Abstract
The primary aim was to study the central respiratory effects of cannabinoids (CB). To this end, the cannabinoid receptor agonist WIN55212-2 was injected into the cisterna magna of urethane-anaesthetised rats and changes in respiratory parameters were observed. The secondary aim was to observe the centrally elicited cardiovascular actions of WIN55212-2. Involvement of opioid mechanisms in the central effects of WIN55212-2 was also studied.
Intracisternal (i.c.) application of WIN55212-2 (1, 3, 10 and 30 μg kg−1) dose-dependently decreased the respiratory rate and minute volume. Tidal volume was slightly increased, whereas peak inspiratory flow remained unchanged. In addition, WIN55212-2 increased mean arterial pressure and the plasma noradrenaline concentration and decreased heart rate. I.c. injection of WIN55212-3 (1, 3, 10 and 30 μg kg−1), an enantiomer of WIN55212-2 lacking affinity for cannabinoid receptors, elicited no effects. All effects of WIN55212-2 were prevented by the CB1 receptor antagonist SR141716 (2 mg kg−1 i.v.).
I.c. administration of the opioid receptor agonist DAMGO (0.1, 0.3, 1 and 3 μg kg−1) markedly lowered the respiratory rate, tidal volume, minute volume and peak inspiratory flow. These effects were attenuated by the opioid receptor antagonist naloxone (0.2 mg kg−1 i.v.). In contrast, naloxone did not affect the respiratory and cardiovascular effects of i.c. administered WIN55212-2.
Our results show that activation of CB1 cannabinoid receptors in the brain stem depresses respiration and enhances sympathetic tone and cardiac vagal tone. Opioid mechanisms are not involved in these central cannabinoid effects.
Keywords: Airflow, blood pressure, cannabinoids, cardiovascular regulation, CB1 cannabinoid receptor, intracisternal administration, opioid receptor, respiratory rate, respiratory regulation, tidal volume
Introduction
Cannabinoids are known to depress respiration in laboratory animals (Graham & Li, 1973; Moss & Friedman, 1976; Doherty et al., 1983; Estrada et al., 1987). Recent results indicated that systemically administered synthetic and natural cannabinoid agonists produced their respiratory depressant effect by activating CB1 cannabinoid receptors (Vivian et al., 1998; Schmid et al., 2003). The consequences of the strong decrease in respiratory rate in the rat were hypoxaemia, hypercapnia and blood acidosis (Schmid et al., 2003). It is not yet known whether cannabinoids depress respiration by acting directly on respiratory centres in the brain stem or by acting at peripheral sites. The first aim of the present study was, therefore, to determine whether cannabinoids are able to depress respiration by acting in the brain stem. To this end, we administered the mixed CB1/CB2 cannabinoid receptor agonist WIN55212-2 into the cisterna magna (i.e., into the vicinity of brain stem respiratory regulatory centres) in anaesthetised rats and observed the changes in the respiratory parameters respiratory rate, peak inspiratory flow, tidal volume and respiratory minute volume.
Several recent studies suggested that in some cannabinoid actions, like antinociception and drug dependence, endogenous opioids and opioid receptors are involved (Tanda et al., 1997; Valverde et al., 2000; Lichtman et al., 2001; Navarro et al., 2001). Our second aim was to determine whether the same holds true for the respiratory depressant effects of cannabinoids. To this end, we compared the effects of WIN55212-2 on respiratory parameters to those of the μ opioid receptor agonist [D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO), and assessed the interaction between WIN55212-2 and the opioid receptor antagonist naloxone.
Cannabinoid agonists elicit complex cardiovascular changes (for reviews, see Wagner et al., 1998; Niederhoffer & Szabo, 1999; Kunos et al., 2000). The role of cannabinoid action on cardiovascular centres in these changes is not clear. While the present study was mainly focused on the respiratory effects of cannabinoids, we also recorded blood pressure, heart rate and the plasma noradrenaline concentration, and thus observed the central effects of cannabinoids on cardiovascular regulation. Preliminary accounts of the results were published in abstract form (Szabo et al., 2002; Pfitzer et al., 2003).
Methods
Experiments were carried out on 59 male Wistar rats (300–400 g), obtained from Charles River (Sulzfeld, Germany). The experiments conformed to the rules of the German law regulating the use of animals in Biomedical Research (Tierschutzgesetz), and were approved by a local ethical committee.
Surgical preparation
Rats were anaesthetised with urethane (1.5 g kg−1 i.p.). A polyethylene catheter (inner diameter, 0.5 mm; outer diameter, 1.0 mm) was implanted into the right femoral artery for recording blood pressure and for blood sampling (for determination of plasma noradrenaline concentration). Arterial blood pressure was measured using a low-volume pressure transducer (Baxter, Bentley Laboratories, Uden, The Netherlands) coupled to a bridge amplifier (Hugo Sachs, Hugstetten, Germany). Heart rate was calculated from the pulsatile blood pressure signal by a cardiotachometer (Hugo Sachs, Hugstetten, Germany). The right femoral vein was cannulated for drug administration. A tracheotomy was performed to allow artificial ventilation during implantation of the intracisternal (i.c.) catheter (see below) and measurement of respiratory parameters during the experiment proper. Additional urethane was then given i.v. (0.2 g kg−1). A further catheter was implanted into the cisterna magna for i.c. administration of drugs.
