Abstract
Heptanol, 18α-glycyrrhetinic acid (18αGA) and 18β-glycyrrhetinic acid (18βGA) are known blockers of gap junctions, and are often used in vascular studies. However, actions unrelated to gap junction block have been repeatedly suggested in the literature for these compounds. We report here the findings from a comprehensive study of these compounds in the arterial wall.
Rat isolated mesenteric small arteries were studied with respect to isometric tension (myography), [Ca2+]i (Ca2+-sensitive dyes), membrane potential and – as a measure of intercellular coupling – input resistance (sharp intracellular glass electrodes). Also, membrane currents (patch-clamp) were measured in isolated smooth muscle cells (SMCs). Confocal imaging was used for visualisation of [Ca2+]i events in single SMCs in the arterial wall.
Heptanol (150 μM) activated potassium currents, hyperpolarised the membrane, inhibited the Ca2+ current, and reduced [Ca2+]i and tension, but had little effect on input resistance. Only at concentrations above 200 μM did heptanol elevate input resistance, desynchronise SMCs and abolish vasomotion.
18βGA (30 μM) not only increased input resistance and desynchronised SMCs but also had nonjunctional effects on membrane currents. 18αGA (100 μM) had no significant effects on tension, [Ca2+]i, total membrane current and synchronisation in vascular smooth muscle.
We conclude that in mesenteric small arteries, heptanol and 18βGA have important nonjunctional effects at concentrations where they have little or no effect on intercellular communication. Thus, the effects of heptanol and 18βGA on vascular function cannot be interpreted as being caused only by effects on gap junctions. 18αGA apparently does not block communication between SMCs in these arteries, although an effect on myoendothelial gap junctions cannot be excluded.
Keywords: Heptanol, membrane potential, [Ca2+]i, ion current, vasomotion, glycyrrhetinic acid, gap junctions, smooth muscle
Introduction
In vascular smooth muscle (VSM), gap junctions with varying densities have been demonstrated by immunohistochemistry (Yeh et al., 1997; Ko et al., 1999; Berman et al., 2002; Hill et al., 2002) and electron microscopy (Hill et al., 2002). The presence of gap junctions or connexins in the media of arteries has been further substantiated through evidence of mRNA for connexins (Haefliger et al., 1997a; Rummery et al., 2002; Sandow et al., 2003) and connexin proteins (Haefliger et al., 1997b; Ko et al., 1999; Sandow et al., 2003). Functionally, it is well known that the VSM cells are electrically coupled, and it has been inferred that gap junctions mediate this intercellular communication (Bény & Pacicca, 1994; Xia & Duling, 1995; Welsh & Segal, 1998).
To obtain functional evidence for the role of gap junctions, a number of drugs with effects on gap junctions have been used, including the long-chain alcohol heptanol and derivatives of glycyrrhetinic acid. Heptanol and 18β-glycyrrhetinic acid (18βGA) are commonly used to block electrical cell-to-cell communication in a number of cells including smooth muscle cells. The effects of heptanol and 18βGA on vascular tone have often been interpreted in terms of the role of gap junctions in maintaining vascular tone (Tsai et al., 1995; Watts & Webb, 1996; Christ et al., 1999; Christ & Brink, 2000; Jiang et al., 2001; Leite & Webb, 2001; Coleman et al., 2002; Lagaud et al., 2002). However, there are concerns that heptanol and 18βGA have effects unrelated to their supposed effects on gap junctions in concentrations close to those needed to block gap junctions (Javid et al., 1996; Chaytor et al., 1997; 1998; Yamamoto et al., 1998; Murai et al., 1999; Santicioli & Maggi, 2000; Coleman et al., 2001; Tare et al., 2002). The α-form of glycyrrhetinic acid (18αGA) has been suggested to be a more specific and less toxic inhibitor of gap junctions than other glycyrrhetinic acid compounds (Davidson et al., 1986; Davidson & Baumgarten, 1988), although its specificity has also been questioned (Chaytor et al., 2000; Schuster et al., 2001; Tare et al., 2002). Surprisingly, despite the suggested nonjunctional effects of heptanol and glycyrrhetinic acid derivates in vascular preparations, these compounds are still extensively used in vascular studies to block gap junctions without providing direct evidence of specificity.
In the present study we tested the specificity of heptanol and glycyrrhetinic acid derivates by investigating their concentration-dependent effects on smooth muscle cell input resistance (as an indicator of gap junctional conductance), force, intracellular free calcium ([Ca2+]i), and membrane potential in rat mesenteric small arteries. In addition, we measured membrane currents in isolated smooth muscle cells from these arteries. The results demonstrate that heptanol and 18βGA block intercellular communication in mesenteric small arteries, but that at lower concentrations several nonjunctional effects appear. These findings strongly indicate that caution is necessary when ascribing an inhibitory effect on vascular tone to a purely junctional effect of these compounds. Moreover, these results have general implications when interpreting works published with these compounds.
Methods
Preparation
All experiments were conducted in accordance with the Danish national guidelines for animal research. Male Wistar rats, 12–18 weeks old, were killed with CO2. The mesentery was removed from the rats and placed into ice-cold physiological salt solution (PSS). Third-generation branches of small mesenteric arteries were dissected out and cleaned of fat and connective tissue. The isolated arterial segments were used either for mounting into a myograph or for cell isolation for patch-clamp studies.
Isometric force, [Ca2+]i and membrane potential measurements
Simultaneous measurements of isometric force and [Ca2+]i or isometric force and membrane potential were made at 37°C in isolated rat mesenteric resistance arteries. An approx. 2-mm long arterial segment was mounted as a ring preparation in the myograph (Danish Myo Technology, Aarhus, Denmark) for isometric force development. The internal circumference of the mounted artery was normalised on the basis of the passive tension–length curve to a value that gives maximal force development (Mulvany & Halpern, 1977). [Ca2+]i in the outer layers of the smooth muscle cells was measured with Fura-2 after loading with 2–5 μM of the acetoxymethyl ester form of the dye (we have previously demonstrated that applying Fura-2/AM to the myograph bath causes loading of only the outer smooth muscle cells (Peng et al., 1998)). Membrane potential was measured using KCl-filled microelectrodes with resistance in the range of 40–100 MΩ. In these experiments cell input resistance was semiquantitatively evaluated by injecting 1 nA current pulses (25 ms) and measuring the subsequent potential change; electrode resistance was routinely compensated by balancing the Wheatstone bridge of the amplifier (Intro-710, WPI) before impalements. All arteries were stimulated with 10 μM noradrenaline (NA), which is a supramaximal NA concentration in these arteries (Mulvany et al., 1980). NA in concentrations of 0.7–2 μM was used to elicit a submaximal response.
