Abstract
We investigated the ability of N-benzyl-N-ethyl-2-(7,8-dihydro-7-methyl-8-oxo-2-phenyl-9H-purin-9-yl)acetamide (AC-5216), a novel mitochondrial benzodiazepine receptor (MBR) ligand, to produce anti-anxiety and antidepressant-like effects in various animal models.
AC-5216 showed high affinity for MBRs prepared from rat whole brain (Ki 0.297 nM), rat glioma cells (IC50 3.04 nM) and human glioma cells (IC50 2.73 nM), but only negligible affinity for the other main receptors including central benzodiazepine receptors.
AC-5216 produced anti-anxiety effects in the Vogel-type conflict test in rats, and in the light/dark box and social interaction tests in mice at 0.1–3, 0.003–0.01 and 0.01–0.3 mg kg−1, p.o., respectively. These effects of AC-5216 were antagonized by PK11195, an MBR antagonist. In the forced swimming test in rats, AC-5216 (3–30 mg kg−1, p.o.) reduced the immobility time, and this effect was blocked by PK11195.
AC-5216 had no myorelaxant effects, did not affect the memory or prolong hexobarbitone-induced sleep in mice, even at doses as high as 1000 mg kg−1, p.o. Although it did slightly prolong the ethanol-induced sleep time at 1000 mg kg−1, AC-5216 (1–100 mg kg−1, p.o.) produced no distinct change in the rat electroencephalogram.
These results indicate that AC-5216 produces anti-anxiety and antidepressant-like effects that are mediated by MBR, but does not cause the side effects normally associated with conventional benzodiazepines. Hence, AC-5216 shows potential for the treatment of stress-related disorders including anxiety and depression.
Keywords: AC-5216, mitochondrial benzodiazepine receptor, anti-anxiety, antidepressant
Introduction
Benzodiazepine (BZD) derivatives have been widely used for the treatment of anxiety. Although BZDs are relatively safe drugs, they produce undesirable side effects (ataxia, amnesia, ethanol and barbiturate potentiation and physical dependence) which limit their usefulness. BZDs produce their therapeutic and undesirable effects by allosterically modulating the action of GABA via the central benzodiazepine receptor (CBR), located on the extracellular domain of the GABAA receptor (Möhler et al., 2002). In contrast, the mitochondrial benzodiazepine receptor (MBR), also commonly called the peripheral BZD receptor or ω3 receptor, was first identified as a peripheral binding site for diazepam, and has subsequently been demonstrated to be functionally and structurally different from the CBR (Beurdeley-Thomas et al., 2000; Casellas et al., 2002). The MBR is widely found in peripheral tissues as well as in the CNS, and its subcellular localization has been described in the mitochondrial membrane (Anholt et al., 1986; Basile & Skolnick, 1986). Functionally, MBR has been shown to be involved in a variety of biological processes including steroidogenesis (Krueger & Papadopoulos, 1990; Papadopoulos et al., 1997), cell growth and apoptosis (Chelli et al., 2001; Casellas et al., 2002). In addition, several series of compounds have been shown to be selective ligands for the MBR, including BZD derivatives (e.g., Ro5-4864, Schoemaker et al., 1983), isoquinoline derivatives (e.g., PK11195, Le Fur et al., 1983), 2-aryl-3-indoleacetamide derivatives (e.g., FGIN1-27, Romeo et al., 1992), DAA1097 and DAA1106 (Okuyama et al., 1999), 2-phenyl-imidazo[1,2-a]pyridine derivatives (e.g., CB 34, Serra et al., 1999) and a pyridazinoindole derivative (SSR180575, Ferzaz et al., 2002). These ligands have been shown to produce a variety of pharmacological actions such as anti-anxiety, anticonvulsant, antiapoptotic, anti-inflammatory and neuroprotective effects.
It has been reported that acute stress tends to increase MBR density while chronic stress induces a decrease in MBR density in the brain and peripheral organs in rodents (Weizman & Gavish, 1993). Similarly, in humans, a decrease in platelet and lymphocyte MBR levels in patients with generalized anxiety disorder, panic disorder, post-traumatic stress disorder or social phobia has been reported (Rocca et al., 1998; Gavish et al., 1999). These findings suggest that the MBR has an important role in stress-response and stress-related disorders such as anxiety. In the CNS, the MBR is mainly located in the glial cells (Gallager et al., 1981; Schoemaker et al., 1982), but can also be found in neurones (Anholt et al., 1984; Doble et al., 1987). MBRs on glial cells are thought to regulate the biosynthesis of neurosteroids, and appropriate MBR ligands are known to stimulate neurosteroidogenesis, both in vitro (Guarneri et al., 1992) and in vivo (Korneyev et al., 1993; Serra et al., 1999), by facilitating the translocation of cholesterol from the outer to the inner mitochondrial membrane, a process known to be the rate-limiting step of steroidogenesis. Neurosteroids serve as neuromodulators at several neurotransmitter receptors, such as GABA, glutamate and acetylcholine receptors, and influence emotion, memory/learning and stress responses (Kulkarni & Reddy, 1995; Compagnone & Mellon, 2000). For example, pregnane neurosteroids such as pregnanolone are known to produce anti-anxiety effects by positively modulating GABAA receptor functions (Bitran et al., 1991). These findings suggest that MBR ligands exert their anti-anxiety effect via newly synthesized neurosteroids. Although, as mentioned above, selective MBR ligands have been reported to produce anti-anxiety effects in animal models of anxiety, only a few compounds are active when administered orally (Romeo et al., 1993; Okuyama et al., 1999). Thus, potent and orally active MBR ligands could provide a new treatment for anxiety disorders. Furthermore, as MBR is known to be involved in a variety of stress responses, MBR ligands also have the potential to prevent other psychiatric disorders arising from stress-induced imbalance of CNS functions.
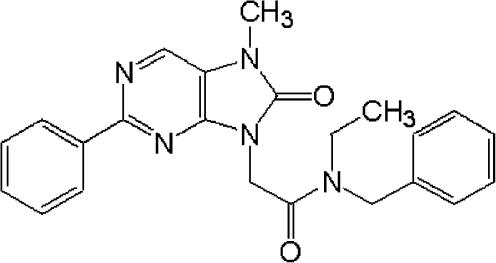
In our search for a new class of orally-active MBR ligands for the therapeutic treatment of stress-related disorders, we synthesized AC-5216, an 8-oxopurine derivative (N-benzyl-N-ethyl-2-(7,8-dihydro-7-methyl-8-oxo-2-phenyl-9H-purin-9-yl)acetamide) (Figure 1), and evaluated its pharmacological profile in receptor binding and behavioural studies. In this study, we present evidence for AC-5216-binding affinity and selectivity for rat and human MBRs, and its anti-anxiety and antidepressant-like effects in mice and rats. In addition, we investigated its potential to produce the undesirable effects associated with conventional BZDs, which include muscle relaxation, memory impairment, prolongation of sleeping time induced by hexobartitone or ethanol, and effects on the cortical electroencephalogram (EEG) pattern. In all experiments, the effects of AC-5216 were evaluated and compared with those of two anti-anxiety drugs in clinical use, that is, diazepam, a typical BZD, and tandospirone, a 5-HT1A agonist. In addition, the antidepressant-like effects of AC-5216 were evaluated and compared with desipramine, a tricyclic antidepressant.
Figure 1.
Chemical structure of AC-5216.
Methods
Animals
Male ddY mice and Wistar rats were obtained from Japan SLC Inc. (Shizuoka, Japan), and male SD rats were purchased from Clea Japan Inc. (Tokyo, Japan). Animals were housed in groups, except for the EEG study where rats were housed singly after surgery. The animals were kept, for at least 5 days before the experiments, in temperature- and humidity-controlled animal rooms under a 12 : 12 h light/dark cycle (light on 06:00–18:00 h) with free access to food and water. Behavioural studies were carried out during the light phase. All experimental procedures used were approved by the Institutional Animal Care and Use Committee at Dainippon Pharmaceutical Co., Ltd.
In vitro binding studies
MBR-binding assay
MBR-binding assays were performed using crude mitochondrial fractions prepared separately from rat whole brain, rat C6 glioma cells and human Hs683 glioma cells.
Receptor preparation: Male Wistar rats weighing 210–250 g were decapitated, and the whole brain was dissected and homogenized in 10 volumes of ice-cold HS buffer (10 mM HEPES buffer pH 7.4 containing 320 mM sucrose) with a Potter–Elvehjem tissue grinder. The homogenate was centrifuged at 770 × g for 10 min, and the supernatant was centrifuged at 9000 × g for 10 min. The pellet was suspended in HS buffer and centrifuged at 9000 × g for 10 min. The resultant pellet was then washed with 50 mM HEPES buffer (pH 7.4) by centrifugation at 12,000 × g for 10 min. Finally, the crude mitochondrial pellet was resuspended in 50 mM HEPES buffer (pH 7.4) at a concentration of 100 mg original wet tissue ml−1 and used for binding assays.