The head of the rat was fixed in a stereotaxic frame. After a midline skin incision, neck muscles were bluntly separated to expose the atlanto-occipital membrane. A polyethylene catheter (inner diameter, 0.28 mm; outer diameter, 0.61 mm; length, 6 cm) was inserted 1 mm into the cisterna magna and glued to the atlanto-occipital membrane with cyanoacrylate (Loctite 414, Loctite, München, Germany). The muscles and the skin were sutured around the free end of the catheter. Animals were removed from the stereotaxic frame and artificial ventilation was stopped. The tracheal catheter was then connected to a pneumotachometer (type 378/1.2) coupled to a bridge amplifier (Hugo Sachs, Hugstetten, Germany). Body temperature was maintained at 37°C throughout the experiment using an electrical heating pad controlled by a rectal probe.
Protocol and evaluation
Recording of parameters began after a 45-min stabilisation period following surgery (t=0 min). At first, a 15-min period for determination of baseline values followed (PRE period). Then, the solvent (25 μl kg−1) or increasing doses of the cannabinoids WIN55212-2 (1, 3, 10 and 30 μg kg−1) and WIN55212-3 (1, 3, 10 and 30 μg kg−1) or of the μ opioid receptor agonist DAMGO (0.1, 0.3, 1 and 3 μg kg−1) were injected i.c. at t=20, 40, 60 and 80 min. In two further WIN55212-2 groups, animals were pretreated with the CB1 cannabinoid receptor antagonist SR141716 (2 mg kg−1 i.v.) or the opioid receptor antagonist naloxone (0.2 mg kg−1 i.v.) at t=−20 min. In one further DAMGO group, naloxone (0.2 mg kg−1 i.v.) was given at t=−20 min.
Airflow, mean arterial blood pressure and heart rate were recorded continuously from t=0 min until the end of the experiment, using the Polyview Software (Grass Instruments, Astro-Med, Rodgau, Germany). Respiratory rate, peak inspiratory flow and tidal volume were calculated by Polyview from the recorded airflow signal (tidal volume was calculated as the area under the airflow curve during inspiration; see Figure 2). Minute volume was calculated as the product of respiratory rate and tidal volume. The parameters were determined every 2 min (as the average of values within a 20-s interval). The concentration of noradrenaline in plasma was determined twice during the PRE period (i.e., at t=0 and 15 min) and 15 min after each drug injection (i.e., at t=35, 55, 75 and 95 min). In each experiment, values for all parameters measured at t=0 and 15 min were averaged to yield initial absolute values before i.c. administration of drugs (PRE). All further values were expressed as percentages of PRE.
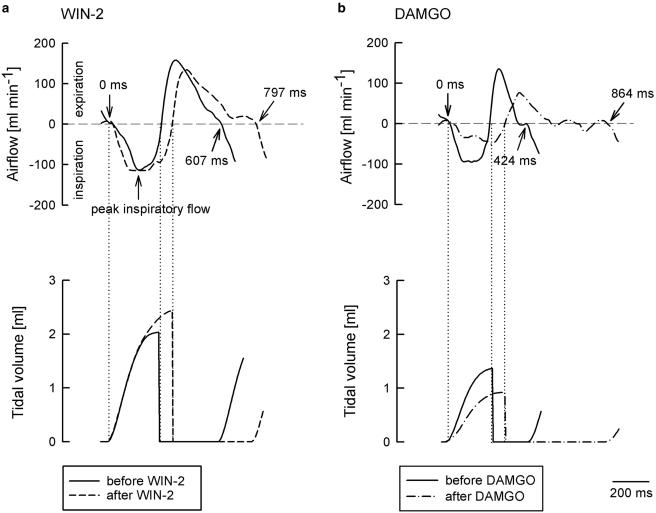
Figure 2.
Original recordings showing the effects of WIN55212-2 (WIN-2) and DAMGO on airflow and tidal volume within one respiratory cycle. The tracings were recorded before and 15 min after i.c. injection of WIN-2 (30 μg kg−1; a) or DAMGO (3 μg kg−1; b). WIN-2 prolonged the respiratory cycle from 607 to 797 ms (i.e., respiratory rate decreased from 99 to 75 min−1) and increased the tidal volume. DAMGO prolonged the respiratory cycle from 424 to 864 ms (i.e., respiratory rate decreased from 141 to 69 min−1) and depressed the peak inspiratory flow and tidal volume. Tracings represent eight (WIN-2) and eight (DAMGO) experiments with similar results.
At the end of the experiments, saline containing Evans blue was injected via the i.c. catheter (0.2% w v−1; 25 μl kg−1). After 15 min, the animals were killed by an overdose of urethane. The skull was opened, and the brain stem was removed and inspected. In three experiments, no staining of the brain stem was observed; these experiments were excluded from statistical analysis. In the remaining experiments, blue staining was observed on the dorsal and ventral surface of the brain stem.
Determination of the plasma noradrenaline concentration
Blood (500 μl) was sampled from the arterial catheter and immediately centrifuged (12,000 × g; 3 min; 0°C). Plasma (200 μl) was obtained and frozen (−20°C) in tubes containing 10 μl Na2SO3 (12.5%) and 10 μl Na2EDTA (10%). Erythrocytes were resuspended by adding 200 μl 0.9% saline and reinjected after the next blood sampling. The plasma noradrenaline concentration was determined from the 200-μl plasma samples by alumina chromatography, followed by high-performance liquid chromatography and electrochemical detection as described by Szabo et al. (2001a).