Confocal measurements
Arteries, mounted as described above for isometric force development, were loaded with 3 μM Calcium Green-1 acetoxymethyl ester. Calcium Green-1/AM was dissolved in DMSO with 0.1% (w v−1) cremophor and 0.02% (w v−1) Pluronic F-127 and the arteries were loaded (final DMSO concentration 0.5%) for 1.5 h at 37°C. The myograph was placed on the stage of an inverted confocal laser scanning microscope (ODYSSEY XL, Noran). Confocal optical sections were acquired with a water immersion objective (× 60, NA 1.2, Nikon). A 77 × 58-μm image (640 × 480 8-bit pixels) was obtained every 266 ms by using a 100-ns time scan mode and 8-frame averaging (in some experiments 16-frame averaging with 533 ms time interval was used). The emission signals at 530 nm (after excitation at 488 nm) were stored on a computer together with simultaneous force measurements. For image analysis, the programs Intervision (Noran) and Image Space (Molecular Dynamics) were used. The [Ca2+]i changes within cells were estimated as changes in the mean intensity of Calcium Green-1 fluorescence within regions of interests (ROIs) in which all pixel values were averaged (Peng et al., 2001).
Patch-clamp recordings
To assess membrane currents, smooth muscle cells were isolated from rat mesenteric small arteries as described previously (Matchkov et al., 2004). A few branches of mesenteric small arteries were placed into a microtube containing an enzyme solution and stored overnight at 4°C. The enzyme solution contained (in mM): NaCl 110, KCl 5, MgCl2 2, KH2PO4 0.5, NaH2PO4 0.5, NaHCO3 10, CaCl2 0.16, EDTA 0.49, Na-HEPES 10, glucose 10, taurine 10 at pH 7.0, as well as 1.5 mg ml−1 papain, 1.6 mg ml−1 albumin, and 0.4 mg ml−1 DL-dithiothreitol. On the following day, the microtube with the vessels was incubated for 5–10 min at 37°C. The vessels were removed from the enzyme solution and stored in standard bath solution at 4°C. Single cells were released by trituration with a polyethylene pipette into the bath solution.
To assess calcium currents, smooth muscle cells were isolated from rat mesenteric resistance arteries by a method modified from Petkov et al. (2001). A few branches of mesenteric resistance arteries were placed into a microtube containing the patch clamp bath solution (for compositions see below) for 30 min at 37°C gently bubbled with 95% O2/5% CO2. The solution was then replaced with 1 ml of calcium-free collagenase/elastase enzyme solution for 30–50 min at 37°C gently bubbled with 95% O2/5% CO2. The latter solution contained (in mM): NaCl 110, KCl 5.6, MgCl2 1.2, Na-HEPES 10, glucose 20, taurine 20, Na-pyruvate 5 at pH 7.4, as well as 1 mg ml−1 collagenase type 1 (Worthington Biochemical Corporation, Lakewood, NJ, U.S.A.), 4.85 μg ml−1 elastase type I (Sigma Chemical, Denmark), 1 mg ml−1 soybean trypsin inhibitor and 1 mg ml−1 albumin. Single cells were released by trituration with a polyethylene pipette into the standard bath solution.
All patch-clamp recordings were made at room temperature (22–24°C). Whole-cell recordings were made with patch pipettes having resistances in the range of 2–5 MΩ. For these experiments, only cells with low access resistance (5–10 MΩ) were used.
Recordings were made with an Axopatch 200B amplifier (Axon Instruments, U.S.A.) in whole-cell configuration. Data were sampled at a rate of 2 kHz. Data acquisition and analysis were carried out with the software package Clampex 7 for Windows (Axon Instruments). Series resistance and capacitative current were routinely compensated. To compare ionic currents from different recordings, the current amplitudes (recorded in pA) were normalised to the cell capacitance (recorded in pF) and expressed as current density (A F−1).
Currents were recorded at a holding potential of −60 mV, and current–voltage characteristics were obtained from ramp protocols. The voltage ramps were performed from −60 to +20 mV with duration of 140 ms.
Solutions
The PSS used contained (in mM): NaCl 119, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 25, CaCl2 2.5, EDTA 0.026, glucose 5.5, gassed with 5% CO2/95% O2 and pH 7.4. K-PSS was PSS where NaCl was substituted with an equimolar amount of KCl and 1 μM phentolamine was added.
In the patch clamp experiments the standard bath solution contained (in mM): NaCl 135, KCl 6, Na-HEPES 10, MgCl2 1, CaCl2 0.1 at pH 7.4. The standard pipette solution contained (in mM): NaCl 10, KCl 122, K-HEPES 10, MgCl2 1, CaCl2 0.01, BAPTA 0.1 at pH 7.4. In potassium-free solution the bath solution contained (in mM): HEPES 10, CsCl 140, CaCl2 0.1 and pH was 7.4, whereas the pipette solution contained (in mM): HEPES 10, BAPTA 10, CsCl 140, CaCl2 0.01 and pH was 7.4.
Calcium currents were recorded using the following solutions (Petkov et al., 2001). Bath solution contained (in mM): NaCl 110, KCl 5.6, CaCl2 5, MgCl2 1.2, Na-HEPES 10, glucose 20, taurine 20, Na-pyruvate 5 at pH 7.4. The pipette solution contained (in mM): CsCl 102, HEPES 10, EGTA 11, CaCl2 1, Na-pyruvate 5, succinic acid 5, oxalacetic acid 5, phosphocreatine 5, MgATP 3 at pH 7.4.
All stock solutions were at least 1000-fold more concentrated than final bath concentration. Stock solutions of 18α-GA (100 mM), 18β-GA (10–100 mM) and glibenclamide (10 mM) were dissolved in DMSO (final DMSO concentration ⩽0.1%). Stock solutions of heptanol (10–100 mM) and nifedipine (10 mM) were dissolved in ethanol (final ethanol concentration ⩽0.5%). Stock solutions of charybdotoxin (0.1 mM) and iberiotoxin (0.1 mM) were made up in 150 mM NaCl solutions.
