Abstract
Irbesartan is a promising antihypertensive drug with beneficial effects on atherosclerotic processes. In the progression of atherosclerosis, human T-lymphocytes play an important role, but it is not yet known how irbesartan modulates human T-lymphocytes activation. To gain insight into the mechanisms by which irbesartan acts, we investigated its effects on human T-lymphocytes.
Primary human T-lymphocytes were isolated from whole blood. Cytokines were determined by ELISA. Activator protein-1 (AP-1) and related protein activities were determined by electrophoretic mobility shift assays, kinase assays, Western blotting and transfection assays.
Irbesartan inhibited the production of both tumor necrosis factor-alpha and interferon-gamma by activated T-cells, especially at therapeutic concentrations. Further investigation at the molecular level indicated that the inhibition of activated human T-lymphocytes specifically correlated with the downregulation of AP-1 DNA-binding activity. In the Jurkat T-cell line, irbesartan also inhibited AP-1 transcriptional activity. Finally, we revealed that irbesartan is unique in its ability to inhibit the activation of both c-Jun NH2-terminal protein kinase and p38 MAPK.
Our studies show that irbesartan may modulate inflammation-based atherosclerotic diseases through a cell-mediated mechanism involving suppression of human T-lymphocytes activation via downregulation of AP-1 activity.
Keywords: Activator protein-1, atherosclerosis, irbesartan, T-lymphocyte
Introduction
Atherosclerosis, a chronic disease mainly involving inflammatory infiltrates such as macrophages and T-lymphocytes, is associated with activation of the immune system (Ross, 1999; Laurat et al., 2001). Specifically, T-lymphocytes are among the key inflammatory cells attracted to the sites of atherosclerosis. Together with monocytes, they can pass through the arterial intima at an early stage of the disease (Libby & Hansson, 1991). Moreover, as shown by expression of activation markers, considerable numbers of these cells are activated (Hansson et al., 1989b). They are also thought to replicate in atherosclerotic plaques through recognition of local antigens. In turn, these activated cells may modulate the functions of other cells in the atherosclerotic plaque and play a vital role in atherogenesis by being the initiators and regulators of immune reactions (Zhou et al., 2000).
In the pathogenesis of cardiovascular diseases such as atherosclerosis, hypertension and myocardial infarction, angiotensin II (AII) plays an important role (Berk et al., 1989). Many of the proatherosclerotic effects of AII are mediated by the binding of the peptide to the type 1 angiotensin receptors (AT1 receptors). Further, AII contributes to disease progression by virtue of its toxic effects, which interfere with the normal functions of the cardiovascular system (Lonn et al., 1994; Keidar et al., 1997; Brasier et al., 2002). In addition, the production of reactive oxygen species (ROS) can be induced by the action of AII, thus promoting early pathogenesis of atherosclerosis (Griendling et al., 1994; Ushio-Fukai et al., 1996; Harrison, 1997; Kunsch & Medford, 1999). AII also activates transcription factors that mediate the expression of the tumor necrosis factor (TNF) gene and thus induces biosynthesis of TNF, which has been identified in atherosclerotic lesions and plays a role in atherogenesis (Chua et al., 1998; Klahr & Morrissey, 1998; Kalra et al., 2002).
Irbesartan can inhibit the action of AII via specific, selective noncompetitive antagonism of AT1 receptors, which mediate most of the known physiological activities of AII (Timmermans et al., 1993). Therefore, irbesartan provides good 24-h blood-pressure control in patients with mild to moderate hypertension (Schiffrin, 2002). The efficacy of irbesartan in patients with heart failure has produced encouraging results (Havranek et al., 1999). In a genetically hypercholesterolemic rabbit model, irbesartan causes inhibition of atherosclerosis in aorta (Hope et al., 1999). Several studies have also proved that it could suppress the atherosclerotic processes by inhibiting the intravascular oxidative state, the inflammatory components of lesion progression and even the aggregation of platelets (Dol et al., 2001; Khan et al., 2001; Navalkar et al., 2001; Li et al., 2000). Thus, the therapeutic effects of irbesartan may potentially include anti-inflammatory properties specifically linked to human T-lymphocytes, which have been reported to possess AT1 receptors (Rodriguez-Iturbe et al., 2001). From the above data, it is reasonable to propose the hypothesis that irbesartan may produce its beneficial clinical results, in part, by modulating the function of human T-cells. In the present study, we demonstrated that irbesartan suppressed, to varying degrees, the production of specific cytokines and their associated activator protein-1 (AP-1) signaling pathways in activated human T-cells.
Methods
Preparation of irbesartan
Irbesartan was kindly provided by Sanofi-Synthelabo Pharmaceutical, France. Prior to experiments, the drug was dissolved in dimethyl sulfoxide (DMSO) to make a concentrated stock solution. For the experiments, the required concentrations of irbesartan were achieved by further dilution of the concentrated stock solution with culture medium. The final concentration of DMSO in the experimental solutions was consistently less than 0.05% and at this concentration no cytotoxic effects on the viability of T-cells were detected. As previously reported, at this concentration DMSO also had no effect on the signaling pathways of human T-cells (Cheng et al., 2003; Yang et al., 2003).
Preparation of human peripheral T lymphocytes
Human T-lymphocytes were isolated and purified from whole blood as previously reported (Lai et al., 1995). Briefly, buffy coat from the blood bank (Taipei, Taiwan) was mixed with Ficoll-Hypaque and after centrifugation the layer of mononuclear cells was collected. After lysis of red blood cells, the peripheral blood mononuclear cells (PBMC) were readily obtained. The PBMC were then incubated with L243 (anti-DR; American Type Culture Collection (ATCC), Rockville, MD, U.S.A.), OKM1 (anti-CD11b; ATCC) and LM2 (anti-Mac1; ATCC) antibodies for 30 min at 4°C. The cells were then washed with medium containing 0.1% fetal bovine serum and incubated with magnetic beads conjugated with goat anti-mouse IgG (R&D, Minneapolis, MN, U.S.A.). The antibody-coated cells were then removed with a magnet. Following repetition of the above procedures, the T-cells obtained were more than 98% pure, as determined by the percentage of CD3+ cells using flow cytometry (Beckton Dickinson, Mountain View, CA, U.S.A.).
Measurement of nonspecific cytotoxicity
Drug toxicity in human peripheral blood T-cells was examined using the trypan blue exclusion method, the LDH detection assay (according to the manufacturer's instructions (Roche, Indianapolis, IN, U.S.A.)) and the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay (Mosmann, 1983).
Cell stimulation
To activate the cells, we used the following stimuli at the concentrations indicated: phorbol 12-myristate 13-acetate (PMA, Sigma, U.S.A.) at 5–20 ng ml−1; ionomycin (Sigma, U.S.A.) at 1 μM; phytohemagglutinin (PHA, Sigma, U.S.A.) at 10 μg ml−1; immobilized anti-CD3 mAb (OKT3, ATCC) at 10 μg ml−1; soluble anti-CD28 mAb (clone 9.3, kindly provided by Dr Carl June, Naval Institute, Bethesda, NIH) at 1 μg ml−1; H2O2 (Sigma, U.S.A.) at 10 μM (Gilston et al., 2001); TNF-α (Sigma, U.S.A.) at 2 ng ml−1 and IL-1β (Sigma, U.S.A.) at 10 ng ml−1. The cells were incubated with a series of stimuli for the times indicated and then cells or supernatants were collected for further analysis.
Cytokine production assay
The determination of cytokine concentrations was performed, with modifications, according to the manufacturer's instructions (R&D Systems, Inc.) and our previous report (Lai et al., 1999).
