Abstract
In an effort to identify endogenous, native mammalian urotensin-II (U-II) receptors (UT), a diverse range of human, primate and rodent cell lines (49 in total) were screened for the presence of detectable [125I]hU-II binding sites.
UT mRNA (Northern blot, PCR) and protein (immunocytochemistry) were evident in human skeletal muscle tissue and cells.
[125I]hU-II bound to a homogenous population of high-affinity, saturable (Kd 67.0±11.8 pM, Bmax 9687±843 sites cell−1) receptors in the skeletal muscle (rhabdomyosarcoma) cell line SJRH30. Radiolabel was characteristically slow to dissociate (⩽15% dissociation 90 min). A lower density of high-affinity U-II binding sites was also evident in the rhabdomyosarcoma cell line TE671 (1667±165 sites cell−1, Kd 74±8 pM).
Consistent with the profile recorded in human recombinant UT-HEK293 cells, [125I]hU-II binding to SJRH30 cells was selectively displaced by both mammalian and fish U-II isopeptides (Kis 0.5±0.1–1.2±0.3 nM) and related analogues (hU-II[4-11]>[Cys5,10]Acm hU-II; Kis 0.4±0.1 and 864±193 nM, respectively).
U-II receptor activation was functionally coupled to phospholipase C-mediated [Ca2+]i mobilization (EC50 6.9±2.2 nM) in SJRH30 cells.
The present study is the first to identify the presence of ‘endogenous' U-II receptors in SJRH30 and TE671 cells. SJRH30 cells, in particular, might prove to be of utility for (a) investigating the pharmacological properties of hU-II and related small molecule antagonists at native human UT and (b) delineating the role of this neuropeptide in the (patho)physiological regulation of mammalian neuromuscular function.
Keywords: Urotensin-II, skeletal muscle, G-protein-coupled receptor, GPR14, UT, rhabdomyosarcoma
Introduction
Human urotensin-II (U-II), a vasoactive cyclic undecapeptide, is the endogenous ligand for the human G-protein-coupled receptor UT (formerly termed GPR14, SENR; Ames et al., 1999; Douglas & Ohlstein, 2000). Interestingly, although the U-II/UT system is purported to influence a diverse range of organ systems (cardiorenal, pulmonary, endocrine, CNS, etc.; Douglas & Ohlstein, 2001), the ability to study the detailed pharmacological interactions between U-II ligands (including UT antagonists) and the ‘native' human receptor has been limited by the lack of suitable model cellular systems. To date, no ‘endogenous' U-II binding sites have been described in a simple, cell-based system. In an attempt to address this issue, the present study describes the screening of a large and diverse collection of primate and rodent cell lines (49 in total) culminating in the identification of endogenous, functional human UT (coupled to [Ca2+]i mobilization) in the human ‘striated muscle' cell lines SJRH30 and TE671. The characterization of ‘endogenous' UT in these cell lines might facilitate a greater understanding of the biochemical and pharmacological characteristics of U-II (and related antagonists) at its cognate, endogenous receptor and, ultimately, might also assist in the elucidation of the physiological role of this novel human neuropeptide in the control of mammalian musculoskeletal function.
Methods
Northern blot analysis of human UT expression in major human organs
Tissue distribution of hUT mRNA was assessed using Clontech (Palo Alto, CA, U.S.A.) Northern blot membranes. Blots were pretreated with denatured ssDNA (2 h, 42°C) and hybridized with denatured full-length hUT cDNA probe (labeled with α[32P]dCTP using standard T7 DNA polymerase/(dN)9-random primers; Pharmacia Biotech, Piscataway, NJ, U.S.A.) overnight at 42°C in 50% deionized formamide, 1.5 M NaCl, 1% SDS and 10% dextran sulphate. Membranes were washed under low stringency (3 × 15 min washes in 1XSSC, 0.1% SDS at 25°C), followed by a 30 min high-stringency wash (0.1XSSC, 0.1% SDS at 55°C). Hybridization was detected using conventional autoradiography (Hyperfilm, Amersham Life Science, U.K.). Once stripped, membranes were probed with human β-actin cDNA as a positive control.
Human UT expression in human cell lines by polymerase chain reaction (PCR)
RT–PCR was performed using DNase-I-treated total RNA (2 μg) isolated from selected human cell lines. cDNA samples were subjected to PCR amplification (33 cycles: 94°C, 30 s; 60°C, 30 s; 72°C, 30 s) in standard buffer containing 0.2 μM hUT primers (5′-GCA ACC CTC AAC AGC TCC TG-3′ and 5′-AAG TCC AGG CCG AAG AGC AC-3′). All cDNA samples produced the predicted amplicon using human GAPDH primers. Amplification fidelity was confirmed by the observation that (a) no amplicon was detected if either Taq polymerase or reverse transcriptase were omitted from the RT–PCR reaction and (b) subsequently by Southern blot analysis. Briefly, upon completion of cDNA electrophoresis, agarose gels were denatured in 0.5 M NaOH/1.5 M NaCl (1 h). Following neutralization (0.5 mM Tris–HCl/1.5 M NaCl [pH 7.5]), PCR amplicons were transferred to GeneScreen Hybridization membranes (NEN Life Science, Boston, MA, U.S.A.) in 10XSSC and, after UV crosslinking, membranes were hybridized (2 h, 42°C) with 1 × 106 c.p.m. ml−1 hUT cDNA probe.
Selection of mammalian cell lines for native urotensin-II binding site screening
A selection of cells, purchased either from ATCC (Manassas, VA, U.S.A.) or the Clonetics Corporation (Walkersville, MD, U.S.A.) and cultured in strict accordance with the supplier's recommendations, were screened for the presence of native U-II binding sites.
Skeletal muscle cell lines
Based on the tissue distribution of human UT mRNA expression determined previously (where transcript encoding the UT was readily detected in human skeletal muscle; Tal et al., 1995; Ames et al., 1999; Liu et al., 1999; Maguire et al., 2000), [125I]hU-II binding was assessed in five cell lines of skeletal muscle origin, namely hSKMC (primary cells) and rat L6 myoblast cells and the human rhabdomyosarcomas cell lines SJRH30, A204 and TE671 (subline 2).
Non-striated sarcoma cell lines
Since [125I]hU-II binding was detected in two rhabdomyosarcoma cell lines (see Section 3), binding was evaluated in ‘non-striated muscle' human sarcomas using vulva leiomyo- (SKLMS-1), osteo- (MG-63), chondro- (SW-1353), synovial (SW-982), fibro- (HT-1080) and lipo- (SW-872) sarcoma cell lines.
Cardiovascular cell lines
U-II exerts pronounced effects in cardiovascular tissue (vasoconstriction/dilation, cardiac inotropy, hypertrophy; Bottrill et al., 2000; Douglas et al., 2000; Stirrat et al., 2001; Russell et al., 2001; Sauzeau et al., 2001; Watanabe et al., 2002; Tzanidis et al., 2003). Thus, [125I]hU-II binding was profiled in human coronary artery (hCoASMC, primary cells) and human (T/G HA-SMC) and rat (SV40LT-SMC, A-10, A7r5) aortic smooth muscle cells, human brain microvascular endothelial cells (hBMVEC, primary cells) and rat embryonic cardiomyocytes (H9c2 [2-1]).
Endocrine cell lines
U-II regulates glucose-induced insulin release from the rat isolated pancreas (Silvestre et al., 2001) and influences catecholamine, cortisol, prolactin and fatty acid synthesis/release in lower vertebrates (Bern et al., 1985; Rivas et al., 1986; Sheridan & Bern, 1986; Conlon et al., 1997). As such, binding was determined in cells of endocrine origin including those derived from rat and human pancreas (BxPC-3 and RIN-m) and those from adrenal (PC-12 cells), thyroid (TT, SW579 and 6–23 cells) and pituitary (MMQ, GH1 and GH3 cells) gland.
Gastrointestinal cells
U-II is a spasmogen of isolated intestinal smooth muscle and modulates transepithelial [Na+]/[Cl−] ion transport and neurotransmission in the gut (Bern et al., 1985; Loretz et al., 1985; Conlon et al., 1996; 1997; Horie et al., 2003). To this end, epithelial cells from the stomach (NCI-SNU-16 and AGS), small intestine (IEC-6, Fhs 74 Int cells) and colon (CaCo-2 and SW-480) were assessed for [125I]hU-II binding.