Charybdotoxin and iberiotoxin were used to block large-conductance, calcium-activated potassium channels (KCa) in smooth muscle cells. These drugs were always used at a concentration of 100 nM, which is known to produce substantial block of KCa in smooth muscle (Nelson & Quayle, 1995). Glibenclamide at a concentration of 10 μM was used to block ATP-sensitive potassium channels (KATP) (Nelson & Quayle, 1995).
Drugs and chemicals
Glibenclamide, heptanol, nifedipine, NA, 18α-GA, 18β-GA and papain were obtained from Sigma (Sigma-Aldrich, Denmark), charybdotoxin from Alamone Labs (Alamone Labs Ltd, Israel), iberiotoxin from Latoxan (Latoxan, France) and phentolamine from Ciba-Geigy (Basel, Switzerland).
Statistics
All data are mean±s.e.m. In the patch clamp experiments only one experimental recording was taken from each cell, thus n is the number of cells; in other experiments n is the number of arteries. Differences between means were tested with a paired two tailed Student's t-test; P<0.05 was considered significant.
Results
Effects on membrane potential and input resistance
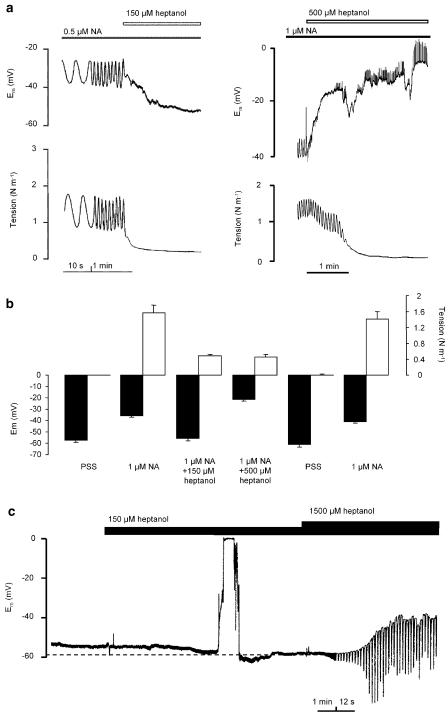
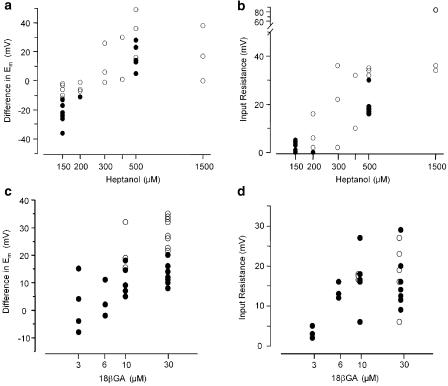
Arteries stimulated with a submaximal concentration of NA responded by oscillating in force and membrane potential, as described previously (Peng et al., 2001). Heptanol relaxed the arteries and abolished oscillation in both force and membrane potential (Figure 1). The effect of heptanol was reversible: depolarisation and contraction to a second NA stimulation after a 20-min washout period was not changed significantly (Figure 1b; n=4). The relaxation induced by 150 μM heptanol in the presence of a submaximal NA concentration was associated with repolarisation (change in membrane potential (ΔUm) was −23±3.2 mV, n=6 cells/6 arteries). There was no measurable increase of cell input resistance in this situation (Figure 1a and 2b). In nonstimulated arteries 150 μM heptanol caused hyperpolarisation (ΔUm=−6.5±1.6 mV, n=6 cells/4 arteries) (Figure 1c and 2a) but had no effect on input resistance (Figure 1c and 2b). With 500 μM heptanol arteries depolarised (Figure 1 and 2a) by 16.6±4.0 mV (n=5 cells/5 arteries) and by 33.7±9.6 mV (n=3 cells/3 arteries) for NA activated and nonactivated arteries, respectively. In both NA-activated and nonactivated arteries input resistance increased substantially (Figure 1 and 2b) at concentrations higher than 200 μM.
Figure 1.
Effect of heptanol on membrane potential, cell input resistance and tension. (a) Original recordings of simultaneous measurements of membrane potential and isometric tension in isolated mesenteric small arteries activated with noradrenaline (NA). (b) Bars illustrate the reversibility of heptanol responses on membrane potential and tension: arteries (n=4) were stimulated with NA, superfused with 150 and 500 μM heptanol, rinsed for 20 min and stimulated with NA again. No significant difference between first and second NA stimulation was found. (c) Original recordings of the effects of heptanol on membrane potential and cell input resistance (rapid deflections) in isolated mesenteric small arteries under resting conditions. Heptanol was present as indicated. Note the change in time scale.
Figure 2.
Concentration–response relations for effects of heptanol (a, b) and 18β-glycyrrhetinic acid (c, d) on membrane potential (a, c) (differences in membrane potential after and before addition of chemicals) and on cell input resistance (b, d) (the increase in amplitude of voltage responses to 1 nA current injection). Experiments were performed under resting conditions (open circles) and during activation with noradrenaline (filled circles).