Nuclear extract preparation
Nuclear extracts were prepared as previously described (Lai et al., 1995). Briefly, cells (2 × 106 ml−1) were incubated at 4°C in 70 μl of buffer A (10 mM HEPES, pH 7.9; 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM PMSF and 3.3 μg ml−1 aprotinin) for 15 min, with occasional gentle vortexing. The swollen cells were then centrifuged at 14,000 r.p.m. for 3 min. After removal of the supernatant (cytoplasmic extract), the pelleted nuclei were washed with 70 μl buffer A and centrifuged at 14,000 r.p.m. for 3 min. Subsequently, the cell pellet was resuspended in 25 μl buffer C (20 mM HEPES, pH 7.9; 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 1 mM DTT, 0.5 mM PMSF and 3.3 μg ml−1 aprotinin) and incubated at 4°C for 30 min with occasional vigorous vortexing. Then, the mixture was centrifuged at 14,000 r.p.m. for 20 min and the supernatant was used as the nuclear extract.
Electrophoretic mobility shift assay (EMSA)
The oligonucleotides containing the NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; 3′-TGA ACT CCC CTG AAA GGG TCC G-5′) and AP-1 (5′-CGC TTG ATG ACT CAG CCG GAA-3′; 3′-GCG AAC TAC TGA GTC GGC CTT-5′) binding sites were purchased and used as the DNA probes (Promega). The DNA probes were radiolabeled with [γ-32P]ATP using T4 kinase (Promega). For the binding reaction, the radiolabeled NF-κB or AP-1 probe was incubated with 5 μg of nuclear extract. The binding buffer contained 10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 5% glycerol and 2 μg poly(dI-dC). The reaction mixtures were left at room temperature for 20 min to allow the binding reaction to proceed. The specificity of binding was also examined using competition assays with a 100-fold molar excess of unlabeled oligonucleotide. If competitor DNAs were added, they were preincubated for 10 min before the addition of the radiolabeled probes. The final reaction mixtures were analyzed in a 6.6% nondenaturing polyacrylamide gel with 0.5 × Tris-borate/EDTA (TBE) as the electrophoresis buffer.
Transient transfections and luciferase assays
The transfection assays were performed as previously described, with some modifications (Lai et al., 1995). In order to reduce cell damage, we used the transfection reagent TransFast™ (Promega) to transfect plasmids into cells instead of using electroporation. Briefly, on the day of the transfection, Jurkat T-cells were adjusted to 1 × 106 ml−1 in serum-free media and were evenly mixed with 2 μg of reporter plasmid pNF-κB-Luc or pAP-1-Luc (Stratagene, La Jolla, CA, U.S.A.) and TransFast™ transfectant (6 μl) in triplicate. At 48 h after transfection, the cells were equally distributed and pretreated or not with various dosages of irbesartan and then stimulated with or without PMA and ionomycin for 24 h. Subsequently, the cell pellets were collected, total cell lysates were prepared and luciferase activity was established by protein determination according to the manufacturer's instructions (Promega). Jurkat cells transfected with reporter construct alone were used to calculate basal activity. Transfections were performed in at least three independent experiments, each consisting of triplicate determinations. Data are expressed relative to control luciferase activity, normalized to protein, and presented as means±s.d.
Western blotting
ECL Western blotting (Amersham, Arlington Heights, IL, U.S.A.) was performed according to the manufacturer's instructions, with some modifications. Briefly, equal amounts of total cell extract were analyzed by 10% SDS–PAGE. The proteins were then transferred to a nitrocellulose filter. For immunoblotting, the nitrocellulose filter was incubated with TBS-T containing 5% nonfat milk (milk buffer) for 2 h, and then blotted with antisera against JNK (Santa Cruz Biotechnology, U.S.A.) for 60–120 min. After washing with milk buffer for 20 min, the filter was incubated with antibodies conjugated to horseradish peroxidase at a dilution of 1 : 500 for 60 min. Finally, the filter was reacted with the substrate for 10–60 s and exposed to an X-ray film.
Immunoprecipitation kinase assays
The immunoprecipitation kinase assays were performed, with modifications, according to the manufacturer's instructions (Cell Signaling Technology, Inc.) The c-Jun N-terminal kinase (JNK) substrate, GST-c-Jun fusion protein, was a kind gift from Dr S.-F. Yang (Academia Sinica, Taiwan). Myelin basic protein (MBP) used as a substrate for extracellular signal-regulated protein kinase (ERK) and p38 MAPK were purchased from Sigma, St Louis, U.S.A. The JNK antibodies for kinase assays were kindly provided by Dr Tse-Hua Tan (Baylor College of Medicine, TX, U.S.A.), and both ERK and p38 antibodies were purchased from Cell Signaling Technology. Briefly, 30 μg of total cellular extract was incubated with 3 μl of specific antibody (JNK, p38 or p44/42 ERK) in 1 ml of incubation buffer (25 mM HEPES (pH 7.7), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 2 μM leupeptin, 400 μM PMSF) for over 2 h. The mixture was then immunoprecipitated by addition of 30 μg of protein A beads and rotated at 4°C overnight. After extensive washing twice with 1 ml HEPES washing buffer (20 mM HEPES, 50 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100), twice with 1 ml LiCl washing buffer (500 mM LiCl, 100 mM Tris (pH 7.6), 0.1% Triton X-100, 1 mM DTT) and twice with 1 × kinase buffer (20 mM MOPS (pH 7.2), 2 mM EDTA, 10 mM MgCl2 0.1% Triton X-100, 1 mM DTT) for JNK, or with 1 ml incubation buffer and 0.5 ml 1 × kinase buffer for p38 and ERK, the pellet was resuspended in 2 × kinase buffer (40 mM MOPS (pH 7.2), 4 mM EDTA, 20 mM MgCl2, 0.2% Triton X-100, 2 mM DTT). The mixture was incubated for 30 min at room temperature with GST-c-Jun or MBP as substrate, cold ATP (250 μM) and [γ-32P]ATP. Reactions were stopped by the addition of 10 μl of 5 × SDS sample buffer, and the reaction mixture was boiled for 5–10 min. The final reaction mixture was analyzed in 12–15% SDS–PAGE. The dried gel was visualized by exposure to X-ray film.
Statistics
The results are expressed as means±s.d. A paired or unpaired Student's t-test was used to determine the significance of differences; a value of P<0.05 was considered statistically significant.
Results
Cytotoxic effects of irbesartan on human T lymphocytes
The results of trypan blue exclusion, LDH and MTT assays performed on human T-lymphocytes indicated that neither therapeutic concentrations of irbesartan nor DMSO concentrations less than 0.05% (DMSO was used as a vehicle in the experiments) was cytotoxic or had any effect on cell viability (data not shown).
Effects of irbesartan on cytokine production by activated human T-lymphocytes
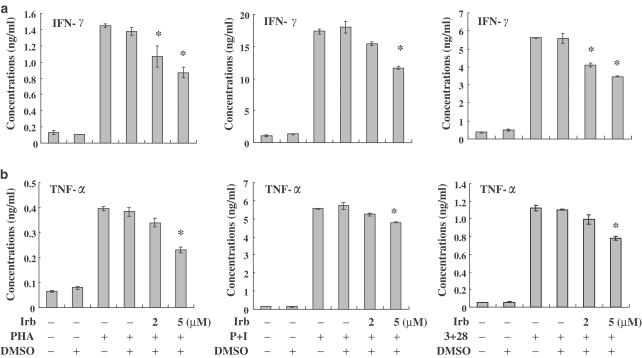
T-cells were pretreated with irbesartan for 2 h and then PHA, PMA+ionomycin or anti-CD3+anti-CD28 were used to activate human cells for another 24 h. We found that irbesartan inhibited the stimulus-induced production of the cytokines IFN-γ and TNF-α by human T-cells in a dose-dependent manner (Figure 1a and b), with IFN-γ being more susceptible to inhibition by irbesartan than TNF-α. However, irbesartan had neutral effects on the production of IL-2 and IL-4 (data not shown). These data show that the potential therapeutic benefits of irbesartan might include the ability to differentially regulate the production of cytokines. Figure 1 also shows that the DMSO concentrations used in the study do not interfere with the interpretation of results.
