Abstract
Previous studies showed that nicotine induces adrenergic nerve-dependent vasodilation that is mediated by endogenous calcitonin gene-related peptide (CGRP) released from CGRP-containing (CGRPergic) nerves. The mechanisms underlying the nicotine-induced vasodilation were further studied.
Rat mesenteric vascular beds without endothelium were contracted by perfusion with Krebs solution containing methoxamine, and the perfusion pressure was measured with a pressure transducer.
Perfusion of nicotine (1–100 μM) for 1 min caused concentration-dependent vasodilation. Capsazepine (vanilloid receptor-1 antagonist; 1–10 μM) and ruthenium red (inhibitor of vanilloid response; 1–30 μM) concentration-dependently inhibited the nicotine-induced vasodilation without affecting the vasodilator response to exogenous CGRP.
Nicotine-induced vasodilation was not inhibited by treatment with 3,4-dihydroxyphenylalanine (DOPA) receptor antagonist (L-DOPA cyclohexyl ester; 0.001–10 μM), dopamine D1 receptor-selective antagonist (SCH23390; 1–10 μM), dopamine D2 receptor antagonist (haloperidol; 0.1–0.5 μM), ATP P2x receptor-desensitizing agonist (α,β-methylene ATP; 1–10 μM), adenosine A2 receptor antagonist (8(p-sulfophenyl)theophylline; 10–50 μM) or neuropeptide Y (NPY)-Y1 receptor antagonist (BIBP3226; 0.1–0.5 μM).
Immunohistochemical staining of the mesenteric artery showed dense innervation of CGRP- and vanilloid receptor-1-positive nerves, with both immunostainings appearing in the same neuron. The mesenteric artery was also densely innervated by NPY-positive nerves. Double immunostainings showed that both NPY and CGRP immunoreactivities appeared in the same neuron of the artery.
These results suggest that nicotine acts on presynaptic nicotinic receptors to release adrenergic neurotransmitter(s) or related substance(s), which then stimulate vanilloid receptor-1 on CGRPergic nerves, resulting in CGRP release and vasodilation.
Keywords: Nicotine, vasodilation, calcitonin gene-related peptide-containing nerves, nicotinic receptor, vanilloid receptor-1, adrenergic nerves, rat mesenteric resistance artery
Introduction
It is widely accepted that peripheral vascular tone is mainly regulated by vascular adrenergic nerves from which noradrenaline, neuropeptide Y (NPY) and ATP are released when these nerves are stimulated (Lundberg et al., 1982). However, recent studies have revealed that the mesenteric resistance arteries are innervated not only by adrenergic nerves but also by nonadrenergic noncholinergic nerves (Bevan & Brayden, 1987; Kawasaki et al., 1988). Previously, we reported that periarterial nerve stimulation (PNS) in rat mesenteric resistance arteries produces nonadrenergic, noncholinergic vasodilation, which is sensitive to tetrodotoxin, a neurotoxin, and to capsaicin, a depletor of neuropeptides in primary sensory nerves (Kawasaki et al., 1988). In addition, PNS-induced vasodilation is inhibited by treatment with the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP[8–37] (Kawasaki et al., 1991). Therefore, neurogenic vasodilation in the rat mesenteric artery is mediated by nonadrenergic, noncholinergic nerves in which CGRP, a potent vasodilator neuropeptide, acts as a neurotransmitter (Kawasaki et al., 1988).