Rat C6 glioma cells (CCL-107, American Type Culture Collection, Rockville, MD, U.S.A.) were cultivated in Ham's F10 medium supplemented with 2.5% heat-inactivated foetal bovine serum, 15% heat-inactivated horse serum, 50 IU ml−1 penicillin and 50 μg ml−1 streptomycin until confluence. The cells were washed twice with phosphate-buffered saline and harvested from the tissue culture flask with a cell scraper. The cell pellet collected by centrifugation at 800 r.p.m. for 5 min was dispersed in HS buffer and sonicated with 10-s bursts using an ultrasonic disruptor. Cell debris was removed by centrifugation at 770 × g for 10 min, and the supernatant was centrifuged at 9000 × g for 10 min. The pellet was suspended in HS buffer and centrifuged at 9000 × g for 10 min. The resultant pellet was then washed with 50 mM HEPES buffer (pH 7.4) by centrifugation at 12,000 × g for 10 min. Finally, the crude mitochondrial pellet was resuspended in 50 mM HEPES buffer (pH 7.4) at a concentration of 0.4 mg protein ml−1 and used for binding assay.
Human Hs683 glioma cells (HTB-138, American Type Culture Collection, Rockville, MD, U.S.A.) were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated foetal bovine serum, 50 IU ml−1 penicillin and 50 μg ml−1 streptomycin, until confluence. Using the same procedure as that described above, human Hs683 glioma cells were collected and the crude mitochondrial fraction was prepared at a concentration of 0.5 mg protein ml−1 and used for binding assays.
All procedures were performed at 4°C after dissection of rat brain or collection of cell pellet. The protein concentration of each receptor preparation was measured with a DC protein assay kit (Bio-Rad).
Binding assay: Each crude mitochondrial preparation (100 μl) was incubated with [3H]-PK11195 (final concentration 0.5 nM) and various concentrations of the test compounds in a total volume of 1 ml for 90 min at 4°C, and the reaction was terminated by rapid filtration through 0.3% polyethylenimine-pretreated Whatman GF/C glass fibre filters, using a cell harvester (MB-48, Brandel). The filters were immediately washed three times with 5 ml ice-cold HEPES buffer (50 mM), and the filter-bound radioactivity was quantified using a liquid scintillation analyzer (Tri-Carb 2700TR, Packard) after addition of 10 ml scintillator (ACS II, Amercham). Nonspecific binding was determined in the presence of 10 μM PK11195. All assays were carried out in duplicate except the total binding and nonspecific binding, which were in quadruplicate. The IC50 values for each test compound were determined according to a nonlinear least-square curve-fitting method using the SAS® system (SAS Institute Inc., Cary, NC, U.S.A.). In the assay with rat whole brain MBRs, Ki values were calculated according to the following formula: Ki=IC50/(1+[L]/KD) where [L] and KD are the concentration of [3H]-PK11195 and the dissociation constant of PK11195 calculated by Scatchard analysis, respectively. Data are expressed as the mean±s.e.m. of three separate experiments with [3H]-PK11195 KD and Bmax values of 0.233±0.005 nM and 285±5 fmol mg−1 protein, respectively.
CBR-binding assay
Receptor preparation: Male Wistar rats weighing 190–210 g were decapitated, and the cerebellum (for CBR type I) and spinal cord (for CBR type II) were dissected. Each tissue was homogenized in 20 volumes of ice-cold Tris-citrate buffer (50 mM, pH 7.1) with a Potter-Elvehjem tissue grinder. The homogenate was then centrifuged at 40,000 × g for 15 min and washed three times with the same buffer. The pellet, after being stored frozen, was resuspended in Tris-citrate buffer (pH 7.1), and centrifuged at 40,000 × g for 15 min. The resulting pellet was resuspended in assay buffer (concentration in mM: NaCl 120, KCl 5, CaCl2 2, MgCl2 1, Tris-HCl 50, pH 7.4) at a concentration of 25 mg wet tissue ml−1, and stored at −80°C until used for binding assays. All procedures were performed at 4°C after tissue dissection.
Binding assay: Receptor preparations (150 μl) were incubated with [3H]-flumazenil (Ro 15-1788) at a final concentration of 0.3 nM for CBR type I and 1 nM for CBR type II, 100 μM (+)-bicuculline and various concentrations of test compounds, in a total volume of 1 ml for 30 min at 37°C. The reaction was terminated by rapid filtration through Whatman GF/B glass fibre filters using a cell harvester. The filters were immediately washed three times with 5 ml ice-cold Tris-HCl buffer (50 mM, pH 7.7), and the filter-bound radioactivity was quantified using a liquid scintillation analyzer after addition of 10 ml scintillator. Nonspecific binding was determined in the presence of 10 μM flunitrazepam. All assays were done in duplicate except the total binding and nonspecific binding, which were in quadruplicate. The IC50 and Ki values for each test compound were calculated according to the same procedure as that described for MBR binding. Data are expressed as the mean±s.e.m. of three separate experiments with [3H]-flumazenil KD and Bmax values of 8.63±0.15 nM and 1233±11 fmol mg−1 protein, respectively (CBR type I) or 8.84±0.04 nM and 246±1 fmol mg−1 protein, respectively (CBR type II).
Non-target-binding assays
To determine AC-5216 binding to nonselective targets, a large number of receptor-, transporter- and ion channel-binding assays were performed by Daiichi Pure Chemicals Co., Ltd (Ibaraki, Japan).
Behavioural studies
Vogel-type conflict test in rats
For this test, the method of Vogel et al. (1971) with a minor modification was used in male SD rats weighing 160–240 g. The test chamber consisted of an acrylic cage (30 × 30 × 27 cm3) with a grid floor (5-mm interval). A water bottle with a metal drinking tube was set on the outside of the chamber, and the drinking tube was placed 0.5 cm into a side wall at a height of 7.5 cm above the floor. The grid floor and the drinking tube were connected to a drinko-meter (Bio Medica, Ltd, Osaka, Japan) that was used to record the number of licks; the rat completed the circuit whenever it licked the tube. When rats licked and had contact with the drinking tube continuously, the drinko-meter was connected to a circuit that produced seven pulses per second, and each pulse was counted as equivalent to one lick. Electric shocks (4 mA, 50 Hz, 10 ms duration) were administered to each rat by automatically switching the connections to the drinking tube and the grid floor from the drinko-meter to an electric stimulator. Rats were individually placed in the test chamber and allowed to explore the chamber for 5 min. They were then returned to their home cages and deprived of water for 48 h. After the first 24 h of water deprivation, rats were placed in the test chamber again and allowed to drink water through the drinking tube without being given an electric shock. When the rats completed 200 licks, they were returned to their home cages and further deprived of water. Only rats that did 200 licks within 5 min were used for the test session and they were allocated to treatment groups by the stratified random allocation method, based on the time required to complete 200 licks. After another 24 h of water deprivation, the rats were individually placed in the test chamber. When a rat had done 20 licks and had received its first electric shock, the test session started. The number of shocks that each rat received after every 20 licks was recorded for 5 min. Test compounds were orally administered 1 h before the test session. In the antagonism study, PK11195 and flumazenil were administered i.p. 30 min before the test session in a volume of 3 ml kg−1.
Light/dark box test
The method of Costall et al. (1989) was used with a minor modification. Male ddY mice weighing 24–30 g were randomly allocated to treatment groups. The acrylic test box consisted of a light compartment (20 × 15 × 17.5 cm3) and a dark one (15 × 15 × 17.5 cm3) that were connected by an opening (4.5 × 4.5 cm) located in the centre of the partition at floor level. The light compartment with an open top had transparent walls and was brightly illuminated with a light source (ca. 1400 lx at floor level), whereas the dark compartment had a covered top and black, opaque walls. A video camera was set above the test box to record mice behaviour. Mice were individually placed in the centre of the light compartment facing the opening and allowed to explore the test box freely for 5 min. The time that each mouse spent in the light compartment was recorded. Three paws placed in a compartment constituted an entry into that compartment. Test compounds were orally administered 1 h before testing. In the antagonism study, PK11195 and flumazenil were administered i.p. 30 min before testing.
Social interaction test
The method used for this test was based on that of File (1980). Experiments were performed under bright-light unfamiliar environmental test conditions. Male ddY mice weighing 22–32 g were randomly allocated to treatment groups and randomly paired for testing. The apparatus consisted of a glass beaker inverted onto a frosted glass plate (Frances, 1988), and was brightly illuminated with a light source (ca. 1200 lx at table level). A video camera was set diagonally above the apparatus and was connected to a monitor placed about 3 m away from the apparatus. Two mice that were housed in separate home cages received the same treatment and were returned to their home cages. The two mice were then placed together in the test apparatus, and the amount of time spent in social interaction by the two mice during a 15-min period was manually recorded. Social interaction was defined as sniffing and grooming the partner, genital investigation of the partner, and climbing over or crawling under the partner. After each test, the beaker and plate were washed and wiped. Test compounds were orally administered 1 h before testing. In the time-course study, separate groups of mice were used for each time point, and test compounds were orally administered 0.5, 1, 2, 4 or 6 h before testing. In the antagonism study, PK11195 and flumazenil were administered i.p. 20 min before the start of the experiment.
Forced swimming test
The method used for the forced swimming test was based on that of Porsolt et al. (1978). Male Wistar rats weighing 95–120 g at the start of experiments were used. The apparatus was a clear acrylic cylinder (33 cm high, 24.5 cm in diameter) containing 15 cm of water at 24–25°C. Rats were daily given a 10-min swimming session for four consecutive days before testing. On day 4, rats that were immobile for more than 180 s during the first 6 min of the swimming session were selected for the test session and randomly allocated to treatment groups. On day 5, the selected rats were placed in the water, and the total period of time they were immobile (immobility time) during a 6-min test session was recorded. Immobility was defined as the absence of active, escape-oriented behaviours such as swimming, jumping, struggling and diving. After each session, the water was changed to fresh water. Test compounds were orally administered 1 h before testing. In the antagonism study, PK11195 was administered (i.p.) 30 min before the start of the experiment in a volume of 3 ml kg−1.