Statistics
Means±s.e.m. of n experiments are given throughout. Statistical differences were evaluated by one-way analysis of variance. Post hoc comparisons were carried out by a modified t-test based on the analysis of variance and Bonferroni correction was applied because of multiple comparisons (SPSS for Windows v.10.0; SPSS, München, Germany). P<0.05 was taken as the limit of significance, and only this level is indicated even if P was < 0.01 or 0.001.
Drugs
Drugs were obtained from the following sources: Research Biochemicals International (Köln, Germany): R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55212-2) and S(−)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55212-3); Rhône-Poulenc (Cranbury, NJ, U.S.A.): Alkamuls EL-620; Sanofi-Synthelabo (Montpellier, France): N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide (SR141716; recently named rimonabant); Tocris (Bristol, U.K.): [D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO) and naloxone hydrochloride.
WIN55212-2, WIN55212-3 and SR141716 were dissolved and further diluted in a solvent containing ethanol/Alkamuls EL-620/saline (1/1/8, v/v/v). DAMGO, naloxone and urethane were dissolved and further diluted in 0.9% saline. I.c. injections had a volume of 25 μl kg−1 and were administered within 1.5 min. I.v. injections had a volume of 1 ml kg−1. Doses refer to the salts.
Results
Initial values (PRE values)
Values for parameters determined at t=0 and 15 min were averaged to obtain the initial values before i. c. drug injections (PRE). PRE values in the different groups are given in Table 1. Pretreatment with the CB1 cannabinoid receptor antagonist SR141716 (2 mg kg−1 i.v.) did not modify the respiratory rate and the cardiovascular parameters. However, peak inspiratory flow, tidal volume and respiratory minute volume were higher in the SR141716-pretreated group. Pretreatment with the opioid receptor antagonist naloxone (0.2 mg kg−1 i.v.) did not elicit respiratory or cardiovascular changes, except a slight increase in peak respiratory flow. For evaluation of agonist-evoked changes, all further values were expressed as percentages of PRE.
Table 1.
Initial values for parameters before i.c. injection of solvent, cannabinoid or opioid ligands (PREa)
| Pretreatment | No pretreatment | SR141716b | Naloxonec |
|---|---|---|---|
| Number (n) of animals | 40 | 7 | 12 |
| Respiratory parameters | |||
| Respiratory rate (min−1) | 122±3 | 114±4 | 114±6 |
| Peak inspiratory flow (ml min−1) | 133±2 | 169±5* | 150±5* |
| Tidal volume (ml (100 g)−1) | 0.63±0.01 | 0.79±0.04* | 0.62±0.02 |
| Minute volume (ml (100 g min)−1) | 77±2 | 90±5* | 70±3 |
| Cardiovascular parameters | |||
| Mean arterial pressure (mmHg) | 77±2 | 76±4 | 79±2 |
| Heart rate (min−1) | 399±7 | 415±20 | 389±10 |
| Plasma noradrenaline concentration (pg ml−1) | 816±62 | 1012±87 | 706±73 |
PRE values are averages of values measured at t=0 and 15 min.
SR141716 (2 mg kg−1) was injected i.v. at t=−20 min.
naloxone (0.2 mg kg−1) was injected i.v. at t=−20 min.
P< 0.05 versus ‘no pretreatment'.
Control experiments
In one control group, the solvent for cannabinoids was injected intracisternally (i.c.) four times (25 μl kg−1). Except transient (<6 min) and small ( ∼5 mmHg) decreases in blood pressure, the measured parameters remained stable in these animals (Figures 3 and 4). In the other control group, the solvent for DAMGO was injected four times (25 μl kg−1). There was a slight decrease in respiratory rate and a slight increase in tidal volume in this solvent group (Figure 5). Cardiovascular parameters remained stable (not shown).
Figure 3.
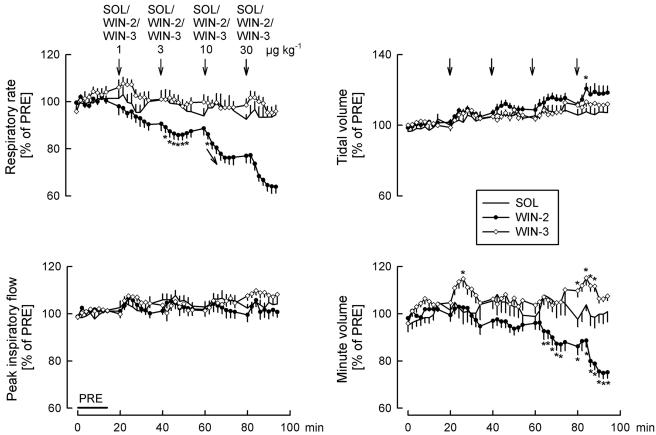
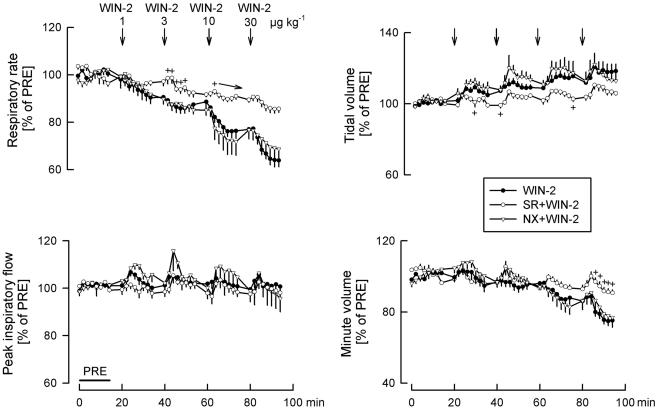
Effects of solvent (SOL), WIN55212-2 (WIN-2) and WIN55212-3 (WIN-3) on respiratory rate, peak inspiratory flow, tidal volume and minute volume. SOL (25 μl kg−1), WIN-2 (1, 3, 10 and 30 μg kg−1) and WIN-3 (1, 3, 10 and 30 μg kg−1) were injected i.c. as indicated by the arrows. Values are given as percentages of PRE. Means±s.e.m. from eight (SOL), eight (WIN-2) and eight (WIN-3) experiments. *P<0.05 versus SOL (arrows in the graphs indicate that all further values were significantly different from SOL).