Neuronal cells
Reverse-phase HPLC reveals the presence of U-II in porcine spinal cord, bovine hypothalamus and non-human primate brain (Mori et al., 1999; Nothacker et al., 1999). Accordingly, UT mRNA is clearly expressed within specific anatomical regions of the mammalian CNS (Tal et al., 1995; Liu et al., 1999; Gartlon et al., 2001). As such, [125I]hU-II binding was assessed in several cell lines of neuronal origin (HCN-1A, D341 Med, NT-2 and Sk-N-MC cells).
Pulmonary cells
Although Tal et al. (1995) reported a lack of rat UT expression in lung (epithelia), U-II influences pulmonary/respiratory function by regulating contractile tone in vitro and in vivo (Douglas et al., 2000; Hay et al., 2000; de Garavilla et al., 2001; Stirrat et al., 2001). Since UT mRNA expression has been reported in human epithelial cells of both vascular (i.e. venous and arterial vascular endothelium; Ames et al., 1999) and non-vascular (sensory [retinal and olfactory]; Tal et al., 1995) origin, binding was profiled in human lung epithelia cell lines (DMS-79 and NCI-H727).
Hepatic cells
The presence of putative [125I]hU-II radioligand binding sites was investigated in HepG2 human hepatoblastoma cells since UT mRNA has been observed previously in primate liver (Elshourbagy et al., 2002).
Leukocytes
U-II ligand has been recorded in human atheroma (Ames et al., 1999). As such, binding was investigated in several human leukocytic cell lines including Raji (B-cells), TPA/PMA-differentiated THP-1 (monocytic/macrophage lineage) and K-562 (a multipotent/hematopoietic chronic myologenous leukemia line) cells.
Sensory epithelia
Rat UT is expressed in sensory epithelia (olfactory tissue and retina; Tal et al., 1995). Consequently, U-II binding has been assessed in several cell lines of retinal origin (epithelia/retinoblastoma; WERI-RB-1, Y-79, HCE-1, RF/6A and RPE-J cells).
Screening for specific, high-affinity [125I]human urotensin-II radioligand binding sites
Preliminary binding was performed in a crude ‘screening' mode in intact cells (adherent cells and cell suspensions) using ([125I]Tyr9)hU-II (2000 Ci mmol−1; Amersham, Arlington Heights, IL, U.S.A.). Confluent cells were washed with DPBS+ (Dulbecco's phosphate-buffered saline, pH 7.4, with 10 mM MgCl2, 0.7 mM CaCl2, 1.4 mM glucose and 0.2% BSA) and incubated with 150 pM [125I]hU-II (1 ml DPBS+, 37°C, 30 min in triplicate). After incubation, cells were washed with cold DPBS+ (4 × 1 ml), solubilized with 1 ml NaOH (1 M) and transferred to 12 × 75 mm glass tubes. Radioactivity was measured with a Packard Cobra γ-counter (the limit of detection for [125I]hU-II binding was estimated to be ⩾500 sites cell−1). Non-specific binding was defined using 1 μM cold hU-II. Any intact cell line that exhibited appreciable specific binding under these conditions was subjected to secondary, detailed analysis. The explicit aim of this approach was to identify a cell line(s) expressing the highest ‘relative abundance' of [125I]hU-II binding sites. As such, it should be recognized that a ‘negative' binding signal in any given cell line was not necessarily indicative of a lack of [125I]hU-II binding sites, rather that if such levels existed they were beyond the level of detection utilizing the approach employed herein.
Immunocytochemical identification of human UT protein in SJRH30 cells
SJRH30 cells (90% confluent) were harvested following serum removal and washed in PBS. Following cell pelleting (centrifugation at 2500 × g, 10 min), supernatant was removed and replaced with 10% neutral buffered formalin (with care being taken not to resuspend the cells). This pellet was then used to generate formalin-fixed, paraffin-embedded cell blocks suitable for immunocytochemical processing. Vulva leiomyosarcoma (SK-LMS-1) and bone osteosarcoma (MG-63) cells were subjected to the same protocol.
UT immunoreactivity was assessed using an affinity-purified rabbit anti-hUT pAb raised using a KLH-conjugated synthetic peptide antigen corresponding to residues 1–15 in the N-terminus of the human UT (H-MALTPESPSSFPGLA-OH; Research Genetics Inc., Huntsville, AL, U.S.A.) which was administered with Freund's adjuvant to an adult New Zealand White rabbit (0.1 mg in three s.c. sites on days 0, 14, 28 and 56). Serum was collected on day 70.
Anti-hUT pAb (1 μg ml−1) immunostaining was detected using an avidin/biotin-immunoperoxidase procedure (streptavidin-horseradish peroxidase, with DAB as the [brown] chromogen counterstained with hematoxylin [blue nuclear stain]). Briefly, cell pellets were blocked using normal goat serum (5 min), following which they were incubated with primary rabbit anti-hUT pAb (25 min). Pellets were then exposed to secondary (biotinylated goat-anti-rabbit) pAb for an additional 25 min, at which point endogenous peroxidase blocking (3 × 90 s) and streptavidin/horse radish peroxidase incubation was performed (10 min). Cells were exposed to DAB (3 × 5 min) and counterstained with hematoxylin (1 min). Specificity of anti-hUT pAb staining was defined as that signal which was inhibited by preincubation with excess antigen peptide.
Pharmacological characterization of [125I]hU-II binding in human rhabdomyosarcoma cells
Saturation (5–300 pM [125I]hU-II) and competition binding (150 pM [125I]hU-II in the presence of increasing concentrations of U-II isopeptides and analogues) was evaluated in SJRH30 cells. Selectivity of hU-II binding was established by determining the effect for numerous unrelated peptides such as somatostatin, urotensin-I, endothelin-1, angiotensin-II, adrenomedullin, MCH, NPY, CGRP and AVP at 1 μM. Non-specific binding was defined using 1 μM unlabeled hU-II.
Association and dissociation studies of [125I]hU-II were also performed. Cells were washed twice with DPBS+ and binding was initiated upon the addition of [125I]hU-II (100 pM) in DPBS+. Cells were incubated at 37oC for various periods in the absence or the presence (non-specific binding) of 1 μM cold hU-II. Dissociation of [125I]hU-II binding was initiated by the addition of 1 μM of unlabeled hU-II at equilibrium (45 min), after which specific binding was determined at various time points.
Intracellular [Ca2+]-signaling in SJRH30 human rhabdomyosarcoma cells
SJRH30 cells were loaded with 2 μM Fura-2/AM in growth medium for measurement of [Ca2+]i. Following a 45 min incubation period, fresh growth medium was added for 15 min to facilitate residual ester hydrolysis. Cells were then rinsed in Dulbecco's PBS, trypsinized, resuspended in growth medium, centrifuged and resuspended into modified (bicarbonate replaced with 25 mM HEPES, pH 7.4) Kreb's Ringer's Henseleit (KRH) buffer containing 0.1% gelatin. Cells were diluted to 1 × 106 cells ml−1 (KRH buffer, 37°C) immediately prior to use. Initially, Fura-2 fluorescence was measured using cell suspensions in a dual-channel fluorometer (U. Pennsylvania Biomedical Instruments Group; Igor software, version 1.28; WaveMetrics, Lake Oswego, OR, U.S.A.).
In addition, single-cell fluorescence microscopy techniques were also applied since it was suspected that only a small subpopulation of parent cells responded to hU-II. As such, cells were grown for ∼3 days at low density on poly-D-Lys-coated, glass-bottom 35 mm culture dishes (MatTek Corp., Ashland, MA, U.S.A.). Cells were loaded with 1 μM Fura-2/AM (Molecular Probes, Eugene, OR, U.S.A.) in RPMI (SJRH30) or DMEM-H (TE671) for 30 min. Cells were rinsed once with buffer (1 ml HBSS plus Ca2+/Mg2+) and incubated for 10 min to allow residual ester hydrolysis. Agonist-induced changes in [Ca2+]i were measured (fluorescence at 510 nm following excitation at 340/380 nm from a 300 W xenon lamp) in individual cells from a single visual field using an integrating CCD camera coupled to a Nikon Eclipse TS100 inverted fluorescence microscope. The 340/380 nm fluorescence emission was converted to [Ca2+]i using a fluorescence/Ca2+ standard curve (Molecular Probes, Eugene, OR, U.S.A.). Data were recorded and processed and pseudocolor images generated using an InCyt Im2/Pm2 imaging system (Intracellular Imaging Inc., Cincinnati, OH, U.S.A.). [Ca2+] signaling was studied in the presence/absence of 1 μM thapsigargin, nifedipine or ryanodine and the role of phospholipase C and Gi/o-protein was investigated by pretreating SJRH30 cells with U-73122, U-73343 (10 μM for 10 min) or pertussis toxin (100 ng ml−1 for 18 h).
