Abstract
We have examined the effect of the histone deacetylase inhibitors apicidin, trichostatin A (TSA) and n-butyrate on the histone acetylation and the differentiation of human eosinophilic leukemia HL-60 clone 15 cells into eosinophils.
Viability of the cells incubated with apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) did not change significantly, but higher concentrations of apicidin (⩾300 nM) or TSA (⩾100 nM) decreased the viability when examined at day 1.
Apicidin (100 nM) as well as n-butyrate (500 μM) induced continuous acetylations of histone H4 and lysine14 residue on histone H3, while TSA (30 nM) induced transient acetylations.
After 6 days incubation, eosinophilic cells stained by Luxol-fast-blue were generated by apicidin (100 nM) and n-butyrate (500 μM) but not by TSA (30 nM). Other markers for differentiation into eosinophils such as changes in intracellular structure, and expressions of integrin β7 and major basic protein, and the inhibition of cell proliferation were also induced by apicidin and n-butyrate but not by TSA.
Continuous acetylation of histone H4 achieved by repeated treatment with TSA (30 nM) at an interval of 12 h for more than three times induced such changes when examined on day 6. In addition, the induction was impaired by shortening the period of incubation with apicidin (100 nM) or n-butyrate (500 μM).
CCAAT/enhancer binding protein was continuously activated by apicidin (100 nM) and n-butyrate (500 μM), but was transiently activated by TSA (30 nM).
These findings suggest that the continuous acetylation of histones H3 and H4 is necessary for the differentiation of HL-60 clone 15 cells into eosinophils.
Keywords: HL-60 clone 15 cells, eosinophils, cellular differentiation, histone deacetylase, apicidin, n-butyrate, trichostatin A
Introduction
Chronic allergic inflammation including bronchial asthma and atopic dermatitis is difficult to treat and the number of patients suffering from such diseases is increasing, probably reflecting environmental changes to lifestyle. Eosinophils are one of the cells that play a critical role in the pathogenesis of allergic diseases (Corrigan & Kay, 1992). In bronchial asthma, eosinophils increase in number in bone marrow and peripheral blood, and accumulate at inflammatory sites in response to various kinds of cytokines and chemical mediators (Corrigan & Kay, 1992; Renauld, 2001). Studies have demonstrated the mechanism by which eosinophils prolong their survival, infiltrate inflammatory sites and release granule proteins (Martin et al., 1996; van der Bruggen & Koenderman, 1996; Wardlaw, 1999). However, the mechanism behind the differentiation of stem cells such as CD34+ cells into mature eosinophils has not been clarified. Clarification of this mechanism is important if one is to understand the generation and maturation of white blood cells, especially myeloid lineages, and may contribute to the development of novel medications for allergic inflammation. Inhibition of the differentiation toward the eosinophil lineage might be useful for the control of allergic inflammation as a preventive measure.
Studies in the last two decades have established the eosinophilic cell lines, HL-60 clone 15, EoL-1 and so on (Fischkoff et al., 1984; Fischkoff & Condon, 1985; Saito et al., 1985; Paul et al., 1993). These cell lines have contributed to the understanding of the regulation and function of eosinophils. n-Butyrate is the most powerful chemical inducer of the differentiation of these cells into mature eosinophils (Fischkoff & Condon, 1985). However, the mechanism by which HL-60 clone 15 cells differentiate into eosinophils on exposure to n-butyrate has remained to be elucidated.
Chromatin comprises repeating units of nucleosome core particles and linker DNA (Klug et al., 1980). The particles consist of an octamer of core histone, containing two molecules each of the histones H2A, H2B, H3 and H4, around which 145 base pairs of DNA are wrapped in 1.75 turns (Csordas, 1990; Grunstein, 1997). Histones in the nucleosome core particle are reversibly acetylated and deacetylated at the ɛ-amino group of the specific lysine residues of the histone N-termini by histone acetyltransferase (HAT) and histone deacetylase (HDAC), respectively (Grunstein, 1997). The neutralization of positively charged histone by acetylation reduces the affinity of histone to the genomic DNA, allowing transcription factors to access target genes, and the balance of acetylation by HATs and deacetylation by HDACs tightly regulates a cell's fate (McGhee & Felsenfeld, 1980; Norton et al., 1990; Lee et al., 1993; Pazin & Kadonaga, 1997). In many cells, n-butyrate inhibits the activity of HDAC and induces the gene expression (Riggs et al., 1977; Cioe et al., 1981; Marks et al., 2000). Therefore, we hypothesized that the inhibition of HDAC induces differentiation of HL-60 clone 15 cells into eosinophils. To test our hypothesis, we have analyzed the relationship between the inhibition of deacetylation and differentiation into eosinophils using the histone deacetylase inhibitors apicidin (Darkin-Rattray et al., 1996), trichostatin A (TSA) (Yoshida et al., 1987) and n-butyrate (Riggs et al., 1977).
Methods
Cell culture
HL-60 clone 15 cells (CRL-1964, American Type Culture Collection, Rockville, MD, U.S.A.) were maintained in RPMI-1640 medium (pH 7.8) (Sigma, St. Louis, MO, U.S.A.) supplemented with 10% FBS (Sigma). For the experiments, 1 × 105 cells were incubated in each well of a 24-well plate (Corning, Corning, NY, U.S.A.) at 37°C for specified periods in 1 ml of medium containing 10% FBS and various concentrations of drugs unless otherwise noted.
Proliferation assay
After incubation for specified periods, the cells were enumerated using a hemocytometer.
Cell staining
Cells were smeared on a glass slide and stained with Luxol-fast-blue (EM Science, Cherry Hill, NJ, U.S.A.) and hematoxylin (Merck, Darmstadt, Germany).