Figure 4.
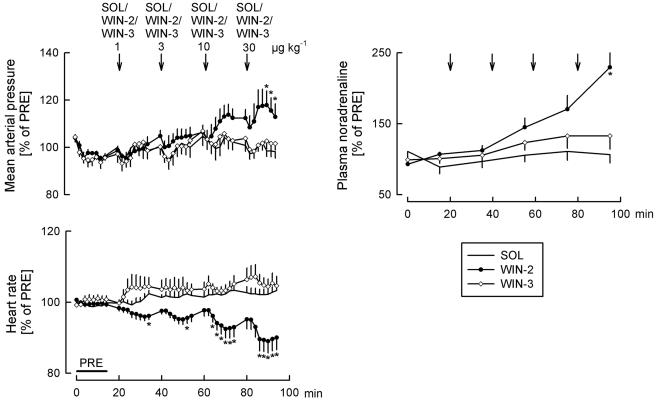
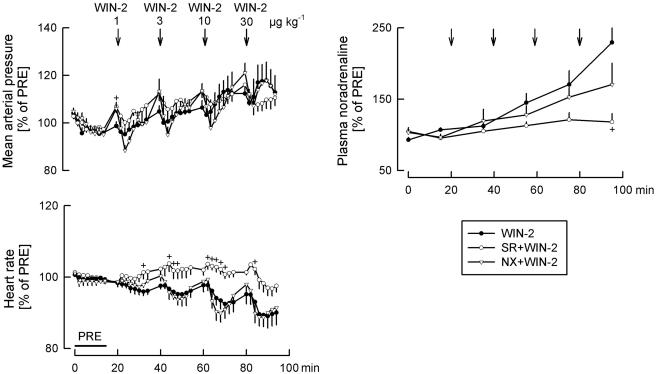
Effects of solvent (SOL), WIN55212-2 (WIN-2) and WIN55212-3 (WIN-3) on mean arterial pressure, heart rate and the plasma noradrenaline concentration. SOL (25 μl kg−1), WIN-2 (1, 3, 10 and 30 μg kg−1) and WIN-3 (1, 3, 10 and 30 μg kg−1) were injected i.c. as indicated by the arrows. Values are given as percentages of PRE. Means±s.e.m. from eight (SOL), eight (WIN-2) and eight (WIN-3) experiments. *P< 0.05 versus SOL.
Figure 5.
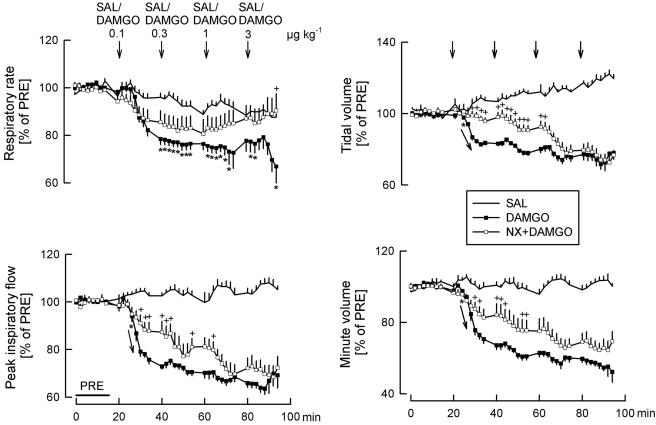
Effects of saline (SAL), DAMGO and naloxone (NX) on respiratory rate, peak inspiratory flow, tidal volume and minute volume. SAL (25 μl kg−1) and DAMGO (0.1, 0.3, 1 and 3 μg kg−1) were injected i.c. as indicated by the arrows. At t=−20 min, one of the two DAMGO groups was pretreated with NX (0.2 mg kg−1 i.v.). Values are given as percentages of PRE. Means±s.e.m. from eight (SAL), eight (DAMGO) and seven (NX+DAMGO) experiments. *P<0.05 DAMGO versus SAL (arrows in the graphs indicate that all further values were significantly different from SAL); +P<0.05 NX+DAMGO versus DAMGO alone.
Effects of the cannabinoid enantiomers WIN55212-2 and WIN55212-3
Four doses of the cannabinoid agonist WIN55212-2 (1, 3, 10 and 30 μg kg−1) and of the inactive enantiomer WIN55212-3 (1, 3, 10 and 30 μg kg−1) were injected i.c. Original tracings from typical experiments with WIN55212-2 are shown in Figures 1 and 2, whereas statistical analysis is given in Figures 3 and 4.