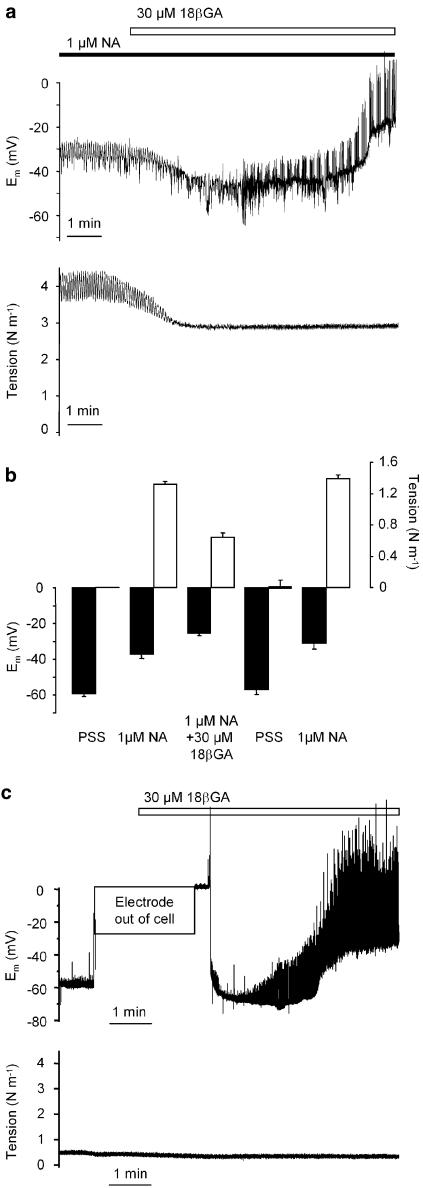
18βGA had a biphasic effect on the membrane potential. When arteries were activated with NA (membrane potential −37±1.7 mV, n=21 cells/12 arteries) 30 μM 18βGA evoked a transient repolarisation by −13±6.3 mV (n=4 cells/3 arteries) followed by depolarisation to membrane potential −25±2.3 mV (n=11 cells/7 arteries) (Figure 3a). In nonactivated arteries membrane potential was −57±1.4 mV (n=23 cells/12 arteries) and 30 μM 18βGA evoked hyperpolarisation (ΔUm=−7±2.1 mV, n=4 cells/3 arteries) followed by depolarisation to membrane potential −27±2.7 mV (n=9 cells/7 arteries) (Figure 3c). 18βGA depolarised and increased input resistance in a concentration-dependent manner (Figure 2c and d). The input resistance was increased significantly by concentrations of 18βGA higher than 3 μM. At 6 μM 18βGA there was no significant change in membrane potential (−37±1.7 mV (n=21 cells/12 arteries) and −32±1.5 mV (n=3 cells/3 arteries) in control NA conditions and in the presence of 6 μM 18βGA, respectively), but a significant increase in input resistance (Figure 2d). The effects of 18βGA on the membrane potential and force were reversible (Figure 3b; n=7).
Figure 3.
Effects of 18β-glycyrrhetinic acid on membrane potential, cell input resistance (rapid deflections) and tension. (a) Original recordings of simultaneous measurements of membrane potential and isometric tension of isolated mesenteric small arteries activated with noradrenaline (NA). (b) Bars illustrate the reversibility of 18β-glycyrrhetinic acid responses on membrane potential and tension: arteries (n=7) were stimulated with NA, superfused with 18β-glycyrrhetinic acid, rinsed for 15 min and stimulated with NA again. No significant difference between first and second NA stimulation was found. (c) Effects of 18β-glycyrrhetinic acid on membrane potential and cell input resistance in isolated mesenteric small arteries under resting conditions. 18β-glycyrrhetinic acid was present as indicated.
Effects on membrane currents
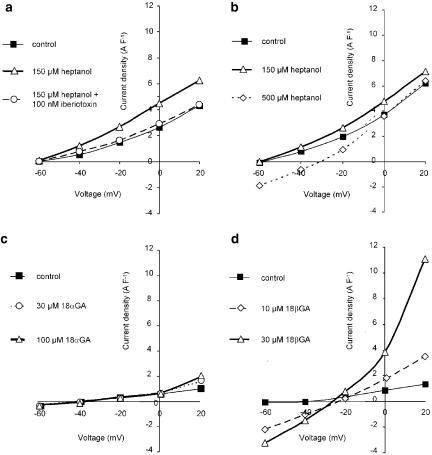
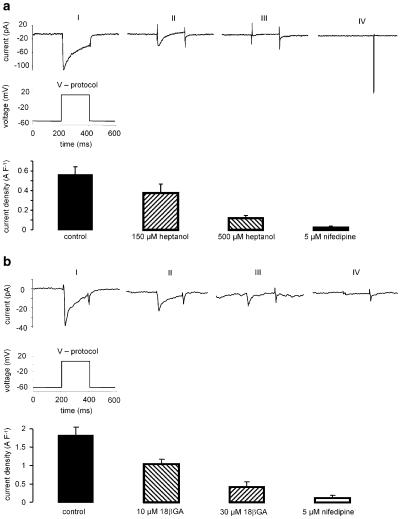
Whole-cell membrane currents were used to determine the ionic currents that are involved in the membrane potential changes seen with heptanol and glycyrrhetinic acid. At 150 μM heptanol activated an outward current that reversed below −60 mV and was inhibited by 100 nM iberiotoxin (Figure 4a). The current densities at a membrane potential of +20 mV were 2.5±0.4, 4.7±0.8 and 2.0±0.3 A F−1 under control conditions, after superfusion with 150 μM heptanol and following application of 100 nM iberiotoxin, respectively (n=5, cell capacitance was 22.1±1.7 pF). This indicates that heptanol at this concentration activates large-conductance, Ca2+-activated potassium channels. At 500 μM heptanol, another membrane current was evoked which reversed at −34±7.6 mV (n=5) and, therefore, could depolarise the membrane at physiological membrane potentials: inward current densities at −60 mV were −0.3±0.1 A F−1 in control and −1.7±0.4 A F−1 after 500 μM heptanol application (cell capacitance 17.5±2.2 pF; n=5) (Figure 4b).
Figure 4.
Current–voltage relations obtained in isolated smooth muscle from rat mesenteric small arteries. (a) I/V relation under control conditions, in the presence of 150 μM heptanol and in the presence of 150 μM heptanol and 100 nM iberiotoxin. (b) I/V relation under control conditions, in the presence of 150 μM heptanol and in the presence of 500 μM heptanol. (c) I/V relation under control conditions, in the presence of 30 μM 18α-glycyrrhetinic acid and in the presence of 100 μM 18α-glycyrrhetinic acid. (d) I/V relation under control conditions, in the presence of 10 μM 18β-glycyrrhetinic acid and in the presence of 30 μM 18β-glycyrrhetinic acid.
At concentrations up to 100 μM 18α-GA did not modify membrane conductance at membrane potentials between −60 and +20 mV (Figure 4c; n=4). However, at more positive membrane voltages 18αGA had a tendency to activate outward currents: current densities were 3.7±0.5, 11.9±3.9 and 16.8±5.2 A F−1 at +60 mV membrane potential under control conditions and in the presence of 30 μM and 100 μM 18αGA, respectively (NS; n=4; cell capacitance 26.8±2.6 pF).
In contrast to the α-isomer, 18βGA increased membrane conductance at negative potentials. The net current activated by 18βGA reversed at about −26 mV (Figure 4d). Under physiological conditions this current would lead to membrane depolarisation, which is consistent with the membrane potential measurements. The inward current densities at −60 mV were −0.4±0.1 A F−1 in control and −4.0±1.1 A F−1 after 30 μM 18βGA application (cell capacitance 18.3±3.2 pF; n=3).