Materials
hU–II, hU-II(4-11), ([Cys5,10]Acm)hU-II and porcine U-IIA/B were synthesized by California Peptide Research Inc. (Napa, CA, U.S.A.). All rodent U-II isopeptides (rat and mouse) were obtained from Phoenix Pharmaceuticals Inc. (Mountain View, CA, U.S.A.). All other reagents were from Bachem California Inc. (Torrance, CA, U.S.A.) or Sigma Chemical Co. (St Louis, MO, U.S.A.) with the exception of pertussis toxin, U-73122 and U-73343 which were purchased from Biomol Research Laboratories Inc. (Plymouth Meeting, PA, U.S.A.). All cells used in the study were purchased either from the American Tissue Culture Collection (Manassas, VA, U.S.A.) or Clonetics Corporation (Walkersville, MD, U.S.A.).
Data analysis
Values are expressed as mean±s.e.m. (n represents the number of separate experimental observations made in duplicate or triplicate). Nonlinear curve-fitting (GraphPad Prism, San Diego, CA, U.S.A.) was used to calculate equilibrium binding affinity (Kd) and maximum binding site density (Bmax) from saturation-binding experiments in addition to determining competitor affinity constant (Kis) from competition binding experiments and agonist potency (EC50) and functional efficacy (Rmax) from [Ca2+]i-mobilization assays.
Results
Northern blot and RT–PCR analysis of human UT expression in major human organs and cell lines
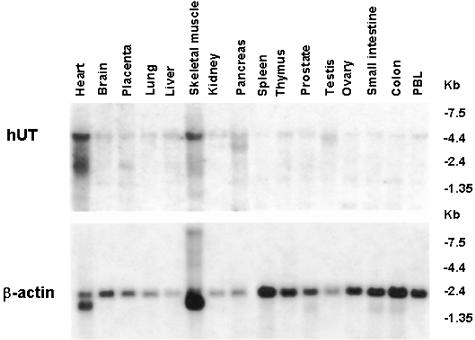
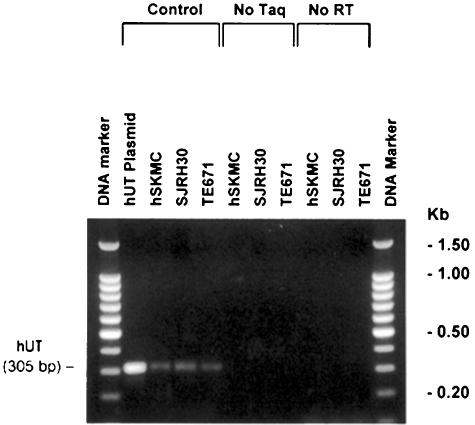
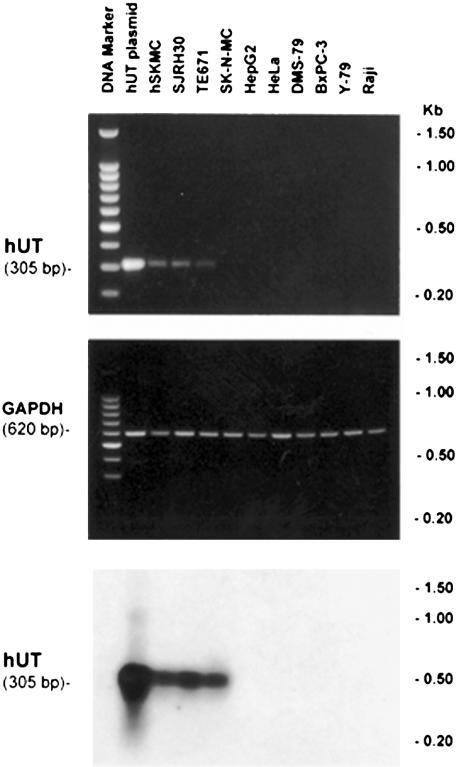
Although evident in many major organs (pancreas, brain, liver, testis, placenta, lung, kidney, thymus, prostate, small intestine, colon, peripheral blood leukocytes, ovary and spleen), expression of hUT mRNA (4.4 kb transcript) was most abundant in tissues of ‘striated muscle' origin, for example, cardiac and skeletal muscle (Figure 1). Interestingly, smaller transcripts were evident in heart, placenta, skeletal muscle (∼3 kb) and pancreas (∼4 kb). Hybridization with human β-actin cDNA (∼2.2 kb transcript) was evident in all tissues (Figure 1). In accord, hUT mRNA was detected in hSKMC (primary skeletal muscle) cells and in two rhabdomyosarcoma cell lines, SJRH30 and TE671, using RT–PCR (generation of the predicted 305 bp amplicon was specific, that is, dependent upon both the presence of Taq polymerase and the generation of cDNA template from mRNA by reverse transciptase and was confirmed by Southern blot analysis; Figures 2 and 3). In contrast, no transcript was detected in ‘non-striated muscle' cells (e.g. SK-N-MC, HepG2, HeLa, DMS-79, BxPC-3, Y-79 and Raji).
Figure 1.
Multiple tissue Northern blot analysis demonstrating hUT mRNA expression (∼4.4 kb transcript) in skeletal muscle and heart, tissues of ‘striated muscle' origin (upper panel). Lower level hUT mRNA expression is evident in pancreas, brain, liver, testis, placenta, lung, kidney, thymus, prostate, small intestine, colon, peripheral blood leukocytes, ovary and spleen. Hybridization with a human β-actin cDNA probe (∼2.2 kb transcript) was evident in all tissues studied (lower panel). Data shown are from a single determination (using pooled tissue mRNA).
Figure 2.
Skeletal muscle cell lines express significant amounts of hUT mRNA. PCR samples were size-fractionated in an ethidium bromide-stained 2% agarose gel (100 bp DNA ladder is shown in lanes 1 and 12). RT–PCR amplification of a specific hUT amplicon (305 bp) was detected using cDNAs generated from hUT plasmid (lane 2) or human skeletal muscle cell lines hSKMC (primary cell line; lane 3), SJRH30 and TE671 (rhabdomyosarcoma cell lines; lanes 4 and 5, respectively). Amplification fidelity was confirmed by demonstrating that no PCR product was generated if Taq DNA polymerase was omitted from the amplification reaction (no Taq; lanes 6–8) or if mRNA was not subjected to reverse transcription (no RT; lanes 9–11) prior to PCR amplification (i.e. amplicons were derived from hUT mRNA, not genomic DNA contamination). Data are shown from a single determination.
Figure 3.
Expression of hUT mRNA is specific to skeletal muscle cells and is not evident in cell lines that are of ‘non-striated muscle' origin. Upper panel: RT–PCR samples were size-fractionated in an ethidium bromide-stained 2% agarose gel (100 bp DNA ladder is shown in lane 1). Amplification of a specific hUT amplicon (305 bp) was detected using cDNAs generated from hUT plasmid (lane 2) and the human skeletal muscle cell lines hSKMC (primary cell line; lane 3), SJRH30 and TE671 (rhabdomyosarcoma cell lines; lanes 4 and 5, respectively). No amplicon was found in a variety of ‘non-skeletal muscle' human cell lines, namely SK-N-MC (supra-orbital neuroblastoma), HepG2 (hepatoblastoma), HeLa (cervical adenocarcinoma), DMS-79 (small cell lung carcinoma), BxPC-3 (pancreatic adenocarcinoma), Y-79 (retinoblastoma) and Raji B-lymphocyte (Burkitt's lymphoma) cells (lanes 6–12, respectively). Middle panel: amplification of GAPDH cDNA (620 bp amplicon) was evident in all samples studied. Lower panel: the presence of hUT gene was confirmed in human skeletal muscle cell lines hSKMC, SJRH30 and TE671 cells by Southern blot analysis (PCR products were tranferred to a nylon membrane and probed with full-length hUT cDNA to generate a 305 bp hybridization product). Data shown one from a single determination.
