Abstract
Ca2+ imaging was used to investigate interactions between responses induced by N-methyl-D-aspartate (NMDA; 15 μM) and (RS)-3,5-dihydroxyphenyl-glycine (DHPG; 30 μM) in human embryonic kidney (HEK) 293 cells, transiently transfected with rat recombinant NR1a, NR2A and mGlu5a cDNA.
Responses to NMDA were reversibly depressed by DHPG from 244±14 to 194±12% of baseline. Treatment with thapsigargin (1 μM, 10 min) prevented this effect.
After thapsigargin pretreatment, repeated applications of NMDA showed a gradual rundown in amplitude over a period of several hours, and were unaffected by DHPG.
Continuous perfusion with staurosporine (0.1 μM), after thapsigargin pretreatment, converted the run-down to a small increase in NMDA responses to 123±6 % of baseline. DHPG induced a further and sustained potentiation of NMDA responses to 174±12% of the initial baseline.
The protein tyrosine kinase (PTK) inhibitors genistein (50 μM) and 3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2; 1 μM) inhibited the staurosporine- and DHPG-induced potentiation of NMDA responses.
The protein phosphatase (PTP) inhibitors orthovanadate (100 μM) and phenyl arsine oxide (PAO, 1 μM) facilitated the staurosporine-evoked potentiation of NMDA responses and occluded DHPG-induced potentiation.
In conclusion, complex interactions can be demonstrated between mGlu5 and NMDA receptors expressed in HEK293 cells. There is a negative inhibitory influence of Ca2+ release and PKC activation. Inhibition of these processes reveals a tonic, mGlu5 receptor and PTK-dependent potentiation of NMDA receptors that can be augmented by either stimulating mGlu5 receptors or by inhibiting PTPs.
Keywords: N-methyl-D-aspartate receptors, metabotropic glutamate receptors, receptor crosstalk, tyrosine phosphorylation, protein kinase C, Ca2+ release, synaptic plasticity, synaptic mechanisms
Introduction
Glutamate is the major excitatory synaptic transmitter in the vertebrate CNS and acts via ionotropic and metabotropic (mGlu) receptors (Watkins & Evans, 1981; Sladeczek et al., 1985; Nicoletti et al., 1986). Much of our knowledge of the role of glutamate receptors in the brain has derived from studies in the hippocampus and, in particular, in the CA1 region. It is known that ionotropic receptors of the N-methyl-D-aspartate (NMDA) subtype mediate a frequency-dependent, slow monosynaptic component of synaptic transmission (Herron et al., 1985; Davies & Collingridge, 1989) and can trigger the induction of bidirectional modifications of synaptic strength: long-term potentiation (LTP; Collingridge et al., 1983) and long-term depression (LTD; Fujii et al., 1991; Dudek & Bear, 1992). At these synapses, mGlu receptors can also be activated synaptically where they too are involved in the induction of LTP (Bashir et al., 1993a) and LTD (Bashir et al., 1993b; Bolshakov & Siegelbaum, 1994; Oliet et al., 1997) under certain conditions. These receptors are also involved in metaplasticity (Abraham & Bear, 1996) since their activation can alter the subsequent induction of synaptic plasticity (Bortolotto et al., 1994).
Since both NMDA and mGlu receptors are important triggers for synaptic plasticity, there has been considerable interest in establishing possible direct interactions between these two classes of glutamate receptor. Indeed, it has been reported that activation of mGlu receptors using the broad spectrum agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid ((1S,3R)-ACPD) results in a pronounced potentiation of NMDA responses that is rapidly reversible upon washout of the mGlu receptor agonist (Harvey et al., 1991; Aniksztejn et al., 1992; Harvey & Collingridge, 1993). This effect is mimicked by the group I (i.e., mGlu1 and mGlu5) selective mGlu receptor agonist 3,5-dihydroxyphenylglycine (DHPG) (Fitzjohn et al., 1996), via an action on mGlu5 receptors (Doherty et al., 1997; Mannaioni et al., 2001; Kotecha et al., 2003). Similar interactions between mGlu receptors and NMDA receptors have also been observed in other brain regions, such as the striatum (Pisani et al., 1997), cortex (Attucci et al., 2001) and spinal cord (Ugolini et al., 1997), suggesting that mGlu/NMDA receptor interactions are of widespread significance.
The mechanism(s) by which activation of mGlu receptors modulate NMDA receptor function are not understood. For example, there is evidence both for (Aniksztejn et al., 1992) and against (Harvey & Collingridge, 1993) a role for PKC as the mediator of this effect in CA1 neurons. However, there are likely to be multiple mechanisms that operate at different synapses and at the same class of synapse under different conditions, which may be determined by the mGlu and NMDA receptor subtypes/splice variants present. In the present study, we have developed a system to study interactions between recombinant mGlu and NMDA receptors in HEK293 cells to facilitate an investigation into the molecular mechanisms involved. We have focused on mGlu5 and NR1/NR2A since these are expressed by CA1 pyramidal neurons (Baude et al., 1993; Monyer et al., 1994; Lujan et al., 1996), which is the neuronal type where interactions between NMDA and mGlu receptors have been most extensively investigated.
Some of these results have appeared in abstract form (Collett & Collingridge, 1998; 2000; 2001).
Methods
Plasmid cDNA
The full-length cDNAs encoding rat NR1a and rat mGlu5a, kindly provided by S. Nakanishi (Institute for Immunology, Kyoto University, Japan), were subcloned from the recombinant phagemid pBluescript SK(−) into the polylinker site of a mammalian expression vector containing a cytomegalovirus (CMV) promoter (pcDNA1/Amp, Invitrogen) and checked by restriction mapping. The NR2A construct, inserted into a CMV promoter-containing vector RK7, was a generous gift from P. Seeburg (University of Heidelberg ZMBH, Heidelberg, Germany).
Cell transfection
HEK293 cells, a human embryonic kidney cell line obtained from the European Collection of Cell Culture (ECACC), were grown as a monolayer using passage numbers less than 25 and maintained in Dulbecco's modified Eagle's medium (DMEM; Life Technologies Ltd), supplemented with 10% dialysed horse serum (Life Technologies Ltd and Labtech International) and 2 mM L-glutamine in a humidified incubator at 37°C with 5% CO2. At 1 day prior to transfection, exponentially growing cells were plated out on coverslips (22 mm diameter, thickness 1, Chance Propper), previously coated with a solution containing poly-D-lysine (25 μg ml−1, Sigma), Type 1 rat tail collagen (25 μg ml−1, Collaborative Biomedical Products) and acetic acid (0.01 M), at a density of 1–2 × 105 per coverslip. The cells were transiently transfected using SuperFect (Qiagen) with a molar ratio of cDNA for NR1a:NR2A of 1 : 3, which had been shown to give optimal expression of NMDARs (Chazot et al., 1994), and in cotransfections with mGlu5a the molar ratio was typically 1 : 3 : 1 (NR1a:NR2A:mGlu5a) using a maximum of 0.5 μg cDNA in 1–2 μl per coverslip. Following transfection, the cells were maintained for 24 h in a glutamine-free medium containing 100 μM D-AP5 and 500 μM (S)-α-methyl-4-carboxyphenylglycine (MCPG) to prevent activation of expressed receptors (Anegawa et al., 1995).