CGRP-containing vasodilator nerves (CGRPergic nerves) have been shown to be activated by various endogenous substances such as acetylcholine (ACh) (Takenaga et al., 1995; Shiraki et al., 2001), prostaglandins (Hua et al., 1994), bradykinin (Nawa et al., 2001) and anandamide, a cannabinoid receptor ligand (Zygmunt et al., 1999). Previously, we reported that ACh produces endothelium-independent neurogenic vasodilation in rat mesenteric resistance arteries by acting on the putative muscarinic receptor located on perivascular CGRPergic nerves causing the release of CGRP (Takenaga et al., 1995). Recently, we reported that ACh induces endothelium-independent vasodilation, which is dependent on intact adrenergic nerves and is mediated by endogenous CGRP released from CGRPergic nerves (Shiraki et al., 2001). Additionally, a previous study showed that nicotine induces endothelium-independent vasodilation, which is also dependent on intact adrenergic nerves via nicotinic receptors present on them and is mediated by endogenous CGRP released from CGRPergic nerves (Shiraki et al., 2000). Therefore, we suggested that nicotine acts on presynaptic nicotinic receptors, causing them to release adrenergic neurotransmitters or unknown related substance(s), which activate CGRPergic nerves, resulting in CGRP release and vasodilation. Nicotine stimulates the release of catecholamines such as dopamine and noradrenaline, NPY (Haass et al., 1991) and ATP (Von Kugelgen & Starke, 1991) from adrenergic nerve terminals. Thus, it is expected that these neurotransmitters might mediate the nicotine-induced vasodilation.
In the present study, therefore, we further investigated the possible mechanisms underlying nicotine-induced vasodilation in rat mesenteric arteries. To assess the possible involvement of receptors on CGRPergic nerves, we used the various antagonists for DOPA receptors, dopamine receptors, NPY receptors, adenosine receptors, ATP receptors and vanilloid receptors. In this report, we show evidence that vanilloid receptors located on CGRPergic nerves are involved in the nicotine-induced vasodilation.
Methods
Animals
Male Wistar rats weighing 250–350 g were used in the present study. All animals were given food and water ad libitum. They were housed in the Animal Research Center of Okayama University at a controlled ambient temperature of 22±2°C with 50±10% relative humidity and a 12-h light/12-h dark cycle (light on 08:00 h).
Perfusion of the mesenteric vascular beds
The animals were anesthetized with pentobarbital-Na (50 mg kg−1, intraperitoneally) and the mesenteric vascular beds were isolated and prepared for perfusion as described previously (Kawasaki et al., 1988; 1991). The superior mesenteric artery was cannulated and flushed gently with Krebs–Ringer bicarbonate solution (Krebs solution) to eliminate blood in the vascular bed. After removal of the entire intestine and associated vascular bed, the mesenteric vascular bed was separated from the intestine by cutting close to the intestinal wall. Only four main arterial branches from the superior mesenteric trunk running to the terminal ileum were perfused. All other branches of the superior mesenteric artery were tied off. The isolated mesenteric vascular bed was then placed in a water-jacketed organ bath maintained at 37°C and perfused with a modified (see below) Krebs solution at a constant flow rate of 5 ml min−1 with a peristaltic pump (model AC-2120, ATTO Co., Tokyo, Japan). The preparation was also superfused with the same solution at a rate of 0.5 ml min−1 to prevent drying. The Krebs solution was bubbled with a mixture of 95% O2–5% CO2 before passage through a warming coil maintained at 37°C. The modified Krebs solution had the following composition (mM): NaCl 119.0; KCl 4.7; CaCl2 2.4; MgSO4 1.2; NaHCO3 25.0; KH2PO4 1.2; disodium EDTA 0.03 and dextrose 11.1 (pH 7.4). Changes in the perfusion pressure were measured with a pressure transducer (model TP-400T, Nihon Kohden, Tokyo, Japan) and recorded using a pen recorder (model U-228, Nippon Denshi Kagaku, Tokyo, Japan).
Chemical removal of the vascular endothelium
After the basal perfusion pressure was allowed to stabilize, the preparations with resting tone were perfused with a 1.8 mg ml−1 solution of sodium deoxycholate (SD) in saline for 30 s to remove the vascular endothelium, as described by Takenaga et al. (1995) and Shiraki et al. (2000). This caused a transient increase (20–30 mmHg) in perfusion pressure. Then, the preparations were rinsed with SD-free Krebs solution for 60 min. After the preparations were contracted by perfusion with Krebs solution containing methoxamine (2 μM), chemical removal of the endothelium was assessed by the lack of a relaxant effect after a bolus injection of 1 nmol ACh, which was injected directly into the perfusate proximal to the arterial cannula with an infusion pump (model 975, Harvard Apparatus, Holliston, MA, U.S.A.). The volume injected was 100 μl over 12 s.