Immunoblotting
After incubation, the cells were washed three times with PBS, suspended in 5 × 106 cells ml−1 in sample buffer (62.5 mM Tris-HCl, pH 6.8, 6 M urea, 10% glycerol, 2% SDS, 0.00125% bromophenol blue and 5% β-mercaptoethanol), sonicated for 15 s using a Handy Sonic Disruptor (Tomy Seiko, Tokyo, Japan) and incubated for 15 min at 65°C. The cell lysate was subjected to electrophoresis on a 17.5% acrylamide gel for 3 h at 125 V and the proteins separated in the gels were transferred onto a nitrocellulose membrane and blocked in blocking solution (Block Ace, Dainippon Pharmaceutical, Osaka, Japan) for 1 h at room temperature. To detect acetylated histone H4, acetylated lysine14 residue on histone H3, major basic protein (MBP), p15INK4b, p21Waf1/Cip1 and p27Kip1, the nitrocellulose membrane was incubated for 12 h at 4°C with rabbit anti-acetylated histone H4 polyclonal antibody (ChiPs grade, Upstate Biotechnology, Waltham, MA, U.S.A.), rabbit anti-acetylated histone H3 (K14) polyclonal antibody (Upstate Biotechnology), mouse anti-eosinophil MBP monoclonal antibody (AHE-2, Chemicon International, Temecula, CA, U.S.A.), rabbit anti-p15INK4b polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), goat anti-p21Waf1/Cip1 polyclonal antibody (Santa Cruz Biotechnology) and rabbit anti-p27Kip1 polyclonal antibody (Santa Cruz Biotechnology), respectively. Thereafter, the membrane was incubated for 3 h at 4°C with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA, U.S.A.), biotinylated anti-mouse IgG (Vector Laboratories) or biotinylated anti-goat IgG (Vector Laboratories). The reaction products were incubated for 30 min at room temperature with Vectastain ABC reagent (Vector Laboratories) and visualized using the Chemiluminescence Detection System (Western Lightning Chemiluminescence Reagent Plus, PerkinElmer Life Sciences, Boston, MA, U.S.A.). The membrane was exposed to Kodak X-Omat AR film (Eastman Kodak, Rochester, NY, U.S.A.). In case of the detection for actin as an internal control, the membrane was incubated with goat anti-actin polyclonal antibody (Santa Cruz Biotechnology) and phosphatase-labeled anti-goat IgG (Vector Laboratories) as described above. The reaction products were visualized with BCIP/NBT color substrate (Promega, Madison, WI, U.S.A.).
Flowcytometry
HL-60 clone 15 cells cultured with HDAC inhibitors were harvested, washed with PBS containing 0.25% BSA (Sigma) and resuspended in the medium at 2 × 106 cells ml−1. For the detection of integrin β7, 20 μl of R-phycoerythrin (R-PE)-conjugated anti-integrin β7 monoclonal antibody solution (FIB504, BD Pharmingen, San Diego, CA, U.S.A.) was added to 50 μl of the cell suspension and incubated for 30 min at 4°C, and then washed three times with 0.25% BSA-PBS. For the analysis of viability, the cells were incubated for 30 min at room temperature in 0.1 ml of PBS containing 2 μg of 7-amino-actinomycin D (Sigma). The fluorescence of the cells stained with R-PE or 7-amino-actinomycin D was analyzed with a flowcytometer (FACScan, Beckton Dickinson, San Jose, CA, U.S.A.).
EMSA
After incubation, the cells were suspended in 400 μl of Tris-buffered KCl solution (20 mM Tris-HCl, pH 7.8, 50 mM KCl, 10 μg ml−1 of leupeptin, 0.1 mM dithiothreitol, and 1 mM phenyl methylsulfonyl fluoride), and lysed by the addition of the same volume of Tris-buffered KCl solution containing 1.2% Nonidet P-40 (Sigma) with vigorous mixing for 10 s. The homogenate was centrifuged at 4°C and 15,000 × g for 30 s, and the nuclear pellet was suspended in 30 μl of cold Tris-buffered KCl solution (20 mM Tris-HCl, pH 7.8, 500 mM KCl, 10 μg ml−1 of leupeptin, 0.1 mM dithiothreitol, and 1 mM phenyl methylsulfonyl fluoride) by mixing at 4°C for 1 min. The suspension was then centrifuged at 4°C and 15,000 × g for 20 min, and the supernatant was stored at −80°C. EMSA was carried out according to the protocol accompanying the Gel Shift Assay System (Promega). The double-stranded oligonucleotide probes (C/EBP consensus oligonucleotide: 5′-TGCAGATTGCGCAATCTGCA-3′ and C/EBP mutant oligonucleotide: 5′-TGCAGAGACTAGTCTCTGCA-3′, Santa Cruz Biotechnology) were end-labeled with 0.37 MBq of [γ-32P] ATP (111 TBq nmol−1, Du Pont New England Nuclear, Boston, MA, U.S.A.) using T4 polynucleotide kinase (Promega). For the competition and the super shift assays, nuclear extract was incubated for 20 min at room temperature with unlabeled consensus oligonucleotide probe at a 50-fold molar ratio against the labeled consensus oligonucleotide probe, and with 2 μg of anti-C/EBPα polyclonal antibody (Santa Cruz Biotechnology), anti-C/EBPβ polyclonal antibody (Santa Cruz Biotechnology), or anti-C/EBPɛ polyclonal antibody (Santa Cruz Biotechnology), respectively. The 32P-labeled probes (1 μl) were incubated for 20 min at room temperature with or without 5 μg of nuclear extract in a gel-shift binding buffer (Promega). DNA/nuclear protein complexes were separated from the DNA probe by electrophoresis on a native 4% acrylamide gel, and the gel was visualized with a GS-250 Molecular Imager (Bio-Rad, Hercules, CA, U.S.A.).
Drugs
Drugs used were apicidin (Calbiochem, San Diego, CA, U.S.A.), trichostatin A (TSA) (Wako, Osaka, Japan), n-butyrate (Wako) and roscovitine (Calbiochem). They were dissolved in dimethylsulfoxide and added to the medium. The concentration of the vehicle in the medium was adjusted to 0.1%.
Statistical analysis
The statistical significance of the results was analyzed using Dunnett's test for multiple comparisons and Student's t-test for unpaired observations.
Results
Differentiation into eosinophils induced by apicidin, n-butyrate and TSA
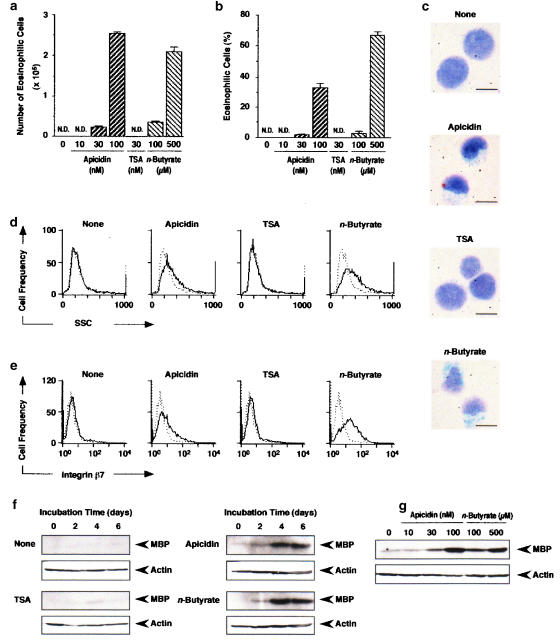
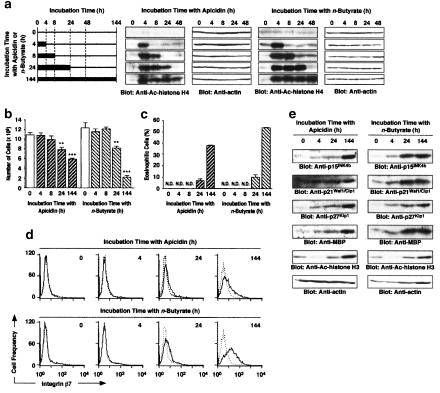
After 6 days incubation of HL-60 clone 15 cells (1 × 105 cells), apicidin at 30 and 100 nM, and n-butyrate at 100 and 500 μM increased the number (Figure 1a) and percentage (Figure 1b) of eosinophilic cells (100 nM apicidin: (2.5±0.1) × 105 cells, (38.0±0.2)%; 500 μM n-butyrate: (2.1±0.1) × 105 cells, (66.0±2.4)%, the means±s.e.m. from four samples). TSA at 30 nM generated no eosinophilic cells (Figure 1a and b). Microscopic observation revealed that 100 nM apicidin and 500 μM n-butyrate but not 30 nM TSA changed the shape of the nucleus and increased the cytoplasmic area in which Luxol-fast-blue-positive granules exist (Figure 1c). Measurement of the side scatter of HL-60 clone 15 cells by flowcytometry after 6 days incubation revealed that 100 nM apicidin and 500 μM n-butyrate induced changes in the intracellular structure of the cells (Figure 1d). The expression of integrin β7 was also induced by 100 nM apicidin and 500 μM n-butyrate (Figure 1e). However, incubation of the cells with 30 nM TSA neither changed the intracellular structure nor induced the expression of integrin β7 (Figure 1d and e). Furthermore, 100 nM apicidin and 500 μM n-butyrate but not 30 nM TSA induced the expression of MBP in a time-dependent manner (Figure 1f). MBP was expressed dependent on the concentration of apicidin and n-butyrate after 6 days incubation (Figure 1g). These findings indicate that apicidin and n-butyrate but not TSA induce the differentiation of HL-60 clone 15 cells into eosinophils.