Profile of undesirable effects
Traction test
Male ddY mice weighing 23–29 g were randomly assigned to treatment groups. The apparatus was a horizontal stainless-steel bar (30 cm long, 2 mm in diameter) set at a height of 15 cm above bench. Mice were suspended from the horizontal bar by their forepaws. Failure to cling to the bar with their hindpaws within 5 s at least twice in three consecutive trials was regarded as positive for myorelaxant effect. This traction test was conducted 0.5, 1 and 2 h after oral administration of test compounds.
Step-down passive avoidance test
Male ddY mice weighing 30–35 g were used. Animals were randomly assigned to treatment groups. The apparatus was a white acrylic open-topped box (30 × 30 × 50 cm3) with a grid floor (2 mm in diameter, 1-cm interval). A wooden platform (4 × 4 × 4 cm3) was fixed at the centre of the grid floor. Electric shocks were delivered via the grid floor by an electric stimulator. Experiments consisted of a training session and a retention session separated by a 24-h interval. In the training session, mice were orally treated with test compounds and 1 h later individually placed on the platform. When the mouse stepped down from the platform and placed all its paws on the grid floor, electric shocks (60 V, 1 Hz, 0.5 s duration) were delivered for 15 s through the grid floor. Training trials were repeated until the mouse stayed on the platform for 60 s. After the training session, mice were returned to their home cages. At 24 h after the training session, mice were placed on the platform again without electric shock, and the step-down latency was measured with a cut-off time of 300 s.
Hexobarbitone- or ethanol-induced sleep
Male ddY mice weighing 23–32 g were randomly assigned to treatment groups. Mice were orally treated with test compounds and, 1 h later, received an injection of hexobarbitone (80 mg kg−1, i.p., 10 ml kg−1). The duration of loss of righting reflex was recorded as sleeping time. The effects of test compounds on ethanol-induced sleep were evaluated using the same procedure as described above except that ethanol (4.2 g kg−1, i.p., 20 ml kg−1) was injected instead of hexobarbitione.
EEG study
Male Wistar rats weighing 290–310 g at the time of surgery were used. Animals were randomly assigned to treatment groups. EEG recording apparatus was a transparent plastic chamber (30 × 30 × 29 cm3) with a slip-ring system on the ceiling placed in a sound-attenuated and electrically shielded box. EEG signals were transmitted through a flexible cable to the slip-ring that was connected to a bioelectric amplifier unit, for amplification, and recorded by a personal computer after A/D conversion.
Rats were anaesthetized with sodium pentobarbitone (50 mg kg−1, i.p.) and mounted on a stereotaxic apparatus. Three screw electrodes were implanted supradurally into the calvarium over the right frontal cortex (AP −3.5, ML 3.0), the ipsilateral occipital cortex (AP 8.0, ML 4.0) and over the cerebellum at the midline as ground electrode. The electrodes were connected to a miniature nine-pin socket, and the socket was fixed securely to the skull with acrylic dental cement. At 1 week after surgery, to habituate them to the experimental environment, animals were placed in the recording box for 6 h a day on two consecutive days, while being connected through the socket to the flexible cable that transmitted EEG signals. At 2 weeks after surgery, EEG recordings were obtained for a 6-h session per day on two consecutive days. At 1 h after the recording had begun, the rats were treated with vehicle on the first day and with AC-5216 or diazepam on the second day.
Analysis of EEG data was carried out using an automatic EEG analysing programme developed in our company. The digitized EEG data were collected continuously by a computer at a rate of 128 Hz 16 bit samples per second. Periodgrams calculated every 1 s by Fast Fourier transform were gathered in groups of 10, averaged and stored as a single epoch (i.e., a 10-s period) of the power spectrum. The relative power (expressed as % of total power) was computed every 30 min after oral administration of test compounds or vehicle for each of the following seven frequency bands: 1–4, 4–6, 6–8, 8–10, 10–12, 12–20 and 20–40 Hz. The difference in relative power between treatment with test compounds and that with vehicle was calculated for each animal.
Drugs
AC-5216, Ro5-4864 and flumazenil were synthesized at our Chemistry Research Laboratories. PK11195, FGIN1-27, desipramine hydrochloride and hexobarbitone were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.), diazepam from Wako Pure Chemical Industries (Osaka, Japan), tandospirone citrate (extracted from Sediel®) from Sumitomo Pharmaceuticals Co., Ltd (Osaka, Japan), [3H]-PK11195 and [3H]-flumazenil from Perkin-Elmer Life Sciences (Boston, MA, U.S.A.). In the in vitro studies, test compounds were initially dissolved in dimethylsulphoxide (DMSO) and diluted with deionized water. The concentration of DMSO was less than 0.1% in all assays. In the behavioural studies, AC-5216, diazepam, tandospirone and desipramine were suspended in 0.5% tragacanth gum aqueous solution for oral administration, and PK11195 and flumazenil were suspended for i.p. injection in saline containing 0.4% Tween 80 in the case of mice or in saline containing 2% DMSO and 0.8% Tween 80 in the case of rats. Hexobarbitone was dissolved in 50 mM NaOH saline solution. Suspensions of drugs were administered in a volume of 10 ml kg−1 (light/dark box and social interaction tests in mice), 20 ml kg−1 (traction test, passive avoidance test and hexobarbitone- or ethanol-induced sleep test in mice), 1 ml kg−1 (Vogel conflict test in rats) and 2 ml kg−1 (forced swimming test and EEG in rats), unless otherwise specified.
Statistical analysis
Data are expressed as mean±s.e.m. (binding assays, Vogel-type conflict test, social interaction test, forced swimming test and hexobarbitone- or ethanol-induced sleep test), box-whisker plots (light/dark box test), median and interquartile values (light/dark box test, passive avoidance test), or incidence rate (traction test). Data from behavioural experiments were analysed by Student's t-test, Dunnett's multiple comparison test, paired t-test, Wilcoxon's rank-sum test, nonparametric Dunnett's multiple comparison test and Fisher's exact probability test, as indicated in the Results section. Values of P<0.05 were considered significant. Statistical analyses were performed using the SAS® system (SAS Institute Inc., Cary, NC, U.S.A.).
Results
In vitro binding assays
AC-5216 showed high affinity for MBR in the crude mitochondrial fraction prepared from rat whole brain with a Ki value of 0.297 nM. AC-5216 potency for binding to this receptor was comparable to that of PK11195, and higher than that of diazepam (Table 1). In the crude mitochondrial fractions prepared from rat C6 glioma cells and human Hs683 glioma cells, AC-5216 showed potent displacement of [3H]-PK11195 binding to MBRs with mean IC50 values from three independent experiments of 3.04±0.21 and 2.73±0.28 nM for rat and human glial MBRs, respectively. PK11195 also showed potent affinity for the rat and human glial MBRs with IC50 values of 5.65±0.37 and 5.55±0.25 nM (mean and s.e.m., n=3), respectively. In contrast, flumazenil had no affinity (IC50>10 μM) for any of the MBRs prepared from the three sources (rat whole brain, rat C6 glioma cells and human Hs683 glioma cells).
Table 1.
Effects of AC-5216 and reference compounds on [3H]-PK11195 binding to the MBR or [3H]-flumazenil binding to the CBR type I and type II
| Compound | MBR | CBR type I | CBR type II | ||
|---|---|---|---|---|---|
| Ki (nM) | % inhibitiona | Ki (nM) | % inhibitiona | Ki (nM) | |
| AC-5216 | 0.297±0.009 | 31.75±0.53 | 21.40±1.16 | ||
| PK11195 | 0.602±0.046 | 21.26±1.05 | 22.59±2.14 | ||
| Ro5-4864 | 1.02±0.08 | 5.13±1.89 | 5.32±1.97 | ||
| FGIN1-27 | 3.25±0.26 | 5.46±1.39 | 10.42±1.77 | ||
| Diazepam | 22.0±1.4 | 98.5±1.7 | 94.8±4.4 | ||
| Flumazenil | Inhibition at 10 μM 2.94±0.88% | 6.25±0.06 | 5.24±0.18 | ||
MBR was prepared from the crude mitochondrial fraction of rat whole brain. CBR type I and type II were prepared from the membrane fraction of rat cerebellum and spinal cord, respectively. Data represent the mean±s.e.m. of three independent experiments.
Inhibition (%) at 10 μM except for FGIN1–27, which was examined at 3 μM.
In the CBR-binding assay, AC-5216 at a concentration of 10 μM slightly displaced [3H]-flumazenil binding to CBR type I and type II (Table 1). Similarly, PK11195 (10 μM) showed weak inhibition at both CBRs. In contrast, diazepam showed high affinity for both CBR type I and type II, and flumazenil showed a more potent affinity for both receptors than that of diazepam.