Perfusion of nicotine
After chemical removal of the endothelium, the basal perfusion pressure was allowed to stabilize and the preparations were perfused with Krebs solution containing methoxamine (α1-adrenoceptor agonist) at a concentration of 2 μM to induce submaximal vasoconstriction. After stabilization of the elevated perfusion pressure, the preparations were subjected to perfusion of nicotine. The nicotine was perfused for 1 min at concentrations of 1, 3, 10, 30 and 100 μM in Krebs solution containing methoxamine. To avoid tachyphylaxis, the perfusion of nicotine was carried out at 20-min intervals.
At the end of each experiment, the preparations were perfused with 100 μM papaverine to induce complete relaxation. Vasodilator activity is expressed as the percentage of perfusion pressure at the maximum relaxation induced by papaverine.
Periarterial nerve stimulation (PNS)
PNS was applied for 30 s using bipolar platinum ring electrodes placed around the superior mesenteric artery. Rectangular pulses of 1 ms and supramaximal voltage (50 V) were applied at 2 Hz using an electronic stimulator (model SEN 3301, Nihon Kohden).
Experimental protocols
To assess the underlying mechanisms involved in the nicotine-induced vasodilation and to assess the possible involvement of receptors on CGRPergic nerves, the effects of various receptor antagonists were examined. After chemical removal of the vascular endothelium by SD perfusion, Krebs solution containing 1, 10 nM or 10 μM L-3,4-dihydroxyphenylalanine (L-DOPA) cyclohexyl ester (L-DOPA CHE; competitive DOPA receptor antagonist), 1 μM SCH23390 (selective dopamine D1 receptor antagonist), 0.1 or 0.3 μM haloperidol (dopamine D2 receptor antagonist), 1 or 10 μM α,β-methylene ATP (α,β-mATP; ATP P2x receptor-desensitizing agonist), 10 or 50 μM 8(p-sulfophenyl)theophylline (8-SPT; adenosine A2 receptor antagonist), 0.1 or 0.5 μM BIBP3226 (selective NPY Y1 receptor antagonist), 1, 5 or 10 μM capsazepine (selective vanilloid receptor-1 antagonist), 1, 10 or 30 μM ruthenium red (an inhibitor of vanilloid response) and methoxamine (2–10 μM) were perfused to produce active tone in the preparations. Then, after the elevated perfusion pressure had stabilized, Krebs solution containing the final concentration of nicotine and L-DOPA CHE, SCH23390, haloperidol, α,β-mATP, 8-SPT, BIBP3226, capsazepine or ruthenium red was perfused for 1 min. In the experiments using haloperidol (0.1 and 0.3 μM) and capsazepine (10 μM), the concentration of methoxamine was increased to 10 μM since these antagonists caused vasodilation and weak active tone.
In the series of experiments using vanilloid receptor antagonists (capsazepine and ruthenium red), PNS at 2 Hz, bolus injections of 100 pmol CGRP and 1-min perfusion of capsaicin (30 nM), which caused long-lasting vasodilation, were carried out during perfusion of each antagonist.
Immunohistochemical study
Confocal laser microscopy
Under pentobarbital-Na anesthesia, the mesenteric artery with the intestine was surgically removed and the third-order branch of the artery was isolated and fixed in Zamboni's fixative containing 2% paraformaldehyde and 15% picric acid in 0.15 M phosphate buffer (PB) for 48 h. After fixation, the specimens were rinsed repeatedly in phosphate-buffered saline (PBS). Immunohistochemical studies were performed using whole mount preparations. The mesenteric artery was immersed in PBS containing 0.5% Triton X-100 overnight, followed by incubation with PBS containing normal goat serum (1 : 100) for 60 min. The tissues were then incubated with the primary antibody: anti-vanilloid receptor-1 (1 : 10,000; raised in rabbit) (Affinity Bio Regents, Golden, CO, U.S.A.) or anti-NPY (1 : 300; raised in rabbit) (Phoenix Pharmaceuticals, Inc., Belmont, CA, U.S.A.), for 72 h at 4°C. After incubation, the tissues were washed in PBS and the site of the antigen–antibody reaction was revealed by incubation with fluorescein-5-isothiocyanate (FITC)-labeled goat anti-rabbit IgG (diluted 1 : 100) (ICN Pharmaceuticals, Inc., Aurora, OH, U.S.A.) for 60 min. The tissues were thoroughly washed in PBS, mounted in glycerol/PBS 2 : 1 (v v−1) and observed under a confocal laser-scanning microscope (CLSM 510, Carl Zeiss, Germany).