Calcium imaging
The transfected cells were loaded with 5 μM fluo3-AM in Hepes-buffered saline (HBS; 150 mM NaCl, 3 mM KCl, 10 mM Hepes, 10 mM D-glucose, 2 mM CaCl2, pH 7.35), containing 1 mg ml−1 of bovine serum albumen (BSA) and incubated at 37°C in the dark. Fluo3-AM-loaded cells were placed in a perfusion chamber and viewed on a confocal microscope (Bio-Rad MRC 600; objective lens × 20, NA 0.75; 520 nm long-pass emission filter) equipped with an argon laser (488 nm). Cells were perfused with HBS containing glycine (10 μM) at a rate of 2 ml min−1. Using brief normal illumination, a field of cells in a monolayer was selected and the cells were rested for 30 min to ensure complete de-esterification of the indicator. Single applications of NMDA and DHPG at the start of each experiment permitted identification of cells expressing either or both types of receptor. The efficiency of transfection was 15±5% with 63±20% of transfected cells expressing both receptor types (2011 cells per 10 experiments). Dose–response relationships were determined using a minimum of six concentrations.
Data analysis and statistics
Acquired images were viewed with the Confocal Assistant program (Bio-Rad) and analysed using the Scion Image program (Scion Corporation, MD, U.S.A.). The fluorescence of individual cells was measured throughout 18 frames associated with each application of agonist. For each recording, preagonist fluorescence levels (mean of responses in frames 1–3) were subtracted from peak levels to give the absolute change in fluorescence for each cell. Each experiment for a particular condition was repeated on at least two separate occasions (x experiments) and response values of all cells (n cells) for that condition were expressed as mean±s.e.m. (n cells per x experiments). Unless otherwise stated, results are expressed relative to the initial experimental baseline determined by averaging the first three responses to NMDA (hence 100%=no change). The effects of DHPG were calculated by expressing the amplitude of the first NMDA response in the presence of DHPG as a percentage of the preceding 1–4 NMDA responses, as appropriate. Statistical analysis was carried out using Student's t-tests, or two-way ANOVA followed by Tukey's test, as appropriate. The value of P<0.05 is considered as significant.
Drugs and chemicals
D-AP5, DHPG, MCPG, MPEP and PP2 were obtained from Tocris Cookson, Bristol, U.K. BSA, fluo3-AM, NMDA and thapsigargin were from Sigma-Aldrich, Poole, Dorset, U.K. and genistein, PAO, sodium orthovanadate and staurosporine from Calbiochem (CN Biosciences Ltd, Nottingham, U.K.). Drugs were dissolved as directed by the manufacturer, stored as frozen stocks, and on the day of experiment, diluted in HBS. In all cases, the solvent : HBS ratio was 1 : 1000 or less.
Results
Functional expression of recombinant NR1a/NR2A and mGlu5a
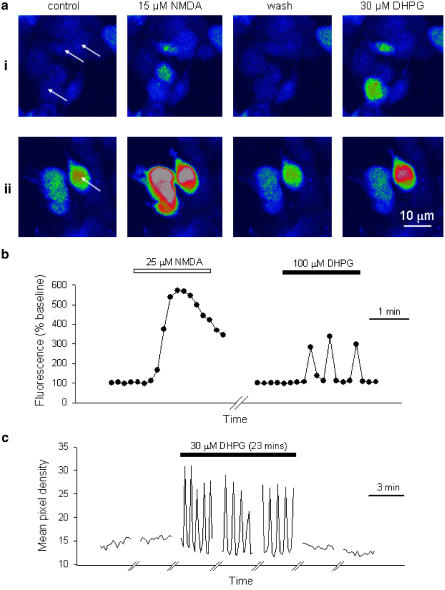
Preliminary experiments showed that in cells that were mock transfected, Ca2+ responses to NMDA (15 μM) or DHPG (30 μM) were negligible (105±1 and 101±0% of baseline, respectively, 28 cells per two experiments). In contrast, transfected cells responded to the agonists with the typical responses shown in Figure 1a and b. Application of NMDA (15 μM) resulted in a transient rise in [Ca2+]i (peak value: 224±11% of baseline, 50 cells per four experiments), which showed some rundown during repeated applications at 15 min intervals (75±4% of baseline after >4.5 h, 25 cells per two experiments; data not shown). The effects of NMDA were concentration dependent over the range 2–100 μM with an EC50 value of 14.9±0.9 μM (25 cells per three experiments). The effects of NMDA were blocked by D-AP5 in a concentration dependent manner (1–300 μM; IC50=2.8±0.5 μM, 14–40 cells per five experiments). Responses to DHPG (30 μM) were oscillatory, which is characteristic of Ca2+ mobilisation by mGlu5a receptors in this expression system (Kawabata et al., 1996) and showed little desensitisation during a prolonged perfusion with DHPG (Figure 1c); thus, peak responses declined from 160±9 to 148±9% of baseline over a 23 min period (24 cells per four experiments). The effects of DHPG were concentration dependent over the range 3–300 μM with an EC50 value of 13.2±1.3 μM (calculated by measuring the peak fluorescence for eight concentrations per cell for 30 cells per three experiments). DHPG-evoked responses were blocked by MPEP in a concentration-dependent manner (3 nM-10 μM; IC50=41±17 nM, 6–20 cells per eight experiments, data not shown). The transfected cells therefore expressed functional NR1a/NR2A and mGlu5a receptors that responded appropriately to their respective agonists and antagonists, whereas nonspecific responses to agonists were not observed in mock-transfected cells. In all the following experiments, cells were transfected with both NR1a/NR2A and mGlu5 receptors, unless stated otherwise.
Figure 1.
Functional expression of NR1a/NR2A and mGlu5a. (a) Two representative fields of cells (i and ii) showing responses to NMDA and DHPG. Arrows indicate cells that responded to both NMDA and DHPG. (b) Response of a single cell to NMDA (25 μM) perfused for 2 min and, after 10 min washout, response of the same cell to DHPG (100 μM) for 2 min. Fluorescence levels are plotted against time and are expressed relative to preagonist levels. (c) Response of another cell to continuous perfusion of DHPG (30 μM) for 23 min. The traces show fluorescence levels over 3 min periods, at 10 min intervals.