Figure 1.
Differentiation of HL-60 clone 15 cells into eosinophils induced by apicidin, TSA and n-butyrate. HL-60 clone 15 cells were incubated in the presence of apicidin, TSA or n-butyrate. (a, b) The number (a) and the percentage (b) of eosinophilic cells after 6 days incubation in the presence of the indicated concentrations of apicidin, TSA or n-butyrate. N.D., not detectable. (c) Microscopic observation of HL-60 clone 15 cells incubated for 6 days in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM). The bar indicates 10 μm. (d, e) The intracellular structure (d) and the expression of integrin β7 (e) after 6 days incubation in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM). Dotted and solid lines represent the histogram before and after incubation with the drug, respectively. (f, g) The expression of MBP in the cells incubated for the periods indicated in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) (f) or for 6 days in the presence of the indicated concentrations of apicidin or n-butyrate (g). Actin in the cell lysate was detected by immunoblotting as an internal control.
Effects of apicidin, TSA and n-butyrate on cell proliferation
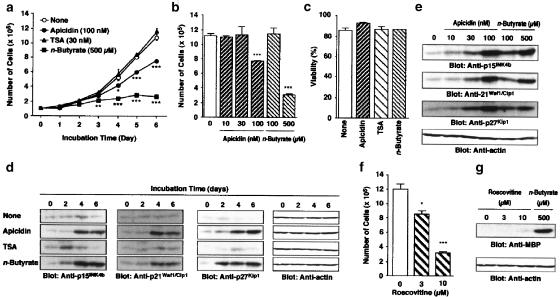
The time-dependent increase in the number of HL-60 clone 15 cells was suppressed by 100 nM apicidin and 500 μM n-butyrate (Figure 2a). Significant inhibition of the cell proliferation by apicidin and n-butyrate was observed after 4 and 3 days incubation, respectively (Figure 2a). However, 30 nM TSA did not suppress the proliferation during 6 days incubation (Figure 2a). After 6 days incubation, 10 and 30 nM apicidin, and 100 μM n-butyrate did not suppress the cell proliferation either (Figure 2b). The viability of the cells after 6 days incubation with 100 nM apicidin, 30 nM TSA or 500 μM n-butyrate did not change significantly compared with that of the control (Figure 2c). At higher concentrations of apicidin (⩾300 nM) or TSA (⩾100 nM), the cell viability was significantly decreased at 24 h incubation due to cytotoxic activity (data not shown). The cyclin-dependent kinase (CDK) inhibitor proteins p15INK4b, p21Waf1/Cip1 and p27Kip1 were expressed in a time-dependent manner on treatment with 100 nM apicidin and 500 μM n-butyrate (Figure 2d). TSA at 30 nM induced a transient expression of these proteins at low levels (Figure 2d). Apicidin and n-butyrate induced the expression of p15INK4b, p21Waf1/Cip1 and p27Kip1 in a concentration-dependent manner at 6 days incubation (Figure 2e). The chemical CDK inhibitor roscovitine at 3 and 10 μM suppressed the proliferation of the cells in a concentration-dependent manner (Figure 2f), but did not induce the expression of MBP (Figure 2g).
Figure 2.
Effects of apicidin, TSA and n-butyrate on the number of cells and the expression of p15INK4b, p21Waf1/Cip1, p27Kip1 and MBP in HL-60 clone 15 cells. (a, b) The number of cells incubated for the periods indicated in the absence and presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) (a), and for 6 days in the presence of the indicated concentrations of apicidin or n-butyrate (b). Values are the means from four samples with s.e.m. Statistical significance: *P<0.05, **P<0.01, ***P<0.001 vs corresponding control. (c) Viability of the cells after 6 days incubation in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM). (d, e) The expression of p15INK4b, p21Waf1/Cip1 and p27Kip1 proteins in the cells incubated for the periods indicated in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) (d) or for 6 days in the presence of the indicated concentrations of apicidin or n-butyrate (e). (f, g) Roscovitine was added to the medium at an interval of 2 days, and the number of cells (f) and the expression of MBP in the cells (g) were determined after 6 days incubation in the presence of the indicated concentrations of roscovitine and n-butyrate. Actin in the cell lysate was detected by immunoblotting as an internal control.
Effects of apicidin, TSA and n-butyrate on the acetylation of histones H3 and H4
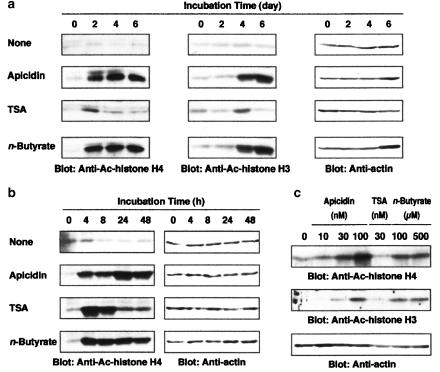
To clarify whether the difference in the action of apicidin, TSA and n-butyrate on the differentiation and proliferation of HL-60 clone 15 cells is due to the difference in histone acetylation, effects of each HDAC inhibitor on the acetylation of histones H3 K14 and H4 were examined. Histone H4 was acetylated by 100 nM apicidin and 500 μM n-butyrate at 2, 4 and 6 days, and acetylation of histone H3 K14 was observed at 4 and 6 days (Figure 3a). n-Butyrate at 500 μM acetylated histone H4, a plateau being reached at 4 h, while apicidin at 100 nM increased the acetylation with time, a maximum being reached at 24 h (Figure 3b). In contrast, acetylation of histone H4 by 30 nM TSA was maximal at 4 h and then declined time dependently (Figure 3a and b), while acetylation of histone H3 K14 by 30 nM TSA was only observed at day 4 (Figure 3b). After 6 days incubation, acetylation of histones H3 K14 and H4 by apicidin and n-butyrate was still detected in a concentration-dependent manner (Figure 3c). These findings indicated that the acetylation of histones H3 and H4 by apicidin and n-butyrate was continuous, while the acetylation by TSA was transient.