Control immunohistochemical staining for vanilloid receptor-1 was done by preabsorbing the blocking peptide for primary antibody to vanilloid receptor-1 (rat) (20 μg ml−1; Alexis Biochemicals, San Diego, CL, U.S.A.) and exhausting the vanilloid receptor-1 antibody with the relevant peptides.
Double immunostainings
For the double-staining immunofluorescence study, the mesenteric arteries were incubated with PBS containing 1% normal goat serum, 1% bovine serum albumin and 0.03% Triton X-100 for 1 h. Following this, the sections were incubated with anti-vanilloid receptor-1 antibody (1 : 10,000; raised in rabbit) or anti-NPY antibody (1 : 300; raised in rabbit) (Phoenix Pharmaceuticals, Inc.) at 4°C for 2 days, followed by incubation with anti-CGRP antibody (1 : 300; raised in guinea-pig) at 4°C for 2 days. Following washes in PBS, the sections were incubated for 2 h with rhodamine-conjugated goat F(ab′)2 fraction anti-guinea-pig IgG (G+L) (diluted 1 : 100) (ICN Pharmaceuticals, Inc.) at 4°C, followed by incubation with FITC-conjugated rabbit IgG fractions (diluted 1 : 100) (ICN Pharmaceuticals, Inc.) at 4°C for 1 h. The samples were then observed under a confocal laser-scanning microscope (CLSM 510, Carl Zeiss) with an exciting filter system (458/488 nm for FITC) and emission filter system (543 nm for rhodamine). Two fluorescence views were obtained from the same microscopic field.
Statistical analysis
Experimental results are presented as mean±s.e.m. Statistical analysis was performed by the Student's unpaired t-test and one-way analysis of variance followed by Tukey's test. A P-value less than 0.05 was considered significant.
Drugs
The following drugs were used: ACh chloride (Daichi Pharmaceutical Co., Tokyo, Japan), α,β-mATP (Sigma Chemical Co., St Louis, MO, U.S.A.), BIBP3226 (Sigma), capsaicin (Sigma), capsazepine (Sigma), L-DOPA CHE (donated by Professor Goshima, Yokohama City University Medical School, Yokohama, Japan), haloperidol (Sigma), rat CGRP (Peptide Institute, Osaka, Japan), methoxamine hydrochloride (Nihon Shinyaku Co., Kyoto, Japan), nicotine tartrate salt (Sigma), papaverine hydrochloride (Sigma), ruthenium red (Sigma), SD (Ishizu Seiyaku, Tokyo, Japan), SCH23390 (Sigma) and 8-SPT (Sigma). All drugs, except for capsaicin and SD, were dissolved in pure water and diluted with Krebs solution containing 2–10 μM methoxamine. Capsaicin was dissolved in 50% ethanol and diluted with Krebs solution (final alcohol concentration, 0.4 mg ml−1). SD was dissolved in 0.9% saline.
Results
Vascular responses to perfusion of nicotine
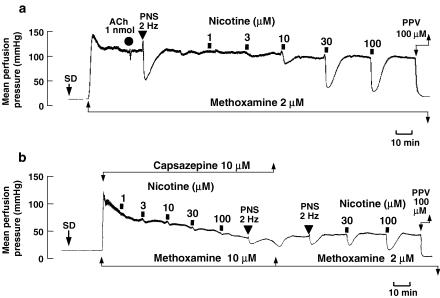
In the preparation without endothelium and with an active tone produced by methoxamine (2 μM), a bolus injection of ACh (1 nmol) did not produce a rapid drop in perfusion pressure due to endothelium-dependent vasodilation (Figure 1a). PNS (2 Hz) caused a transient pressor response followed by long-lasting depressor response (Figure 1a). In this preparation with active tone, perfusion of nicotine (1–100 μM) for 1 min concentration-dependently decreased the perfusion pressure due to vasodilation (Figure 1a). Long-lasting vasodilation induced by nicotine returned to the pre-perfusion level within 5–10 min, as shown in Figure 1a. A very slight vasoconstriction by nicotine at low concentrations of 1–10 μM preceded the vasodilation.