Depression of NMDA responses by mGlu5a receptor activation
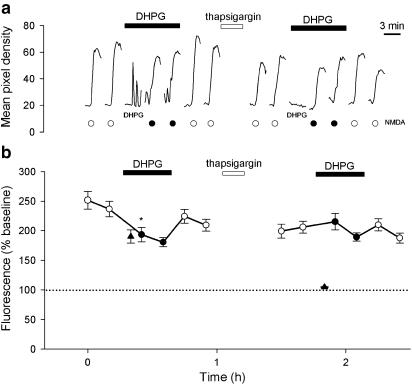
The effects of DHPG (30 μM; 15 min) were tested on responses to NMDA (15 μM, repeated at 10 min intervals; Figure 2). DHPG induced continual oscillations and significantly depressed responses induced by NMDA, applied in its presence, from 244±14 to 194±12%, (35 cells per five experiments; P<0.001). Following washout of DHPG, responses to NMDA recovered to a value (217±10%) that was not significantly different from control. The responses to NMDA in the presence of DHPG were an overestimation of their actual size due to the superimposition of the DHPG response. To remove this complication, we applied thapsigargin (1 μM; 10 min) to deplete intracellular Ca2+ stores. This treatment eliminated DHPG-induced Ca2+ signals for the remainder of the experiment (not illustrated) but did not significantly affect NMDA-induced Ca2+ signals (reduced from 217±10 to 203±11%). These results suggest that all of the mGlu but little, if any, of the NMDA responses are mediated by Ca2+ release from intracellular stores. Under these conditions, DHPG had no significant effect on NMDA responses.
Figure 2.
DHPG inhibits NMDA responses in a thapsigargin-sensitive manner. (a) A single cell to show that DHPG partially inhibits NMDA responses before, but not following, thapsigargin treatment. In this, and subsequent single examples, the timing of applications of NMDA (15 μM, 2 min) are indicated by circles (black-filled circles are NMDA applications in the presence of 30 μM DHPG). NMDA was applied at 15 min intervals; for most of the time, the cells were not illuminated to minimise bleaching. The time scale is therefore compressed between responses. DHPG (30 μM) was applied as indicated by the bar. In this example, the initial part of the response to DHPG is illustrated as indicated. Thapsigargin (1 μM; 10 min) was applied as indicated by the bar. (b) Plots pooled data for 35 cells, from five experiments. In this, and subsequent data pools, each point plots the mean±s.e.m. of the peak fluorescence for each NMDA response vs time (black-filled circles are NMDA applications in the presence of DHPG). In this example, the DHPG response is indicated by a triangle. In this, and subsequent figures, significant effects of DHPG (first response in DHPG relative to immediately preceding baseline response) is indicated; paired t-tests *P<0.05.
Potentiation of NMDA responses by mGlu5a receptor activation
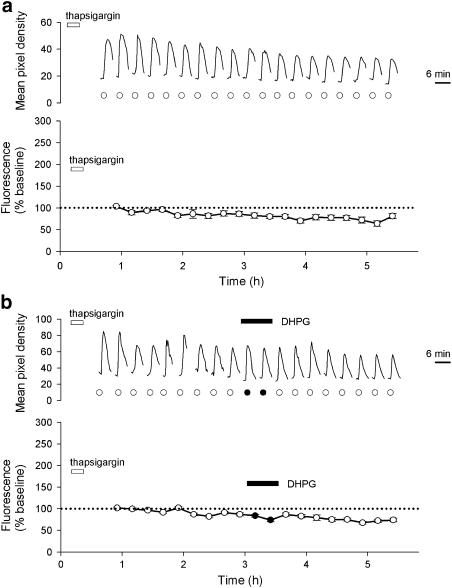
Having eliminated the depressant effects of DHPG, we next investigated whether it was possible to establish conditions under which DHPG was able to potentiate responses induced by NMDA. In these and all subsequent experiments, cells were initially treated with thapsigargin (1 μM, 10 min) and then NMDA (15 μM) was applied at 15 min intervals for several hours. Since thapsigargin treatment is irreversible over this time scale, any complicating effects of release from intracellular stores was eliminated. Under these conditions, NMDA responses showed a gradual run-down in amplitude to a value of 80±5% of baseline by the end of the experiment (Figure 3a, 32 cells per four experiments; P<0.001), a value not significantly different from similar experiments performed in the absence of thapsigargin. Application of DHPG (30 μM; 26 min) had no acute or long-term effects on NMDA responses, followed for at least 2 h after the application of DHPG. At the end of the experiment, the responses were depressed to a value of 74±4% of baseline (Figure 3b, 56 cells per five experiments), a value not significantly different from interleaved experiments performed without DHPG.
Figure 3.
Gradual run-down of NMDA responses and lack of effect of DHPG in thapsigargin-treated cells. (a) A single representative cell (upper graph) and pooled data from 26 cells (lower graph) showing responses to NMDA (15 μM) over a 5 h period. In this, and subsequent experiments, cells were pretreated with thapsigargin (1 μM) at the time indicated. (b) Equivalent experiments (as a) except that DHPG (30 μM) was perfused as indicated. The lower graph plots pooled data from 56 cells.
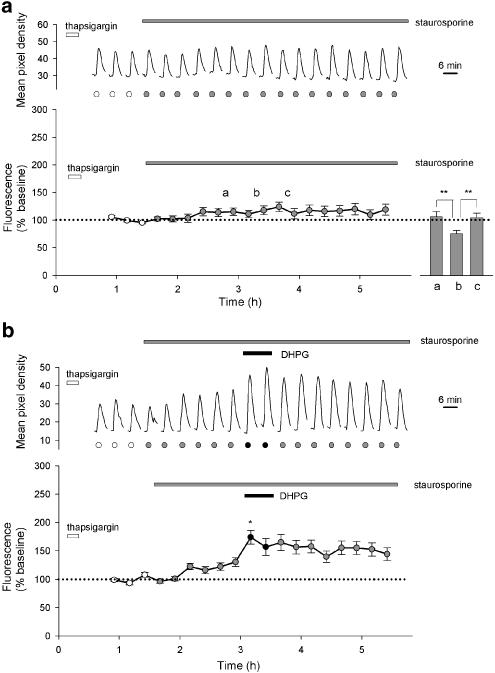
We reasoned that endogenous PKC activity may be interfering with the ability of DHPG to potentiate NMDA responses, since PKC can inhibit mGlu receptors (Aramori & Nakanishi, 1992) and in hippocampal slices treatment with phorbol esters inhibits mGlu receptor-mediated potentiation of NMDA responses (Harvey & Collingridge, 1993). For these studies, we used the broad spectrum kinase inhibitor staurosporine, since it blocks this phorbol ester inhibition without interfering directly with DHPG-induced potentiation of NMDA responses in hippocampal slices (Harvey & Collingridge, 1993). Treatment with staurosporine (0.1 μM) converted the gradual run-down in NMDA responses to a small potentiation such that responses at the end of the experiment were 119±10% of baseline (Figure 4a, 30 cells per three experiments; P<0.05). We also examined the effects of two more specific PKC inhibitors Ro-31-8220 (0.03 μM) and bisindolylmaleimide 1 (0.03 μM), but these compounds directly affected Ca2+ levels in our HEK293 cells.
