T.E. Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 1999;261:218–223. doi: 10.1006/bbrc.1999.1012. [DOI] [PubMed] [Google Scholar]
- DWYER J.H., ALLAYEE H., DWYER K.M., FAN J., WU H., MAR R., LUSIS A.J., MEHRABIAN M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N. Engl. J. Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- FISCHER L., SZELLAS D., RÅDMARK O., STEINHILBER D., AND WERZ O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003;17:949–951. doi: 10.1096/fj.02-0815fje. [DOI] [PubMed] [Google Scholar]
- FUNK C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- GARCIA C., BOYCE B.F., GILLES J., DALLAS M., QIAO M., MUNDY G.R., BONEWALD L.F. Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J. Bone Miner Res. 1996;11:1619–1627. doi: 10.1002/jbmr.5650111105. [DOI] [PubMed] [Google Scholar]
- GHOSH J., MYERS C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJJAR D.P., POMERANTZ K.B. Signal transduction in atherosclerosis: integration of cytokines and the eicosanoid network. FASEB J. 1992;6:2933–2941. doi: 10.1096/fasebj.6.11.1644257. [DOI] [PubMed] [Google Scholar]
- HAMMARBERG T., KUPRIN S., RADMARK O., HOLMGREN A. Epr investigation of the active site of recombinant human 5-lipoxygenase: inhibition by selenide. Biochemistry. 2001;40:6371–6378. doi: 10.1021/bi001595d. [DOI] [PubMed] [Google Scholar]
- JOHNSON G.L., LAPADAT R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- MANCUSO P., STANDIFORD T.J., MARSHALL T., PETERS-GOLDEN M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANO T., OKUMURA Y., SAKAKIBARA M., OKUMURA T., TAMURA T., MIYAMOTO K., STEVENS R.W. 4-[5-Fluoro-3-[4-(2-methyl-1H-imidazol-1-yl)benzyloxy]phenyl]-3,4,5,6-tetrahydro-2H-pyran-4-carboxamide, an orally active inhibitor of 5-lipoxygenase with improved pharmacokinetic and toxicology characteristics. J. Med. Chem. 2004;47:720–725. doi: 10.1021/jm0303554. [DOI] [PubMed] [Google Scholar]
- MANO T., STEVENS R.W., ANDO K., NAKAO K., OKUMURA Y., SAKAKIBARA M., OKUMURA T., TAMURA T., MIYAMOTO K. Novel imidazole compounds as a new series of potent, orally active inhibitors of 5-lipoxygenase. Bioorg. Med. Chem. 2003;11:3879–3887. doi: 10.1016/s0968-0896(03)00436-x. [DOI] [PubMed] [Google Scholar]
- MCMILLAN R.M., WALKER E.R.H. Designing therapeutically effective 5-lipoxygenase inhibitors. Trends Pharmacol. Sci. 1992;13:323–330. doi: 10.1016/0165-6147(92)90100-k. [DOI] [PubMed] [Google Scholar]
- MEHRABIAN M., ALLAYEE H. 5-Lipoxygenase and atherosclerosis. Curr. Opin. Lipidol. 2003;14:447–457. doi: 10.1097/00041433-200310000-00005. [DOI] [PubMed] [Google Scholar]
- NASSER S.M., BELL G.S., HAWKSWORTH R.J., SPRUCE K.E., MACMILLAN R., WILLIAMS A.J., LEE T.H., ARM J.P. Effect of the 5-lipoxygenase inhibitor ZD2138 on allergen-induced early and late asthmatic responses. Thorax. 1994;49:743–748. doi: 10.1136/thx.49.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS-GOLDEN M., BROCK T.G. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487:323–326. doi: 10.1016/s0014-5793(00)02374-7. [DOI] [PubMed] [Google Scholar]
- PROVOST P., DOUCET J., HAMMARBERG T., GERISCH G., SAMUELSSON B., RÅDMARK O. 5-Lipoxygenase interacts with coactosin-like protein. J. Biol. Chem. 2001;276:16520–16527. doi: 10.1074/jbc.M011205200. [DOI] [PubMed] [Google Scholar]