Evaluation of established cell lines for U-II receptors
In an effort to identify an endogenous/native U-II binding site, a wide range of human, primate and rodent cell lines, including those of skeletal muscle origin, were screened for the presence of readily detectable levels of specific U-II binding sites. This was achieved using a crude whole-cell screening approach, whereby both adherent cells and cell suspensions were exposed to 150 pM [125I]hU-II. Using recombinant hUT-HEK293 cells, the limit of detection for [125I]hU-II binding was estimated to be ⩾500 sites cell−1 (hence, a ‘negative' binding signal did not mean that cells lacked UT, only that if such protein was expressed it was at an extremely low density and beyond the level of detection using the current experimental conditions). Of the 49 cell lines assayed, only three (∼6%) exhibited a significant binding signal. Significant [125I]hU-II binding was observed in SJRH30 and TE671 rhabdomyosarcoma cells. In contrast, binding was not detected in the A204 rhabdomyosarcoma cell line, the L6 rat skeletal myoblast cell line or the human primary skeletal muscle cells hSKMC. Similarly, rat H9c2 cells (‘striated' cardiac myoblasts) were also devoid of detectable [125I]hU-II binding. Binding to rhabdomyosarcomas was not an observation associated with ‘connective tissue' sarcomas in general. Specific [125I]hU-II binding was not evident in ‘non-skeletal muscle' sarcomas (namely leiomyo-, osteo-, chondro-, synovial, fibro- and lipo-sarcomas). Similarly, no binding was noted in numerous cardiovascular (vascular smooth muscle and endothelial cells), endocrine (pancreas, adrenal, pituitary gland), gastrointestinal (stomach, small intestine and colon), sensory epithelial (retinal), neuronal, pulmonary (epithelia), hepatic and leukocyte (B-cells, monocyte/macrophage) cell lines. Although binding was evident in a rat medullary thyroid cell line (6–23), albeit at very low density (see below), a similar signal was not noted in the human thyroid cell lines TT (medullary carcinoma) and SW-579 (squamous cell carcinoma).
Characterization of native UT
Since both TE671 and rat thyroid carcinoma (6–23) cells displayed poor U-II binding site densities (∼5–10% of that recorded in SJRH30), detailed hUT characterization (e.g. immunocytochemistry, signal transduction) was performed primarily in SJRH30 cells.
UT immunostaining in the human rhabdomyosarcoma cell line SJRH30
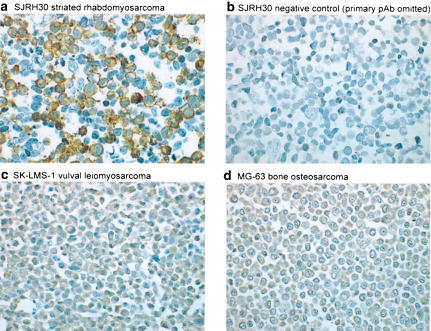
Affinity purified rabbit anti-hUT pAb staining of SJRH30 cells was primarily cell membrane-associated (Figure 4a). Staining, globular/granular in nature, was evident in ∼50% of cells. The specificity of this interaction was confirmed by demonstrating that there was (a) complete inhibition by preincubation with excess (5 μg ml−1) antigen peptide (Figure 4b) and (b) no specific membrane immunostaining evident in ‘non-striated' human sarcoma cell lines prepared under identical conditions (faint, diffuse cytoplasmic staining was observed in SK-LMS-1 and MG-63 cells but this signal was not inhibited by preincubation with excess peptide antigen and, therefore, represented non-specific staining; Figure 4c and d). Control, rabbit IgG staining was run in parallel with anti-hUT pAb in SJRH30 cells and confirmed the specificity of the receptor antibody staining.
Figure 4.
hUT immunoreactivity is detectable in SJRH30 cells using a novel affinity-purified rabbit anti-hUT pAb. (a) Globular/granular staining is observed at the plasma membrane of formalin-fixed, paraffin-embedded SJRH30 cell pellets labeled with 1.0 μg ml−1 hUT pAb. (b) In contrast, staining of the SJRH30 cell pellet is abolished following preincubation of the SJRH30 cells with anti-hUT pAb in the presence of antigen (1 h at 5 μg ml−1). Similarly, no membrane staining is noted in the ‘non-striated' human sarcoma cell lines prepared in an identical manner, including (c) SK-LMS-1 (vulval leiomyosarcoma) and (d) MG-63 (bone osteosarcoma) cell lines.
Radioligand-binding studies in SJRH30 human rhabdomyosarcoma cells
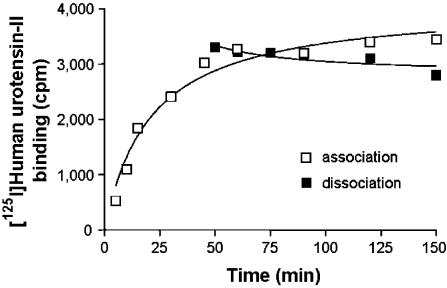
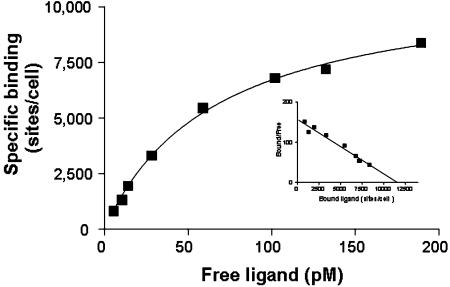
Equilibrium [125I]hU–II binding to intact SJRH30 cells was reached within ∼25 min at 37°C and remained constant over 2 h (Figure 5). Binding was monoexponential, indicating that the radioligand interacted with a single class of homologous sites (non-specific binding was 10–15% of the total binding). Notably, only modest dissociation (∼15%) of bound ligand was evident up to 90 min after the addition of excess cold hU-II (Figure 5). [125I]hU–II binding was concentration-dependent and saturable (Kd 67.0±11.8 pM, Bmax 9687±843 sites cell−1; Figure 6). Scatchard analysis (nonlinear regression) suggested an interaction of the radioligand with a single class of non-cooperative sites (specific binding was >90% at the Kd). In competition studies, all native U-II (human, rat, mouse, pig and fish) isoforms potently inhibited [125I]hU–II binding to SJRH30 cells in a concentration-dependent manner (Figure 7). Ligand affinities (Kis) were similar for all U-II isopeptides studied (0.5–1.2 nM; Table 1) and Hill coefficients approximated unity. The truncated hU-II analog hU-II(4-11) was also equipotent at inhibiting [125I]hU-II binding. In contrast, however, the linear analog ([Cys5,10]Acm)hU-II was approximately three orders of magnitude less potent than hU-II in this system. Unrelated peptides, such as urotensin-I, somatostatin-14, endothelin-1, angiotensin-II, adrenomedullin, MCH, NPY, CGRP and AVP (1 μM), were unable to inhibit binding (>2000-fold less potent than hU-II).
Figure 5.
[125I]hU-II binds (associates) to intact SJRH30 cells in a time-dependent manner, with a slow dissociation off-rate. [125I]hU-II (100 pM) was added in 1 ml of DPBS to wells containing a monolayer of 2 × 106 SJRH30 cells (n=3, duplicate determinations). The incubation was carried out at 37°C for up to 150 min. Association (open squares) was complete within ∼25 min. The dissociation of cell-bound [125I]hU-II from SJRH30 cells (filled squares) was studied after incubation of cells with 100 pM [125I]hU-II for 30 min at which point excess unlabeled hU-II (1 μM) was added to the incubation mixture. Radioligand dissociation, observed over the subsequent 2 h period, was slow (∼15%).
Figure 6.
Saturation equilibrium binding of [125I]hU-II to an intact SJRH30 cell monolayer. Specific binding (>90%) was determined in SJRH30 cells incubated with 5–300 pM [125I]hU-II for 30 min at 37°C in the presence or absence of excess cold hU-II (1 μM). Radioligand bound to a single class of homologous sites as determined by Scatchard analysis (inset). Values are the mean of duplicate determinations from a representative experiment. The experiment was repeated a total of three times (in duplicate) where Kd 67.0±11.8 pM and Bmax 9687±843 sites cell−1.
Figure 7.
U-II isopeptides inhibit [125I]hU-II binding to SJRH30 cells in a specific, competitive and concentration-dependent manner. Cells were grown as a monolayer and were incubated with 0.15 nM radioligand for 30 min at 37°C in the presence of increasing concentrations of competing ligand. [125I]hU-II binding was sensitive to the truncated analogue hU-II(4–11), but not the linear hU-II analogue ([Cys5,10]Acm)hU-II. Binding was also insensitive to all ‘unrelated' peptides studied, namely urotensin-I, somatostatin, endothelin-1, angiotensin-II, adrenomedullin, MCH, NPY, CGRP and Arg-vasopressin (<10% inhibition at 1 μM concentration of peptides). Each point represents the mean of duplicate determinations from a representative experiment and all experiments were repeated a total of three to four times with similar observations (see Table 1) (a) hU-II (open circles), hU-II (4-11) (filled circles) and ([Cys5,10]Acm)hU-II (open squares); (b) Goby U-II (filled squares), rat U-II (open triangles) and mouse U-II (filled triangles); (c) porcine U-IIa (open diamonds) and porcine U-IIb (filled diamonds).