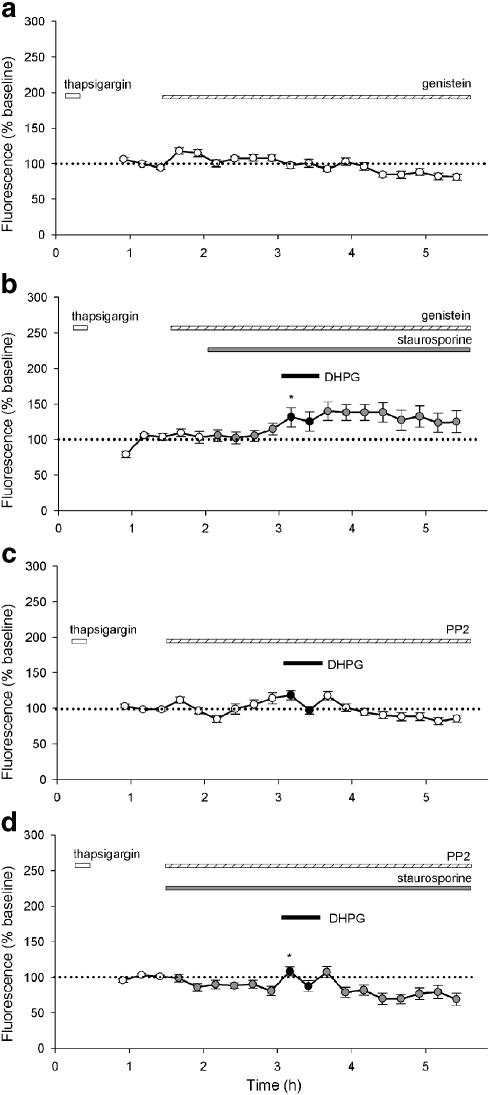
Figure 4.
Staurosporine prevents run-down and enables DHPG-induced potentiation of NMDA responses. (a) A single representative cell (upper graph) and pooled data from 30 cells (lower graph) showing responses to NMDA (15 μM). After three control responses, staurosporine (0.1 μM) was applied for the remainder of the experiment (grey-filled circles indicate NMDA responses in the presence of staurosporine). The inset shows the effect of MPEP (1 μM) perfused at point b for 26 min in similar experiments, performed on a separate occasion. Bars a and c each represent the mean of two responses to NMDA immediately before and after MPEP, respectively, bar b the mean of two responses during perfusion of MPEP (14 cells; **P<0.001). (b) Equivalent experiments (as a) except that DHPG (30 μM) was perfused as indicated. The lower graph plots pooled data from 32 cells.
Application of the noncompetitive mGlu5 antagonist MPEP (1 μM, 26 min) reduced NMDA responses in the presence of staurosporine from 107±10 to 76±6%, an effect that was fully reversible upon washout of MPEP to 105±9% of baseline (14 cells per two experiments, P<0.001; Figure 4a inset). Indeed, MPEP reduced NMDA responses to a value similar to that observed in the absence of staurosporine (see Figure 3a). This suggests that the staurosporine-induced rise may be mediated by constitutive activity of the mGlu5 receptors, and that MPEP is acting as an inverse agonist.
In experiments interleaved with controls, DHPG was applied after the effects of staurosporine on NMDA responses had reached a plateau. Under these conditions, DHPG produced a further potentiation from 123±6 to 174±12% of the initial baseline (P<0.01). When expressed relative to a baseline in the presence of staurosporine, the DHPG-induced potentiation was 142±10% of control (P<0.01). The potentiation induced by DHPG was not reversible, following washout of DHPG (Figure 4b, 32 cells per five experiments) over the time course of these experiments (up to 2 h following washout of DHPG). The potentiating effect of DHPG was concentration dependent over the range 1–100 μM with an EC50 value of 4.5±2.6 μM (10–19 cells per 10 experiments; data not shown).
Role of tyrosine phosphorylation
We next investigated the possibility that the DHPG-induced potentiation of NMDA responses involved tyrosine phosphorylation, given that tyrosine phosphorylation can potentiate NMDA receptor function (Wang & Salter, 1994). We investigated the effects of two tyrosine kinase inhibitors genistein (50 μM; Figure 5a and b) and 3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2; 1 μM; Figure 5c and d), a specific Src family inhibitor.
Figure 5.
Tyrosine kinase inhibitors antagonise DHPG-induced potentiation of NMDA responses. Pooled data for (a) genistein alone (50 μM; 26 cells), (b) genistein (50 μM) with staurosporine (0.1 μM) and DHPG (30 μM; 20 cells), (c) PP2 (1 μM) with DHPG (36 cells) and (d) PP2 (1 μM) with staurosporine and DHPG (25 cells).
Genistein was applied to thapsigargin-treated cells to determine whether NMDA responses were affected directly by inhibition of tyrosine phosphorylation. In the presence of genistein, there was a run-down of NMDA responses that was not significantly different to that observed in the presence of thapsigargin alone (Figure 5a; 26 cells per two experiments). This suggests that genistein is not directly affecting NMDA responses. However, genistein suppressed the potentiation induced by staurosporine and the additional potentiation induced by DHPG. Thus, in the presence of genistein, staurosporine produced a potentiation to 108±8% of baseline and DHPG increased the effect to 128±14% of baseline (Figure 5b; 20 cells per three experiments). The DHPG-induced potentiation (120±13% of control), while still significant (P<0.05), was less than that observed in the absence of genistein.
We also tested PP2 on NMDA responses both in the absence (Figure 5c; 36 cells per four experiments) and presence (Figure 5d; 25 cells per three experiments) of staurosporine. Like genistein, PP2 did not appear to affect NMDA responses directly, since in the absence of staurosporine, there was a run-down of NMDA responses in the presence of PP2 that was not significantly different to that observed in thapsigargin alone. This confirms that inhibition of tyrosine phosphorylation has no direct effect on NMDA responses under the conditions of these experiments (Figure 5c). In this set of experiments, we applied DHPG to see whether a tyrosine kinase inhibitor enabled DHPG to affect NMDA responses in the absence of staurosporine; however, no effect of DHPG was observed. Similar to genistein, PP2 inhibited both the staurosporine- and DHPG-induced potentiation. Indeed, in the presence of PP2, the effect of staurosporine was eliminated thereby revealing the gradual run-down in NMDA responses, seen under control conditions. There was still a significant DHPG-induced potentiation to 124±7% of control (P<0.001), but this was significantly less than that observed in the absence of PP2 (P<0.001). These data indicate that a tyrosine kinase, most probably a Src family member, is involved in the mGlu5a-induced potentiation of NMDA responses, under these conditions.