To further investigate the selectivity of AC-5216 for the MBR, the binding affinity of this compound for other receptors, transporters or ion channels was examined in a total of 90 assays. These were adenosine (A1, A2 and A3) receptors, adrenoceptors (α1, α2, β1, β2 and β3) and receptors for bradykinin BK2, dopamine (D1, D2, D3, D4 and D5), CCKA, GABA (GABAA, GABAB and picrotoxin), glutamate (AMPA, kainite and NMDA), histamine (H1, H2 and H3), muscarinic (M1, M2, M3, M4 and M5), neurokinin (NK1 and NK2), nicotine, opioid (μ, δ and κ), serotonin (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6 and 5-HT7), sigma (σ1 and σ2), vasopressin V1b and hormones (oestrogen, glucocorticoid, progesterone and testosterone) plus the transporters (dopamine, GABA and monoamines) and ion channels (calcium, potassium and sodium). AC-5216 at a concentration of 1 μM showed no noticeable affinity for any of the receptors, transporters or ion channels tested (inhibition <35%).
Anti-anxiety effects
Vogel-type conflict test in rats
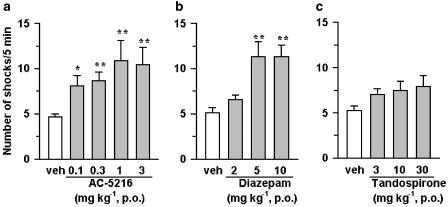
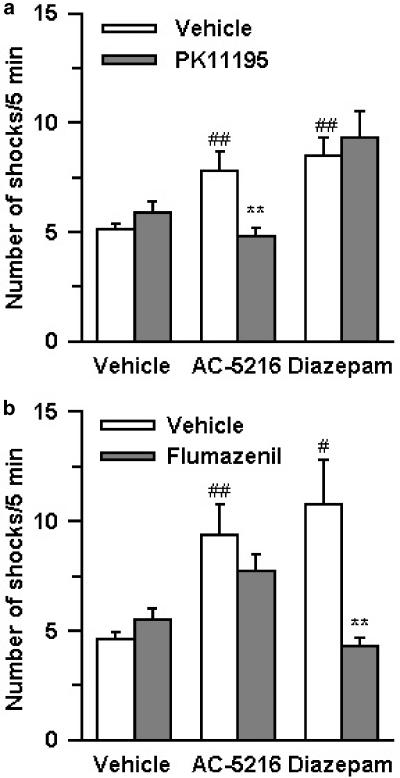
In this test, the anti-anxiety effects of test compounds were indicated by an increase in the number of shocks that rats received, as a result of inhibition of the conflict between the desire to drink water and the aversion to an electric shock. AC-5216, given orally at doses of 0.1–3 mg kg−1, significantly increased the number of shocks that rats received (Figure 2a). Diazepam at oral doses of 5 and 10 mg kg−1 also produced a significant increase in the number of shocks (Figure 2b). Tandospirone (3–30 mg kg−1, p.o.) induced a slight increase in the number of shocks, although its effects did not reach statistical significance (Figure 2c). The anti-conflict effect of AC-5216 (1 mg kg−1, p.o.) was antagonized by PK11195 (3 mg kg−1, i.p.), an MBR antagonist, but not by flumazenil (10 mg kg−1, i.p.), a CBR antagonist, whereas the anti-conflict effect of diazepam (5 mg kg−1, p.o.) was reversed by flumazenil, but not by PK11195 (Figure 3a and b). Neither PK11195 nor flumazenil, when administered alone at the indicated dose, affected the number of shocks.
Figure 2.
Anti-conflict effects of AC-5216 (a), diazepam (b) and tandospirone (c) in the Vogel-type conflict test in rats. Each column represents the mean±s.e.m. (n=20). Test compounds were administered orally 1 h before testing. *P<0.05, **P<0.01, significantly different from the respective vehicle control group (nonparametric Dunnett's multiple comparison test).
Figure 3.
Antagonism by PK11195 (a) and flumazenil (b) of the anti-conflict effects of AC-5216 and diazepam in the Vogel-type conflict test in rats. Each column represents the mean±s.e.m. (n=20). AC-5216 (1 mg kg−1) and diazepam (5 mg kg−1) were administered orally 1 h before testing. PK11195 (3 mg kg−1) and flumazenil (10 mg kg−1) were administered i.p. 30 min before testing. #P<0.05, ##P<0.01, significantly different from the respective vehicle control group; **P<0.01, significantly different from the AC-5216 alone- or diazepam alone-treated group (Wilcoxon's rank-sum test).
Light/dark box test in mice
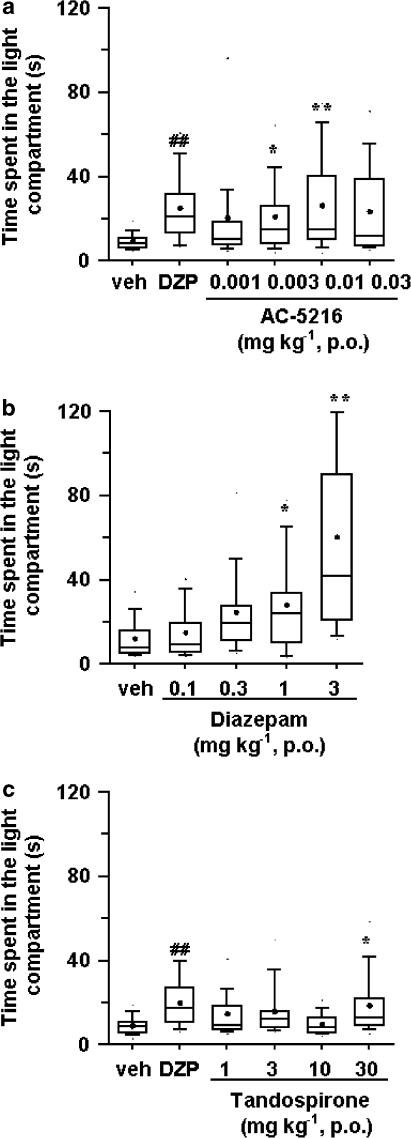
In the light/dark box test, the anti-anxiety effects of test compounds were indicated by an increase in the amount of time that mice spent in the light compartment (aversive area). AC-5216, at oral doses of 0.003 and 0.01 mg kg−1, significantly increased the time spent in the light compartment but only slightly increased that time at 0.03 mg kg−1, p.o. (P<0.1) (Figure 4a). Diazepam (1 and 3 mg kg−1, p.o.) also produced a significant increase in the time spent in the light compartment (Figure 4b). Tandospirone, on the other hand, induced a slight but significant increase in the time spent in the light compartment at an oral dose of 30 mg kg−1 (Figure 4c). The anti-anxiety effect of AC-5216 (0.01 mg kg−1, p.o.) was significantly reduced by PK11195 (1 and 10 mg kg−1, i.p.), but not by flumazenil even at 30 mg kg−1 (i.p.) (Table 2), whereas that of diazepam (1 mg kg−1, p.o.) was blocked by flumazenil (30 mg kg−1, i.p.) but not by PK11195 even at 10 mg kg−1 (i.p.) (Table 2). Neither PK11195 nor flumazenil, when given alone at the doses indicated, affected the amount of time spent in the light compartment.
Figure 4.
Effects of AC-5216 (a), diazepam (b) and tandospirone (c) in the light/dark box test in mice (n=20). Data are represented by box-whisker plots. The rectangular box shows the interquartile range. The median is shown as the bar inside each box. The small filled circle indicates the mean. The lower and upper whiskers extended from the bottom and top of the box show the locations of the 10th and 90th percentiles. veh: vehicle. DZP: diazepam 1 mg kg−1 (positive control group). Test compounds were administered orally 1 h before testing. ##P<0.01, significantly different from the respective vehicle control group (Wilcoxon's rank-sum test). *P<0.05, **P<0.01, significantly different from the respective vehicle control group (nonparametric Dunnett's multiple comparison test).
Table 2.
Effects of PK11195 and flumazenil on the anti-anxiety effect of AC-5216 and diazepam in the light/dark box test in mice
| Treatment | Time spent in light compartment (s)a | |
|---|---|---|
| p.o. | i.p. | |
| Effect of PK11195 | ||
| Vehicle | Vehicle | 7.62 (5.21–9.57) |
| Diazepam (1 mg kg−1) | Vehicle | 17.69 (12.71–32.30)## |
| AC-5216 (0.01 mg kg−1) | Vehicle | 16.55 (10.86–23.35)## |
| AC-5216 (0.01 mg kg−1) | PK11195 (0.1 mg kg−1) | 10.28 (7.98–14.76) |
| AC-5216 (0.01 mg kg−1) | PK11195 (1 mg kg−1) | 8.70 (4.48–11.80)** |
| AC-5216 (0.01 mg kg−1) | PK11195 (10 mg kg−1) | 7.25 (4.41–12.31)** |
| Vehicle | Vehicle | 8.35 (4.67–11.54) |
| Diazepam (1 mg kg−1) | Vehicle | 24.06 (16.88–36.33)## |
| Diazepam (1 mg kg−1) | PK11195 (0.1 mg kg−1) | 18.74 (13.78–25.62) |
| Diazepam (1 mg kg−1) | PK11195 (1 mg kg−1) | 17.36 (11.14–20.35) |
| Diazepam (1 mg kg−1) | PK11195 (10 mg kg−1) | 17.91 (14.66–27.98) |
| Effect of flumazenil | ||
| Vehicle | Vehicle | 6.85 (5.28–10.60) |
| Diazepam (1 mg kg−1) | Vehicle | 26.67 (11.38–34.11)## |
| AC-5216 (0.01 mg kg−1) | Vehicle | 19.34 (7.44–24.12)## |
| AC-5216 (0.01 mg kg−1) | Flumazenil (3 mg kg−1) | 13.25 (9.07–37.23) |
| AC-5216 (0.01 mg kg−1) | Flumazenil (10 mg kg−1) | 14.29 (8.54–26.58) |
| AC-5216 (0.01 mg kg−1) | Flumazenil (30 mg kg−1) | 15.99 (10.06–32.88) |
| Vehicle | Vehicle | 9.30 (7.22–14.37) |
| Diazepam (1 mg kg−1) | Vehicle | 18.80 (11.55–39.04)## |
| Diazepam (1 mg kg−1) | Flumazenil (3 mg kg−1) | 15.66 (10.55–23.45) |
| Diazepam (1 mg kg−1) | Flumazenil (10 mg kg−1) | 13.73 (10.64–23.16) |
| Diazepam (1 mg kg−1) | Flumazenil (30 mg kg−1) | 10.63 (5.75–20.89)* |
AC-5216 and diazepam were administered orally 1 h before testing, and PK11195 and flumazenil were administered i.p. 30 min before testing.