Figure 1.
Effects of irbesartan (Irb) on cytokine production from activated human T-cells. Human peripheral blood T-cells at 1 × 106 ml−1 were pretreated with various concentrations of irbesartan (2–5 μM) for 2 h and then stimulated with PHA, PMA+ionomycin (P+I) or anti-CD3+anti-CD28 mAb (3+28) for another 24 h. The supernatants were collected for measurements of IFN-γ (a) and TNF-α (b). The data are representative of at least three different donor cells and are shown as means±s.d. * denotes the statistical significance (P-value <0.05) compared with stimulated control cells.
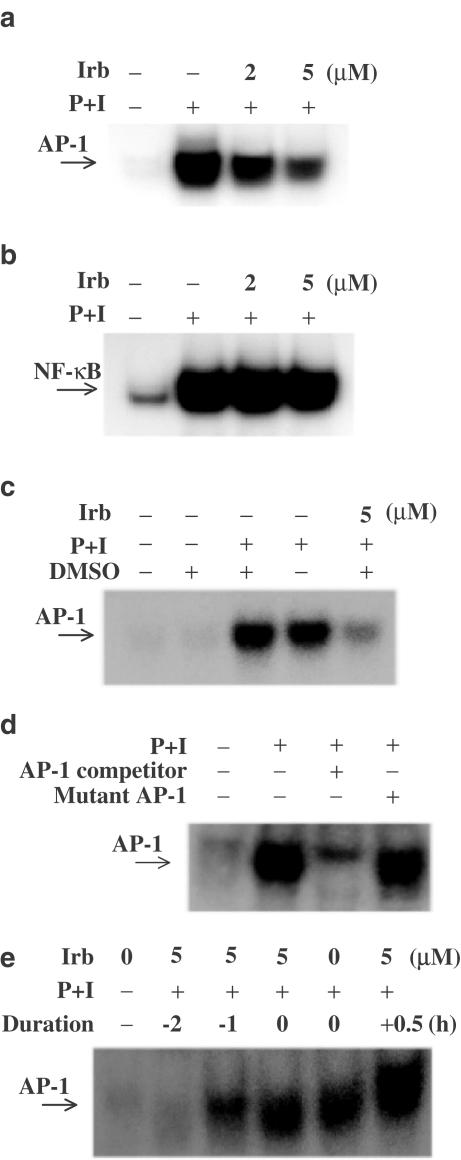
Irbesartan downregulated AP-1, but not NF-κB DNA-binding activity in activated human T lymphocytes
Since irbesartan is able to inhibit cytokine production, it may modulate some of the signaling molecules that are commonly involved in T-lymphocyte activation. We therefore examined the effects of irbesartan on the activation of the transcription factors AP-1 and NF-κB, a family of proteins extensively shared by a variety of signaling pathways. After stimulation with PMA+ionomycin in the presence or absence of irbesartan, the T-lymphocytes were incubated with radiolabeled AP-1 or NF-κB probes.
We showed in a parallel experiment that irbesartan effectively inhibited AP-1, but not NF-κB DNA-binding activity in human T-lymphocytes activated by PMA+ionomycin (Figure 2a and b). We also showed that DMSO used as a vehicle at less than 0.05% did not affect the results (Figure 2c). For the competition assays, cell lysates were preincubated with a 100-fold molar excess of unlabeled AP-1 wild-type or mutant oligonucleotide (5′-CGC TTG ATG ACT TGG CCG GAA-3′; 3′-GCG AAC TAC TGA ACC GGC CTT-5′). The wild-type AP-1 oligonucleotide caused a relatively complete disappearance of the band but the mutant oligonucleotide did not, indicating the specificity of AP-1 (Figure 2d). It was also important to determine the time course of irbesartan pretreatment that would block DNA binding of AP-1 before the addition of the stimulus. Then cells were preincubated at 37°C with 5 μM irbesartan for the times indicated and then tested for AP-1 activation for 60 min with or without PMI+ionomycin. Negative values indicate how long (h) irbesartan was present before the addition of PMI+ionomycin; ‘0' indicates that irbesartan was coincubated with PMI+ionomycin; and ‘+0.5' indicates that irbesartan was added 30 min after PMI+ionomycin. When the cells were pretreated with irbesartan for over 60 min, inhibition of AP-1 was observed. Cotreatment or post-stimulus treatment with irbesartan did not inhibit AP-1 activation significantly (Figure 2e). These data show that inhibition of AP-1 is not due to the cytotoxic effect of irbesartan, and that for irbesartan to suppress AP-1 activation it is necessary to inactivate the signaling molecules associated with AP-1 before initiation of stimulation.
Figure 2.
Irbesartan (Irb) blocked AP-1, but not NF-κB DNA-binding activity stimulated by PMA+ionomycin (P+I). Human peripheral blood T-cells at 2 × 106 ml−1 were pretreated with various concentrations of irbesartan for 2 h and then stimulated or not with P+I for 1 h. (a) Irbesartan inhibited AP-1 DNA-binding activity. (b) The activation of NF-κB was not inhibited by irbesartan up to 5 μM. (c) AP-1 activity induced by P+I was not influenced by addition of DMSO at less than 0.05%. (d) The specificity of AP-1 binding activity was also assessed by competition with 100 × unlabeled AP-1. Coincubation of nuclear extracts with 100-fold molar excess of wild-type AP-1 led to inhibition of the retarded AP-1 band. In contrast, nuclear extracts coincubated with mutant-type AP-1 had little effect on the band density. (e) When the cells were pretreated for over 60 min with irbesartan, marked inhibition of AP-1 was observed. Cotreatment or post-stimulus treatment with irbesartan did not inhibit AP-1 activation significantly. Representative data from three different donor cells are shown.
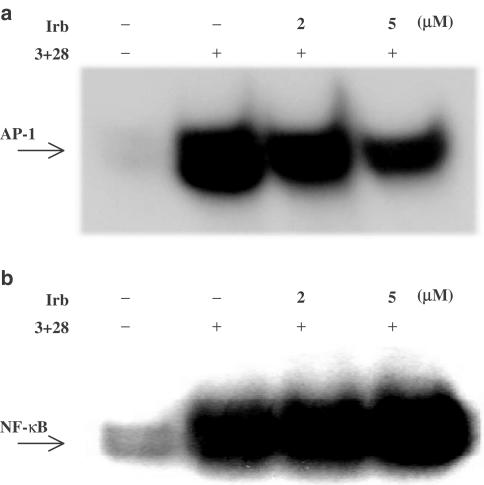
In order to explore the actual induction of the T-cell immune response more clinically, costimulation with anti-CD3+anti-CD28 mAb was performed to enhance T-cell activation. The results show that irbesartan effectively downregulated the AP-1, but not the NF-κB DNA-binding activity stimulated by anti-CD3+anti-CD28 mAb (Figure 3a and b).
Figure 3.
Irbesartan (Irb) blocked AP-1, but not NF-κB DNA-binding activity stimulated by anti-CD3+anti-CD28 mAb. Human peripheral blood T-cells at 2 × 106 ml−1 were pretreated with various concentrations of irbesartan for 2 h and then stimulated or not with anti-CD3+anti-CD28 mAb (3+28) for 14 h. (a) Irbesartan inhibited AP-1 DNA-binding activity. (b) The activation of NF-κB was not inhibited by irbesartan up to 5 μM. Representative data from three different donor cells are shown.