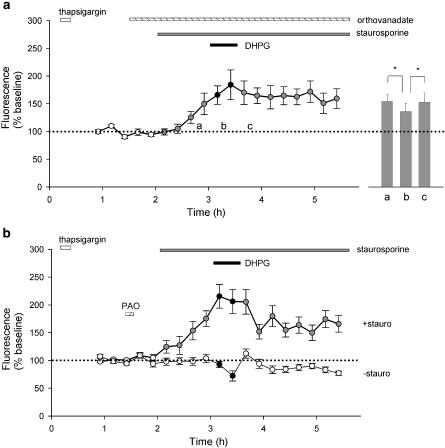
The effects of the tyrosine phosphatase inhibitor sodium orthovanadate (100 μM; perfused continuously; Figure 6a) was also investigated. This compound had no significant effect on NMDA responses during the 30 min period preceding the application of staurosporine. However, it augmented the staurosporine-induced potentiation of NMDA responses and largely occluded any additional effects of DHPG. Thus, in the presence of orthovanadate, the responses immediately prior to and in the presence of DHPG were 150±19% (P<0.05) and 166±17% of baseline (P<0.05; Figure 6a; 16 cells per three experiments). The small residual effect of DHPG in the presence of orthovanadate was not significant. Consistent with the staurosporine-induced rise being due, at least in part, to constitutive activity of mGlu5 receptors, MPEP (1 μM, 26 min) reduced NMDA responses in the presence of staurosporine and orthovanadate from 154±12 to 136±6%, an effect that was fully reversible upon washout of MPEP to 153±17% of baseline (29 cells per three experiments, P<0.05; Figure 6a inset).
Figure 6.
Tyrosine phosphatase inhibitors occlude DHPG-induced potentiation of NMDA responses. (a) Pooled data for orthovanadate (100 μM; 16 cells). The inset shows the effect of MPEP (1 μM); the experiments were performed in an analogous manner to those shown in the inset to Figure 4a (*P<0.05). (b) Pooled data for PAO (1 μM; 28 cells) that show marked potentiation of NMDA (15 μM) responses in staurosporine (0.1 μM) prior to application of DHPG (30 μM). However, in the absence of staurosporine, PAO does not have any significant effect (15 cells).
Next, we tested another PTP inhibitor phenyl arsine oxide (PAO). When applied continuously, this compound led to a gradual increase in basal Ca2+ levels, indicative of toxicity (18 cells per three experiments; data not shown). We therefore applied this compound as a bolus (1 μM for 10 min) according to the protocol adopted by Liao & Leonard (1999). Under these conditions, its effects were similar to those of orthovanadate. Thus, PAO had no significant effects on NMDA responses in the 30 min prior to application of staurosporine (Figure 6b). Furthermore, in cells treated with PAO but not with staurosporine, NMDA responses declined gradually in a manner not significantly different from controls (Figure 6b; 15 cells per three experiments). This confirms that alteration in tyrosine phosphorylation has no appreciable effect on NMDA responses in the absence of staurosporine. However, like orthovanadate, PAO augmented the staurosporine-induced potentiation of NMDA responses and partially occluded any additional effects of DHPG. Thus, in the presence of PAO, the responses immediately prior to and in the presence of DHPG were 175±14% (P<0.01) and 215±21% (P<0.001) of baseline. Again, the residual effect of DHPG in the presence of PAO was not significant (Figure 6b; 28 cells per four experiments).
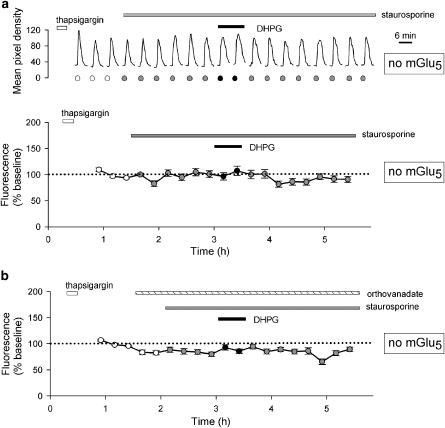
Requirement for mGlu5 receptors
In interleaved experiments using cells that were transfected with NR1a/NR2A but not mGlu5, staurosporine did not cause an increase in basal NMDA responses (mean of last three responses in staurosporine was 100±6% of baseline, 24 cells per two experiments; Figure 7a). Furthermore, DHPG was unable to potentiate NMDA responses in the presence of staurosporine (Figure 7a; first response in DHPG was 97±7% of baseline). In addition, in the absence of mGlu5 receptors, the pronounced effects of orthovanadate were also absent (mean of last three responses in staurosporine was 83±4% of baseline, first response in DHPG was 93±5% of baseline, 26 cells per two experiments; Figure 7b). These data suggest that the increase in NMDA responses, observed under basal conditions, is due to constitutive activity of mGlu5 receptors, which is revealed when the negative regulatory effect of PKC is inhibited. These data also show that DHPG does not directly affect NMDA responses mediated via NR1a/NR2A receptors, under the conditions of our experiments (see, Contractor et al., 1998).
Figure 7.
Staurosporine- and DHPG-induced potentiation of NMDA responses requires mGlu5 receptors. (a) A single representative cell (upper graph) and pooled data from 24 cells (lower graph) showing responses to NMDA (15 μM). After three control responses, staurosporine (0.1 μM) was applied for the remainder of the experiment. (b) Pooled data from 26 cells in which orthovanadate (100 μM) was applied prior to the application of staurosporine. Experiments were performed in an identical manner to those illustrated in Figures 4b and 6a, respectively, except that cells were not transfected with mGlu5.
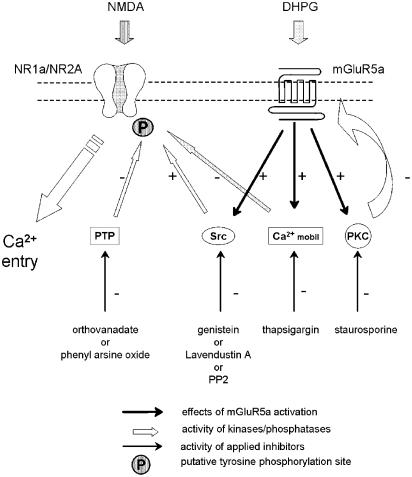
A scheme to explain the results is shown in Figure 8. NMDA receptor function is measured by imaging Ca2+ entry through NR1a/NR2A heteromers. Ca2+ mobilisation from internal stores is blocked by thapsigargin pretreatment. Staurosporine prevents feedback inhibition of mGlu5 receptors via inhibition of a kinase, such as an isoform of PKC. These treatments reveal a constitutive and DHPG-induced potentiation of NMDA responses that involves tyrosine phosphorylation, via an Src family member.