Table 1.
Urotensin-II isopeptides inhibit [125I]hU-II binding to SJRH30 rhabdomyosarcomas (competition whole cell binding assay)
| Competing ligand | Affinity (Ki) nM | Relative potency |
|---|---|---|
| Mammalian U-II isopeptides | ||
| Human U-II | 0.5±0.1 | 1.0 |
| Goby U-II | 0.6±0.1 | 1.2 |
| Rat U-II | 0.7±0.1 | 1.4 |
| Mouse U-II | 1.2±0.3 | 2.4 |
| Porcine U-IIA | 1.1±0.2 | 2.2 |
| Porcine U-IIB | 0.5±0.1 | 1.0 |
| Human U-II analogs | ||
| Human U-11(4-11) | 0.4±0.1 | 0.8 |
| ([Cys5,10]Acm) human U-II | 864.3±192.7 | 1729 |
Values are expressed as mean±s.e.m. (n=3). Relative potency values are determined in comparison to human U-II.
Radioligand-binding studies in human TE671 and rat 6-23 cells
hU-II fully displaced [125I]hU-II binding in human TE671 and rat 6-23 cells in a concentration-dependent manner (Kis 2.2±0.2 and 3.0±0.8 nM, respectively; Figure 8). Scatchard analysis revealed the presence of a single class of high-affinity, low-density binding sites for hU-II in both cell types (Kds 74.0±7.6 and 57.3±6.5 pM and Bmaxs 1667±165 and 493±114 sites cell−1, respectively; Figure 8).
Figure 8.
Saturation equilibrium binding of [125I]hU-II to intact (a) human TE671 and (b) rat 6–23 cell monolayers. Specific binding was determined by incubating cells with 5–300 pM [125I]hU-II for 30 min at 37°C in the presence or absence of excess cold hU-II (1 μM). Radioligand bound to a single class of homologous sites in both cell types, as determined by Scatchard analysis (insets). (c) hU-II isopeptide inhibits [125I]hU-II binding to human TE671 (filled squares) and rat 6–23 (open squares) cells in a specific, competitive and concentration-dependent manner. Cells were grown as a monolayer and were incubated with 0.15 nM radioligand for 30 min at 37°C in the presence of increasing concentrations of hU-II. Each point represents the mean of duplicate determinations from a representative experiment and all experiments were repeated a total of three times with similar observations.
Intracellular [Ca2+]i mobilization in SJRH30 cells
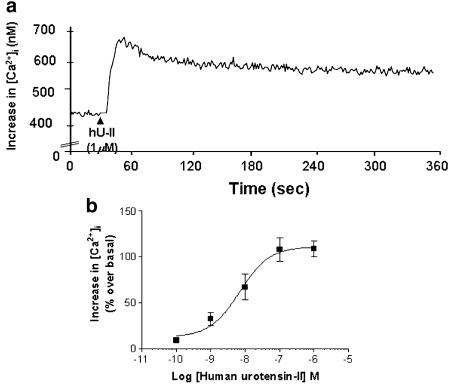
Binding of hU-II to SJRH30 cells resulted in ligand-induced [Ca2+]i mobilization, as determined using the Ca2+-indicator Fura-2. hU-II (1 μM) trebled [Ca2+]i levels from a resting, basal value of approximately 50 nM to an Rmax of 150±30 nM (Figure 9a). hU-II-induced [Ca2+]i increase was concentration-dependent (EC50 6.9±2.2 nM; Figure 9b). Responses were insensitive to both ryanodine and nifedipine, but were attenuated by 1 μM thapsigargin (56.0±15.4 nM increase in [Ca2+]i over basal).
Figure 9.
hU-II stimulates [Ca2+]i transients in SJRH30 cell supensions. (a) [Ca2+]i mobilization to 1 μM hU-II (indicated by the filled triangle) was determined in Fura-2 AM-loaded cells using a fluorescence spectrophotometer. (b) Concentration–response curve for hU-II-mediated [Ca2+]i mobilization in SJRH30 cells (values are mean±s.e.m; n=3).
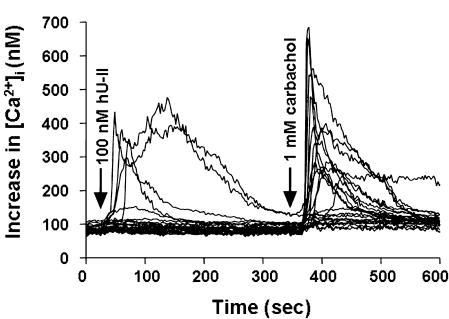
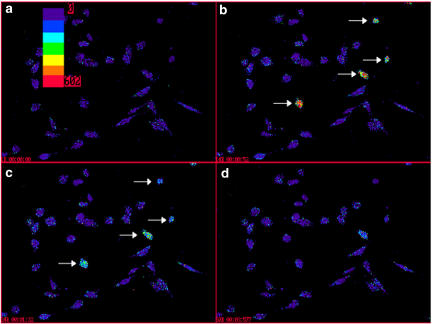
To investigate further the effects of hU-II on signal transduction, [Ca2+]i mobilization was studied on the single-cell level using a digital imaging system. Application of 100 nM hU-II resulted in rapid, transient increases in [Ca2+]i levels but, notably, only in a subset of SJRH30 cells (Figures 10 and 11). A significant hU-II-induced [Ca2+]i response was only observed in 10.3±2.0% (38/370 cells; n=13) of SJRH30 cells tested (233/304 SJRH30 cells (76.7%) responded to 1 mM carbachol; n=11). Similarly, the magnitude of the hU-II-induced [Ca2+]i varied significantly between individual cells from ∼10 nM to several hundred nM over baseline. In contrast, hU-II (⩽1 μM) failed to evoke a [Ca2+]i response in TE671 (0/77 cells, n=3) cells (1 mM carbachol evoked a rapid, robust rise in [Ca2+]i in 52/52 TE671 cells tested). U-II-induced [Ca2+]i release was significantly reduced (90±4%; n=3) when SJRH30 cells were treated with the phospholipase C inhibitor U-73122 (10 μM, 10 min), whereas the inactive structural analogue U-73343 had no effect on U-II-evoked [Ca2+]i release. Pretreatment of SJRH30 cells with pertussis toxin (100 ng ml−1 for 18 h) resulted in a ∼40% reduction in U-II-mediated [Ca2+]i response (basal [Ca2+]i was unaffected). However, neither elevating the concentration of pertussis toxin nor increasing the incubation time enhanced the degree of inhibition.
Figure 10.
Composite of [Ca2+]i-mobilization responses in 23 individual SJRH30 cells from a single microscopic field. Five out of 23 cells exposed to 100 nM hU-II responded (21 of 23 cells responded to 1 mM carbachol). Arrows above the calcium traces indicate the addition of agonists.
Figure 11.
Pseudocolor images illustrating time-dependent increase in [Ca2+]i in SJRH30 cells following exposure to hU-II (100 nM). Upper left panel (a): SJRH30 cells under resting conditions in a single visual field (bar correlates pseudocolor with relative [Ca2+]i levels). Upper right (b): a subset of SJRH30 cells (identified by arrows) responded upon addition of hU-II with rapid, transient increases in [Ca2+]i levels (image taken 52 s following the addition of ligand). Lower left and right panels (c and d): increases in [Ca2+]i levels were transient and reversible (images taken 92 and 237 s following the addition of hU-II).
Discussion
Although U-II was originally identified over three decades ago (Bern & Lederis, 1969), [125I]hU-II binding has been recorded in only a very limited number of tissues (Stratton et al., 1989; Itoh et al., 1988; Ames et al., 1999; Maguire et al., 2000). In the present study, Northern blot and RT-PCR analysis confirmed the presence of UT mRNA in (a) skeletal muscle (and cardiac) tissue specimens, (b) the rhabdomyosarcoma cell lines SJRH30 and TE671 and (c) the human primary skeletal muscle cell line hSKMC. Further to this, radioligand-binding sites for U-II were found to be present in SJRH30 and TE671 cells, transformed rhabdomyosarcoma cell lines that exhibit the cytogenetic, biochemical and ultrastructural characteristics typical of that seen in skeletal muscle (sarcomeres, cytosolic glycogen granules, desmin, myoglobin, bungarotoxin binding sites, muscle-specific transcription/differentiation factor myogenin, etc.; Roberts et al., 1989; Stratton et al., 1989). Such findings are consistent with those of Maguire et al. (2000) using whole tissue quantitative receptor autoradiography. Detailed characterization of SJRH30 cells revealed that this cell line expressed a single, homogenous population of high affinity (Kd 67 pM) and low density (Bmax ∼10,000 sites cell−1) [125I]hU-II binding sites. Lower density binding was evident in TE671 cells. To the best of the Authors' knowledge, the present report is the first to record [125I]hU-II binding in a native cell line. In accord, specific membrane-associated immunoreactivity was noted in SJRH30 cells using an affinity-purified anti-hUT pAb. Such findings are consistent with the hypothesis that U-II might play a physiological role in the regulation of mammalian locomotor function (Gartlon et al., 2001), which requires further study. As such, SJRH30 cells might prove to be of utility for investigating the pharmacological properties of hU-II and related small molecule antagonists at native human UT and delineating the role of this neuropeptide in the (patho)physiological regulation of mammalian neuromuscular function.