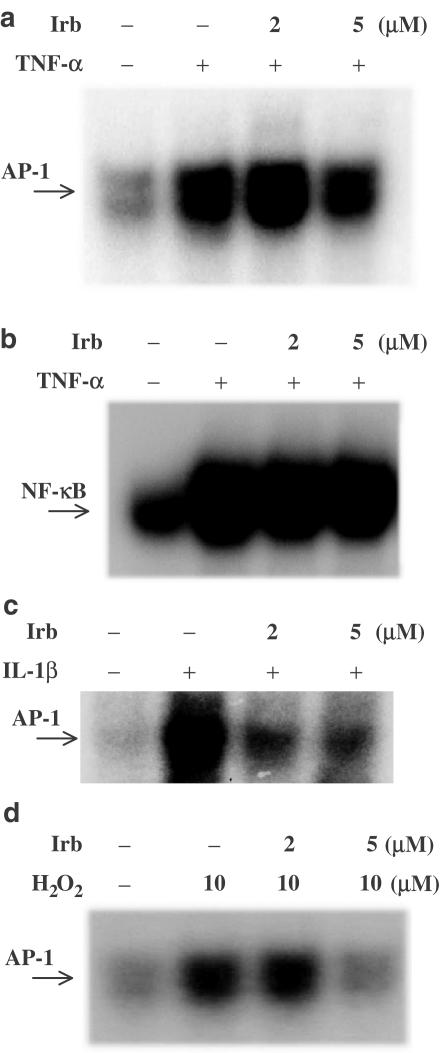
It is known that both TNF-α and IL-1 are present in human atherosclerotic lesions and are powerful inducers of local inflammation in blood vessels and elsewhere (Barath et al., 1990; Moyer et al., 1992; Kishikawa et al., 1993). IL-1 is an important costimulatory factor for T-lymphocyte activation and TNF-α is a pro-apoptotic cytokine. Their activation is involved in the cascade of proinflammatory cytokine pathways, which plays an important role in the progression of atherosclerotic cardiovascular diseases. We were therefore interested in finding whether irbesartan could modulate the AP-1 DNA-binding activity induced by TNF-α or IL-1β. As shown in Figure 4a and b, irbesartan blocked AP-1, but not NF-κB DNA-binding activity induced by TNF-α. We also demonstrated that the optimal time needed to see any effect of irbesartan on IL-1β-stimulated AP-1 DNA-binding activity in activated cells is 2 h (Figure 4c).
Figure 4.
The effects of Irbesartan (Irb) on AP-1 or NF-κB DNA-binding activity stimulated by TNF-α, IL-1β or H2O2. Human peripheral blood T-cells at 2 × 106 ml−1 were pretreated with various concentrations of irbesartan for 2 h and then stimulated or not with TNF-α for 1 h, IL-1β for 2 h or H2O2 for 3–4 h. (a) Irbesartan inhibited AP-1 DNA-binding activity induced by TNF-α. (b) The activation of NF-κB induced by TNF-α was not inhibited by irbesartan up to 5 μM. (c) Irbesartan also blocked AP-1 DNA-binding activity stimulated by IL-1β. (d) Irbesartan blocked AP-1 DNA-binding activity stimulated by H2O2. Representative data from three different donor cells are shown.
As activation of intracellular oxidative signals can modulate vascular proinflammatory processes and irbesartan has been reported to have antioxidant properties, we tested whether it could suppress AP-1 DNA-binding activity induced by H2O2. As shown in Figure 4d, irbesartan inhibited the AP-1 DNA-binding activity induced by H2O2.
Irbesartan inhibited AP-1 transcriptional activity in Jurkat T-cells
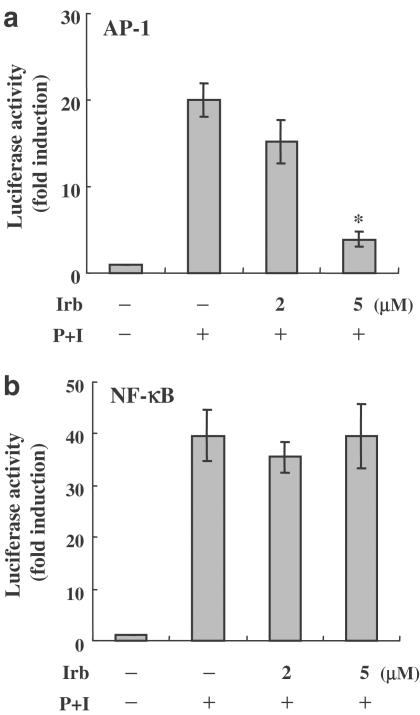
To investigate whether the inhibition of AP-1 activity by irbesartan in vitro could also be seen in vivo, we transiently transfected AP-1-luciferase reporter plasmids or NF-κB-luciferase reporter plasmids into Jurkat T-cells. Consistent with our in vitro observations, irbesartan significantly inhibited the transcriptional activity of AP-1 in a dose-dependent manner, but did not have any effect on NF-κB transcriptional activity (Figure 5a and b).
Figure 5.
The effects of irbesartan (Irb) on the transcriptional activity of both AP-1 and NF-κB in Jurkat T-cells. Transient transfections and luciferase assays were performed as described in Methods. Values were expressed as luciferase activity (fold activation) compared with unstimulated cells. (a) Irbesartan markedly attenuated the transcriptional activity of AP-1. (b) The activation of NF-κB was not inhibited by irbesartan at 5 μM. At least three independent experiments were performed. Representative data are shown as means±s.d. * denotes statistical significance (P-value <0.05) compared with stimulated control cells.
Selective inhibitory effects of irbesartan on both JNK and p38 MAPK from activated human T-cells
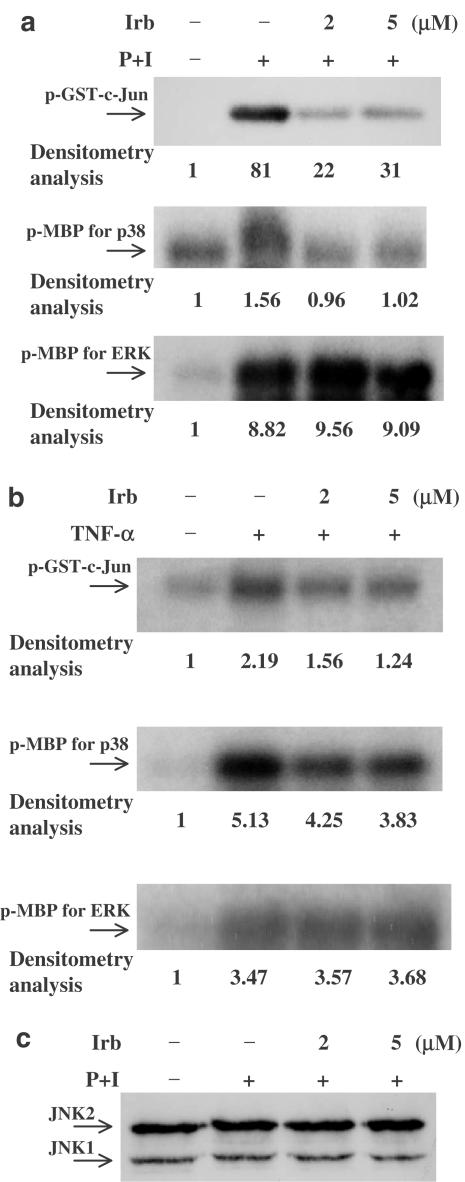
The inhibition of AP-1 DNA-binding activity by irbesartan raises the question of whether mitogen-activated protein kinases (MAPKs) are affected, because MAPKs are the upstream signaling regulators of AP-1 activation. We evaluated the effects of irbesartan on PMA+ionomycin- or TNF-α-stimulated activation of the MAPK cascades, especially JNK, p38 MAPK and ERK activities. As shown in Figure 6a and b, irbesartan inhibited the activation of JNK and p38 MAPK at physiologically effective concentrations, but had less effect on ERK activity. Figure 6c showed that stimulant treatment for a short time, with or without irbesartan pretreatment, did not affect the amount of JNK, indicating that loading of protein was equal.