Median (25–75% values). n=20.
P<0.01, significantly different from the respective vehicle control group (Wilcoxon's rank-sum test).
P<0.05, significantly different from the diazepam alone-treated group
P<0.01, significantly different from the AC-5216 alone-treated group (nonparametric Dunnett's multiple comparison test).
Social interaction test in mice
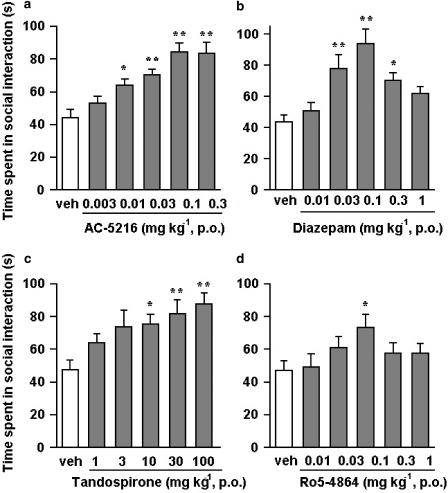
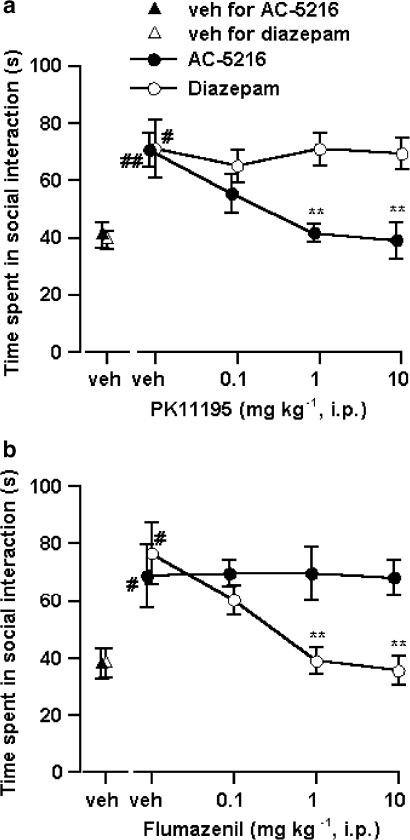
The anti-anxiety activity of test compounds in this test was indicated by an increase in the time that mice spent in social interaction under bright light unfamiliar test conditions. AC-5216, at oral doses of 0.01–0.3 mg kg−1, significantly increased the time spent in social interaction with maximum effect at 0.1 mg kg−1 (Figure 5a). Diazepam (0.03–0.3 mg kg−1, p.o.) also increased the time spent in social interaction with maximum effect at 0.1 mg kg−1, and its effect decreased at 1 mg kg−1 (Figure 5b). However, in an additional experiment carried out to examine the duration of the anti-anxiety effects of AC-5216 and diazepam in separate groups of mice (n=10 pairs per group), the anti-anxiety effect of AC-5216 lasted longer than that of diazepam; that is, AC-5216, given orally at the dose that produced maximum effect in a dose–response study (0.1 mg kg−1), produced a significant increase in the time spent in social interaction at 0.5, 1, 2 and 4 h after administration, but not at 6 h. Diazepam at 0.1 mg kg−1 also produced a significant increase in the time spent in social interaction at 1 and 2 h after its administration, but had no effect at 0.5, 4 or 6 h after its administration. Both tandospirone and Ro5-4864, a potent MBR ligand, produced a significant increase in the time spent in social interaction at oral doses of 10–100 and 0.1 mg kg−1, respectively (Figure 5c and d). However, the effect of Ro5-4864 decreased with higher doses. The anti-anxiety effect induced by AC-5216 (0.1 mg kg−1) was completely antagonized by PK11195 (1 and 10 mg kg−1, i.p.), but not by flumazenil even at 10 mg kg−1 (i.p.) (Figure 6), whereas that induced by diazepam (0.1 mg kg−1) was antagonized by flumazenil (1 and 10 mg kg−1, i.p.) but not by PK11195 even at 10 mg kg−1 (i.p.) (Figure 6). Neither PK11195 nor flumazenil, when administered alone at the doses indicated, affected the time spent in social interaction.
Figure 5.
Effects of AC-5216 (a), diazepam (b), tandospirone (c) and Ro5-4864 (d) on the time spent in social interaction under bright-light unfamiliar test conditions in mice. Each column represents the mean±s.e.m. of the time spent in social interaction during a 15-min period (n=10 pairs). Test compounds were administered orally 1 h before testing. *P<0.05, **P<0.01, significantly different from the respective vehicle control group (Dunnett's multiple comparison test).
Figure 6.
Antagonism by PK11195 (a) and flumazenil (b) of the anti-anxiety effects of AC-5216 and diazepam in the social interaction test in mice. Each symbol represents the mean±s.e.m. of the time spent in social interaction during a 15-min period (n=5 pairs). AC-5216 (0.1 mg kg−1) and diazepam (0.1 mg kg−1) were administered orally 1 h before testing. PK11195 and flumazenil were administered i.p. 20 min before testing. #P<0.05, ##P<0.01, significantly different from the respective vehicle control group (Student's t-test). **P<0.01, significantly different from the AC-5216 alone- or diazepam alone-treated group (Dunnett's multiple comparison test).
Antidepressant-like effects
AC-5216 (3–30 mg kg−1) significantly decreased the amount of time the rats spent being immobile (immobility time) in the forced swimming test in rats (Table 3). Desipramine, a positive control drug, also induced a significant decrease in the immobility time at a dose of 30 mg kg−1, p.o. The AC-5216 (10 mg kg−1)-induced decrease in immobility time was antagonized by PK11195 (3 mg kg−1, i.p.), which itself had no effect on the immobility time (Table 4).
Table 3.
Effects of AC-5216 and desipramine on duration of immobility in the forced swimming test in rats
| Compound | Dose (mg kg−1, p.o.) | Immobility time (s) |
|---|---|---|
| Vehicle | 215.8±10.8 | |
| Desipramine | 30 | 169.8±6.7## |
| AC-5216 | 1 | 197.2±7.0 |
| 3 | 173.7±9.7* | |
| 10 | 156.3±10.4** | |
| 30 | 142.8±15.9** |
Test compounds were administered orally 1 h before testing. Data are expressed as mean±s.e.m. (n=6).
P<0.01, significantly different from the vehicle control group (Student's t-test).
P<0.05
P<0.01, significantly different from the vehicle control group (Dunnett's multiple comparison test).
Table 4.
Effect of PK11195 on the antidepressant-like effect of AC-5216 in the forced swimming test in rats
| Treatment | Immobility time (s) | |
|---|---|---|
| p.o. | i.p. | |
| Vehicle | Vehicle | 222.8±8.8 |
| Desipramine (30 mg kg−1) | Vehicle | 171.5±7.0## |
| AC-5216 (10 mg kg−1) | Vehicle | 152.3±13.8## |
| AC-5216 (10 mg kg−1) | PK11195 (3 mg kg−1) | 219.0±9.7** |
| Vehicle | PK11195 (3 mg kg−1) | 212.5±5.3 |
AC-5216 and desipramine were administered orally 1 h before testing, and PK11195 was administered i.p. 30 min before testing. Data represent the mean±s.e.m. (n=6).
P<0.01, significantly different from the vehicle control group
P<0.01, significantly different from the AC-5216 alone-treated group (Student's t-test). There was no significant difference between the PK11195 alone-treated group and vehicle control group (Student's t-test).
Profile of undesirable effects
Traction test in mice
The muscle-relaxing effects of test compounds were assessed in mice by studying their effect in the traction test. Neither vehicle nor AC-5216 at any of the doses tested (1, 10, 100 and 1000 mg kg−1, p.o., n=10 per group) impaired the traction performance of the mice at any of the time points examined (0.5, 1 and 2 h after drug administration). In contrast, diazepam caused traction impairment in a number of mice; the results, expressed as ‘muscle-relaxation'/number of mice tested, were: 2/10 (0.5 h), 2/10 (1 h) and 0/10 (2 h) at 1 mg kg−1, p.o.; 8/10 (0.5 h, P<0.01 vs vehicle control, Fisher's exact probability test), 7/10 (1 h, P<0.01) and 3/10 (2 h) at 2 mg kg−1, p.o.; 10/10 (0.5 h, P<0.01), 10/10 (1 h, P<0.01) and 9/10 (2 h, P<0.01) at 5 mg kg−1, p.o. Tandospirone also induced traction impairment at oral doses of 100 and 300 mg kg−1, but not at 30 mg kg−1 in a number of mice; ‘muscle-relaxation'/number of mice tested being 2/10 (0.5 h), 3/10 (1 h) and 0/10 (2 h) at 100 mg kg−1, p.o.; 7/10 (0.5 h, P<0.01), 5/10 (1 h, P<0.05) and 5/10 (2 h, P<0.05) at 300 mg kg−1, p.o.