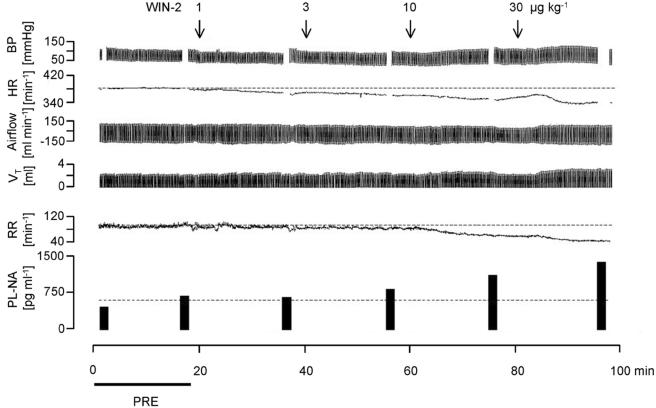
Figure 1.
Original recordings showing the effects of WIN55212-2 (WIN-2) on blood pressure (BP), heart rate (HR), airflow (inspiration: negative values), tidal volume (VT), respiratory rate (RR) and the plasma noradrenaline concentration (PL-NA). WIN-2 (1, 3, 10 and 30 μg kg−1) was injected into the cisterna magna (i.c.) as indicated by the arrows. PRE shows the baseline reference period. Tracings represent eight experiments with similar results.
WIN55212-2 elicited a dose-dependent and marked decrease in respiratory rate (Figures 1, 2 and 3). Peak inspiratory flow was not changed (Figures 1, 2 and 3). Tidal volume increased slightly after the highest WIN55212-2 dose (Figures 1, 2 and 3). Respiratory minute volume was calculated as the product of respiratory rate and tidal volume. WIN55212-2 dose-dependently lowered the respiratory minute volume; the decrease was less than the decrease in respiratory rate, due to the slight increase in tidal volume (Figure 3).
Mean arterial pressure did not change following application of the two lower doses of the cannabinoid agonist WIN55212-2, but increased after the two higher doses (Figures 1 and 4). WIN55212-2 dose-dependently lowered heart rate and increased the plasma noradrenaline concentration (Figures 1 and 4).
WIN55212-3, an enantiomer of WIN55212-2 which has very low affinity for cannabinoid receptors, had no effects on respiratory and cardiovascular parameters (Figures 3 and 4). Only respiratory minute volume increased slightly, the reason for which is not known.
Effects of the opioid agonist DAMGO
We compared effects of the cannabinoid agonist WIN55212-2 also with those of the μ opioid receptor agonist DAMGO. Four doses of DAMGO (0.1, 0.3, 1 and 3 μg kg−1) were injected i.c. Effects on respiratory parameters are shown in Figures 2 and 5. DAMGO markedly depressed the respiratory rate, peak inspiratory flow, tidal volume and minute volume (Figures 2 and 5). Since DAMGO decreased both respiratory rate and tidal volume, the product of these two parameters, respiratory minute volume, decreased more strongly than the individual parameters (Figure 5). Expectedly, the opioid receptor antagonist naloxone attenuated the respiratory effects of DAMGO (Figure 5).
After DAMGO, blood pressure strongly decreased (to 57±6% of PRE after 3 μg kg−1; P<0.05), heart rate remained at initial levels, and there was a marked increase in the plasma noradrenaline concentration (to 446±68% of PRE after 3 μg kg−1; P<0.05). These cardiovascular effects were also attenuated by naloxone (not shown).
Interaction between WIN55212-2 and SR141716 and naloxone
We tested at first the interaction between the selective CB1 receptor antagonist SR141716 and WIN55212-2. All respiratory effects of WIN55212-2 were antagonized by SR141716 (Figure 6). SR141716 prevented the increase in blood pressure elicited by the highest dose of WIN55212-2 (30 μg kg−1), the increase in the plasma noradrenaline concentration and the WIN55212-2-evoked bradycardia (Figure 7).
Figure 6.
Interaction between WIN55212-2 (WIN-2) and SR141716 (SR) and naloxone (NX) on respiratory rate, peak inspiratory flow, tidal volume and minute volume. WIN-2 (1, 3, 10 and 30 μg kg−1) was injected i.c. in all the three groups as indicated by the arrows. At t=−20 min, one group was pretreated with SR (2 mg kg−1 i.v.) and another group with NX (0.2 mg kg−1 i.v.). Values are given as percentages of PRE. Means±s.e.m. from eight (WIN-2), seven (SR+WIN-2) and five (NX+WIN-2) experiments. +P<0.05 versus WIN-2 alone (the arrow in the graph indicates that all further values were significantly different from WIN-2 alone).
Figure 7.
Interaction between WIN55212-2 (WIN-2) and SR141716 (SR) and naloxone (NX) on mean arterial pressure, heart rate and the plasma noradrenaline concentration. WIN-2 (1, 3, 10 and 30 μg kg−1) was injected i.c. in all the three groups as indicated by the arrows. At t=−20 min, one group was pretreated with SR (2 mg kg−1 i.v.) and another group with NX (0.2 mg kg−1 i.v.). Values are given as percentages of PRE. Means±s.e.m. from eight (WIN-2), seven (SR+WIN-2) and five (NX+WIN-2) experiments. +P<0.05 versus WIN-2 alone.