Figure 6.
Irbesartan (Irb) treatment inhibits JNK, p38 MAPK, and, to a lesser extent, ERK signaling pathways. Human peripheral blood T-cells at 2 × 106 ml−1 were pretreated or not with 2–5 μM irbesartan for 2 h and then stimulated with PMA+ionomycin or TNF-α for 15–20 min. MAPK activities were measured based on phosphorylation of GST-c-Jun fusion protein (c-Jun) substrate and MBP substrate. (a) PMA+ionomycin activated JNK, p38 MAPK and ERK. Irbesartan pretreatment suppressed the activity of JNK and p38 MAPK, but had little effect on the activity of ERK. (b) TNF-α also activated JNK, p38 MAPK and ERK and irbesartan pretreatment suppressed the activity of JNK and p38 MAPK, but had little effect on the activity of ERK. (c) Under these conditions, irbesartan had no effect on total JNK protein levels. Lane intensity was determined by densitometry using AlphaEaseFC™ Software Version 3.1.2 (Alpha Innotech Corporation). The lane density of the medium was taken as one and the relative (fold) induction is indicated. Representative data from three different donor cells are shown.
Discussion
Biochemical and clinical studies have indicated that atherosclerosis is associated with the renin–angiotensin system. Therefore, it was postulated that one of the molecular mechanisms by which irbesartan achieves its pharmacological effects is dependent on modulation of the inflammatory factors associated with this system. Some studies have focused on the effects of the interaction between AII and antagonists of AT1 receptors on modulation of the activation of monocytes and production of their related chemokines or cytokines (Strawn et al., 1999; Kintscher et al., 2001; Sakamoto et al., 2001). Here, we particularly explored the regulatory functions of irbesartan on human peripheral T-lymphocytes activated by various stimuli.
Our current data show that irbesartan can inhibit both IFN-γ and TNF-α secretion from activated T-lymphocytes via downregulation of AP-1 DNA-binding activity. We have also shown that irbesartan specifically and significantly inhibits AP-1 DNA-binding activity in a dose-dependent fashion, but has less effect on another transcription factor, NF-κB, although NF-κB is primarily an oxidative stress-responsive transcription factor (Mihm et al., 1995). We next examined the effects of irbesartan on AP-1-dependent transcriptional activation and found that, in transient transfections, irbesartan can diminish stimulus-induced luciferase activity of AP-1. As is known, AP-1 is composed of multiple protein complexes with dimerization between Fos and Jun proteins. AP-1-mediated signaling pathways can be regulated by upstream MAPKs including JNK, ERK and p38 MAPK. The activation of MAPKs and their substrate molecules are related to various external stimuli and may be among the molecular mechanisms underlying the synergism among cardiovascular risk factors and pathogenesis of atherosclerosis (Lo & Cruz, 1995; Sundaresan et al., 1995; Kyriakis, 1999; Gertzberg et al., 2000). Therefore, it is relevant to define further the molecular mechanisms whereby irbesartan acts on MAPK pathways in human T-cells. Our results show that irbesartan can attenuate the activities of both JNK and p38 MAPK, activation of which would be expected to result in phosphorylation of c-Jun and ATF-2 transcription factors, increasing their trans-activating activity. Remarkably, irbesartan is able to suppress AP-1 DNA-binding activity via dual regulation of JNK and p38 MAPK.
As reported, matrix metalloproteinase expression and monocyte chemoattractant protein-1, both of which feature in atherosclerosis-related diseases and precipitation of acute coronary syndrome, are potentially regulated by AP-1 (Dollery et al., 1995; Jormsjö et al., 2000; Viedt et al., 2002). In addition, due to its sensitivity to redox changes, AP-1 responds to low-density lipoprotein treatment, which can also induce vascular cell adhesion molecule-1, implicated in atherogenesis (Meyer et al., 1993; Xanthoudakis et al., 1994; Lin et al., 1996). In neointimal formation after vascular injury, AP-1 plays a critical part (Ahn et al., 2002), and blocking the MAPK-AP-1 signaling pathway is known to be associated with the inhibition of neointima hyperplasia in mouse vein grafts (Hu et al., 1999). Downregulation of AP-1 may be linked to mechanisms that attenuate inflammation-based atherogenesis (Rivard et al., 2000; Maggi-Capeyron et al., 2001). Strategies aimed at pharmacologically manipulating AP-1 activity will be expected to be of therapeutic importance in the future.
It has been known for some time that AP-1 has a role in regulating the gene expression of several cytokines involved in inflammation (Karin et al., 1997). T-lymphocytes, when activated by target cells, can secrete some of these cytokines to recruit other cells, damage target cells and/or provide cell–cell interactions at the sites of inflammation-induced atherosclerosis. Therefore, the cytokine networks may play a vital role in mediating the host inflammatory response. IFN-γ, a secretory product of activated T-cells, can not only downregulate expression of macrophage scavenger receptors in atherosclerotic plaque (Geng et al., 1995), but can also induce some antigens which are not normally present on smooth muscle cells (Hansson et al., 1989a). Thus, a shift towards T-lymphocytes, which are able to secrete high levels of IFN-γ, may contribute to the development of a complicated atherosclerotic lesion (Hansson et al., 1989b; Geng et al., 1995). In addition, TNF-α, a pleiotropic cytokine, has been shown to play a role in both vascular and bone pathophysiology (Tintut et al., 2000). It shares several activities with IFN-γ, such as increasing expression of adhesion molecules on endothelial cells, synthesis of matrix metalloproteinases and expression of the scavenger receptor by smooth muscle cells (Galis et al., 1994; Li et al., 1995). TNF-α can also induce production of ROS in endothelial cells and activate oxidative stress-responsive genes (Larrick & Wright, 1990). In hyperlipidemic rabbits and atherosclerotic plaques, the existence of TNF-α has been demonstrated using immunohistochemical techniques and the polymerase chain reaction (Rus et al., 1991; Fleet et al., 1992). There is no doubt that TNF-α influences many aspects of atherogenesis. From the point of view of their pathological and immunohistochemical features, atherosclerotic lesions are accumulations of inflammatory cells and cytokines, accompanied by a chronic inflammatory process (Hansson et al., 1989b; Stemme & Hansson, 1994). From the above evidence, an increase in cytokine-mediated expression resulting from the overall inflammatory process may be critical. Further, the combined effects of inflammatory cytokines may contribute to more deleterious clinical outcomes. Our present observations emphasize a potential role for irbesartan in local regulation of inflammation in atherosclerotic lesions and providing insights into atherogenesis.