U-II is expressed throughout the CNS across evolutionary levels, from invertebrate molluscs (Gonzalez et al., 1992) to man (Coulouarn et al., 2001). Since ligand expression is associated specifically with somatic motor nuclei and cholinesterase-positive ventral horn motor neurons (Chartrel et al., 1998; Ames et al., 1999; Clark et al., 2001; Coulouarn et al., 2001; Dun et al., 2001), this neuropeptide is purported to regulate mammalian sensory-locomotor activity. Indeed, U-II has been shown recently to stimulate [Ca2+]i influx in rat isolated spinal cord cholinergic neurons (Filipeanu et al., 2002) and to increase habituated motor activity in rodents upon central administration (Gartlon et al., 2001). Since U-II also augments spontaneous neurotransmitter release (in the frog sartorius muscle; Brailoiu et al., 2003), it has been proposed that U-II influences skeletomuscular function via a peripheral site of action, for example, at the neuromuscular junction. Such a proposition is consistent with the present study.
As recorded in recombinant UT systems (Ames et al., 1999), [125I]hU-II was slow to dissociate from SJRH30 cells, consistent with the characterization of U-II isopeptide as a ‘pseudo-irreversible' UT ligand (Douglas, 2003). This phenomenon likely underlies the extremely sustained pharmacodynamic actions of hU-II, for example, vasoconstriction that is resistant to washout (Douglas et al., 2000). Interestingly, such sustained actions are not particularly reminiscent of most neurotransmitter ligand/receptor systems and it remains to be seen whether U-II regulates neuromuscular function in a ‘phasic' manner or whether any such putative actions are more chronic in nature, for example, ‘metabotropic' function.
Exposure of SJRH30 cells to U-II was associated with [Ca2+]i mobilization. The agonist potency of hU-II (EC50 ∼7 nM) was in close agreement both with the binding affinity of the U-II isopeptides (Ki∼1 nM) and with the potency of U-II as a [Ca2+]i-mobilizing agent in rat spinal cord neurons (Filipeanu et al., 2002) and rodent/primate recombinant UT cells and native cells (Ames et al., 1999; Elshourbagy et al., 2002). As such, the interaction described herein is believed to be physiologically and pharmacologically relevant.
Although an appreciable hU-II binding site density was observed in SJRH30 cells, single-cell digital imaging techniques demonstrated that only ∼10% SJRH30 cells exposed to hU-II responded with an appreciable [Ca2+]i response (and the magnitude of this response, where observed, exhibited considerable cell–cell variation). The reason underlying this observation is unclear at present, but perhaps relates either to natural variations in cell-to-cell phenotype (to date, clonal cell lines have not been selected) or it could be simply related to the low receptor density/lack of receptor reserve (subtle fluctuations in receptor expression between cells or varied exposure to endogenous U-II, a pseudo-irreversible ligand, might have profound effects on ‘available' receptor, that is, that free to couple to [Ca2+]i mobilization; Douglas, 2003). This observation might explain why [125I]hU-II binding site densities appear to be so low in cell culture systems (and tissues), since only a small fraction or subpopulation of cells within a culture plate or tissue express cell surface U-II receptors at any given time. Indeed, this observation would go a long way to explaining why the detection (either by binding or function) of endogenous UT in native mammalian cell lines has proven so difficult to date.
Despite the observations that radiolabelled hU-II bound to TE671 cells, cold peptide was unable to induce [Ca2+]i mobilization in this cell line. The reason for the disparity between SJRH30 and TE671 cells is unknown, but likely relates to differences in receptor expression and/or receptor–effector coupling between these two cell lines.
In the present study, no [125I]hU-II binding was observed in either primary human skeletal muscle cells (hSKMC) or rat L6 myoblasts (although UT mRNA was evident, at least in the former). Such findings do not mean that radioligand-binding sites are not present in either cell line, rather that using the binding techniques employed herein the binding site density was below the level of detection in either cell line. Alternatively, the lack of binding in hSKMC cells might simply reflect ‘inter-donor' variability (the hSKMC cells are primary cells obtained from individual donors by Clonetics). Indeed, there is precedence for donor-to-donor variability in the level of skeletal muscle UT mRNA expression in human tissue. In contrast to the present study, in a prior publication from this group (Ames et al., 1999), no appreciable UT mRNA was observed in human skeletal muscle by Northern blot analysis using membranes from the same commercial source (Clontech). Further, in subsequent studies, both Liu et al. (1999) and Elshourbagy et al. (2002) reported significant primate and murine UT mRNA expression in freshly harvested tissue.
The hU-II-induced [Ca2+]i mobilization from thapsigargin-sensitive (sarcoplasmic reticulum) intracellular stores observed in the present study is in accord with Brailoiu et al. (2003), who demonstrated that U-II-induced increases in miniature end-plate potential frequency in the frog sartorius muscle were thapsigargin-sensitive. [Ca2+]i mobilization was insensitive to both blockade of L-type Ca2+ channels (with nifedipine) and inhibition of the Ca2+-sensitive Ca2+ release (with ryanodine). [Ca2+]i mobilization was phospholipase C-dependent (U-73122 sensitive) in agreement with observations made in frog sartorius muscle and rat and rabbit vascular preparations (Gibson, 1987; Saetrum-Opgaard et al., 2000; Rossowski et al., 2002; Watanabe et al., 2002; Brailoiu et al., 2003). Although phospholipase C-mediated [Ca2+]i mobilization is generally transduced by Gi/o or Gq-proteins (e.g. U-II-mediated Gi/o activation in recombinant UT systems), multiple G-proteins appear to be involved in U-II mediated [Ca2+]i release in SJRH30 cells since pertussis toxin pretreatment (Gi/o ADP ribosylation and inactivation) only partially inhibited (∼40%) this signaling event that is, it is mediated by pertussis toxin-sensitive (Gi/o) and pertussis toxin-insensitive (possibly Gq) G-proteins (Ziltener et al., 2002).
The SAR data generated in the present study are consistent with the ‘endogenous' SJRH30 [125I]hU-II binding site being homologous with the human recombinant UT. U-II isopeptides are equipotent UT ligands in SJRH30 cells (all native isoforms possessed ∼1 nM Kis). Such observations imply that the divergent amino-tail of U-II is not required for biological activity at SJRH30 binding sites (as is the case for hUT), rather that the conserved cyclic portion of the U-II ligand confers affinity for UT (Bern & Lederis, 1969; Bhaskaran et al., 1994; Labarrère et al., 2003). Indeed, the truncated peptide analog hU-II(4–11) was equipotent with mature hU-II peptide in SJRH30 cells (Coy et al., 2002; Croston et al., 2002; Kinney et al., 2002). In contrast, ([Cys5,10]Acm)hU-II, a linear ligand, was three orders of magnitude less potent than parent hU-II in an SJRH30 competition binding assay. This is consistent with [1H]NMR studies where cysteinyl disulphide bridge modification (e.g. [Cys5,10] Ser, Ala, D-Cys substitutions) leads to a loss in the UT affinity of resultant ‘linear' peptides (Flohr et al., 2002; Grieco et al., 2002; Labarrère et al., 2003).
Preliminary analysis of hUT mRNA (4.4 kb mRNA) distribution revealed the presence of two additional 3 and 4kb transcripts in skeletal muscle and heart. While it is tempting to speculate that this is suggestive of multiple UT subtypes (Liu et al., 1999; Coy et al., 2000), to date, no molecular evidence exist to support the concept that U-II mediates its biological actions via multiple UT subtypes.