Figure 8.
A model to explain the interactions between mGlu5 and NMDA receptors. mGlu5 receptors are under strong negative regulation by constitutively active PKC. When this is inhibited (by staurosporine), mGlu5 receptors activate Src (or a Src family member), which phosphorylates NR2A, or an associated protein, to enhance NMDA receptor function. Stimulation of mGlu5 receptors (by DHPG) further activates Src to lead to greater phosphorylation and a larger potentiation of NMDA responses. Inhibition of PTP activity enables sufficient tyrosine phosphorylation by constitutively active mGlu5 receptors (in the presence of staurosporine) that DHPG has little additional effect.
Discussion
The purpose of the present experiments was to attempt to reconstitute in an expression system a positive interaction between mGlu and NMDA receptors. Since we are particularly interested in this interaction in the CA1 region of the hippocampus, we chose mGlu5 and the NR1a/NR2A combination of subtypes since these are highly expressed in CA1 pyramidal neurons. We selected HEK293 cells since they do not express endogenous glutamate receptors but do express many components of cell signalling processes, including the G protein subunits Gi, Go, Gs and Gq/11 and enzymes AC, PLA2, PLCβ, PLD, PKA, PKC, Src, PYK2 and ras (Buhl et al., 1995; Toth et al., 1996; Daaka et al., 1997; Della Rocca et al., 1997; Kifor et al., 1997) and the PSD-95 like scaffolding protein SAP102 (Sans et al., 2003). Although the levels of these and other relevant signalling molecules are likely to be different to those expressed in neurons, we have identified several interactions involving Ca2+ release from intracellular stores, PKC and tyrosine phosphorylation.
Depression of NMDA responses by mGlu5 receptor activation
In our first experiments, we observed a depression, as opposed to a potentiation, of NMDA responses, when we applied DHPG. This negative interaction can be attributed to an effect of Ca2+ release from intracellular stores, since it was eliminated by thapsigargin. DHPG would, by mobilising Ca2+ from stores, reduce any contribution of Ca2+ release from intracellular stores to the NMDA responses. However, we observed little, if any, contribution of Ca2+ release from intracellular stores to the NMDA-induced signal, which is consistent with a previous report of NMDA-induced Ca2+ signals in HEK293 cells expressing NR1a/NR2A (Grant et al., 1997). Alternatively, Ca2+ release might contribute to Ca2+-dependent inactivation of NMDA receptors (Rosenmund & Westbrook, 1993; Ehlers et al., 1996; Krupp et al., 1996; Zhang et al., 1998). Since thapsigargin does not interfere with the potentiation of NMDA responses by mGlu receptor activation in hippocampal slices (Harvey & Collingridge, 1993), we routinely pretreated cells with this irreversible inhibitor in an attempt to reveal DHPG-induced potentiation.
Potentiation of NMDA responses by mGlu5 receptor activation
In the presence of thapsigargin, responses to NMDA were reproducible, exhibiting only a gradual run-down over a period of several hours. This system was therefore suitable for the study of their regulation by mGlu5 receptor activation. However, DHPG was unable to cause potentiation of NMDA responses. There were potentially many reasons for this, such as the absence in HEK293 cells of some essential component of the system. However, we wondered whether the failure of DHPG to potentiate NMDA responses may be due to endogenous PKC activity in HEK293 cells, since in hippocampal neurons activation of PKC blocks mGlu regulation of NMDA responses (Harvey & Collingridge, 1993). To test this possibility, we used staurosporine since this is an effective antagonist of the phorbol ester inhibition of the mGlu regulation of NMDA responses. The finding that in the presence of staurosporine, DHPG was able to potentiate NMDA responses suggests that endogenous PKC activity was the cause of the failure to observe regulation in HEK293 cells. In contrast, PKC is presumably more tightly regulated in neurons.
The results demonstrate that HEK293 cells do indeed contain the necessary proteins to enable the positive regulation of NMDA receptors by mGlu5 receptors to be studied. However, there were two differences between the effects observed in HEK293 cells and in hippocampal neurons. First, staurosporine alone caused an enhancement of NMDA responses and, secondly, the effects of DHPG were not readily reversible. Since the enhancement of basal NMDA activity depended on the presence of mGlu5 receptors and was prevented by the noncompetitive mGlu5 receptor antagonist MPEP, it may be due to constitutive activity of these receptors, which is revealed when their negative regulation by PKC is removed. In this context, there is evidence for constitutive activity of group I mGlu receptors in other systems (Parmentier et al., 2002). The sustained effect of DHPG might be explained by a persistent enhancement of this constitutive activity, or some difference between HEK293 cells and neurons in the mechanisms that terminate the potentiation.
Role of tyrosine phosphorylation in mGlu receptor regulation of NMDA responses
Both the staurosporine- and DHPG-induced potentiation of NMDA responses were reduced by the broad-spectrum PTK inhibitor genistein and by the Src-family-specific PTK inhibitor PP2. Furthermore, inhibition of PTP activity, using either orthovanadate or PAO, mimicked and occluded the effects of DHPG. These data suggest a model (Figure 8) whereby NMDA receptor function is regulated by Src (or a Src family member)-dependent tyrosine phosphorylation, which is stimulated by mGlu5 receptors. However, the basal and agonist-activated potentiation is under negative regulation by PKC and so is only observed when this activity is suppressed. In this context, it is worth noting that staurosporine (used in the present study at 0.1 μM) is a potent inhibitor of PKC (e.g., IC50 value of 0.032 μM vs PKCγ) but a weak inhibitor of membrane associated, nonreceptor PTKs, such as Src (IC50 value of 10–40 μM; Elberg et al., 1995). Consistent with this model, there is evidence for the activation of PTKs by group I mGlu receptors in a variety of systems (Peavy & Conn, 1998; Heuss et al., 1999; Kotecha et al., 2003). Similarly, there is considerable evidence for positive modulation of NMDAR channels by PTKs (e.g., Wang & Salter, 1994; Yu et al., 1997; Lancaster & Rogers, 1998; Liao et al., 2000). Of particular relevance to our present work, Kotecha et al. (2003) report that activation of mGlu5 results in a long-lasting potentiation of NMDA responses in hippocampal neurons. In contrast to the present study, and our previous work in hippocampal slices (Harvey & Collingridge, 1993), this effect was dependent on Ca2+ release from intracellular stores and activation of PKC. This may be due to the effects described by Kotecha et al. (2003) being on the peak NMDA current, while we have studied much slower NMDA responses in response to bath application of the agonist.