After oral intake, the absolute bioavailability of irbesartan is around 60–80% (prescription information for irbesartan, Bristol-Myers Squibb Pharma EEIG, Ickenham, England). Its oral bioavailability is higher than losartan or valsartan (prescription information for losartan, package insert, Merk & Co., West Point, PA, U.S.A., 1997; prescription information for valsartan, package insert, Novartis East Hanover, NJ, U.S.A., 1997). It benefits the patients with hypertension or heart failure, improves endothelial function, and decreases markers of inflammation associated with atherosclerosis. These therapeutic effects are related to its plasma concentrations. As reported, the peak plasma concentration of irbesartan attained within 1.5–2 h after a single oral dose of 300 mg and maintained at steady state after once daily oral dosing for 7 days in healthy persons was below 7 μM (Brunner, 1997). The concentrations of irbesartan used in our study, ranging from 2 to 5 μM, are within the physiologically effective levels in humans and can modulate the functions of human T-cells. These results further emphasize the clinical relevance in our study.
A major feature of this study is the use of physiological stimulators such as antigen-presenting cells. Initially, we used nonspecific stimulators (mitogens), PMA+ionomycin and PHA to study the effects of irbesartan on the cytokine production. As is known, the CD28 antigen can be present on human peripheral T-lymphocytes containing CD3 (T-cell receptor) (June et al., 1990). The CD28 antigen functions as the ligand for CD80 (B7-1) and CD86 (B7-2), which are present on antigen-presenting cells (Levine et al., 1995; Fleischer et al., 1996). Interaction of the CD28 antigen with CD80 and/or CD86 antigens co-stimulates CD3 antigen/T-cell antigen receptor (TCR)-dependent T-cell-mediated proliferation and cytotoxicity (Lanier et al., 1995). To extend our study, therefore, we required the specific stimulator, anti-CD3+anti-CD28, to mimic the physiological T-cell activation triggered by antigen-presenting cells. In our study, we obtained similar in vitro results using mitogens or anti-CD3+anti CD28. Further study is needed to clarify these interesting relationships.
A limitation of this study is that we only show the in vitro effects of irbesartan on human T-cells from human healthy donors and the effect of irbesartan on AP-1 transcriptional activity in Jurkat T-cells. Also, we did not investigate the effects of irbesartan in human T-cells activated by AngII, because AngII stimulates NF-κB activation in human monocytes, but not in lymphocytes (Kranzhofer et al., 1999). We also found that AngII only weakly induced the expression of AP-1 (data not shown). However, AngII can induce the expression of both TNF-α and ROS and other inflammation-related molecules, which is relevant and important for understanding how irbesartan modulates human T-cells activated by the various stimuli used in this study. It is worth noting that another AT1 receptor antagonist, losartan, has been reported to reduce the activities of activated T-cells (Sonmez et al., 2001). However, losartan had no effect on lymphocytic angiotensin-converting enzyme and did not show antiproliferative effects in PBMC (Petrov et al., 2001a, 2001b). It is possible that losartan, which binds to PBMC, may have been removed during purification of these cells. Further, as previously reported (Godsel et al., 2003), captopril did not directly influence Ag-specific T-cell responsiveness. We suggest that although irbesartan and captopril act through similar pathways to reduce blood pressure, irbesartan may have different functional groups from captopril, and thus may exert different immunomodulatory effects on T-cells. Irbesartan may also provide a more functionally complete block of the effects of AT1 receptors than ACE inhibitors like captopril. In addition, different stimulators may trigger different kinds of signaling pathways and irbesartan could target specific stimulator-induced activation signals to selectively suppress cytokine production by human peripheral T-cells. Moreover, it is possible that T-cells from different models may produce different results, and differences between the in vitro and in vivo effects of drugs should be also considered (Constantinescu et al., 1998; Peeters et al., 1998; Zhao & Xie, 2001). Further studies are required to delineate the effects of these drugs under various conditions. Nevertheless, our present experiments demonstrated that irbesartan can regulate the processes associated with AP-1 activity in activated human T-lymphocytes, suggesting that irbesartan may reduce inflammation and maintain the integrity of vascular cells. These discoveries may throw new light on the prevention and treatment of atherosclerosis associated with inflammation-induced injuries. We expect that future clinical studies will examine the in vitro effects of irbesartan in patients with cardiovascular diseases.
In conclusion, the present study has investigated possible therapeutic mechanisms for the action of irbesartan in order to obtain a better insight into its immunomodulatory reactions in human T-cells. Clinically, irbesartan is widely used and may exert an atheroprotective action through differential regulation of MAPK-AP-1 pathways in human T-cells, which reduces the inflammatory signals within the vessel walls.
Acknowledgments
This work was supported in part by the National Health Research Institutes (NHRI-EX92-9208SI), Taiwan, ROC. The kind gifts of JNK antibodies, GST-c-Jun fusion protein, soluble anti-CD28 mAbs and irbesartan from Drs T.-H. Tan, S.-F. Yang and Carl June, and Sanofi-Synthelabo Pharmaceutical are highly appreciated.
Abbreviations
- AII
angiotensin II
- AP-1
activator protein-1
- AT1 receptors
type 1 angiotensin receptors
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated protein kinase
- IFN-γ
interferon-gamma
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
- NF-κB
nuclear factor-kappa B
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- TBE
Tris-borate/EDTA
- TNF-α
tumor necrosis factor-alpha
References
- AHN J.D., MORISHITA R., KANEDA Y., LEE S.J., KWON K.Y., CHOI S.Y., LEE K.U., PARK J.Y., MOON I.J., PARK J.G., YOSHIZUMI M., OUCHI Y., LEE I.K. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ. Res. 2002;90:1325–1332. doi: 10.1161/01.res.0000023200.19316.d5. [DOI] [PubMed] [Google Scholar]
- BARATH P., FISHBEIN M.C., CAO J., BERENSON J., HELFANT R.H., FORRESTER J.S. Detection and localization of tumor necrosis factor in human atheroma. Am. J. Cardiol. 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- BERK B.C., VEKSHTEIN V., GORDON H.M., TSUDA T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989;13:305–314. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- BRASIER A.R., RECINOS A., III, ELEDRISI M.S. Vascular inflammation and the renin–angiotensin system. Arterioscler. Thromb. Vasc. Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- BRUNNER H.R. The new angiotensin II receptor antagonist, irbesartan. Am. J. Hypertens. 1997;10:311S–317S. doi: 10.1016/s0895-7061(97)00391-9. [DOI] [PubMed] [Google Scholar]
- CHENG S.M., YANG S.P., HO L.J., TSAO T.P., JUAN T.Y., CHANG D.M., CHANG S.Y., LAI J.H. Down-regulation of c-jun N-terminal kinase-activator protein-1 signaling pathway by Ginkgo biloba extract in human peripheral blood T cells. Biochem. Pharmacol. 2003;66:679–689. doi: 10.1016/s0006-2952(03)00388-5. [DOI] [PubMed] [Google Scholar]