Pretreatment with the opioid receptor antagonist naloxone did not modify the respiratory and cardiovascular responses elicited by WIN55212-2 (Figures 6 and 7). WIN55212-2-evoked changes in respiratory rate, tidal volume, respiratory minute volume, mean arterial pressure and heart rate were impressively identical in the absence and presence of naloxone (and strikingly different from the changes obtained in the presence of SR141716) (Figures 6 and 7).
Discussion
Respiratory effects
When injected i.c., the mixed CB1/CB2 cannabinoid receptor agonist WIN55212-2 (Pertwee, 1999; Howlett et al., 2002) depressed the respiratory rate and respiratory minute volume and slightly increased the tidal volume. Lack of effect of the stereoisomer WIN55212-3 (Pertwee, 1999; Howlett et al., 2002) points to involvement of specific cannabinoid receptors. Antagonism of the effects by the CB1 receptor-selective antagonist SR141716 (Rinaldi-Carmona et al., 1994; Pertwee, 1999; Howlett et al., 2002) identifies the receptor as the CB1 receptor. At least in the case of respiratory rate, SR141716 had no own effects (Table 1); thus, it is unlikely that the observed antagonism was due to a functional antagonism between WIN55212-2 and SR141716.
The intracisternal doses eliciting respiratory effects in the present experiments (1–30 μg kg−1) were much smaller than the intravenous doses leading to respiratory depression in rats (30–1000 μg kg−1; Schmid et al., 2003). This finding argues against a systemic effect of i.c. injected WIN55212-2 (after diffusion into the systemic circulation), and makes it likely that WIN55212-2 acted indeed in centres in the brain stem which are accessible from the intracisternal space. According to a recent observation in rats (Padley et al., 2003), cannabinoids microinjected into the rostroventrolateral reticular nucleus cause a short-lasting depression of the firing of the phrenic nerve (i.e., they cause apnoea; Padley et al., 2003). The respiratory depression was short-lasting in this latter study (<8 s), in contrast to the long-lasting effect in our study (>10 min); therefore, the phenomena observed in the two studies may not be identical. Still, the study of Padley et al. (2003) and our study show for the first time that activation of CB1 receptors in the brain stem leads to respiratory depression. Our study shows this by directly measuring several respiratory dynamical parameters. It is likely that systemically administered cannabinoids also depress respiration by acting in the brain stem, but additional sites of action (also peripheral sites) cannot be excluded.
It is not known which of the brain stem respiratory centres (ventral medullary respiratory group, dorsal medullary respiratory group, pontine respiratory group) was the primary target of the cannabinoids for lowering respiratory rate. But, CB1 receptor protein and mRNA are present in several nuclei belonging to these respiratory centres, that is, nucleus of the solitary tract, dorsal motor nucleus of vagus, area postrema, rostroventrolateral reticular nucleus, ambiguus nucleus and parabrachial nucleus (Herkenham et al., 1991; Mailleux & Vanderhaeghen, 1992; Matsuda et al., 1993; Glass et al., 1997; Tsou et al., 1998; Padley et al., 2003). In contrast to the depression of respiratory rate, WIN55212-2 did not affect peak inspiratory flow. Since peak inspiratory flow is a function of respiratory drive (defined here as the number of muscle fibers actively involved in inspiration) and airway resistance, this observation indicates that WIN55212-2 did not change the medullary regulation of these parameters. It selectively influenced medullary respiratory rhythm generation.
Our second aim was to determine whether endogenous opioids and opioid receptors are involved in the respiratory depressant effects of cannabinoids. The results of two kinds of experiments argue against an involvement of opioids. The pattern of effects of the cannabinoid agonist WIN55212-2 differed from that of the μ opioid receptor agonist DAMGO. Both drugs lowered respiratory rate and respiratory minute volume. The cannabinoid agonist had no effect on peak inspiratory flow, whereas the opioid agonist strongly lowered it. Tidal volume was increased by the cannabinoid, but depressed by the opioid. The more decisive argument against an involvement of endogenous opioids was delivered by the interaction experiments: the opioid receptor antagonist naloxone did not change the respiratory depression evoked by the cannabinoid agonist (while it attenuated responses to the opioid agonist). Since naloxone possesses affinity for μ, δ and κ opioid receptors (Corbett et al., 1993), it is unlikely that any of these receptors was involved in the action of the cannabinoid agonist WIN55212-2. An additional argument against involvement of κ opioid receptors is the fact that κ opioid receptor agonists do not depress respiration (Flórez & Hurlé, 1993). Lack of interaction of an opioid antagonist with the respiratory depressant effects of systemically administered cannabinoids was observed also in monkeys (Vivian et al., 1998).
The CB1 receptor antagonist SR141716, given i.v., increased the peak inspiratory flow, tidal volume and respiratory minute volume (Table 1). It is difficult to attribute these effects to the blockade of effects of endogenous cannabinoids in the brain stem (e.g., in that case a decrease in tidal volume would be expected). An effect of SR141716 in the periphery seems to be more likely. Reduction of airway resistance by SR141716 could explain all the three observed effects. If endogenous cannabinoids tonically inhibit noradrenaline release from sympathetic neurons of the bronchi (like exogenous cannabinoids; Vizi et al., 2001), then it is expected that SR141716 lowers airway resistance.