Figure 3.
Acetylation of histones H3 K14 and H4 by apicidin, TSA and n-butyrate. Acetylated histone H3 K 14 and histone H4 in the cells incubated for the periods indicated in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) (a, b) and for 6 days in the presence of the indicated concentrations of apicidin, TSA or n-butyrate (c). Actin in the cell lysate was detected by immunoblotting as an internal control.
Differentiation into eosinophils on repeated treatment with TSA
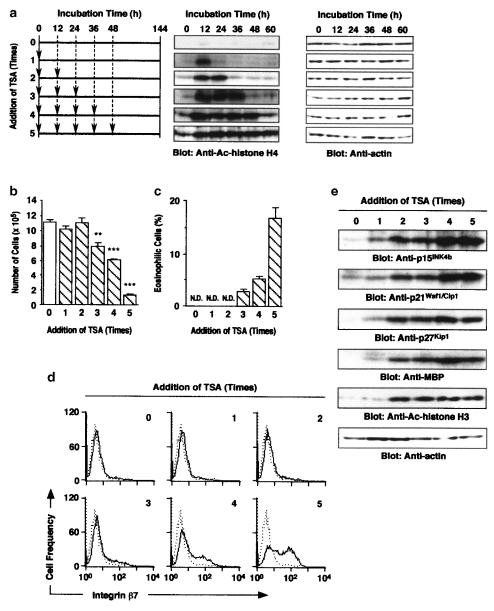
The acetylation of histone H4 was continued by repeated treatment with 30 nM TSA at an interval of 12 h (Figure 4a). The repeated treatment with TSA decreased the number of cells, but increased the percentage of eosinophilic cells (Figure 4b and c). The decrease in the number of cells and increase in eosinophilic cells were induced by more than three treatments with TSA (Figure 4b and c). Expression of integrin β7 on the cell surface was also induced by more than three treatments with TSA (Figure 4d). Furthermore, TSA induced the expression of p15INK4b, p21Waf1/Cip1, p27Kip1 and MBP, and the acetylation of histone H3 K14 at 6 days on repeated treatment (Figure 4e).
Figure 4.
Differentiation of HL-60 clone 15 cells into eosinophils on repeated treatment with TSA. TSA (30 nM) was added to HL-60 clone 15 cells up to 5 times at an interval of 12 h (a, arrowheads). (a) The acetylation of histone H4 in the cells at the indicated periods of incubation. (b, c) Effects of the repeated treatment with TSA (30 nM) on the total number of cells (b) and the percentage of eosinophilic cells (c) after 6 days incubation. N.D., not detectable. Values are the means from three samples with s.e.m. Statistical significance: **P<0.01, ***P<0.001 vs control. (d) Effects of the repeated treatment with TSA (30 nM) on the expression of integrin β7 on the cells after 6 days incubation. Dotted and solid lines represent the histogram before and after 6 days incubation, respectively. (e) Effects of the repeated treatment with TSA (30 nM) on the expression of p15INK4b, p21Waf1/Cip1, p27Kip1, MBP and acetylated histone H3 K14 after 6 days incubation. Actin in the cell lysate was detected by immunoblotting as an internal control.
Impairment of differentiation into eosinophils by shortening the period of incubation with apicidin or n-butyrate
Effects of the period of incubation with apicidin or n-butyrate on the deacetylation of histone H4 and the differentiation of HL-60 clone 15 cells into eosinophils were examined. Acetylation of histone H4 continued throughout the culture in the presence of 100 nM apicidin or 500 μM n-butyrate, but shortening the incubation period with apicidin or n-butyrate decreased the acetylation time dependently (Figure 5a). In parallel with the decrease in histone H4 acetylation, the number of cells recovered and the percentage of eosinophilic cells decreased at day 6 (Figure 5b and c). The expression of integrin β7, p15INK4b, p21Waf1/Cip1, p27Kip1 and MBP, and the acetylation of histone H3 K14 were also decreased by shortening the period of incubation with apicidin or n-butyrate (Figure 5d and e).
Figure 5.
Impairment of the differentiation of HL-60 clone 15 cells into eosinophils by shortening the period of incubation with apicidin or n-butyrate. HL-60 clone 15 cells were incubated for 0, 4, 8, 24 or 144 h in the presence of 100 nM apicidin or 500 μM n-butyrate (a, bold lines), and further incubated for 144, 140, 136, 120 or 0 h in the absence of apicidin or n-butyrate till 6 days, respectively (a, thin lines). (a) The acetylation of histone H4 in the cells at the indicated periods of incubation. (b, c) Effects of the incubation period with apicidin (100 nM) or n-butyrate (500 μM) on the number of cells (b) and the percentage of eosinophilic cells (c) after 6 days. N.D., not detectable. Values are the means from three samples with s.e.m. Statistical significance: **P<0.01, ***P<0.001 vs control. (d) Effects of the incubation period with apicidin (100 nM) or n-butyrate (500 μM) on the expression of integrin β7 after 6 days. Dotted and solid lines represent the histogram before and after 6 days incubation, respectively. (e) Effects of the incubation period with apicidin (100 nM) or n-butyrate (500 μM) on the expression of p15INK4b, p21Waf1/Cip1, p27Kip1, MBP and acetylated histone H3 K14 after 6 days. Actin in the cell lysate was detected by immunoblotting as an internal control.
Activation of C/EBP by apicidin, TSA and n-butyrate
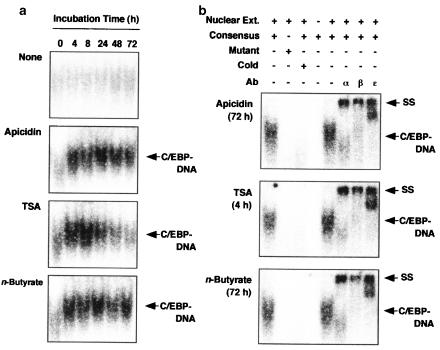
On treatment with apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM), the amount of the transcription factor C/EBP bound to the DNA probe was increased (Figure 6a). Apicidin and n-butyrate activated C/EBP until 72 h, while TSA induced a transient activation, a maximum being reached at 4–8 h followed by a time-dependent decline until 72 h (Figure 6a). The binding of C/EBP to DNA was inhibited when the nuclear extracts were incubated with 32P-labeled mutant probe or excess unlabeled-consensus DNA (Figure 6b). The addition of anti-C/EBPα antibody, anti-C/EBPβ antibody or anti-C/EBPɛ antibody to the nuclear extracts specifically supershifted the C/EBP-DNA complex (Figure 6b). These findings indicated that the activation of C/EBPα, C/EBPβ and C/EBPɛ by apicidin and n-butyrate was continuous, while the activation by TSA was transient.