Activation of potassium channels by heptanol was also observed in membrane potential measurements on artery segments. NA depolarised arteries from −62.0±3.6 to −35.4±1.6 mV; addition of 150 μM heptanol repolarised the membrane to −55.4±4.4 mV, a membrane potential change of −20.0±4.3 mV (n=6 cells/6 arteries). The repolarisation by heptanol was antagonised by 100 nM of either iberiotoxin or charybdotoxin: the change in potential in response to heptanol was −12.0±1.7 mV (n=7 cells/6 arteries). The remaining repolarisation may partly be due to activation of KATP channels, since the repolarisation was only −9.3±3.7 mV (n=4 cells/4 arteries) in the presence of 10 μM glibenclamide together with either 100 nM iberiotoxin or 100 nM charybdotoxin.
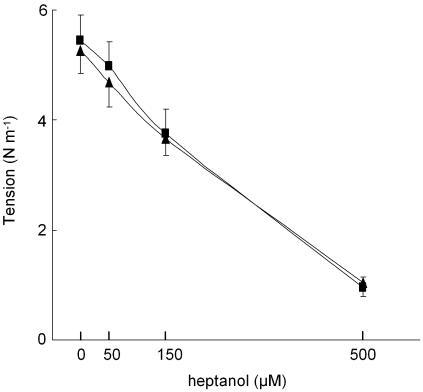
On the basis of these results we expected the opening of KCa channels to contribute to the relaxation induced by 150 μM heptanol: blocking these channels would, therefore, reduce the heptanol relaxant effect. However, as seen in Figure 5, this was not the case. Force was similarly affected by heptanol in the absence and presence of 100 nM iberiotoxin. This observation prompted us to investigate additional effects of heptanol in these arteries.
Figure 5.
Concentration–tension relation for heptanol in arteries activated with 10 μM noradrenaline (triangles) and with 10 μM noradrenaline in the presence of 100 nM iberiotoxin (squares), n=4. Vertical bars indicate s.e.m.
Effects on force and [Ca2+]i
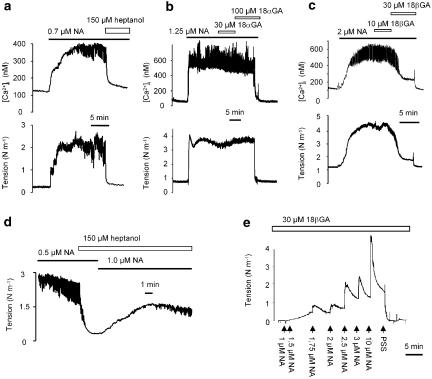
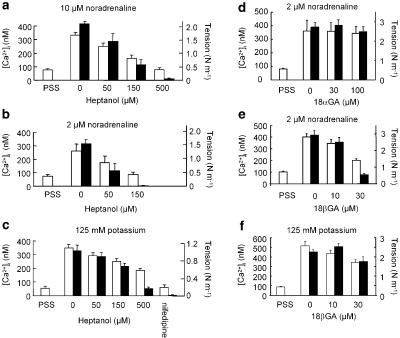
Arteries stimulated with a submaximal concentration of NA responded with an increase in force and [Ca2+]i with superimposed oscillations on both (Figure 6a–c). Both force and [Ca2+]i elevations were inhibited by 150 μM heptanol (Figure 6a) and by 30 μM 18βGA (Figure 6c) but were not affected by 18αGA at concentrations up to 100 μM (Figure 6b). Heptanol reduced both tension and [Ca2+]i in a concentration-dependent manner, and was less potent in arteries activated with high NA concentrations (compare 10 μM in Figure 7a with 2 μM in Figure 7b). In potassium-activated arteries heptanol also reduced both tension and [Ca2+]i (Figure 7c) although this inhibition was less than in NA-activated arteries. 18βGA also concentration-dependently reduced both tension and [Ca2+]i in arteries activated with NA or potassium (Figure 7e and f). In contrast, 30–100 μM 18αGA had no effect on either tension or [Ca2+]i (Figure 7d).
Figure 6.
Original recordings of the effects of heptanol (a), 18αGA (b) and 18βGA (c) on tension and [Ca2+]i in arteries stimulated with a submaximal concentration of noradrenaline (NA). (d) Addition of 150 μM heptanol relaxes the artery and inhibits NA-induced oscillations, but force can be recovered by increasing the NA concentration; oscillation reappeared, but with reduced amplitude. (e) In the presence of 30 μM 18βGA NA evokes only tonic contraction.
Figure 7.
Concentration-dependent effects of heptanol (a–c), 18αGA (d) and 18βGA (e–f) on [Ca2+]i (open bars) and tension (filled bars). Arteries were activated with 10 μM noradrenaline, n=5 (a), 2 μM noradrenaline, n=4 (b), 125 mM potassium, n=5 (c), 2 μM noradrenaline, n=7 (d), 2 μM noradrenaline, n=10 (e) or 125 mM potassium, n=9 (f). Vertical bars indicate s.e.m.
In the presence of 150 μM heptanol tension could be recovered by increasing the NA concentration: oscillations also reappeared in four out five experiments but with reduced amplitude (Figure 6d). With 500 μM heptanol present oscillations did not reappear (not shown), nor was it possible to restore oscillations in the presence of 30 μM 18βGA (Figure 6e). 18αGA at concentrations 30–100 μM had no effect on oscillation amplitude or frequency (n=9).
Effects on calcium currents
Since iberiotoxin did not reverse the effect of 150 μM heptanol on tension in NA-activated arteries, and because 150 μM heptanol reduced [Ca2+]i (and tension) in potassium-activated arteries, it seemed possible that heptanol could inhibit Ca2+ entry in these arteries by a mechanism distinct from its effect on membrane potential. We therefore tested the effect of heptanol on whole-cell voltage-sensitive Ca2+ currents (Figure 8a). These experiments showed that heptanol concentration-dependently inhibited the nifedipine-sensitive Ca2+ current, and that this effect was significant at 150 μM (P<0.05, n=5). 18βGA also inhibited the whole-cell voltage-sensitive Ca2+ current in a concentration-dependent manner (P<0.05, n=5, Figure 8b) with a significant effect at 10 μM.
Figure 8.