Passive avoidance test in mice
Since BZDs induce anterograde amnesia, test compounds were administered to mice before the training session in which they acquired a passive avoidance response. The retention test was carried out 24 h after the training session. AC-5216 and tandospirone induced no significant decrease in the step-down latency in the retention test at any of the doses tested (Table 5). Diazepam, on the other hand, significantly decreased the step-down latency in the retention test at oral doses of 3 and 10 mg kg−1, p.o.
Table 5.
Effects of AC-5216, diazepam and tandospirone on passive avoidance response in mice
| Compound | Dose (mg kg−1, p.o.) | Step-down latency (s)a in 24-h retention test |
|---|---|---|
| Vehicle | 200 (150–300) | |
| AC-5216 | 1 | 247 (116–300) |
| 10 | 230 (175–267) | |
| 100 | 274 (189–300) | |
| 1000 | 215 (189–300) | |
| Vehicle | 262 (223–300) | |
| Diazepam | 0.3 | 233 (171–293) |
| 1 | 223 (98–267) | |
| 3 | 120 (41–192)* | |
| 10 | 21 (8–57)** | |
| Vehicle | 273 (231–300) | |
| Tandospirone | 3 | 208 (120–281) |
| 10 | 266 (209–300) | |
| 30 | 300 (185–300) | |
| 100 | 223 (97–283) | |
| 300 | 150 (109–297) |
Test compounds were administered orally 1 h before training session.
Median (25–75% values) (n=12).
P<0.05
P<0.01, significantly different from the respective vehicle control group (nonparametric Dunnett's multiple comparison test).
Hexobarbitone-induced sleep in mice
The interaction between the test compounds and barbiturates was assessed by measuring the sleeping time induced by hexobarbitone in mice. AC-5216, at all of the doses tested (1–1000 mg kg−1, p.o.), had no effect on hexobarbitone-induced sleeping time (Table 6). In contrast, both diazepam (1 and 3 mg kg−1, p.o.) and tandospirone (300 mg kg−1, p.o.) significantly prolonged the sleeping time induced by hexobarbitone in mice.
Table 6.
Effects of AC-5216, diazepam and tandospirone on hexobarbitone- and ethanol-induced sleep in mice
| Compound | Dose (mg kg−1, p.o.) | Sleeping time (min) | |
|---|---|---|---|
| Hexobarbitone | Ethanol | ||
| Vehicle | 36.9±3.4 | 38.9±4.0 | |
| AC-5216 | 1 | 32.5±4.1 | 59.8±8.1 |
| 10 | 32.1±3.3 | 48.5±5.6 | |
| 100 | 39.1±2.3 | 48.8±7.7 | |
| 1000 | 36.1±3.1 | 70.2±9.1* | |
| Vehicle | 34.1±3.1 | 52.8±10.9 | |
| Diazepam | 0.1 | 33.5±3.6 | 80.1±9.7 |
| 0.3 | 41.9±3.0 | 75.8±12.5 | |
| 1 | 53.4±4.4* | 135.0±11.1** | |
| 3 | 73.4±7.6** | 223.5±20.1** | |
| Vehicle | 35.1±2.4 | 42.8±6.7 | |
| Tandospirone | 10 | 31.2±3.3 | 43.3±7.6 |
| 30 | 35.3±2.1 | 46.4±7.8 | |
| 100 | 42.6±2.4 | 64.6±10.3 | |
| 300 | 67.7±7.6** | 108.0±13.6** | |
Test compounds were administered orally 1 h before i.p. injection of hexobarbitone (80 mg kg−1) or ethanol (4.2 g kg−1). Data represent the mean±s.e.m. (n=10).
P<0.05
P<0.01, significantly different from the respective vehicle control group (nonparametric Dunnett's multiple comparison test).
Ethanol-induced sleep in mice
As with hexobarbitone, the interaction between the test compounds with ethanol was also assessed by measuring the sleeping time induced by ethanol in mice. AC-5216, at oral doses of 1–100 mg kg−1, had no effect on ethanol-induced sleep, but at the highest dose used (1000 mg kg−1, p.o.) it increased the sleeping time (Table 6). In contrast, both diazepam (1 and 3 mg kg−1, p.o.) and tandospirone (300 mg kg−1, p.o.) significantly increased ethanol-induced sleeping time in mice. However, it should be noted that the AC-5216 (1000 mg kg−1, p.o.)-induced increase in sleeping time was much shorter than that induced by diazepam or tandospirone.
Effect on EEG in rats
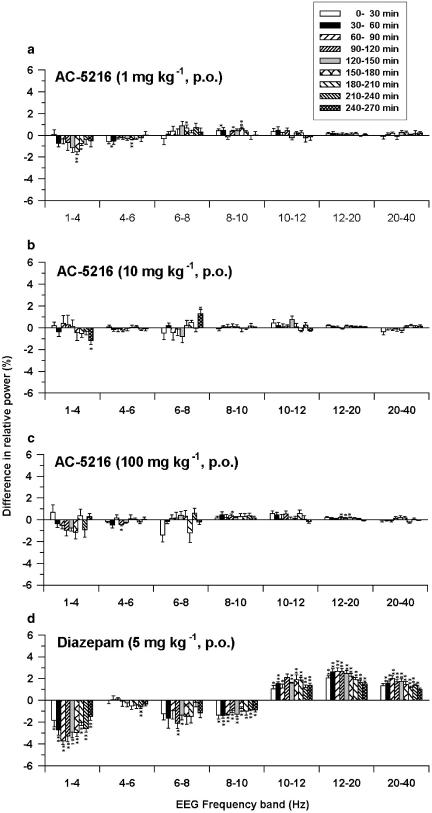
To investigate the possible effect of AC-5216 on the general function of the CNS, the ability of this compound to alter the EEG was evaluated in freely moving rats. AC-5216, at doses of 1, 10 and 100 mg kg−1, p.o., slightly changed the EEG power density distribution. However, these changes were not dose-related, frequency-specific or time-dependent (Figure 7a–c). Diazepam, at an oral dose of 5 mg kg−1, decreased the power of the low frequencies (below 10 Hz) and increased the power of the high frequencies (above 10 Hz) (Figure 7d).
Figure 7.
Effects of AC-5216 (a–c) and diazepam (d) on EEG power density distribution in rats. Each column represents the mean±s.e.m. of the difference in relative power between the drug session and the vehicle session during a 30-min period (n=6). *P<0.05, **P<0.01, significantly different from the vehicle session (paired t-test).
Discussion
In the present study, we evaluated the pharmacological profile of AC-5216, an 8-oxopurine derivative, in both receptor binding and behavioural studies. Our results demonstrate that AC-5216 has a potent and selective affinity for the MBR and can produce anti-anxiety and antidepressant-like effects in animal models, without causing the undesirable effects associated with conventional BZDs.
In the in vitro binding study, AC-5216 showed high affinity for the MBR from rat whole brain with a subnanomolar Ki value, indicating that it is comparable to or more potent than the other MBR ligands tested. AC-5216 bound to both the human glial MBR and the rat glial MBR with similar affinity, indicating that its binding properties do not differ between species. MBR is known to be a heterotrimeric complex of at least three different subunits, and some MBR ligands exhibit species differences in their binding affinity. For instance, it has been reported that PK11195 consistently has a potent affinity for MBRs from a variety of species, whereas Ro5-4864 exhibits much lower affinity for the human MBR than that for rodent MBRs (Gavish et al., 1999). In line with these findings, PK11195, in the present study, showed a similar affinity for both rat and human MBRs. It is possible that the binding site of AC-5216 in the MBR may be closer to that of PK11195 rather than that of Ro5-4864. AC-5216 had only negligible affinity for both type I and type II CBR. Diazepam, on the other hand, showed similar affinity for both the MBR and CBR. AC-5216 was also found not to exhibit any noticeable binding to a large number of other receptors, monoamine transporters or ion channels. These results demonstrate that AC-5216 has a high affinity and selectivity for the MBR.