Figure 6.
DNA binding activity of C/EBP in apicidin-, TSA- and n-butyrate-treated cells. (a) HL-60 clone 15 cells were incubated for the periods indicated in the presence of apicidin (100 nM), TSA (30 nM) or n-butyrate (500 μM) and the nuclear proteins were extracted. The amount of C/EBP in 5 μg of the nuclear proteins bound to the 32P-labeled DNA probe containing the C/EBP binding site was detected by EMSA. (b) The nuclear extracts were preincubated with unlabeled-consensus probe, anti-C/EBPα (α), anti-C/EBPβ (β) or anti-C/EBPɛ (ɛ). The arrowheads indicate the complex of C/EBP with DNA (C/EBP-DNA), and C/EBP-DNA supershifted by the antibody (SS).
Discussion
Eosinophils are characterized by morphological features, basic granule proteins such as MBP, and the cell-surface expression of certain proteins (Gleich et al., 1973; Shoham et al., 1974; Lundahl et al., 2000). n-Butyrate is the most powerful chemical inducer of the differentiation of HL-60 clone 15 cells into mature eosinophils (Fischkoff & Condon, 1985) and has contributed to the understanding of the characterization, regulation and function of eosinophils (Fischkoff et al., 1984; Fischkoff & Condon, 1985; Tiffany et al., 1998; Lundahl et al., 2000). In this study, we showed that apicidin generated eosinophil-specific features including positive staining with Luxol-fast-blue, changes in the intracellular structure, and the expression of MBP and integrin β7, indicating that apicidin induced the differentiation of HL-60 clone 15 cells into eosinophils. However, TSA did not induce the differentiation into eosinophils. We therefore analyzed the difference in the acetylation pattern of histones H3 K14 and H4 among these HDAC inhibitors, because they elevate HAT activity resulting in the hyperacetylation of molecules including histones H3 and H4 in the cells (Riggs et al., 1977; Yoshida et al., 1987; Darkin-Rattray et al., 1996; Grunstein, 1997; Hong et al., 2003). As shown in Figure 3, apicidin and n-butyrate induced continuous acetylation of histones H3 K14 and H4, whereas TSA induced a transient acetylation. Furthermore, the repeated treatment but not a single treatment with TSA induced the continuous acetylation of histones H3 K14 and H4 and differentiation of HL-60 clone 15 cells into eosinophils (Figures 1, 2, 3 and 4), and shortening the period of incubation with apicidin and n-butyrate reduced the extent of acetylation and differentiation (Figure 5). These findings suggest that the continuous acetylation of histones H3 K14 and H4 is necessary for HL-60 clone 15 cells to differentiate into eosinophils. However, the acetylation pattern of histones H3 K14 and H4 was different; namely, the acetylation of histone H4 was followed by the acetylation of histone H3 K14 (Figure 3). In the expression of various genes, bindings of the activated transcription factors to the promoter region of the target gene lead to the recruitment of HATs such as GCN5 and CBP/p300 coactivators, followed by the recruitment of remodeling factors such as SWI/SNF, and then basal transcription factors including TFIID (Agalioti et al., 2000; Merika & Thanos, 2001; Agalioti et al., 2002). Acetylation of histones needs to install each component of the transcriptional factor complex to the promoter region, because the factor selectively recognizes the acetylated lysine residue in the histone by its bromodomain (Dhalluin et al., 1999; Jacobson et al., 2000; Agalioti et al., 2002). Especially, the acetylated histones H4 K8 and H3 K9 and K14 are critical for the recruitment of the SWI/SNF complex and TFIID, respectively (Agalioti et al., 2002). Our findings suggest that the continuous acetylation of histones H3 K14 and H4 by apicidin and n-butyrate contributes the continuous recruitment and activation of transcriptional factor complex to express various genes composing eosinophils. Again, one of the mechanisms by which single treatment of TSA did not induce the differentiation of HL-60 clone 15 cells into eosinophils might be the pulse-activation of the transcriptional factor complex by transient acetylation of histones H4 and H3 K14. Although many reports (Cioe et al., 1981; Marks et al., 2000; Witt et al., 2003) have described the difference in apoptosis- or differentiation-inducing activity of HDAC inhibitors, the mechanism responsible for the different activities has not been clarified. Our findings might explain the mechanism for the different biological activities among HDAC inhibitors.
Accompanying the inhibition of cell proliferation and the induction of differentiation, the expression of p15INK4b, p21Waf1/Cip1 and p27Kip1, which negatively regulate the progression of the cell cycle by inhibiting the activity of cyclin/CDK (Lee & Yang, 2001), increased (Figures 2, 4 and 5). These findings suggest that such CDK inhibitor proteins are implicated in the inhibition of proliferation of HL-60 clone 15 cells. However, lower concentrations of apicidin (30 nM) and n-butyrate (100 μM) weakly induced the expression of such CDK inhibitor proteins (Figure 2) and the differentiation into eosinophils (Figure 1), but did not reduce the cell proliferation (Figure 2). Recently, it has been reported that levels of p21Waf1/Cip1 and p27Kip1 increase during cell differentiation and are implicated in the differentiation of the pro-B cell line Ba/F3, myelomonocytic cell line U937 and so on (Liu et al., 1996; Nosaka et al., 1999; Hisatake et al., 2001; McArthur et al., 2002; Buitenhuis et al., 2003). The finding that roscovitine, which inhibits CDK2 and cdc2 and arrests the cell cycle (Meijer et al., 1997; McArthur et al., 2002), did not induce expression of MBP but inhibited the cell proliferation (Figure 2), suggested that the inhibition of cell proliferation mediated by CDK inhibitor proteins did not trigger the cell differentiation. The CDK inhibitor proteins p15INK4b, p21Waf1/Cip1 and p27Kip1 whose expression is induced by HDAC inhibitors might contribute to the cell differentiation by some mechanism other than the inhibition of cell proliferation.