While the present study focused on characterizing U-II binding and function in skeletal muscle cell types, the rat thyroid medullary carcinoma cell line 6-23 was shown to express an appreciable density of [125I]hU-II binding sites (no binding was detectable in TT and SW-579 thyroid carcinomas). Although receptor density was extremely low (∼5% of that seen in SJRH30 cells), the observation of high-affinity binding (74 pM and 3 nM Kd and Ki, respectively) was interesting given the putative endocrine function ascribed for U-II in mammals, for example, regulation of TSH (insulin, glucose, ACTH, catecholamine and prolactin) secretion (Gartlon et al., 2001; Silvestre et al., 2001; Watson et al., 2003). Notably, U-II and UT mRNA have been identified in primate thyroid tissue in the acinar cells lining the thyroid follicles (Ames et al., 1999; Elshourbagy et al., 2002). As such, the identification of U-II binding sites in rat 6-23 cells might assist future attempts to establish a (patho)physiological role for U-II in the regulation of mammalian thyroid function.
In conclusion, the present study demonstrates that skeletal muscle cell lines (SJRH30 and TE671) express appreciable numbers of hU-II binding sites, receptors functionally coupled to second messenger generation (phospholipase C-mediated [Ca2+]i-mobilization). The utility of identifying a native U-II binding site in a cell line such as SJRH30 is two-fold. SJRH30 cells might facilitate advancements in delineating the role of U-II in the (patho)physiological regulation and/or integration of mammalian sensory-locomotor activity and they might also assist in the pharmacological evaluation of novel ligands (i.e. antagonists) at an endogenous human UT expressed in its ‘native' environment.
Acknowledgments
We thank Gwendolyn E. Townsend, David J. Behm and Douglas G. Johns (Vascular Biology and Thrombosis, GlaxoSmithKline) for their skillful technical assistance during the preparation of this manuscript.
Abbreviations
- Acm
acetamidomethyl
- ATCC
American Type Culture Collection
- AVP
Arg-vasopressin
- Bmax
maximum binding site density
- BSA
bovine serum albumin
- [Ca2+]i/e
intracellular/extracellular calcium
- CCD
charge-coupled device
- CGRP
calcitonin gene-related peptide
- DAB
3,3′diaminobenzidine
- DMEM/-H
Dulbecco's modified Eagles medium with HEPES buffer
- DPBS+
Dulbecco's phosphate-buffered saline
- EC50
effective agonist concentration required to give a half-maximal response
- Fura-2
1-[6-amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N′,N′-tetraacetic acid, pentapotassium salt
- G-protein
GTP-binding protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPCR
G-protein coupled receptor
- GPR14
G-protein receptor 14
- HBSS
Hank's balanced salt solution
- HEK293 cells
human embryonic kidney cells
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HPLC
high-performance liquid chromatography
- i.c.v.
intracerebroventricular
- IgG
immunoglobulin G
- IUPHAR
International Union of Pharmacology
- Kd/i
equilibrium binding affinity/competitor affinity constant
- KRH
Kreb's Ringer's Henseleit buffer
- MCH
melanin concentrating hormone
- NMR
nuclear magnetic resonance
- NPY
neuropeptide Y
- pAb
polyclonal antibody
- PBS
phosphate-buffered saline
- Rmax
maximum functional response (efficacy)
- RPMI
Roswell Park Memorial Institute medium
- RT–PCR
reverse transcription–polymerase chain reaction
- SAR
structure–activity relationship
- s.c.
subcutaneous
- SDS/SSC
sodium chloride, sodium citrate/sodium dodecyl sulphate solution
- s.e.m.
standard error of the mean
- SENR
sensory epithelial neuropeptide-like receptor
- ssDNA
salmon sperm DNA
- TSH
thyroid-stimulating hormone
- U-II
urotensin-II
- (h)UT
(human) urotensin-II receptor
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.-S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II: a potent vasoconstrictor and agonist for an orphan receptor. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BERN H.A., LEDERIS K.A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts J. Endocrinol. 196945xi–xii [PubMed] [Google Scholar]
- BERN H.A., PEARSON D., LARSON B.A., NISHIOKA R.S. Neurohormones from fish tails: the caudal neurosecretory system I: ‘Urophysiology' and the caudal neurosecretory system of fishes. Rec. Prog Horm. Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- BHASKARAN R., ARUNKUMAR A.I., YU C. NMR and dynamic simulated annealing studies on the solution conformation of urotensin II. Biochim. Biophys. Acta. 1994;1199:115–122. doi: 10.1016/0304-4165(94)90105-8. [DOI] [PubMed] [Google Scholar]
- BOTTRILL F.E., DOUGLAS S.A., HILEY C.R., WHITE R. Human U-II is an endothelium-dependent vasodilator in rat small arteries. Br. J. Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAILOIU E., BRAILOIU C.G., MIYAMOTO M.D., DUN N.J. The vasoactive peptide urotensin II stimulates spontaneous release from frog motor nerve terminals. Br. J. Pharmacol. 2003;138:1580–1588. doi: 10.1038/sj.bjp.0705204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARTREL N., CONLON J.M., COLLIN F., BRAUN B., WAUGH D., VALLARINO M., VAUDRY H. Urotensin II in the central nervous system of the frog Rana ridibunda: biochemical characterization and immunohistochemical localization. Ann. N.Y. Acad. Sci. 1998;839:506–507. doi: 10.1111/j.1749-6632.1998.tb10851.x. [DOI] [PubMed] [Google Scholar]
- CLARK S.D., NOTHACKER H.P., WANG Z., SAITO Y., LESLIE F.M., CIVELLI O. The urotensin II receptor is expressed in the cholinergic mesopontine tegmentum of the rat. Brain Res. 2001;923:120–127. doi: 10.1016/s0006-8993(01)03208-5. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., TOSTIVINT H., VAUDRY H. Somatostatin- and urotensin II-related peptides: molecular diversity and evolutionary perspectives. Reg. Pept. 1997;69:95–103. doi: 10.1016/s0167-0115(97)02135-6. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., YANO K., WAUGH D., HAZON N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J. Exp. Zool. 1996;275:226–238. [PubMed] [Google Scholar]
- COULOUARN Y., FERNEX C., JEGOU S., HENDERSON C.E., VAUDRY H., LIHRMANN I. Specific expression of the urotensin II gene in sacral motoneurons of developing rat spinal cord. Mech. Dev. 2001;101:187–190. doi: 10.1016/s0925-4773(00)00548-7. [DOI] [PubMed] [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., HOCART S.J., TAYLOR J.E. Novel urotensin-II (UII) antagonists point to multiple receptor involvement in UII bioactivity. Reg. Pept. 2000;94:48. [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., TAYLOR J.E. Structural requirements at the N-terminus of urotensin octapeptides. Peptides. 2002;23:2259–2264. doi: 10.1016/s0196-9781(02)00266-8. [DOI] [PubMed] [Google Scholar]
- CROSTON G.E., OLSSON R., CURRIER E.A., BURSTEIN E.S., WEINER D., NASH N., SEVERANCE D., ALLENMARK S.G., THUNBERG L., MA J.-N., MOHELL N., O'DOWD B., BRANN M.R., HACKSELL U. Discovery of the first nonpeptide agonist of the GPR14/urotensin-II receptor: 3-(4-chlorophenyl)-3-(2-(dimethylamino)ethyl)isochroman-1-one (AC-7954) J. Med. Chem. 2002;45:4950–4953. doi: 10.1021/jm025551+. [DOI] [PubMed] [Google Scholar]
- DE GARAVILLA L., QI J., SANTULLI R., ANDRADE-GORDON P., ABRAHAM W.M. Inhalation of urotensin-II increases airway resistance in sheep. Am. J. Resp. Crit. Care Med. 2001;163:A924. [Google Scholar]