- CHUA C.C., HAMDY R.C., CHUA B.H.L. Upregulation of vascular endothelial growth factor by angiotensin II on rat heart endothelial cells. Biochim. Biophys. Acta. 1998;1401:187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- CONSTANTINESCU C.S., GOODMAN D.B., VENTURA E.S. Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immunol. Lett. 1998;62:25–31. doi: 10.1016/s0165-2478(98)00025-x. [DOI] [PubMed] [Google Scholar]
- DOL F., MARTIN G., STAELS B., MARES A.M., CAZAUBON C., NISATO D., BIDOUARD J.P., JANIAK P., SCHAEFFER P., HERBERT J.M. Angiotensin AT1 receptor antagonist irbesartan decreases lesion size, chemokine expression, and macrophage accumulation in apolipoprotein-E-deficient mice. J. Cardiovasc. Pharmacol. 2001;38:395–405. doi: 10.1097/00005344-200109000-00008. [DOI] [PubMed] [Google Scholar]
- DOLLERY C.M., MCEWAN J.R., HEMMEY A.M. Matrix metalloproteinases and cardiovascular disease. Circ. Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- FLEET J.C., CLINTON S.K., SALOMON R.N., LOPPNOW H., LIBBY P. Atherogenic diets enhance endotoxin-stimulated interleukin-1 and tumor necrosis factor gene expression in rabbit aortae. J. Nutr. 1992;122:294–305. doi: 10.1093/jn/122.2.294. [DOI] [PubMed] [Google Scholar]
- FLEISCHER J., SOETH E., REILING N., GRAGE-GRIEBENOW E., FLAD H.D., ERNST M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996;89:592–598. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALIS Z.S., MUSZYNSKI M., SUKHOVA G.K. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ. Res. 1994;75:181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- GENG Y.J., HOLM J., NYGREN S., BRUZELIUS M., STEMME S., HANSSON G.K. Expression of the macrophage scavenger receptor in atheroma. Relationship to immune activation and the T-cell cytokine interferon-gamma. Arterioscler. Thromb. Vasc. Biol. 1995;15:1095–1102. doi: 10.1161/01.atv.15.11.1995. [DOI] [PubMed] [Google Scholar]
- GERTZBERG N., CLEMENTS R., JASPERS I., FERRO T.J., NEUMANN P., FLESCHER E., JOHNSON A. Tumor necrosis factor-α-induced activating protein-1 activity is modulated by nitric oxide-mediated protein kinase G activation. Am. J. Resp. Cell Mol. Biol. 2000;22:105–115. doi: 10.1165/ajrcmb.22.1.3801. [DOI] [PubMed] [Google Scholar]
- GILSTON V., WILLIAMS M.A., NEWLAND A.C., WINYARD P.G. Hydrogen peroxide and tumor necrosis factor-alpha induce NF-kappa B-DNA binding in primary human T lymphocytes in addition to T cell lines. Free Radic. Res. 2001;35:681–691. doi: 10.1080/10715760100301201. [DOI] [PubMed] [Google Scholar]
- GODSEL L.M., LEON J.S., WANG K., FORNEK J.L., MOLTENI A., ENGMAN D.M. Captopril prevents experimental autoimmune myocarditis. J. Immunol. 2003;171:346–352. doi: 10.4049/jimmunol.171.1.346. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., MINIERI C.A., OLLERENSHAW J.D., ALEXANDER R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- HANSSON G.K., HELLSTRAND M., RYMO L., RUBBIA L., GABBIANI G. Interferon-γ inhibits both proliferation and expression of differentiation-specific α-smooth muscle actin in arterial smooth muscle cells. J. Exp. Med. 1989a;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSSON G.K., HOLM J., JONASSON L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am. J. Pathol. 1989b;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- HARRISON D.G. Cellular and molecular mechanisms of endothelial cell dysfunction. J. Clin. Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVRANEK E.P., THOMAS I., SMITH W.B., PONCE G.A., BILSKER M., MUNGER M.A., WOLF R.A. Dose-related beneficial long-term hemodynamic and clinical efficacy of irbesartan in heart failure. J. Am. Coll. Cardiol. 1999;33:1174–1181. doi: 10.1016/s0735-1097(98)00695-0. [DOI] [PubMed] [Google Scholar]
- HOPE S., BRECHER P., CHOBANIAN A.V. Comparison of the effects of AT1 receptor blockade and angiotensin converting enzyme inhibition on atherosclerosis. Am. J. Hypertens. 1999;12:28–34. doi: 10.1016/s0895-7061(98)00203-9. [DOI] [PubMed] [Google Scholar]
- HU Y., ZOU Y., DIETRICH H., WICK G., XU Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999;100:861–868. doi: 10.1161/01.cir.100.8.861. [DOI] [PubMed] [Google Scholar]
- JORMSJÖ S., YE S., MORITZ J., WATER D.H., DIMMELER S., ZEIHER A.M., HENNEY A., HAMSTERN A., ERIKSSON P. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ. Res. 2000;86:998–1003. doi: 10.1161/01.res.86.9.998. [DOI] [PubMed] [Google Scholar]
- JUNE C.H., LEDBETTER J.A., LINSLEY P.S., THOMPSON C.B. Role of the CD28 receptor in T-cell activation. Immunol. Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- KALRA D., SIVASUBRAMANIAN N., MANN D.L. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation. 2002;105:2198–2205. doi: 10.1161/01.cir.0000015603.84788.47. [DOI] [PubMed] [Google Scholar]
- KARIN M., LIU Z., ZANDI E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- KEIDAR S., ATTIAS J., SMITH J., BRESLOW J., HAYEK T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 1997;236:622–625. doi: 10.1006/bbrc.1997.6844. [DOI] [PubMed] [Google Scholar]
- KHAN B.V., NAVALKAR S., KHAN Q.A., RAHMAN S.T., PARTHASARATHY S. Irbesartan, an angiotensin type I receptor inhibitor, regulates the vascular oxidative state in patients with coronary artery disease. J. Am. Coll. Cardiol. 2001;38:1662–1667. doi: 10.1016/s0735-1097(01)01615-1. [DOI] [PubMed] [Google Scholar]
- KINTSCHER U., WAKINO S., KIM S., FLECK E., HSUEH W.A., LAW R.E. Angiotensin II induces migration and pyk2/paxillin phosphorylation of human monocytes. Hypertension. 2001;37:587–593. doi: 10.1161/01.hyp.37.2.587. [DOI] [PubMed] [Google Scholar]
- KISHIKAWA H., SHIMOKAMA T., WATANABE T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima: role of cell-mediated immunity in human atherogenesis. Virchows Arch. A Pathol. Anat. Histopathol. 1993;423:433–442. doi: 10.1007/BF01606532. [DOI] [PubMed] [Google Scholar]
- KLAHR S., MORRISSEY J. Angiotensin II and gene expression in the kidney. Am. J. Kidney Dis. 1998;31:171–176. doi: 10.1053/ajkd.1998.v31.pm9428470. [DOI] [PubMed] [Google Scholar]
- KRANZHOFER R., BROWATZKI M., SCHMIDT J., KUBLER W. Angiogensin II activates the proinflammatory transcription factor nuclear factor-κB in human monocytes. Biochem. Biophys. Res. Commun. 1999;257:826–828. doi: 10.1006/bbrc.1999.0543. [DOI] [PubMed] [Google Scholar]
- KUNSCH C., MEDFORD R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- KYRIAKIS J.M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr. 1999;7:217–231. [PMC free article] [PubMed] [Google Scholar]
- LAI J.H., HO L.J., KWAN C.Y., CHANG D.M., LEE T.C. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: potential immunosuppressants in transplantation immunology. Transplantation. 1999;68:1383–1392. doi: 10.1097/00007890-199911150-00027. [DOI] [PubMed] [Google Scholar]