In this study, we demonstrated the importance of the balance of HAT/HDAC activity for the differentiation of HL-60 clone 15 cells into eosinophils. However, the mechanism behind the cell differentiation induced by HDAC inhibitors might be specific to the type of cell, because as we reported, in HL-60 cells, apicidin and TSA induced the early stage differentiation into CD11b-expressing cells and not the complete maturation to neutrophils or monocytes (Hong et al., 2003). In erythroleukemia K562 cells, apicidin and n-butyrate induce differentiation into fetal hemoglobinproducing cells but not into CD11b-expressing cells (Cioe et al., 1981; Hong et al., 2003; Witt et al., 2003), and the differentiation of K562 cells stimulated by apicidin is irreversible (Witt et al., 2003) whereas the differentiation of HL-60 clone 15 cells was reversible (Figure 5). These findings suggest that the mechanism behind the cell differentiation is regulated by not only the acetylation of histone but also the endogenous cell-specific ability to differentiate into each lineage.
C/EBPα, C/EBPβ and C/EBPɛ are important transcription factors for eosinophilic differentiation, because C/EBP inhibits the cell proliferation by associating with Rb (Gery et al., 2004), E2F (Gery et al., 2004), CDK (Wang et al., 2001) or p21Waf1/Cip1 (Timchenko et al., 1997) and induces the expression of eosinophilic-specific genes (Müller et al., 1995; Zhang et al., 1997; Yamaguchi et al., 1998; 1999; Du et al., 2002; McNagny & Graf, 2002; Gombart et al., 2003). The expression of MBP is controlled under the P2 promoter from –117 to +1 bp region (Yamaguchi et al., 1998; 1999; Du et al., 2002; Gombart et al., 2003). In this region, two binding sites for each GATA, PU.1 and C/EBP are identified, and the bindings of GATA1, PU.1 C/EBPα, C/EBPβ and C/EBPɛ synergistically regulate the MBP expression (Yamaguchi et al., 1998; 1999; Du et al., 2002; Gombart et al., 2003). Eosinophil-derived neurotoxin/RNS2, one of the eosinophil granule proteins, is also induced by the activation of C/EBPα, C/EBPβ and C/EBPɛ (Baltus et al., 1999). Therefore, the activation of C/EBP plays an important role in the differentiation into mature eosinophils. We showed that the DNA-binding activity of C/EBP was increased by apicidin, TSA and n-butyrate, and the activation of C/EBP by apicidin and n-butyrate was continuous, while the activation by TSA was transient (Figure 6a). The continuous effect of apicidin and n-butyrate and the transient effect of TSA were also observed in histones H4 and H3 K14 acetylation (Figure 3), and the differentiation of HL-60 clone 15 cells into eosinophils was induced by apicidin and n-butyrate but not TSA. These findings suggested that the C/EBP activation is related to the HDAC inhibition and the differentiation into eosinophils. We also showed that the antibodies against C/EBPα, C/EBPβ and C/EBPɛ supershifted each C/EBP-DNA complex from HL-60 clone 15 cells treated with apicidin for 72 h, TSA for 4 h or n-butyrate for 72 h (Figure 6b), suggesting that the mechanism for the activation of each C/EBP by these HDAC inhibitors is the same.
The generation of eosinophils from stem cells is regulated by cytokines such as IL-3, IL-5 and GM-CSF (de Groot et al., 1998). Receptors for IL-3, IL-5 and GM-CSF are heterodimers consisting of an α-subunit specific to each cytokine and a β-subunit common to these cytokines (Hayashida et al., 1990; Kitamura et al., 1991; Tavernier et al., 1991; Woodcock et al., 1996; de Groot et al., 1998). In mature eosinophils isolated from peripheral blood or inflammatory sites, IL-3, IL-5 and GM-CSF activate kinases including Janus protein kinases, STAT5 and p44/42 MAPK via each cytokine receptor, and have almost the same biological roles such as the prolongation of survival (Rothenberg et al., 1988; de Groot et al., 1998; Miike et al., 1999; Ishihara et al., 2001). However, the response of stem cells to these cytokines is different; CD34−CD33+ cells, which are more differentiated than CD34+ cells, have the ability to differentiate into eosinophils in the presence of IL-5, and into eosinophils, neutrophils and macrophages in the presence of IL-3 (Ema et al., 1990). Furthermore, MBP is expressed during the maturation process, but not in mature eosinophils (Gruart et al., 1992). Therefore, it is possible that the signal transduction system involved in IL-3-, IL-5- and GM-CSF-induced differentiation of stem cells is different, and that IL-5 transduces different intracellular signals between immature eosinophils and mature eosinophils. Recently, it has been reported that the transcription factors GATA, C/EBP, PU.1 and STAT5a are involved in the differentiation of stem cells into eosinophils (Müller et al., 1995; Chumakov et al., 1997; Yamanaka et al., 1997; Zhang et al., 1997; Yamaguchi et al., 1998; 1999; Du et al., 2002; Hirasawa et al., 2002; McNagny & Graf, 2002; Gombart et al., 2003). However, the mechanism for the differentiation induced by IL-5 has not been clarified in detail. Here, we focused on the differentiation of HL-60 clone 15 cells into eosinophils and found that the continuous acetylation of histones H3 K14 and H4 is necessary for the differentiation of HL-60 clone 15 cells into eosinophils. Furthermore, we showed that the transcription factor C/EBP was activated in parallel with the histone H4 acetylation. Our findings will contribute to the understanding of the specific differentiation of the eosinophil lineage from stem cells.
Acknowledgments
This work was supported in part by Research on Health Sciences focusing on Drug Innovation from Japan Health Sciences Foundation (to K.I.), by the Research Fund from Kyonggi Pharmaceutical Research Center (KPRC), Sungkyunkwan University, Suwon, Korea, and by Grant-in-Aid for Scientific Research (B) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) (14370738 to K.O.).