Whole-cell calcium currents from smooth muscle cells isolated from rat mesenteric small arteries. Traces in (a) show (I) control current, with (II) 150 μM and (III) 500 μM heptanol and with (IV) 5 μM nifedipine; (V) shows the voltage protocol applied. n=5. Traces in (b) show (I) control current, with (II) 10 μM and (III) 30 μM 18βGA and (IV) 5 μM nifedipine; (V) shows the voltage protocol applied; n=4. Vertical bars indicate s.e.m.
Effects on calcium transient synchronisation – confocal experiments
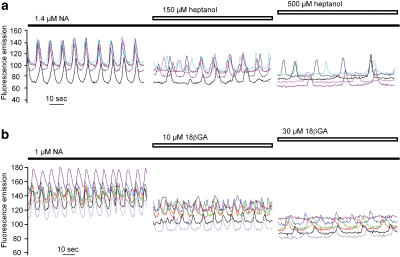
Heptanol and 18βGA disrupted the synchronisation of calcium signals between smooth muscle cells (Figure 9). Synchronised [Ca2+]i oscillations in smooth muscle cells were observed in arteries activated with a submaximal NA concentration (n=10). Heptanol at 150 μM partly desynchronised the [Ca2+]i oscillations while 500 μM heptanol led to complete desynchronisation (Figure 9a, n=4). In the presence of 10 μM 18βGA [Ca2+]i transients were partly desynchronised and 30 μM 18βGA completely abolished synchronisation (Figure 9b, n=6).
Figure 9.
Confocal recordings of [Ca2+]i in regions of interest (ROI, see Methods). Each trace represents one ROI corresponding to a single smooth muscle cell in the arterial wall. (a) Effects of 150 and 500 μM heptanol on an artery activated with noradrenaline (NA). (b) Effects of 10 and 30 μM 18βGA.
Discussion
We evaluated the specificity of heptanol, 18αGA and 18βGA in blocking gap junctions in mesenteric small artery smooth muscle. The specificity for gap junctions of heptanol (Javid et al., 1996; Chaytor et al., 1997; 1998; Yamamoto et al., 1998; Murai et al., 1999), 18αGA (Chaytor et al., 2000; Schuster et al., 2001; Tare et al., 2002) and 18βGA (Davidson & Baumgarten, 1988; Santicioli & Maggi, 2000; Coleman et al., 2001; 2002; Tare et al., 2002) has often been questioned but there have been few direct measurements of their specificity in the intact artery wall (Hashitani & Suzuki, 1997; Yamamoto et al., 1998; Murai et al., 1999; Tare et al., 2002). The present study demonstrates that heptanol and 18βGA increase input resistance and prevent synchronisation between single smooth muscle cells in the arterial wall – observations consistent with inhibition of gap junctions – whereas 18αGA is without significant effect. Most importantly, it also demonstrates nonjunctional effects of heptanol and 18βGA on ion currents, [Ca2+]i and force that are present at concentrations lower than those required for significant inhibition of gap junctions.
We showed that 150 μM heptanol causes hyperpolarisation, decreases [Ca2+]i and tension, but has no significant effect on input resistance. At higher concentrations input resistance increased, oscillation in [Ca2+]i became desynchronised and vasomotion stopped – all effects consistent with blockade of gap junctions. The most likely reason for the hyperpolarising effect of 150 μM heptanol is the opening of KCa and possibly KATP channels, although other mechanisms may contribute. In non-vascular smooth muscle heptanol has been reported to lack effect on membrane potential (Blennerhassett & Garfield, 1991; Manchanda & Venkateswarlu, 1997), or to cause a depolarisation at concentrations ⩾200 μM (Huizinga et al., 1988). Despite recent findings that 1 mM heptanol hyperpolarises VSM cells in cerebral arteries (Lagaud et al., 2002) and is without effect on the membrane potential in submucosal arterioles (Hashitani & Suzuki, 1997), we found that in concentrations ⩾500 μM heptanol depolarises mesenteric small arteries. It therefore seems likely that the nonjunctional effects of heptanol are concentration-dependent and may vary between preparations. 18βGA at 5–10 μM increased input resistance and partly desynchronised the calcium transients in smooth muscle cells, consistent with inhibition of gap junctions (Chaytor et al., 1997; Dhein, 1998; Saez et al., 2003). At these concentrations it also depolarised the membrane, reduced [Ca2+]i and relaxed the arteries. A depolarising effect of 18βGA on smooth muscle cells has been reported previously in submucosal arterioles (Imaeda et al., 2000; Coleman et al., 2001) and in tail artery (Tare et al., 2002). Consistent with the depolarisation, we found both heptanol and 18βGA to be associated with an increase of a net inward (depolarising) current in isolated cells at physiological membrane potentials. This increase of membrane conductance may be due to the lipophilic nature of heptanol (Burt & Spray, 1989; Bastiaanse et al., 1993; Guan et al., 1997; Yamamoto et al., 1998) and 18βGA (Davidson & Baumgarten, 1988; Itoh et al., 1989; Yamamoto et al., 1998; Coleman et al., 2001) but cannot be due to an effect on intercellular communication as increased membrane conductance was seen in isolated smooth muscle cells.
It seems unlikely that potassium channel activation is the only mechanism responsible for the relaxation to 150 μM heptanol since iberiotoxin was without effect on the relaxation and because 150 μM heptanol also reduced [Ca2+]i in potassium-activated arteries. These findings could suggest a direct action of heptanol on Ca2+ channels, which is consistent with our patch-clamp measurements, showing inhibition of Ca2+ channels by heptanol. The same principle could apply to the observation with 18βGA. Therefore, we suggest that blockade of Ca2+ channels is a common mechanism by which these compounds relax depolarised arteries.
In contrast to heptanol and 18βGA, 100 μM 18αGA did not have any detectable effect on smooth muscles. 18αGA has been suggested previously to be a specific gap junction blocker at concentrations up to 100 μM (Davidson et al., 1986; Davidson & Baumgarten, 1988) where it effectively blocks myoendothelial gap junctions (Taylor et al., 1998). In preliminary experiments we also found 18αGA to attenuate acetylcholine-induced responses, indicating possible gap junction block between endothelial and smooth muscle cells (data not shown). These findings are in accordance with previous reports describing 18αGA as myoendothelial uncoupler (Taylor et al., 1998; Kagota et al., 1999; Hill et al., 2000). Although 18αGA has been used to block communication between smooth muscle cells in pressurised rat middle cerebral arteries (Lagaud et al., 2002), this contrasts with many others previous reports (Taylor et al., 1998; Chaytor et al., 2000; Schuster et al., 2001) as well as with the present study where 100 μM 18αGA did not change vascular tone and [Ca2+]i in the smooth muscle. 18αGA failed to affect the oscillation in tone and [Ca2+]i observed in intact arteries nor did it affect the ionic currents in isolated smooth muscle cells, which is consistent with a lack of effect on membrane potential observed in guinea pig coronary artery smooth muscle (Tare et al., 2002). Thus, 18αGA seems to have neither junctional nor nonjunctional effects on mesenteric small arteries smooth muscle.