Cardiovascular effects
There is general agreement that one important component of the cannabinoid action on the cardiovascular system is the inhibition of noradrenaline release from postganglionic sympathetic neurons by activation of presynaptic CB1 receptors (Varga et al., 1996; Malinowska et al., 1997; 2001; Niederhoffer & Szabo, 1999; Szabo et al., 2001b; Niederhoffer et al., 2003).
The picture on the effects of cannabinoids on cardiovascular regulatory centres is less clear. Administration of Δ9-tetrahydrocannabinol into the head circulation of dogs or into the lateral cerebral ventricle of cats caused sympathoinhibition (Cavero et al., 1973a, 1973b; Vollmer et al., 1974). In a recent study in dogs, cannabinoid agonists injected into the nucleus of the solitary tract did not change the gain of the baroreceptor reflex (Rademacher et al., 2003). In conscious rabbits, injection of cannabinoids into the cisterna magna elicited sympathoexcitation and vagally mediated bradycardia (Niederhoffer & Szabo, 1999; 2000). In rats, microinjection of cannabinoids into the nucleus of the solitary tract elicited no effects, whereas injection into the rostroventrolateral reticular nucleus elicited a small sympathoinhibition (Niederhoffer et al., 2003). In another study in the rat, cannabinoid microinjection into the rostroventrolateral reticular nucleus caused sympathoexcitation (Padley et al., 2003).
In the present experiments, i.c. injection of the cannabinoid receptor agonist WIN55212-2 elicited three effects: plasma noradrenaline concentration and blood pressure increased and heart rate decreased. Lack of effect of the stereoisomer WIN55212-3 and abolishment of the effects by the CB1 receptor-selective antagonist SR141716 point to involvement of CB1 receptors in the effects of WIN55212-2.
It is very likely that the i.c. injected cannabinoid agonist WIN55212-2 acted in the brain stem and not systemically (after diffusion into the systemic circulation). First, because the intracisternal doses eliciting cardiovascular effects in the present experiments (1–30 μg kg−1) were much smaller than the intravenous doses leading to cardiovascular changes in rats (30–1000 μg kg−1; Lake et al., 1997; Niederhoffer et al., 2003; Schmid et al., 2003). Second, because the pattern of effects elicited by i.c. administered cannabinoids differed from the pattern of effects observed after i.v. administration of cannabinoids. Thus, WIN55212-2 increased plasma noradrenaline and blood pressure after i.c. administration, whereas these parameters decreased after i.v. administration (Lake et al., 1997; Niederhoffer et al., 2003; Schmid et al., 2003). WIN55212-2 elicited its cardiovascular effects probably by directly acting in cardiovascular centres. But, since respiration was depressed simultaneously, it cannot be excluded that cardiovascular changes in response to hypoxaemia, hypercapnia and acidosis contributed to the overall cardiovascular response.
The increase of the plasma noradrenaline concentration indicates that, in the rat (similarly as in rabbits; see Niederhoffer & Szabo, 1999; 2000), cannabinoid agonists enhance sympathetic tone by acting in the brain stem. Intracisternal injection of WIN55212-2 also elicited dose-dependent bradycardia (similarly as in rabbits; see Niederhoffer & Szabo, 1999; 2000). This is probably due to an enhancement of cardiac vagal tone, because the bradycardia elicited by systemically administered WIN55212-2 in rats is vagally mediated (Niederhoffer et al., 2003; Schmid et al., 2003).
In conclusion, our results show that activation of CB1 cannabinoid receptors depresses the respiratory rhythm generator in the brain stem. Sympathoexcitation and enhancement of cardiac vagal tone are additional consequences of the activation of central CB1 receptors. Opioid mechanisms are not involved in the central respiratory and cardiovascular effects of cannabinoids. The observed central respiratory and cardiovascular effects of cannabinoids may represent unwanted side effects if cannabinoid agonists are used, for example, in the treatment of asthma, pain, nausea and muscle spasticity (Kumar et al., 2001).
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (Ni 644/1-1; Sz 72/5-1). We thank Claudia Schurr for the catecholamine analysis. The support and advice of Klaus Starke are gratefully acknowledged. We thank Sanofi-Synthelabo (Montpellier, France) for generous supply of SR141716.