Figure 1.
Typical record showing vascular responses to nicotine (a) and the effect of capsazepine (b) in rat perfused mesenteric vascular beds without endothelium and with active tone produced by methoxamine (2 μM). SD, perfusion of SD for 30 s. ACh, bolus injection of ACh (1 nmol; closed circles). PNS, periarterial nerve stimulation (2 Hz; closed inverted triangles). Nicotine (1–100 μM) was perfused for 1 min at times indicated by the horizontal bars. PPV, perfusion of 100 μM papaverine.
Effects of capsazepine and ruthenium red on vasodilator response to perfusion of nicotine
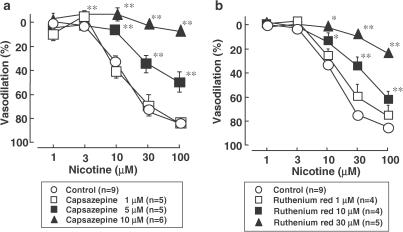
As shown in Figures 1b and 2, in the preparation without endothelium, the vanilloid receptor-1 antagonist capsazepine (1–10 μM) markedly attenuated the vasodilator response to perfusion of nicotine in a concentration-dependent manner. The inhibition was reversed by washing out with capsazepine-free Krebs solution (Figure 1b). An inhibitor of the vanilloid response, ruthenium red (1–30 μM), also concentration-dependently inhibited the nicotine-induced vasodilation (Figure 2). Neither capsazepine nor ruthenium red affected the vasodilation induced by exogenous CGRP injection (Table 1), whereas the PNS-induced vasodilation was significantly inhibited by capsazepine and ruthenium red. Both capsazepine and ruthenium red markedly inhibited the vasodilation induced by perfusion of capsaicin (30 nM) (Table 1).
Figure 2.
Line graph showing the effects of capsazepine (a) and ruthenium red (b) on the nicotine-induced vasodilation in rat perfused mesenteric vascular beds without endothelium. *P<0.05, **P<0.01, compared with control.
Table 1.
Effects of capsazepine and ruthenium red on vasodilator responses induced by periarterial nerve stimulation (PNS, 2 Hz), bolus injection of CGRP (100 pmol) and perfusion of capsaicin (30 nM) in rat mesenteric vascular beds without endothelium
| Vasodilation (%) induced by | |||
|---|---|---|---|
| PNS (2 Hz) (n) | CGRP (100 pmol) (n) | Capsaicin (30 nM) (n) | |
| Control | 80.7±4.3 (5) | 91.4±3.6 (4) | 90.4±1.1 (4) |
| Capsazepine | |||
| 1 μM | 81.2±3.2(5) | 96.1±0.7 (3) | 30.7±8.9** (3) |
| 5 μM | 63.0±5.2* (5) | 90.4±3.4 (3) | 6.9±3.6** (3) |
| 10 μM | 43.4±6.7** (6) | 85.3±2.4(5) | 2.5±0.5** (3) |
| Ruthenium red | |||
| 1 μM | 87.5±1.8(6) | 84.2±5.9(4) | 63.6±6.8** (4) |
| 10 μM | 54.1±9.2* (5) | 82.6±5.4 (5) | 0.5±4.1** (4) |
| 30 μM | 25.4±1.7** (6) | 79.2±4.8 (5) | 0.5±0.8** (4) |
(n): number of animals.
P<0.05,
P<0.01, compared with control.