In conclusion, these findings suggest that the vasodilator effects of heptanol and 18βGA can be mediated through several mechanisms unrelated to an effect on gap junctions. These nonjunctional effects appear at concentrations lower than those required to significantly increase input resistance (which signifies the inhibition of gap junctional communication). Our data suggest that 18αGA cannot be used to block gap junctions between smooth muscle cells in mesenteric small arteries (although it might be effective in inhibiting myoendothelial communication), and that caution is needed when interpreting the effects of using heptanol and 18βGA in vascular preparations. Ascribing the tension reduction seen with these compounds solely as a response to gap junction inhibition is not justified in the absence of more direct evidence, and this should be kept in mind when interpreting data obtained with these compounds.
Acknowledgments
Donna Briggs is thanked for comments on the manuscript. Jørgen Andresen and Kirsten Skaarup are thanked for excellent technical assistance. This work was supported by the Danish Research Council and the Danish Heart Foundation (Grant No. 03-1-2-20A-22056). The Water and Salt Research Center at the University of Aarhus is established and supported by the Danish National Research Foundation (Danmarks Grundforskningsfond).
Abbreviations
- 18αGA
18α-glycyrrhetinic acid
- 18βGA
18β-glycyrrhetinic acid
- NA
noradrenaline
References
- BASTIAANSE E.M., JONGSMA H.J., VAN DER L.A., TAKENS-KWAK B.R. Heptanol-induced decrease in cardiac gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J. Membr. Biol. 1993;136:135–145. doi: 10.1007/BF02505758. [DOI] [PubMed] [Google Scholar]
- BÉNY J.L., PACICCA C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am. J. Physiol. 1994;266:H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- BERMAN R.S., MARTIN P.E., EVANS W.H., GRIFFITH T.M. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc. Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- BLENNERHASSETT M.G., GARFIELD R.E. Effect of gap junction number and permeability on intercellular coupling in rat myometrium. Am. J. Physiol. 1991;261:C1001–C1009. doi: 10.1152/ajpcell.1991.261.6.C1001. [DOI] [PubMed] [Google Scholar]
- BURT J.M., SPRAY D.C. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ. Res. 1989;65:829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARSH W.L., HUTCHESON I.R., GRIFFITH T.M. Comparison of glycyrrhetinic acid isoforms and carbenoxolone as inhibitors of EDHF-type relaxations mediated via gap junctions. Endothelium. 2000;7:265–278. doi: 10.3109/10623320009072213. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., BRINK P.R. Gap junctions in isolated rat aorta: evidence for contractile responses that exhibit a differential dependence on intercellular communication. Braz. J. Med. Biol. Res. 2000;33:423–429. doi: 10.1590/s0100-879x2000000400008. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., SPEKTOR M., BRINK P.R., BARR L. Further evidence for the selective disruption of intercellular communication by heptanol. Am. J. Physiol. 1999;276:H1911–H1917. doi: 10.1152/ajpheart.1999.276.6.H1911. [DOI] [PubMed] [Google Scholar]
- COLEMAN H.A., TARE M., PARKINGTON H.C. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J. Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN H.A., TARE M., PARKINGTON H.C. Myoendothelial electrical coupling in arteries and arterioles and its implications for endothelium-derived hyperpolarizing factor. Clin. Exp. Pharmacol. Physiol. 2002;29:630–637. doi: 10.1046/j.1440-1681.1999.03701.x. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J.S., BAUMGARTEN I.M. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J. Pharmacol. Exp. Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- DAVIDSON J.S., BAUMGARTEN I.M., HARLEY E.H. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem. Biophys. Res. Commun. 1986;134:29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- DHEIN S. Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol. Sci. 1998;19:229–241. doi: 10.1016/s0165-6147(98)01192-4. [DOI] [PubMed] [Google Scholar]
- GUAN X., CRAVATT B.F., EHRING G.R., HALL J.E., BOGER D.L., LERNER R.A., GILULA N.B. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAEFLIGER J.A., CASTILLO E., WAEBER G., AUBERT J.F., NICOD P., WAEBER B., MEDA P. Hypertension differentially affects the expression of the gap junction protein connexin43 in cardiac myocytes and aortic smooth muscle cells. Adv. Exp. Med. Biol. 1997a;432:71–82. doi: 10.1007/978-1-4615-5385-4_8. [DOI] [PubMed] [Google Scholar]
- HAEFLIGER J.A., CASTILLO E., WAEBER G., BERGONZELLI G.E., AUBERT J.F., SUTTER E., NICOD P., WAEBER B., MEDA P. Hypertension increases connexin43 in a tissue-specific manner. Circulation. 1997b;95:1007–1014. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., SUZUKI H. K+ channels which contribute to the acetylcholine-induced hyperpolarization in smooth muscle of the guinea-pig submucosal arteriole. J. Physiol. 1997;501:319–329. doi: 10.1111/j.1469-7793.1997.319bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL C.E., HICKEY H., SANDOW S.L. Role of gap junctions in acetylcholine-induced vasodilation of proximal and distal arteries of the rat mesentery. J. Autonom. Nerv. System. 2000;81:122–127. doi: 10.1016/s0165-1838(00)00113-2. [DOI] [PubMed] [Google Scholar]
- HILL C.E., RUMMERY N., HICKEY H., SANDOW S.L. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin. Exp. Pharmacol. Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- HUIZINGA J.D., SHIN A., CHOW E. Electrical coupling and pacemaker activity in colonic smooth muscle. Am. J. Physiol. 1988;255:C653–C660. doi: 10.1152/ajpcell.1988.255.5.C653. [DOI] [PubMed] [Google Scholar]