- LAI J.H., HORVATH G., SUBLESKI J., BRUDER J., GHOSH P., TAN T.H. RelA is a potent transcriptional activator of the CD28 response element within the interleukin 2 promoter. Mol. Cell. Biol. 1995;15:4260–4271. doi: 10.1128/mcb.15.8.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANIER L.L., O'FALLON S., SOMOZA C., PHILLIPS J.H., LINSLEY P.S., OKUMURA K., ITO D., AZUMA M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- LARRICK J.W., WRIGHT S.C. Cytotoxic mechanism of tumor necrosis factor-α. FASEB J. 1990;4:3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- LAURAT E., POIRIER B., TUPIN E., CALIGIURI G., HANSSON G.K., BARIETY J., NICOLETI A. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- LEVINE B.L., UEDA Y., CRAIGHEAD N., HUANG M.L., JUNE C.H. CD28 ligands CD80 (B7-1) and CD 86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int. Immunol. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- LI H., FREEMAN M.W., LIBBY P. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J. Clin. Invest. 1995;95:122–133. doi: 10.1172/JCI117628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI P., FUKUHARA M., DIZ D.I., FERRARIO C.M., BROSNIHAN K.B. Novel angiotensin II AT(1) receptor antagonist irbesartan prevents thromboxane A(2)-induced vasoconstriction in canine coronary arteries and human platelet aggregation. J. Pharmacol. Exp. Ther. 2000;292:238–246. [PubMed] [Google Scholar]
- LIBBY P., HANSSON G.K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab. Invest. 1991;64:5–15. [PubMed] [Google Scholar]
- LIN J.H.C., ZHU Y., LIAO H.L., KOBARI Y., GROSZEK L., STEMERMAN M.B. Induction of vascular cell adhesion molecule-1 by low-density lipoprotein. Atherosclerosis. 1996;127:185–194. doi: 10.1016/s0021-9150(96)05951-5. [DOI] [PubMed] [Google Scholar]
- LO Y.Y.C., CRUZ T.F. Involvement of reactive oxygen species in cytokine and growth induction of c-fos expression in chondrocytes. J. Biol. Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- LONN E.M., YUSUF S., JHA P., MONTAGUE T.J., TEO K.K., BENEDICT C.R., PITT B. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–2069. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- MAGGI-CAPEYRON M.F., CEBALLOS P., CRISTOL J.P., DELBOSC S., LE DOUCEN C., PONS M., LEGER C.L., DESCOMPS B. Wine phenolic antioxidants inhibit AP-1 transcriptional activity. J. Agric. Food Chem. 2001;49:5646–5652. doi: 10.1021/jf010595x. [DOI] [PubMed] [Google Scholar]
- MEYER M., SCHRECK R., BAEUERLE P.A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHM S., GALTER D., DROGE W. Modulation of transcription factor NF-κB activity by intracellular glutathine levels and by variations of the extracellular cysteine supply. FASEB J. 1995;9:246–252. doi: 10.1096/fasebj.9.2.7781927. [DOI] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MOYER C.F., SAJUTHI D., TULLI H., WILLIAMS J.K. Synthesis of IL-1α and IL-1β by arterial cells in atherosclerosis. Am. J. Pathol. 1992;138:951–960. [PMC free article] [PubMed] [Google Scholar]
- NAVALKAR S., PARTHASARATHY S., SANTANAM N., KHAN B.V. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosis. J. Am. Coll. Cardiol. 2001;37:440–444. doi: 10.1016/s0735-1097(00)01138-4. [DOI] [PubMed] [Google Scholar]
- PEETERS A.C., NETEA M.G., KULLBERG B.J., THIEN T., VAN DER MEER J.W. The effect of renin–angiotensin system inhibitors on pro- and anti-inflammatory cytokine production. Immunology. 1998;94:376–379. doi: 10.1046/j.1365-2567.1998.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETROV V.V., FAGARD R.H., LIJNEN P.J. Long-term treatment with enalapril or losartan does not show antiproliferative effects in peripheral blood mononuclear cells. Methods Find. Exp. Clin. Pharmacol. 2001a;23:149–152. doi: 10.1358/mf.2001.23.3.627949. [DOI] [PubMed] [Google Scholar]
- PETROV V.V., FAGARD R.H., LIJNEN P.J. T-lymphocyte and plasma angiotensin-converting enzyme activity during enalapril and losartan administration in humans. J. Cardiovasc. Pharmacol. 2001b;38:578–583. doi: 10.1097/00005344-200110000-00010. [DOI] [PubMed] [Google Scholar]
- RIVARD A., PRINCIPE N., ANDRES V. Age-dependent increase in c-fos activity and cyclin A expression in vascular smooth muscle cells. A potential link between aging, smooth muscle cell proliferation and atherosclerosis. Cardiovasc. Res. 2000;45:1026–1034. doi: 10.1016/s0008-6363(99)00385-5. [DOI] [PubMed] [Google Scholar]
- ROSS R. Atherosclerosis – an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-ITURBE B., PONS H., HERRERA-ACOSTA J., JOHNSON R.J. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–1640. doi: 10.1046/j.1523-1755.2001.0590051626.x. [DOI] [PubMed] [Google Scholar]
- RUS H.G., NICULESCU F., VLAICU R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991;89:247–254. doi: 10.1016/0021-9150(91)90066-c. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO H., AIKAWA M., HILL C.C., WEISS D., TAYLOR W.R., LIBBY P., LEE R.T. Biomechanical strain induces class A scavenger receptor expression in human monocyte/macrophages and THP-1 cells. Circulation. 2001;104:109–114. doi: 10.1161/hc2701.091070. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L. Vascular changes in hypertension in response to drug treatment: effects of angiotensin receptor blockers. Can. J. Cardiol. 2002;18 Suppl A:15A–18A. [PubMed] [Google Scholar]
- SONMEZ A., KISA U., UCKAYA G., EYILETEN T., COMERT B., KOC B., KOCABALKAN F., OZATA M. Effects of losartan treatment on T-cell activities and plasma leptin concentrations in primary hypertension. JRAAS. 2001;2:112–116. doi: 10.3317/jraas.2001.011. [DOI] [PubMed] [Google Scholar]
- STEMME S., HANSSON G.K. Immune mechanisms in atherogenesis. Ann. Med. 1994;26:141–146. doi: 10.3109/07853899409147881. [DOI] [PubMed] [Google Scholar]
- STRAWN W.B., GALLAGHER P.E., TALLANT A., GANTEN D., FERRARIO C.M. Angiotensin II AT1-receptor blockade inhibits monocyte activation and adherence in transgenic (mRen2) 27 rats. J. Cardiovasc. Pharmacol. 1999;33:341–351. doi: 10.1097/00005344-199903000-00001. [DOI] [PubMed] [Google Scholar]
- SUNDARESAN M., YU Z.X., FERRANS V.J., IRANI K., FINKEL T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- TIMMERMANS P.B., WONG P.C., CHIU A.T., HERBLIN W.F., BENFIELD P., CARINI D.J., LEE R.J., WEXLER R.R., SAYE J.A., SMITH R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- TINTUT Y., PATEL J., PARHAMI F., DEMER L.L. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- USHIO-FUKAI M., ZAFARI A.M., FUKUI T., ISHIZAKA N., GRIENDLING K.K. P22phox is a critical component of superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- VIEDT C., VOGEL J., ATHANASIOU T., SHEN W., ORTH S.R., KUBLER W., KREUZER J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler. Thromb. Vasc. Biol. 2002;22:914–920. doi: 10.1161/01.atv.0000019009.73586.7f. [DOI] [PubMed] [Google Scholar]
- XANTHOUDAKIS S., MIAO G.G., CURRAN T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc. Natl. Acad. Sci. U.S.A. 1994;91:23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S.P., HO L.J., LIN Y.L., CHENG S.M., TSAO T.P., CHANG D.M., HSU Y.L., SHIH C.Y., JUAN T.Y., LAI J.H. Carvedilol, a new antioxidant β-blocker, blocks in vitro human peripheral blood T cell activation by downregulation NF-êB activity. Cardiovasc. Res. 2003;59:776–787. doi: 10.1016/s0008-6363(03)00459-0. [DOI] [PubMed] [Google Scholar]
- ZHAO S.P., XIE X.M. Captopril inhibits the production of tumor necrosis factor-alpha by human mononuclear cells in patients with congestive heart failure. Clin. Chim. Acta. 2001;304:85–90. doi: 10.1016/s0009-8981(00)00405-8. [DOI] [PubMed] [Google Scholar]
- ZHOU X., NICOLETTI A., ELHAGE R., HANSSON G.K. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]