Activation of mGlu1α receptors has also been shown to potentiate NMDA responses expressed in Xenopus oocytes; however, unlike in the present study, this involved activation of PKC (Skeberdis et al., 2001). In contrast, activation of mGlu1 receptors in cortical neurons resulted in a PKC-independent, tyrosine phosphorylation of NMDA receptors and potentiation of NMDA responses (Heidinger et al., 2002). In mGlu1α-expressing HEK293 cells, DHPG was able to phosphorylate NR2A via a process dependent upon Pyk2 and Src (Heidinger et al., 2002). Our observations with mGlu5 receptors are consistent with such a process, although we did not need to transfect either Pyk2 or Src to obtain functional modulation.
It seems likely that activation of mGlu receptors can, under different conditions, result in either PKC-dependent or PTK-dependent regulation of NMDA receptors, the latter involving enzymes such as Pyk2 and Src (Huang et al., 2001). In addition, under certain circumstances, regulation may be dependent upon both PKC and PTK (Lu et al., 1999; Kotecha et al., 2003). The understanding of the regulation of NMDA receptors is further complicated since the peak and steady-state components of NMDA response are differentially modulated by phosphorylation, the latter of which will dominate the Ca2+ signals measured in the present investigation. Further studies will be required to determine the relative importance of the different forms of regulation of NMDA receptors for synaptic transmission and plasticity and to establish the molecular mechanisms involved.
Acknowledgments
This study is supported by the MRC. We are most grateful to Zafar Bashir, Andrew Doherty, Stephen Fitzjohn and Andy Irving for helpful suggestions.
Abbreviations
- (1S,3R)-ACPD
(1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid
- D-AP5
D-2-amino-5-phosphonopentanoic acid
- CMV
cytomegalovirus
- DHPG
(RS)-3,5-dihydrophenylglycine
- DMEM
Dulbecco's modified Eagle's medium
- HBS
HEPES-buffered saline
- HEK
human embryonic kidney
- LTD
long-term depression
- LTP
long-term potentiation
- MCPG
(S)-α-methyl-4-carboxyphenylglycine
- mGlu
metabotropic
- MPEP
2-methyl-6-(phenylethynyl)pyridine
- PAO
phenyl arsine oxide
- PP2
3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
References
- ABRAHAM W.C., BEAR M.F. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- ANEGAWA N.J., LYNCH D.R., VERDOORN T.A., PRITCHETT D.B. Transfection of N-methyl-D-aspartate receptors in a non-neuronal cell line leads to cell death. J. Neurochem. 1995;64:2004–2012. doi: 10.1046/j.1471-4159.1995.64052004.x. [DOI] [PubMed] [Google Scholar]
- ANIKSZTEJN L., OTANI S., BEN-ARI Y. Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. Eur. J. Neurosci. 1992;4:500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- ARAMORI I., NAKANISHI S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- ATTUCCI S., CARLA V., MANNAIONI G., MORONI F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br. J. Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASHIR Z.I., BORTOLOTTO Z.A., DAVIES C.H., BERRETTA N., IRVING A.J., SEAL A.J., HENLEY J.M., JANE D.E., WATKINS J.C., COLLINGRIDGE G.L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993a;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- BASHIR Z.I., JANE D.E., SUNTER D.C., WATKINS J.C., COLLINGRIDGE G.L. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur. J. Pharmacol. 1993b;239:265–266. doi: 10.1016/0014-2999(93)91009-c. [DOI] [PubMed] [Google Scholar]
- BAUDE A., NUSSER Z., ROBERTS J.D.B., MULVIHILL E., McILHINNEY R.A.J., SOMOGYI P. The metabotropic receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- BOLSHAKOV V.Y., SIEGELBAUM S.A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- BORTOLOTTO Z.A., BASHIR Z.I., DAVIES C.H., COLLINGRIDGE G.L. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- BUHL A.M., OSAWA S., JOHNSON G.L. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J. Biol. Chem. 1995;270:19828–19832. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- CHAZOT P.L., COLEMAN S.K., CIK M., STEPHENSON F.A. Molecular characterization of NMDA receptors expressed in mammalian cells yields evidence for the coexistence of 3 subunit types within a discrete receptor molecule. J. Biol. Chem. 1994;269:24403–24409. [PubMed] [Google Scholar]
- COLLETT V.J., COLLINGRIDGE G.L. Potentiation of recombinant NR1a/NR2A receptors by mGluR5a activation in HEK293 cells. Eur. J. Neurosci. Abst. 1998;10:344. [Google Scholar]
- COLLETT V.J., COLLINGRIDGE G.L. Modulation of recombinant NR1a/NR2A receptors by mGluR5a activation in HEK293 cells. Eur. J. Neurosci. Abst. 2000;12:371. [Google Scholar]
- COLLETT V.J., COLLINGRIDGE G.L. Potentiation of recombinant NR1a/NR2A receptors by mGluR5a activation in HEK293 cells may involve tyrosine phosphorylation. Soc. Neurosci. Abst. 2001;31:45.4. [Google Scholar]
- COLLINGRIDGE G.L., KEHL S.J., McLENNAN H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J. Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTRACTOR A., GEREAU R.W., GREEN T., HEINEMANN S.F. Direct effects of metabotropic glutamate receptor compounds on native and recombinant N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8969–8974. doi: 10.1073/pnas.95.15.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAAKA Y., LUTTRELL L.M., LEFKOWITZ R.J. Switching of the coupling of β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- DAVIES S.N., COLLINGRIDGE G.L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc. R. Soc. London B Biol. Sci. 1989;236:373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- DELLA ROCCA G.J., VAN BIESEN T., DAAKA Y., LUTTRELL D.K., LUTTRELL L.M., LEFKOWITZ R.J. Ras-dependent mitogen activated kinase activation by G protein-coupled receptors. J. Biol. Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- DOHERTY A.J., PALMER M.J., HENLEY J.M., COLLINGRIDGE G.L., JANE D.E. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- DUDEK S.M., BEAR M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHLERS M.D., ZHANG S., BERNHARDT J.P., HUGANIR R.L. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- ELBERG G., LI J., LEIBOVITCH A., SCHECHTER Y. Non-receptor cytosolic protein tyrosine kinase from various rat tissues. Biochim. Biophys. Acta. 1995;1269:299–306. doi: 10.1016/0167-4889(95)00124-8. [DOI] [PubMed] [Google Scholar]