Abbreviations
- C/EBP
CCAAT/enhancer binding protein
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- MBP
major basic protein
- TSA
trichostatin A
References
- AGALIOTI T., CHEN G., THANOS D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111:381–392. doi: 10.1016/s0092-8674(02)01077-2. [DOI] [PubMed] [Google Scholar]
- AGALIOTI T., LOMVARDAS S., PAREKH B., YIE J., MANIATIS T., THANOS D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- BALTUS B., BUITENHUIS M., VAN DIJK T.B., VINSON C., RAAIJMAKERS J.A., LAMMERS J.W., KOENDERMAN L., DE GROOT R.P. C/EBP regulates the promoter of the eosinophil-derived neurotoxin/RNS2 gene in human eosinophilic cells. J. Leukoc. Biol. 1999;66:683–688. doi: 10.1002/jlb.66.4.683. [DOI] [PubMed] [Google Scholar]
- BUITENHUIS M., BALTUS B., LAMMERS J.-W.J., COFFER P.J., KOENDERMAN L. Signal transducer and activator of transcription 5a (STAT5a) is required for eosinophil differentiation of human cord blood-derived CD34+ cells. Blood. 2003;101:134–142. doi: 10.1182/blood-2002-03-0740. [DOI] [PubMed] [Google Scholar]
- CHUMAKOV A.M., GRILLIER I., CHUMAKOVA E., CHIH D., SLATER J., KOEFFLER H.P. Cloning of the novel human myeloid-cell-specific C/EBP-ɛ transcription factor. Mol. Cell. Biol. 1997;17:1375–1386. doi: 10.1128/mcb.17.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIOE L., MCNAB A., HUBBELL H.R., MEO P., CURTIS P., ROVERA G. Differential expression of the globin genes in human leukemia K562(S) cells induced to differentiate by hemin or butyric acid. Cancer Res. 1981;41:237–243. [PubMed] [Google Scholar]
- CORRIGAN C.J., KAY A.B. T cells and eosinophils in the pathogenesis of asthma. Immunol. Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- CSORDAS A. On the biological role of histone acetylation. Biochem. J. 1990;265:23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARKIN-RATTRAY S.J., GURNETT A.M., MYERS R.W., DULSKI P.M., CRUMLEY T.M., ALLOCCO J.J., CANNOVA C., MEINKE P.T., COLLETTI S.L., BEDNAREK M.A., SINGH S.B., GOETZ M.A., DOMBROWSKI A.W., POLISHOOK J.D., SCHMATZ D.M. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GROOT R.P., COFFER P.J., KOENDERMAN L. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell. Signal. 1998;10:619–628. doi: 10.1016/s0898-6568(98)00023-0. [DOI] [PubMed] [Google Scholar]
- DHALLUIN C., CARLSON J.E., ZENG L., HE C., AGGARWAL A.K., ZHOU M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- DU J., STANKIEWICZ M.J., LIU Y., XI Q., SCHMITZ J.E., LEKSTROM-HIMES J.A., ACKERMAN S.J. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPɛ isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J. Biol. Chem. 2002;277:43481–43494. doi: 10.1074/jbc.M204777200. [DOI] [PubMed] [Google Scholar]
- EMA H., SUDA T., NAGAYOSHI K., MIURA Y., CIVIN C.I., NAKAUCHI H. Target cells for granulocyte colony-stimulating factor, interleukin-3, and interleukin-5 in differentiation pathways of neutrophils and eosinophils. Blood. 1990;76:1956–1961. [PubMed] [Google Scholar]
- FISCHKOFF S.A., CONDON M.E. Switch in differentiative response to maturation inducers of human promyelocytic leukemia cells by prior exposure to alkaline conditions. Cancer Res. 1985;45:2065–2069. [PubMed] [Google Scholar]
- FISCHKOFF S.A., POLLAK A., GLEICH G.J., TESTA J.R., MISAWA S., REBER T.J. Eosinophilic differentiation of the human promyelocytic leukemia cell line, HL-60. J. Exp. Med. 1984;160:179–196. doi: 10.1084/jem.160.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERY S., GOMBART A.F., FUNG Y.K., KOEFFLER H.P. C/EBP interacts with retinoblastoma and E2F1 during granulopoiesis. Blood. 2004;103:828–835. doi: 10.1182/blood-2003-01-0159. [DOI] [PubMed] [Google Scholar]
- GLEICH G.J., LOEGERING D.A., MALDONADO J.E. Identification of a major basic protein in guinea pig eosinophil granules. J. Exp. Med. 1973;137:1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMBART A.F., KWOK S.H., ANDERSON K.L., YAMAGUCHI Y., TORBETT B.E., KOEFFLER H.P. Regulation of neutrophil and eosinophil secondary granule gene expression by transcription factors C/EBP and PU.1. Blood. 2003;101:3265–3273. doi: 10.1182/blood-2002-04-1039. [DOI] [PubMed] [Google Scholar]
- GRUART V., TRUONG M.J., PLUMAS J., ZANDECKI M., KUSNIERZ J.P., PRIN L., VINATIER D., CAPRON A., CAPRON M. Decreased expression of eosinophil peroxidase and major basic protein messenger RNAs during eosinophil maturation. Blood. 1992;79:2592–2597. [PubMed] [Google Scholar]
- GRUNSTEIN M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- HAYASHIDA K., KITAMURA T., GORMAN D.M., ARAI K., YOKOTA T., MIYAJIMA A. Molecular cloning of a second subunit of the receptor for human granulocyte–macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRASAWA R., SHIMIZU R., TAKAHASHI S., OSAWA M., TAKAYANAGI S., KATO Y., ONODERA M., MINEGISHI N., YAMAMOTO M., FUKAO K., TANIGUCHI H., NAKAUCHI H., IWAMA A. Essential and instructive roles of GATA factors in eosinophil development. J. Exp. Med. 2002;195:1379–1386. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HISATAKE J., O'KELLY J., USKOKOVIC M.R., TOMOYASU S., KOEFFLER H.P. Novel vitamin D3 analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D3, that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood. 2001;97:2427–2433. doi: 10.1182/blood.v97.8.2427. [DOI] [PubMed] [Google Scholar]
- HONG J.J., ISHIHARA K., YAMAKI K., HIRAIZUMI K., OHNO T., AHN J.W., ZEE O.P., OHUCHI K. Apicidin, a histone deacetylase inhibitor, induces differentiation of HL-60 cells. Cancer Lett. 2003;189:197–206. doi: 10.1016/s0304-3835(02)00500-1. [DOI] [PubMed] [Google Scholar]
- ISHIHARA K., SATOH I., MUE S., OHUCHI K. Possible participation of a JAK2 signaling pathway in recombinant rat interleukin-5-induced prolongation of rat eosinophil survival. Biochim. Biophys. Acta. 2001;1536:73–84. doi: 10.1016/s0925-4439(01)00035-7. [DOI] [PubMed] [Google Scholar]
- JACOBSON R.H., LADURNER A.G., KING D.S., TJIAN R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- KITAMURA T., SATO N., ARAI K., MIYAJIMA A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991;66:1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- KLUG A., RHODES D., SMITH J., FINCH J.T., THOMAS J.O. A low resolution structure for the histone core of the nucleosome. Nature. 1980;287:509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- LEE D.Y., HAYES J.J., PRUSS D., WOLFFE A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- LEE M.H., YANG H.Y. Negative regulators of cyclin-dependent kinases and their roles in cancers. Cell. Mol. Life Sci. 2001;58:1907–1922. doi: 10.1007/PL00000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M., LEE M.H., COHEN M., BOMMAKANTI M., FREEDMAN L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Gene Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- LUNDAHL J., SEHMI R., HAYES L., HOWIE K., DENBURG J.A. Selective upregulation of a functional β7 integrin on differentiating eosinophils. Allergy. 2000;55:865–872. doi: 10.1034/j.1398-9995.2000.00574.x. [DOI] [PubMed] [Google Scholar]