- RÅDMARK O. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2002;68–69:211–234. doi: 10.1016/s0090-6980(02)00032-1. [DOI] [PubMed] [Google Scholar]
- ROMANO M., CLARIA J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J. 2003;17:1986–1995. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- ROUZER C.A., SAMUELSSON B. The importance of hydroperoxide activation for the detection and assay of mammalian 5-lipoxygenase. FEBS Lett. 1986;204:293–296. doi: 10.1016/0014-5793(86)80831-6. [DOI] [PubMed] [Google Scholar]
- SAILER E.R., SCHWEIZER S., BODEN S.E., AMMON H.P.T., SAFAYHI H. Characterization of an acetyl-11-keto-beta-boswellic acid and arachidonate-binding regulatory site of 5-lipoxygenase using photoaffinity labeling. Eur. J. Biochem. 1998;256:364–368. doi: 10.1046/j.1432-1327.1998.2560364.x. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B., DAHLÉN S.-E., LINDGREN J.-Å., ROUZER C.A., SERHAN C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- SMITH W.G., SHAFFER A.F., CURRIE J.L., THOMPSON J.M., KIM S., RAO T., ISAKSON P.C. Characterization of 5-lipoxygenase inhibitors in biochemical and functional in vivo assays. J. Pharmacol. Exp. Ther. 1995;275:1332–1338. [PubMed] [Google Scholar]
- STEINHILBER D. 5-lipoxygenase: a target for antiinflammatory drugs revisited. Curr. Med. Chem. 1999;6:69–83. [PubMed] [Google Scholar]
- TURNER C.R., SMITH W.B., ANDRESEN C.J., EGGLER J.F., WATSON J.W. The effect of 5-lipoxygenase inhibition on Ascaris antigen (Ag)-induced responses in atopic monkeys. Inflamm. Res. 1996;45:42–49. doi: 10.1007/BF02263504. [DOI] [PubMed] [Google Scholar]
- URSINI F., MAIORINO M., BRIGELIUSFLOHE R., AUMANN K.D., ROVERI A., SCHOMBURG D., FLOHE L. Diversity of glutathione peroxidases. Biothiols, Pt B. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- WERZ O., BUERKERT E., SAMUELSSON B., RÅDMARK O., STEINHILBER D. Activation of 5-lipoxygenase by cell stress is calcium-independent in human polymorphonuclear leukocytes. Blood. 2002a;99:1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- WERZ O., BURKERT E., FISCHER L., SZELLAS D., DISHART D., SAMUELSSON B., RADMARK O., STEINHILBER D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002b;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- WERZ O., KLEMM J., SAMUELSSON B., RADMARK O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERZ O., SCHNEIDER N., BRUNGS M., SAILER E.R., SAFAYHI H., AMMON H.P.T., STEINHILBER D. A test system for leukotriene synthesis inhibitors based on the in-vitro differentiation of the human leukemic cell lines HL-60 and Mono Mac 6. Naunyn Schmied. Arch. Pharmacol. 1997;356:441–445. doi: 10.1007/pl00005074. [DOI] [PubMed] [Google Scholar]
- WERZ O., STEINHILBER D. Selenium-dependent peroxidases suppress 5-lipoxygenase activity in B-lymphocytes and immature myeloid cells – the presence of peroxidase-insensitive 5-lipoxygenase activity in differentiated myeloid cells. Eur. J. Biochem. 1996;242:90–97. doi: 10.1111/j.1432-1033.1996.0090r.x. [DOI] [PubMed] [Google Scholar]
- WERZ O., SZELLAS D., HENSELER M., STEINHILBER D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol. Pharmacol. 1998;54:445–451. doi: 10.1124/mol.54.2.445. [DOI] [PubMed] [Google Scholar]