Our results from behavioural studies demonstrate that AC-5216 as well as diazepam induce anti-anxiety effects in one conditioned model of anxiety (Vogel-type conflict test) and two unconditioned models of anxiety (light/dark box and social interaction tests). In the Vogel-type conflict test, AC-5216 produced its anti-conflict effects at doses lower than those of diazepam, although AC-5216 did not exhibit as steep a dose–response curve as that seen with diazepam. However, tandospirone (3–30 mg kg−1, p.o.) had no significant effect in this model, in contrast with results from a previous study that showed this drug at doses of 20–40 mg kg−1 (p.o.) produces anti-conflict effects comparable to those of diazepam (Shimizu et al., 1987). This discrepancy may be due to differences in the methods used, such as the presence or absence of electric shocks during the pre-test session. In the light/dark box test, AC-5216 at doses much lower than those of diazepam produced anti-anxiety effects with a bell-shaped dose–response curve, although the maximum effect of AC-5216 was less than that of diazepam. The modest activity of AC-5216 in this test may be explained by differences in effects on motor activity; this test is based on exploratory behaviour and we have previously found that AC-5216, unlike diazepam, does not increase spontaneous locomotor activity in the same strain of mice as that used in the present study (data not shown). Tandospirone also produced anti-anxiety effects in the light/dark box test although its maximum effect was much less than that of diazepam. In the social interaction test, AC-5216 showed anti-anxiety effects comparable to those of diazepam, with the duration longer than that of diazepam at the same dose. Ro5-4864, which is known to be a potent MBR ligand, induced anti-anxiety effects only at one dose (0.1 mg kg−1, p.o.). Ro5-4864 has been reported to produce anxiety effects that are not mediated by the MBR (Pellow & File, 1984) and later found to produce anti-anxiety effects mediated by the MBR at relatively low doses (Reddy & Kulkarni, 1996). These contrasting effects of Ro5-4864 may be the cause of the narrow range of effective doses observed in the present study. A comparison of the effective doses of each compound in the two anxiety models in mice revealed differences between the anti-anxiety effects of each agent used in the present study. The effective doses of AC-5216 in the social interaction test were higher than those in the light/dark box test, whereas the order was reversed with diazepam. Tandospirone produced anti-anxiety effects at the same dose level in both tests. Since anxiety is thought to be mediated by different neuronal pathways in each model, the different profile for AC-5216 compared to that of diazepam or tandospirone, as described above, may be due to differences in the mechanism of action between AC-5216 and each of the other two agents. In all the three anxiety models used in this study, the AC-5216-induced anti-anxiety effects were antagonized by the MBR antagonist PK11195, but not by the CBR antagonist flumazenil. These results indicate that the anti-anxiety effects of AC-5216, irrespective of the dose range used, are mediated by the MBR. In contrast to AC-5216, diazepam-induced anti-anxiety effects were completely antagonized by flumazenil but not by PK11195, consistent with previously reported results (Romeo et al., 1992; Przegalinski et al., 2000), even though diazepam possesses binding affinity for the MBR as well as the CBR. However, it has been reported that diazepam at 10 mg kg−1 (i.p.) produces an anti-inflammatory effect which is blocked by PK11195 in rats (Lazzarini et al., 2001). These findings together with the results of the present study suggest that the anti-anxiety effects of diazepam at the doses tested are mediated by the CBR but not by the MBR, even though diazepam can produce MBR-mediated effects in peripheral tissues. In the present study, PK11195 at doses of 0.1–10 mg kg−1 (i.p.) had no anti-anxiety effects in any of the in vivo tests used, but antagonized the effects of AC-5216, demonstrating that it is an antagonistic for MBR ligands. These results are consistent with those obtained previously, showing that PK11195 at an i.v. dose of 4.2 μmol kg−1 (ca. 1.5 mg kg−1) has no anti-anxiety effects and blocks the effects of FGIN1–27, an MBR ligand, but not those of diazepam in rats (Romeo et al., 1992). However, high doses of PK11195 (25–50 mg kg−1 i.p.) have been reported to produce anti-conflict effects in rats, the mechanism of which is not clear (Mizoule et al., 1985). In addition, a pilot study has shown that this compound, at doses of 200–400 mg daily, ameliorates anxiety in psychiatric patients (Ansseau et al., 1991). It is assumed that differences in the dose of PK11195 used is, at least in part, the reason for the conflicting results obtained in the various studies.
Depression, as well as anxiety, is thought to be often associated with stress. As MBRs have been shown to be involved in stress responses, we investigated the ability of AC-5216 to produce antidepressant-like effects in the rat forced swimming test. AC-5216 showed antidepressant-like effects with a maximum effect comparable to that of desipramine. Additionally, the antidepressant-like effects of AC-5216 were completely blocked by PK11195, indicating that AC-5216-induced antidepressant-like effects are also mediated by the MBR. In support of these findings, two MBR ligands Ro5-4864 and FGIN1-27 have been reported to produce antidepressant-like effects in the mouse forced swimming test (Khisti et al., 2000). These results, together with those of the present study, demonstrate that MBR ligands have the potential to produce antidepressant-like effects based on a novel mechanism distinct from existing antidepressants.
Other MBR ligands such as FGIN1–27 and CB 34 have been reported to produce anti-anxiety effects by facilitating the synthesis of neurosteroids (Romeo et al., 1993; Serra et al., 1999). In addition, some neurosteroids have been found to produce anti-anxiety effects in several models of anxiety (e.g., Romeo et al., 1993) and induce antidepressant-like effects in the forced swimming test in mice (Khisti et al., 2000); these effects were observed within 30 min after their administration. These findings suggest that newly synthesized neurosteroids are involved in the anti-anxiety effects of AC-5216. However, further studies are needed to determine the level of endogenous neurosteroids after administration of AC-5216, in order to substantiate this hypothesis.
BZDs cause several adverse effects through their action on the CBR (Möhler et al., 2002). In the present study, diazepam produced myorelaxant effects in the traction test, memory impairment in the passive avoidance test and prolonged hexobarbitone- and ethanol-induced sleep, at doses (1–10 mg kg−1, p.o.) close to those that produced anti-anxiety effects. Tandospirone also induced myorelaxant effects and potentiated the hexobarbitone- and ethanol-induced sleep at 300 mg kg−1, a dose that is 10–30 times higher than those used to induce anti-anxiety effects in the light/dark box and social interaction tests. In contrast, AC-5216 did not induce myorelaxant effects, memory impairment or potentiation of hexobarbitone-induced sleep even at doses as high as 1000 mg kg−1 (p.o.). Although AC-5216 significantly prolonged ethanol-induced sleep at the highest dose used (1000 mg kg−1, p.o.), the extent of this effect was much less than that induced by diazepam. In the frequency analysis of rat EEG, diazepam decreased the spectral power below 10 Hz and increased that above 10 Hz at 5 mg kg−1, a dose that produced anti-conflict effects in rats. AC-5216, on the other hand, did not produce dose-dependent or time-dependent changes in rat EEG patterns at doses of 1–100 mg kg−1, doses effective in the anxiety and depression models.
As discussed above, it is possible that endogenous neurosteroids are involved in the pharmacological effects of MBR ligands including AC-5216. Neurosteroids that positively modulate the function of the GABAA receptor such as allopregnanolone and pregnanolone have been found, in rodents, to induce muscle relaxation and to have a reduced propensity to interact with ethanol as compared with BZDs (Vanover et al., 1999). However, unlike exogenously administered neurosteroids, AC-5216 did not produce these undesirable effects. One possibility for this difference is that the change in endogenous neurosteroid levels induced by MBR ligands is small compared with that after their exogenous administration. Another possibility is that various neurosteroids, which have multiple, occasionally opposite, actions, interfere with one another, preventing their excessive actions. Although the exact reason remains to be elucidated, the results of the present study suggest that AC-5216, even when used across a wide dose range, induces neither inhibition nor excitation of general CNS functions, and that AC-5216 has few side effects due to its high selectivity for the MBR.
In conclusion, the present study demonstrates that AC-5216 is a potent and highly selective ligand for the MBR, and possesses potent oral anti-anxiety and antidepressant activity, without causing BZD-like undesirable effects. These results suggest that AC-5216 has the potential to be a therapeutic drug for the treatment of stress-related disorders including anxiety and depression.
Acknowledgments
We thank Teruya Murata, Kaoru Masumoto, Katsunori Kondoh and Dr Kazunori Ono for the chemical synthesis of AC-5216 and reference compounds.