Abbreviations
- CB
cannabinoid
- PRE
average of initial values (before drug application)
References
- CAVERO I., SOLOMON T., BUCKLEY J.P., JANDHYALA B.S. Studies on the bradycardia induced by (−)- -trans-tetrahydrocannabinol in anesthetized dogs. Eur. J. Pharmacol. 1973a;22:263–269. doi: 10.1016/0014-2999(73)90025-3. [DOI] [PubMed] [Google Scholar]
- CAVERO I., SOLOMON T., BUCKLEY J.P., JANDHYALA B.S. Studies on the hypotensive activity of (–)-delta9-trans-tetrahydrocannabinol in anesthetized dogs. Res. Commun. Chem. Pathol. Pharmacol. 1973b;6:527–540. [PubMed] [Google Scholar]
- CORBETT A.D., PATERSON S.J., KOSTERLITZ H.W.Selectivity of ligands for opioid receptors Opioids I. Handbook of Experimental Pharmacology 1993104/IHeidelberg: Springer; 645–667.ed. Herz, A., pp [Google Scholar]
- DOHERTY P.A., MCCARTHY L.E., BORISON H.L. Respiratory and cardiovascular depressant effects of nabilone, N-methyllevonantradol and delta 9-tetrahydrocannabinol in anesthetized cats. J. Pharmacol. Exp. Ther. 1983;227:508–516. [PubMed] [Google Scholar]
- ESTRADA U., BRASE D.A., MARTIN B.R., DEWEY W.L. Cardiovascular effects of delta 9- and delta 9(11)-tetrahydrocannabinol and their interaction with epinephrine. Life Sci. 1987;41:79–87. doi: 10.1016/0024-3205(87)90559-5. [DOI] [PubMed] [Google Scholar]
- FLÓREZ J., HURLÉ M.A.Opioids in respiration and vomiting Opioids II. Handbook of Experimental Pharmacology 1993104/IIHeidelberg: Springer; 263–292.ed. Herz, A. pp [Google Scholar]
- GLASS M., DRAGUNOW M., FAULL R.L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- GRAHAM J.D., LI D.M. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973;49:1–10. [PMC free article] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. Classification of cannabinoid receptors. International Union of Pharmacology. XXVII. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- KUMAR R.N., CHAMBERS W.A., PERTWEE R.G. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–1068. doi: 10.1046/j.1365-2044.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- KUNOS G., JARAI Z., VARGA K., LIU J., WANG L., WAGNER J.A. Cardiovascular effects of endocannabinoids – the plot thickens. Prostaglandins Other Lipid Mediat. 2000;61:71–84. doi: 10.1016/s0090-6980(00)00056-3. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LICHTMAN A.H., SHEIKH S.M., LOH H.H., MARTIN B.R. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J. Pharmacol. Exp. Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- MAILLEUX P., VANDERHAEGHEN J.J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., GODLEWSKI G., BUCHER B., SCHLICKER E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., PISZCZ J., KONECZNY B, HRYNIEWICZ A, SCHLICKER E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:233–241. doi: 10.1007/s002100100450. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., BONNER T.I., LOLAIT S.J. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- MOSS I.R., FRIEDMAN E. Delta9-tetrahydrocannabinol: depression of ventilatory regulation; other respiratory and cardiovascular effects. Life Sci. 1976;19:99–104. doi: 10.1016/0024-3205(76)90379-9. [DOI] [PubMed] [Google Scholar]
- NAVARRO M., CARRERA M.R., FRATTA W., VALVERDE O., COSSU G., FATTORE L., CHOWEN J.A., GOMEZ R., DEL ARCO I., VILLANUA M.A., MALDONADO R., KOOB G.F., DE FONSECA F.R. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SCHMID K., SZABO B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- PADLEY J.R., LI Q., PILOWSKY P.M., GOODCHILD A.K. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 2003;140:384–394. doi: 10.1038/sj.bjp.0705422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- PFITZER T., NIEDERHOFFER N., SZABO B. Respiratory effects of cannabinoids in rats: comparison with opioids. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:R105. doi: 10.1007/s00210-003-0787-3. [DOI] [PubMed] [Google Scholar]
- RADEMACHER D.J., PATEL S., HOPP F.A., DEAN C., HILLARD C.J., SEAGARD J.L. Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1570–H1576. doi: 10.1152/ajpheart.00772.2002. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIÉ P., BRELIÈRE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- SCHMID K., NIEDERHOFFER N., SZABO B. Analysis of the respiratory effects of cannabinoids in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;368:301–308. doi: 10.1007/s00210-003-0787-3. [DOI] [PubMed] [Google Scholar]
- SZABO B., FRITZ T., WEDZONY K. Effects of imidazoline antihypertensive drugs on sympathetic tone and noradrenaline release in the prefrontal cortex. Br. J. Pharmacol. 2001a;134:295–304. doi: 10.1038/sj.bjp.0704237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO B., NORDHEIM U., NIEDERHOFFER N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J. Pharmacol. Exp. Ther. 2001b;297:819–826. [PubMed] [Google Scholar]
- SZABO B., PFITZER T., NIEDERHOFFER N.Central respiratory effects of cannabinoids in rats 2002 Symposium on the Cannabinoids 2002International Cannabinoid Research Society, Burlington Vermont; S157p [Google Scholar]
- TANDA G., PONTIERI F.E., DI CHIARA G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- TSOU K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- VALVERDE O., MALDONADO R., VALJENT E., ZIMMER A.M., ZIMMER A. Cannabinoid withdrawal syndrome is reduced in pre-proenkephalin knock-out mice. J. Neurosci. 2000;20:9284–9289. doi: 10.1523/JNEUROSCI.20-24-09284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA K., LAKE K.D., HUANGFU D., GUYENET P.G., KUNOS G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- VIVIAN J.A., KISHIOKA S., BUTELMAN E.R., BROADBEAR J., LEE K.O., WOODS J.H. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J. Pharmacol. Exp. Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- VIZI E.S., KATONA I., FREUND T.F. Evidence for presynaptic cannabinoid CB(1) receptor-mediated inhibition of noradrenaline release in the guinea pig lung. Eur. J. Pharmacol. 2001;431:237–244. doi: 10.1016/s0014-2999(01)01413-3. [DOI] [PubMed] [Google Scholar]
- VOLLMER R.R., CAVERO I., ERTEL R.J., SOLOMON T.A., BUCKLEY J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (–)-delta 9-trans-tetrahydrocannabinol. J. Pharm. Pharmacol. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., KUNOS G. Cardiovascular actions of cannabinoids and their generation during shock. J. Mol. Med. 1998;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]