- FITZJOHN S.M., IRVING A.J., PALMER M.J., HARVEY J., LODGE D., COLLINGRIDGE G.L. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- FUJII S., SAITO K., MIYAKAWA H., ITO K., KATO H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea-pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- GRANT E.R., BACSKAI B.J., PLEASURE D.E., PRITCHETT D.B., GALLAGHER M.J., KENDRICK S.J., KRICKA L.J., LYNCH D.R. N-methyl-D-aspartate receptors expressed in a non-neuronal cell line mediate subunit-specific increases in free intracellular calcium. J. Biol. Chem. 1997;272:647–656. doi: 10.1074/jbc.272.1.647. [DOI] [PubMed] [Google Scholar]
- HARVEY J., COLLINGRIDGE G.L. Signal transduction pathways involved in the acute potentiation of NMDA responses by 1S,3R-ACPD in rat hippocampal slices. Br. J. Pharmacol. 1993;109:1085–1090. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J., FRENGUELLI B.G., SUNTER D.C., WATKINS J.C., COLLINGRIDGE G.L. The actions of 1S,3R-ACPD, a glutamate metabotropic receptor agonist, in area CA1 of rat hippocampus. Br. J. Pharmacol. 1991;104:75P. [Google Scholar]
- HEIDINGER V., MANZERRA P., WANG X.Q., STRASSER U., YU S.-P., CHOI D.W., BEHRENS M.M. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk/Src-family kinase pathway in cortical neurons. J. Neurosci. 2002;22:5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRON C.E., LESTER R.A., COAN E.J., COLLINGRIDGE G.L. Intracellular demonstration of an N-methyl-D-aspartate receptor mediated component of synaptic transmission in the rat hippocampus. Neurosci. Lett. 1985;60:19–23. doi: 10.1016/0304-3940(85)90375-1. [DOI] [PubMed] [Google Scholar]
- HEUSS C., SCANZIANI M., GAHWILER B.H., GERBER U. G-protein-independent signalling mediated by metabotropic glutamate receptors. Nat. Neurosci. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- HUANG Y.-Q., LU W.-Y., ALI D.W., PELKEY K.A., PITCHER G.M., LU Y.M., AOTO H., RODER J.C., SASAKI T., SALTER M.W., MACDONALD J.F. CAKβ/Pyk2 kinase is a signalling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- KAWABATA S., TSUTSUMI R., KOHARA A., YAMAGUCHI T., NAKANISHI S., OKADA M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- KIFOR O., DIAZ R., BUTTERS R., BROWN E.M. The Ca2+-sensing receptor (CaR) activates phospholipases C, A2 and D in bovine parathyroid and CaR-transfected human embryonic kidney 293 cells. J. Bone Miner. Res. 1997;12:715–725. doi: 10.1359/jbmr.1997.12.5.715. [DOI] [PubMed] [Google Scholar]
- KOTECHA S.A., JACKSON M.F., AL-MAHROUKI A., RODER J.C., ORSER B.A., MACDONALD J.F. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J. Biol. Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- KRUPP J.J., VISSEL B., HEINEMANN S.F., WESTBROOK G.L. Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol. Pharmacol. 1996;50:1680–1688. [PubMed] [Google Scholar]
- LANCASTER B., ROGERS M.V. A peptide activator of endogenous tyrosine kinase enhances synaptic currents mediated by NMDA receptors. Eur. J. Neurosci. 1998;10:2302–2308. doi: 10.1046/j.1460-9568.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- LIAO G.-Y., KREITZER M.A., SWEETMAN B.J., LEONARD J.P. The postsynaptic density protein PSD-95 differentially regulates insulin- and Src-mediated current modulation of mouse NMDA receptors expressed in Xenopus oocytes. J. Neurochem. 2000;75:282–287. doi: 10.1046/j.1471-4159.2000.0750282.x. [DOI] [PubMed] [Google Scholar]
- LIAO G.-Y., LEONARD J.P. Insulin modulation of cloned mouse NMDA receptor currents in Xenopus oocytes. J. Neurochem. 1999;73:1510–1519. doi: 10.1046/j.1471-4159.1999.0731510.x. [DOI] [PubMed] [Google Scholar]
- LU W.-Y., XIONG Z.-G., LEI S., ORSER B.A., DUDEK E., BROWNING M.D., MACDONALD J.F. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- LUJAN R., NUSSEN Z., ROBERTS J.D.B., SHIGEMOTO R., SOMOGYI P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- MANNAIONI G., MARINO M.J., VALENTI O., TRAYNELIS S.F., CONN P.J. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONYER H., BURNASHEV N., LAURIE D.J., SAKMANN B., SEEBURG P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- NICOLETTI F., MEEK J.L., IADAROLA M.J., CHUANG D.M., ROTH B.L., COSTA E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J. Neurochem. 1986;46:40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- OLIET S.H.R., MALENKA R.C., NICOLL R.A. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- PARMENTIER M.-L., PREZEAU L., BOCKAERT J., PIN J.-P. A model for the functioning of family 3 GPCRs. Trends Pharmacol. Sci. 2002;23:268–274. doi: 10.1016/s0165-6147(02)02016-3. [DOI] [PubMed] [Google Scholar]
- PEAVY R.D., CONN P.J. Phosphorylation of mitogen-activated protein kinase in cultured rat cortical glia by stimulation of metabotropic glutamate receptors. J. Neurochem. 1998;71:603–612. doi: 10.1046/j.1471-4159.1998.71020603.x. [DOI] [PubMed] [Google Scholar]
- PISANI A., CALABRESI P., CENTONZE D., BERNARDI G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurons. Br. J. Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENMUND C., WESTBROOK G.L. Rundown of NMDA channels during whole-cell recording in rat hippocampal neurons: role of Ca2+ and ATP. J. Physiol. 1993;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANS N., PRYBYLOWSKI K., PETRALIA R.S., CHANG K., WANG Y.-X., RACCA C., VICINI S., WENTHOLD R.J. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- SKEBERDIS V.A., LAN J., OPITZ T., ZHENG X., BENNETT M.V.L., ZUKIN R.S. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40:856–865. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- SLADECZEK F., PIN J.-P., RECASENS M., BOCKAERT J., WEISS S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- TOTH P.T., SCHEKTER L.R., MA G.H., PHILIPSON L.H., MILLER R.J. Selective G-protein regulation of neuronal calcium channels. J. Neurosci. 1996;16:4617–4624. doi: 10.1523/JNEUROSCI.16-15-04617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UGOLINI A., CORSI M., BORDI F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- WANG Y.T., SALTER M.W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- WATKINS J.C., EVANS R.H. Excitatory amino-acid transmitters. Annu. Rev. Pharmacol. Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- YU X.-M., ASKALAN R., KEIL G.J., II, SALTER M.W. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- ZHANG S., EHLERS M.D., BERNHARDT J.P., SU C.T., HUGANIR R.L. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]