Abbreviations
- AC-5216
N-benzyl-N-ethyl-2-(7,8-dihydro-7-methyl-8-oxo-2-phenyl-9H-purin-9-yl)acetamide
- BZD
benzodiazepine
- CB 34
N,N-dipropyl-2-(4-chlorophenyl)-6,8-dichloro-imidazo[1,2-a]pyridine-3-acetamide
- CBR
central benzodiazepine receptor
- DAA1097
N-(4-chloro-2-phenoxyphenyl)-N-(2-isopropoxybenzyl)acetamide
- DAA1106
N-(2,5-dimethoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl)acetamide
- EEG
electroencephalogram
- FGIN1–27
N,N-di-n-hexyl-2-(4-fluorophenyl)indole-3-acetamide
- MBR
mitochondrial benzodiazepine receptor
- PK11195
1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide
- Ro5-4864
4′-chlorodiazepam
- SSR180575
7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide
References
- ANHOLT R.R., MURPHY K.M., MACK G.E., SNYDER S.H. Peripheral-type benzodiazepine receptors in the central nervous system: localization to olfactory nerves. J. Neurosci. 1984;4:593–603. doi: 10.1523/JNEUROSCI.04-02-00593.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANHOLT R.R., PEDERSEN P.L., DE SOUZA E.B., SNYDER S.H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J. Biol. Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- ANSSEAU M., VON FRENCKELL R., CERFONTAINE J.L., PAPART P. Pilot study of PK 11195, a selective ligand for the peripheral-type benzodiazepine binding sites, in inpatients with anxious or depressive symptomatology. Pharmacopsychiatry. 1991;24:8–12. doi: 10.1055/s-2007-1014425. [DOI] [PubMed] [Google Scholar]
- BASILE A.S., SKOLNICK P. Subcellular localization of ‘peripheral-type' binding sites for benzodiazepines in rat brain. J. Neurochem. 1986;46:305–308. doi: 10.1111/j.1471-4159.1986.tb12965.x. [DOI] [PubMed] [Google Scholar]
- BEURDELEY-THOMAS A., MICCOLI L., OUDARD S., DUTRILLAUX B., POUPON M.F. The peripheral benzodiazepine receptors: a review. J. Neurooncol. 2000;46:45–56. doi: 10.1023/a:1006456715525. [DOI] [PubMed] [Google Scholar]
- BITRAN D., HILVERS R.J., KELLOGG C.K. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- CASELLAS P., GALIEGUE S., BASILE A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- CHELLI B., FALLENI A., SALVETTI F., GREMIGNI V., LUCACCHINI A., MARTINI C. Peripheral-type benzodiazepine receptor ligands: mitochondrial permeability transition induction in rat cardiac tissue. Biochem. Pharmacol. 2001;61:695–705. doi: 10.1016/s0006-2952(00)00588-8. [DOI] [PubMed] [Google Scholar]
- COMPAGNONE N.A., MELLON S.H. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- COSTALL B., JONES B.J., KELLY M.E., NAYLOR R.J., TOMKINS D.M. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- DOBLE A., MALGOURIS C., DANIEL M., DANIEL N., IMBAULT F., BASBAUM A., UZAN A., GUEREMY C., LE FUR G. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK11195: anatomical and subcellular distribution. Brain Res. Bull. 1987;18:49–61. doi: 10.1016/0361-9230(87)90033-5. [DOI] [PubMed] [Google Scholar]
- FERZAZ B., BRAULT E., BOURLIAUD G., ROBERT J.P., POUGHON G., CLAUSTRE Y., MARGUET F., LIERE P., SCHUMACHER M., NOWICKI J.P., FOURNIER J., MARABOUT B., SEVRIN M., GEORGE P., SOUBRIE P., BENAVIDES J., SCATTON B. SSR180575 (7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide), a peripheral benzodiazepine receptor ligand, promotes neuronal survival and repair. J. Pharmacol. Exp. Ther. 2002;301:1067–1078. doi: 10.1124/jpet.301.3.1067. [DOI] [PubMed] [Google Scholar]
- FILE S.E. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- FRANCES H. New animal model of social behavioral deficit: reversal by drugs. Pharmacol. Biochem. Behav. 1988;29:467–470. doi: 10.1016/0091-3057(88)90005-6. [DOI] [PubMed] [Google Scholar]
- GALLAGER D.W., MALLORGA P., OERTEL W., HENNEBERRY R., TALLMAN J. [3H]Diazepam binding in mammalian central nervous system: a pharmacological characterization. J. Neurosci. 1981;1:218–225. doi: 10.1523/JNEUROSCI.01-02-00218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVISH M., BACHMAN I., SHOUKRUN R., KATZ Y., VEENMAN L., WEISINGER G., WEIZMAN A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- GUARNERI P., PAPADOPOULOS V., PAN B., COSTA E. Regulation of pregnenolone synthesis in C6-2B glioma cells by 4'-chlorodiazepam. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5118–5122. doi: 10.1073/pnas.89.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHISTI R.T., CHOPDE C.T., JAIN S.P. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- KORNEYEV A., PAN B.S., POLO A., ROMEO E., GUIDOTTI A., COSTA E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J. Neurochem. 1993;61:1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- KRUEGER K.E., PAPADOPOULOS V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J. Biol. Chem. 1990;265:15015–15022. [PubMed] [Google Scholar]
- KULKARNI S.K., REDDY D.S. Neurosteroids: a new class of neuromodulators. Drugs Today. 1995;31:433–455. [Google Scholar]
- LAZZARINI R., MALUCELLI B.E., PALERMO-NETO J. Reduction of acute inflammation in rats by diazepam: role of peripheral benzodiazepine receptors and corticosterone. Immunopharmacol. Immunotoxicol. 2001;23:253–265. doi: 10.1081/iph-100103864. [DOI] [PubMed] [Google Scholar]
- LE FUR G., PERRIER M.L., VAUCHER N., IMBAULT F., FLAMIER A., BENAVIDES J., UZAN A., RENAULT C., DUBROEUCQ M.C., GUEREMY C. Peripheral benzodiazepine binding sites: effect of PK11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983;32:1839–1847. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- MIZOULE J., GAUTHIER A., UZAN A., RENAULT C., DUBROEUCQ M.C., GUÉREMY C, LE FUR G. Opposite effects of two ligands for peripheral type benzodiazepine binding sites, PK 11195 and Ro5-4864, in a conflict situation in the rat. Life Sci. 1985;36:1059–1068. doi: 10.1016/0024-3205(85)90491-6. [DOI] [PubMed] [Google Scholar]
- MÖHLER H., FRITSCHY J.M., RUDOLPH U. A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- OKUYAMA S., CHAKI S., YOSHIKAWA R., OGAWA S., SUZUKI Y., OKUBO T., NAKAZATO A., NAGAMINE M., TOMISAWA K. Neuropharmacological profile of peripheral benzodiazepine receptor agonists, DAA1097 and DAA1106. Life Sci. 1999;64:1455–1464. doi: 10.1016/s0024-3205(99)00079-x. [DOI] [PubMed] [Google Scholar]
- PAPADOPOULOS V., AMRI H., BOUJRAD N., CASCIO C., CULTY M., GARNIER M., HARDWICK M., LI H., VIDIC B., BROWN A.S., REVERSA J.L., BERNASSAU J.M., DRIEU K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- PELLOW S., FILE S.E. Behavioural actions of Ro 5-4864: a peripheral-type benzodiazepine. Life Sci. 1984;35:229–240. doi: 10.1016/0024-3205(84)90106-1. [DOI] [PubMed] [Google Scholar]
- PORSOLT R.D., ANTON G., BLAVET N., JALFRE M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- PRZEGALIÑSKI E., TATARCZYÑSKA E., CHOJNACKA-WÓJCIK E. The influence of the benzodiazepine receptor antagonist flumazenil on the anxiolytic-like effects of CGP 37849 and ACPC in rats. Neuropharmacology. 2000;39:1858–1864. doi: 10.1016/s0028-3908(00)00023-x. [DOI] [PubMed] [Google Scholar]
- REDDY D.S., KULKARNI S.K. Role of GABA-A and mitochondrial diazepam binding inhibitor receptors in the anti-stress activity of neurosteroids in mice. Psychopharmacology (Berl.) 1996;128:280–292. doi: 10.1007/s002130050136. [DOI] [PubMed] [Google Scholar]
- ROCCA P., BEONI A.M., EVA C., FERRERO P., ZANALDA E., RAVIZZA L. Peripheral benzodiazepine receptor messenger RNA is decreased in lymphocytes of generalized anxiety disorder patients. Biol. Psychiatry. 1998;43:767–773. doi: 10.1016/s0006-3223(97)00279-5. [DOI] [PubMed] [Google Scholar]
- ROMEO E., AUTA J., KOZIKOWSKI A.P., MA D., PAPADOPOULOS V., PUIA G., COSTA E., GUIDOTTI A. 2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR) J. Pharmacol. Exp. Ther. 1992;262:971–978. [PubMed] [Google Scholar]
- ROMEO E., CAVALLARO S., KORNEYEV A., KOZIKOWSKI A.P., MA D., POLO A., COSTA E., GUIDOTTI A. Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamide derivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J. Pharmacol. Exp. Ther. 1993;267:462–471. [PubMed] [Google Scholar]
- SCHOEMAKER H., BOLES R.G., HORST W.D., YAMAMURA H.I. Specific high-affinity binding sites for [3H]Ro 5-4864 in rat brain and kidney. J. Pharmacol. Exp. Ther. 1983;265:61–69. [PubMed] [Google Scholar]
- SCHOEMAKER H., MORELLI M., DESHMUKH P., YAMAMURA H.I. [3H]Ro5-4864 benzodiazepine binding in the kainate lesioned striatum and Huntington's diseased basal ganglia. Brain Res. 1982;248:396–401. doi: 10.1016/0006-8993(82)90602-3. [DOI] [PubMed] [Google Scholar]
- SERRA M., MADAU P., CHESSA M.F., CADDEO M., SANNA E., TRAPANI G., FRANCO M., LISO G., PURDY R.H., BARBACCIA M.L., BIGGIO G. 2-Phenyl-imidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br. J. Pharmacol. 1999;127:177–187. doi: 10.1038/sj.bjp.0702530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU H., HIROSE A., TATSUNO T., NAKAMURA M., KATSUBE J. Pharmacological properties of SM-3997: a new anxioselective anxiolytic candidate. Jpn. J. Pharmacol. 1987;45:493–500. doi: 10.1254/jjp.45.493. [DOI] [PubMed] [Google Scholar]
- VANOVER K.E., SURUKI M., ROBLEDO S., HUBER M., WIELAND S., LAN N.C., GEE K.W., WOOD P.L., CARTER R.B. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology (Berl.) 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- VOGEL J.R., BEER B., CLODY D.E. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- WEIZMAN R., GAVISH M. Molecular cellular and behavioral aspects of peripheral-type benzodiazepine receptors. Clin. Neuropharmacol. 1993;16:401–417. doi: 10.1097/00002826-199310000-00003. [DOI] [PubMed] [Google Scholar]