- IMAEDA K., YAMAMOTO Y., FUKUTA H., KOSHITA M., SUZUKI H. Hyperpolarization-induced dilatation of submucosal arterioles in the guinea-pig ileum. Br. J. Pharmacol. 2000;131:1121–1128. doi: 10.1038/sj.bjp.0703689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOH K., HARA T., SHIRAISHI T., TANIGUCHI K., MORIMOTO S., ONISHI T. Effects of glycyrrhizin and glycyrrhetinic acid on (Na+/K+)-ATPase of renal basolateral membranes in vitro. Biochem. Int. 1989;18:81–89. [PubMed] [Google Scholar]
- JAVID P.J., WATTS S.W., WEBB R.C. Inhibition of nitric oxide-induced vasodilation by gap junction inhibitors: a potential role for a cGMP-independent nitric oxide pathway. J. Vasc. Res. 1996;33:395–404. doi: 10.1159/000159168. [DOI] [PubMed] [Google Scholar]
- JIANG Z.G., SI J.Q., LASAREV M.R., NUTTALL A.L. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. J. Physiol. 2001;537:829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGOTA S., YAMAGUCHI Y., NAKAMURA K., KUNITOMO M. Characterization of nitric oxide- and prostaglandin-independent relaxation in response to acetylcholine in rabbit renal artery. Clin. Exp. Pharmacol. Physiol. 1999;26:790–796. doi: 10.1046/j.1440-1681.1999.03123.x. [DOI] [PubMed] [Google Scholar]
- KO Y.S., YEH H.I., HAW M., DUPONT E., KABA R., PLENZ G., ROBENEK H., SEVERS N.J. Differential expression of connexin43 and desmin defines two subpopulations of medial smooth muscle cells in the human internal mammary artery. Arterioscler. Thromb. Vasc. Biol. 1999;19:1669–1680. doi: 10.1161/01.atv.19.7.1669. [DOI] [PubMed] [Google Scholar]
- LAGAUD G., KARICHETI V., KNOT H.J., CHRIST G.J., LAHER I. Inhibitors of gap junctions attenuate myogenic tone in cerebral arteries. Am. J. Physiol. 2002;283:H2177–H2186. doi: 10.1152/ajpheart.00605.2001. [DOI] [PubMed] [Google Scholar]
- LEITE R., WEBB R.C. Increased dilator response to heptanol and octanol in aorta from DOCA-salt-hypertensive rats. Pharmacology. 2001;62:29–35. doi: 10.1159/000056069. [DOI] [PubMed] [Google Scholar]
- MANCHANDA R., VENKATESWARLU K. Effects of heptanol on electrical activity in the guinea-pig vas deferens. Br. J. Pharmacol. 1997;120:367–370. doi: 10.1038/sj.bjp.0700900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHKOV V.V., AALKJAER C., NILSSON H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J. Gen. Physiol. 2004;123:121–134. doi: 10.1085/jgp.200308972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., AALKJAER C., CHRISTENSEN J. Changes in noradrenaline sensitivity and morphology of arterial resistance vessels during development of high blood pressure in spontaneously hypertensive rats. Hypertension. 1980;2:664–671. doi: 10.1161/01.hyp.2.5.664. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- MURAI T., MURAKI K., IMAIZUMI Y., WATANABE M. Levcromakalim causes indirect endothelial hyperpolarization via a myo-endothelial pathway. Br. J. Pharmacol. 1999;128:1491–1496. doi: 10.1038/sj.bjp.0702956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PENG H., MATCHKOV V., IVARSEN A., AALKJAER C., NILSSON H. Hypothesis for the initiation of vasomotion. Circ. Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- PENG H.L., IVARSEN A., NILSSON H., AALKJAER C. On the cellular mechanism for the effect of acidosis on vascular tone. Acta. Physiol. Scand. 1998;164:517–525. doi: 10.1111/j.1365-201x.1998.tb10701.x. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., FUSI F., SAPONARA S., GAGOV H.S., SGARAGLI G.P., BOEV K.K. Characterization of voltage-gated calcium currents in freshly isolated smooth muscle cells from rat tail main artery. Acta. Physiol. Scand. 2001;173:257–265. doi: 10.1046/j.1365-201X.2001.00907.x. [DOI] [PubMed] [Google Scholar]
- RUMMERY N.M., HICKEY H., MCGURK G., HILL C.E. Connexin37 is the major connexin expressed in the media of caudal artery. Arterioscler. Thromb. Vasc. Biol. 2002;22:1427–1432. doi: 10.1161/01.atv.0000028814.45706.e5. [DOI] [PubMed] [Google Scholar]
- SAEZ J.C., BERTHOUD V.M., BRANES M.C., MARTINEZ A.D., BEYER E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., LOOFT-WILSON R., DORAN B., GRAYSON T.H., SEGAL S.S., HILL C.E. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc. Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Effect of 18beta-glycyrrhetinic acid on electromechanical coupling in the guinea-pig renal pelvis and ureter. Br. J. Pharmacol. 2000;129:163–169. doi: 10.1038/sj.bjp.0703004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUSTER A., OISHI H., BÉNY J.L., STERGIOPULOS N., MEISTER J.J. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am. J. Physiol. 2001;280:H1088–H1096. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- TARE M., COLEMAN H.A., PARKINGTON H.C. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am. J. Physiol. 2002;282:H335–H341. doi: 10.1152/ajpheart.2002.282.1.H335. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18-alpha glycyrrhetinic acid. Br. J. Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI M.L., WATTS S.W., LOCH-CARUSO R., WEBB R.C. The role of gap junctional communication in contractile oscillations in arteries from normotensive and hypertensive rats. J. Hypertens. 1995;13:1123–1133. doi: 10.1097/00004872-199510000-00007. [DOI] [PubMed] [Google Scholar]
- WATTS S.W., WEBB R.C. Vascular gap junctional communication is increased in mineralocorticoid-salt hypertension. Hypertension. 1996;28:888–893. doi: 10.1161/01.hyp.28.5.888. [DOI] [PubMed] [Google Scholar]
- WELSH D.G., SEGAL S.S. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am. J. Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- XIA J., DULING B.R. Electromechanical coupling and the conducted vasomotor response. Am. J. Physiol. 1995;269:H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., FUKUTA H., NAKAHIRA Y., SUZUKI H. Blockade by 18beta-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J. Physiol. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEH H.I., LUPU F., DUPONT E., SEVERS N.J. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler. Thromb. Vasc. Biol. 1997;17:3174–3184. doi: 10.1161/01.atv.17.11.3174. [DOI] [PubMed] [Google Scholar]