- DOUGLAS S.A. Human urotensin-II as a novel cardiovascular target: ‘heart' of the matter or simply a fishy ‘tail'. Curr. Opin. Pharmacol. 2003;3:159–167. doi: 10.1016/s1471-4892(03)00012-2. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H.Urotensin receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000London: IUPHAR Media; 365–372.ed. Girdlestone, D. pp [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2001;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human U-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset, and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUN S.L., BRAILOIU G.C., YANG J., CHANG J.K., DUN N.J. Urotensin II-immunoreactivity in the brainstem and spinal cord of the rat. Neurosci. Lett. 2001;305:9–12. doi: 10.1016/s0304-3940(01)01804-3. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N., DOUGLAS S.A., SHABON U., HARRISON S., DUDDY G., SECHLER J.L., AO Z., MALEEFF B.E., NASELSKY D., DISA J., AIYAR N.V. Molecular and pharmacological characterization of genes encoding urotensin-II peptides and their cognate G-protein-coupled receptors from the mouse and monkey. Br. J. Pharmacol. 2002;136:9–22. doi: 10.1038/sj.bjp.0704671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPEANU C.M., BRAILOIU E., LE DUN S., DUN N.J. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J. Neurochem. 2002;83:879–884. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- FLOHR S., KURZ M., KOSTENIS E., BRKOVICH A., FOURNIER A., KLABUNDE T. Identification of nonpeptide urotensin II receptor antagonists by virtual screening based on a pharmacophore model derived from structure–activity relationships and nuclear magnetic resonance studies on urotensin-II. J. Med. Chem. 2002;45:1799–1805. doi: 10.1021/jm0111043. [DOI] [PubMed] [Google Scholar]
- GARTLON J., PARKER F., HARRISON D.C., DOUGLAS S.A., ASHMEADE T.E., RILEY G.J., HUGHES Z.A., TAYLOR S.G., MUNTON R.P., HAGAN J.J., HUNTER A.J., JONES D.N.C. Central effects of human U-II following i.c.v. administration in rats. Psychopharmacology. 2001;155:426–433. doi: 10.1007/s002130100715. [DOI] [PubMed] [Google Scholar]
- GIBSON A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br. J. Pharmacol. 1987;91:205–212. doi: 10.1111/j.1476-5381.1987.tb09000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ G.C., MARTINEZ-PADRÓN M., LEDERIS K., LUKOWIAK K. Distribution and coexistence of urotensin I and urotensin II peptides in the cerebral ganglia of Aplysia californica. Peptides. 1992;13:695–703. doi: 10.1016/0196-9781(92)90175-3. [DOI] [PubMed] [Google Scholar]
- GRIECO P., CAROTENUTO A., CAMPIGLIA P., ZAMPELLI E., PATACCHINI R., MAGGI C.A., NOVELLINO E., ROVERO P. A new, potent urotensin II receptor peptide agonist containing a Pen residue at the disulfide bridge. J. Med. Chem. 2002;45:4391–4394. doi: 10.1021/jm025549i. [DOI] [PubMed] [Google Scholar]
- HAY D.W.P., LUTTMANN M.A., DOUGLAS S.A. Human U-II is a potent spasmogen of primate airway smooth muscle. Br. J. Pharmacol. 2000;131:10–12. doi: 10.1038/sj.bjp.0703533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIE S., YASUDA S., TSURUMAKI Y., SOMEYA A., SAITO T., OKUMA Y., NOMURA Y., HIRABAYASHI T., MURAYAMA T. Contraction of isolated guinea-pig ileum by urotensin II via activation of ganglionic cholinergic neurons and acetylcholine release. Neuropharmacology. 2003;45:1019–1027. doi: 10.1016/s0028-3908(03)00272-7. [DOI] [PubMed] [Google Scholar]
- ITOH H., MCMASTER D., LEDERIS K. Functional receptors for fish neuropeptide urotensin II in major rat arteries Eur. J. Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- KINNEY W.A., ALMOND H.R., JR, QI J., SMITH C.E., SANTULLI R.J., DE GARAVILLA L., ANDRADE-GORDON P., CHO D.S., EVERSON A.M., FEINSTEIN M.A., LEUNG P.A., MARYANOFF B.E. Structure–function analysis of urotensin II and its use in the construction of a ligand-receptor working model. Angew. Chem. Int. Ed. Engl. 2002;41:2940–2944. doi: 10.1002/1521-3773(20020816)41:16<2940::AID-ANIE2940>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- LABARRÈRE P., CHATENET D., LEPRINCE J., MARIONNEAU C., LOIRAND G., TONON M.-C., DUBESSY C., SCALBERT E., PFEIFFER B., RENARD P., CALAS B., PACAUD P., VAUDRY H. Structure–activity relationships of human urotensin II and related analogues on rat aortic ring contraction. J. Enz. Inhib. Med. Chem. 2003;18:77–88. doi: 10.1080/1475636031000093507. [DOI] [PubMed] [Google Scholar]
- LIU Q., PONG S.S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., JR, DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVANS J.F., CASKEY T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- LORETZ C.A., HOWARD M.E., SIEGEL A.J. Ion transport in goby intestine: cellular mechanism of urotensin II stimulation. Am. J. Physiol. 1985;249:G284–G293. doi: 10.1152/ajpgi.1985.249.2.G284. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI M., SUGO T., ABE M., SHIMOMURA Y., KURIHARA M., KITADA C., KIKUCHI K., SHITANI Y., KUROKAWA T., ONDA H., NISHIMURA O., FUJINO M. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14) Biochem. Biophys. Res. Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.-P., WANG Z., MCNEILL A.M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nat. Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- RIVAS R.J., NISHIOKA R.S., BERN H.A. In vitro effects of somatostatin and urotensin II on prolactin and growth hormone secretion in tilapia, Oreochromis mossambicus. Gen. Comp. Endocrinol. 1986;63:245–251. doi: 10.1016/0016-6480(86)90162-0. [DOI] [PubMed] [Google Scholar]
- ROBERTS W.M., DOUGLASS E.C., PEIPER S.C., HOUGHTON P.J., LOOK A.T. Amplification of the gli gene in childhood sarcomas. Cancer Res. 1989;49:5407–5413. [PubMed] [Google Scholar]
- ROSSOWSKI W.J., CHENG B.-L., TAYLOR J.E., DATTA R., COY D.H. Human urotensin II-induced aorta ring contractions are mediated by protein kinase C, tyrosine kinases and Rho-kinase: inhibition by somatostatin receptor antagonists. Eur. J. Pharmacol. 2002;438:159–170. doi: 10.1016/s0014-2999(02)01341-9. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., MOLENAAR P., O'BRIEN D.M. Cardiostimulant effects of U-II in human heart in vitro. Br. J. Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAETRUM-OPGAARD O.S., NOTHACKER H., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE MELLIONNEC E., BERTOGLIO J., SCALBERT E., PACAUD P., LOIRAND G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- SHERIDAN M.A., BERN H.A. Both somatostatin and the caudal neuropeptide, urotensin II, stimulate lipid mobilization from coho salmon liver incubated in vitro. Reg. Pept. 1986;14:333–344. doi: 10.1016/0167-0115(86)90175-8. [DOI] [PubMed] [Google Scholar]
- SILVESTRE R.A., RODRIGUEZ-GALLARDO J., EGIDO E.M., MARCO J. Inhibition of insulin release by urotensin II: a study on the perfused rat pancreas. Horm. Metab. Res. 2001;33:379–381. doi: 10.1055/s-2001-15414. [DOI] [PubMed] [Google Scholar]
- STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., KIRK A., BERRY C., RICHARDSON M., MACLEAN M.R. Potent vasodilator responses to human U-II in human pulmonary and abdominal resistance arteries: comparison with adrenomedullin and other vasodilators in pulmonary arteries. Am. J. Physiol. 2001;280:H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- STRATTON M.R., REEVES B.R., COOPER C.S. Misidentified cell. Nature. 1989;337:311–312. doi: 10.1038/337311c0. [DOI] [PubMed] [Google Scholar]
- TAL M., AMMAR D.A., KARPUJ M., KRIZHANOVSKY V., NAIM M., THOMPSON D.A. A novel putative neuropeptide receptor expressed in neural tissue, including sensory epithelia. Biochem. Biophys. Res. Commun. 1995;209:752–759. doi: 10.1006/bbrc.1995.1563. [DOI] [PubMed] [Google Scholar]
- TZANIDIS A., HANNAN R.D., THOMAS W.G., ONAN D., AUTELITANO D.J., SEE F., KELLY D.J., GILBERT R.E., KRUM H. Direct actions of urotensin-II on the heart: implications for cardiac fibrosis and hypertrophy. Circ. Res. 2003;93:246–253. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- WATANABE T., KOBA S., KATAGIRI T., PAKALA R., BENEDICT C.R. Lysophosphatidylcholine potentiates the mitogenic effect of various vasoactive compounds on rabbit aortic smooth muscle cells. Jap. Heart J. 2002;43:409–416. doi: 10.1536/jhj.43.409. [DOI] [PubMed] [Google Scholar]
- WATSON A.M., LAMBERT G.W., SMITH K.J., MAY C.N. Urotensin II acts centrally to increase epinephrine and ACTH release and cause potent inotropic and chronotropic actions. Hypertension. 2003;42:373–379. doi: 10.1161/01.HYP.0000084633.85427.E6. [DOI] [PubMed] [Google Scholar]
- ZILTENER P., MUELLER C., HAENIG B., SCHERZ M.W., NAYLER O. Urotensin II mediates ERK1/2 phosphorylation and proliferation in GPR14-transfected cell lines. J. Recept. Signal Transduct. Res. 2002;22:155–168. doi: 10.1081/rrs-120014593. [DOI] [PubMed] [Google Scholar]