- MARKS P.A., RICHON V.M., RIFKIND R.A. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- MARTIN L.B., KITA H., LEIFERMAN K.M., GLEICH G.J. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int. Arch. Allergy Immunol. 1996;109:207–215. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- MCARTHUR G.A., FOLEY K.P., FERO M.L., WALKLEY C.R., DEANS A.J., ROBERTS J.M., EISENMAN R.N. MAD1 and p27KIP1 cooperate to promote terminal differentiation of granulocytes and to inhibit Myc expression and cyclin E-CDK2 activity. Mol. Cell. Biol. 2002;22:3014–3023. doi: 10.1128/MCB.22.9.3014-3023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGHEE J.D., FELSENFELD G. Nucleosome structure. Ann. Rev. Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- MCNAGNY K., GRAF T. Making eosinophils through subtle shifts in transcription factor expression. J. Exp. Med. 2002;195:F43–F47. doi: 10.1084/jem.20020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIJER L., BORGNE A., MULNER O., CHONG J.P., BLOW J.J., INAGAKI N., INAGAKI M., DELCROS J.G., MOULINOUX J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- MERIKA M., THANOS D. Enhanceosomes. Curr. Opin. Genet. Dev. 2001;11:205–208. doi: 10.1016/s0959-437x(00)00180-5. [DOI] [PubMed] [Google Scholar]
- MIIKE S., NAKAO A., HIRAGURI M., KURASAWA K., SAITO Y., IWAMOTO I. Involvement of JAK2, but not PI 3-kinase/Akt and MAP kinase pathways, in anti-apoptotic signals of GM-CSF in human eosinophils. J. Leukoc. Biol. 1999;65:700–706. doi: 10.1002/jlb.65.5.700. [DOI] [PubMed] [Google Scholar]
- MÜLLER C., KOWENZ-LEUTZ E., GRIESER-ADE S., GRAF T., LEUTZ A. NF-M (chicken C/EBP β) induces eosinophilic differentiation and apoptosis in a hematopoietic progenitor cell line. EMBO J. 1995;14:6127–6135. doi: 10.1002/j.1460-2075.1995.tb00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON V.G., MARVIN K.W., YAU P., BRADBURY E.M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J. Biol. Chem. 1990;265:19848–19852. [PubMed] [Google Scholar]
- NOSAKA T., KAWASHIMA T., MISAWA K., IKUTA K., MUI A.L., KITAMURA T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL C.C., TOLBERT M., MAHRER S., SINGH A., GRACE M.J., BAUMANN M.A. Cooperative effects of interleukin-3 (IL-3), IL-5, and granulocyte–macrophage colony-stimulating factor: a new myeloid cell line inducible to eosinophils. Blood. 1993;81:1193–1199. [PubMed] [Google Scholar]
- PAZIN M.J., KADONAGA J.T. What's up and down with histone deacetylation and transcription. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- RENAULD J.-C. New insights into the role of cytokines in asthma. J. Clin. Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS M.G., WHITTAKER R.G., NEUMANN J.R., INGRAM V.M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- ROTHENBERG M.E., OWEN W.F., JR, SILBERSTEIN D.S., WOODS J., SOBERMAN R.J., AUSTEN K.F., STEVENS R.L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J. Clin. Invest. 1988;81:1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., BOURINBAIAR A., GINSBURG M., MINATO K., CERESI E., YAMADA K., MACHOVER D., BREARD J., MATHE G. Establishment and characterization of a new human eosinophilic leukemia cell line. Blood. 1985;66:1233–1240. [PubMed] [Google Scholar]
- SHOHAM D., DAVID E.B., ROZENSZAJN L.A. Cytochemical and morphologic identification of macrophages and eosinophils in tissue cultures of normal human bone marrow. Blood. 1974;44:221–233. [PubMed] [Google Scholar]
- TAVERNIER J., DEVOS R., CORNELIS S., TUYPENS T., VAN DER HEYDEN J., FIERS W., PLAETINCK G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- TIFFANY H.L., ALKHATIB G., COMBADIERE C., BERGER E.A., MURPHY P.M. CC chemokine receptors 1 and 3 are differentially regulated by IL-5 during maturation of eosinophilic HL-60 cells. J. Immunol. 1998;160:1385–1392. [PubMed] [Google Scholar]
- TIMCHENKO N.A., HARRIS T.E., WILDE M., BILYEU T.A., BURGESS-BEUSSE B.L., FINEGOLD M.J., DARLINGTON G.J. CCAAT/enhancer binding protein α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol. Cell. Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER BRUGGEN T., KOENDERMAN L. Signal transduction in eosinophils. Clin. Exp. Allergy. 1996;26:880–891. [PubMed] [Google Scholar]
- WANG H., IAKOVA P., WILDE M., WELM A., GOODE T., ROESLER W.J., TIMCHENKO N.A. C/EBPα arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol. Cell. 2001;8:817–828. doi: 10.1016/s1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- WARDLAW A.J. Molecular basis for selective eosinophil trafficking in asthma: A multistep paradigm. J. Allergy Clin. Immunol. 1999;104:917–926. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- WITT O., MONKEMEYER S., RONNDAHL G., ERDLENBRUCH B., REINHARDT D., KANBACH K., PEKRUN A. Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood. 2003;101:2001–2007. doi: 10.1182/blood-2002-08-2617. [DOI] [PubMed] [Google Scholar]
- WOODCOCK J.M., BAGLEY C.J., ZACHARAKIS B., LOPEZ A.F. A single tyrosine residue in the membrane-proximal domain of the granulocyte–macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 receptor common β-chain is necessary and sufficient for high affinity binding and signaling by all three ligands. J. Biol. Chem. 1996;271:25999–26006. doi: 10.1074/jbc.271.42.25999. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI Y., ACKERMAN S.J., MINEGISHI N., TAKIGUCHI M., YAMAMOTO M., SUDA T. Mechanisms of transcription in eosinophils: GATA-1, but not GATA-2, transactivates the promoter of the eosinophil granule major basic protein gene. Blood. 1998;91:3447–3458. [PubMed] [Google Scholar]
- YAMAGUCHI Y., NISHIO H., KISHI K., ACKERMAN S.J., SUDA T. C/EBPβ and GATA-1 synergistically regulate activity of the eosinophil granule major basic protein promoter: implication for C/EBPβ activity in eosinophil gene expression. Blood. 1999;94:1429–1439. [PubMed] [Google Scholar]
- YAMANAKA R., KIM G.D., RADOMSKA H.S., LEKSTROM-HIMES J., SMITH L.T., ANTONSON P., TENEN D.G., XANTHOPOULOS K.G. CCAAT/enhancer binding protein ɛ is preferentially upregulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6462–6467. doi: 10.1073/pnas.94.12.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA M., NOMURA S., BEPPU T. Effects of trichostatins on differentiation of murine erythroleukemia cells. Cancer Res. 1987;47:3688–3691. [PubMed] [Google Scholar]
- ZHANG D.E., ZHANG P., WANG N.D., HETHERINGTON C.J., DARLINGTON G.J., TENEN D.G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]