Immunohistochemical study
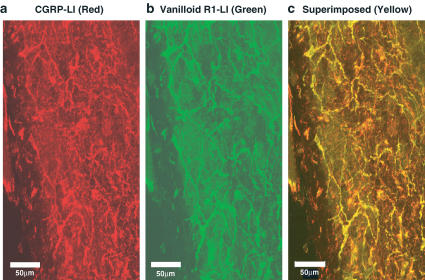
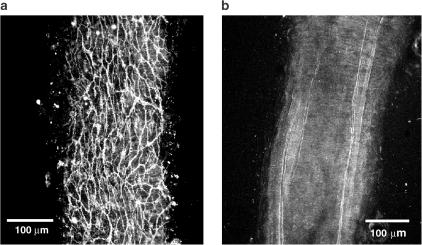
As shown in Figure 3a, the mesenteric artery was innervated by dense CGRP-like immunoreactivities (LI)-positive nerves. Also, dense innervations of the vanilloid receptor-1-positive nerves were observed in the same area of the artery (Figure 3b). As shown in double immunostainings in Figure 3c, CGRP-LI-positive nerves were colocalized with vanilloid receptor-1-LI-positive nerves. When the vanilloid receptor-1 antibody was pre-absorbed with the blocking peptide, no immunostainings were observed in the control experiment (Figure 4).
Figure 3.
Confocal laser micrograph showing CGRP (a; red)-like immunoreactive (LI)- and vanilloid receptor-1 (b; green)-LI-containing fibers in the mesenteric artery. (a) and (b) are photographs of the same area in the whole mount of the artery. (c) is a superimposed image of (a) and (b). Colocalization of CGRP-LI and vanilloid receptor-1-LI is recognized as yellow in many fibers. The scale bar in (a)–(c) indicates 50 μm.
Figure 4.
Confocal laser micrograph showing vanilloid receptor-1-like immunoreactivities (a; green) and their absence (b) after pre-absorption with the blocking peptide for primary antibody to vanilloid receptor-1 (rat) in the mesenteric artery. The scale bar in (a) and (b) indicates 100 μm.
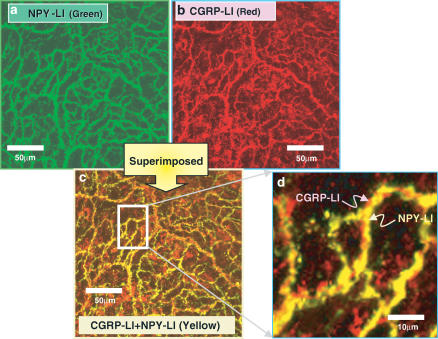
Dense innervation of NPY-LI (Figure 5a) – and CGRP-LI (Figure 5b) – positive nerves were observed in the same area of the mesenteric artery. As shown in double immunostainings in Figure 5c, some of the NPY-LI positive nerves were colocalized with CGRP-LI-positive nerves. Higher magnification (Figure 5d) showed that both CGRP and NPY immunostainings did not fuse entirely and CGRP-LI-positive nerves closely contacted with NPY-LI-positive nerves.
Figure 5.
Confocal laser photomicrograph showing NPY (a; green)-like immunoreactive (LI)- and CGRP (b; red)-LI-containing fibers in the mesenteric artery. (a) and (b) are photographs of the same area in the whole mount of the artery. (c) is a superimposed image of (a) and (b). Colocalization of CGRP-LI and NPY-1-LI is recognized as yellow in many fibers. (d) shows higher magnification of the rectangular area in (c). The scale bar in (c) and (d) indicates 50 and 10 μm, respectively.
Effects of various receptor antagonists on vasodilator response to nicotine perfusion
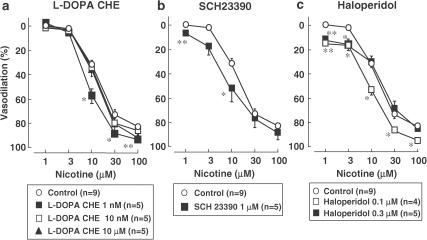
To assess the possible mechanisms underlying the vasodilator response to perfusion of nicotine, the effects of various receptor antagonists were further examined in preparations without endothelium. As shown in Figure 6, the nicotine-induced vasodilation was not inhibited by the competitive DOPA receptor antagonist, L-DOPA CHE (1, 10 or 10 μM), but a low concentration of the antagonist (1 nM) augmented the response (Figure 6a). In addition, neither a selective dopamine D1 receptor antagonist, SCH23390 (1 μM) (0.1 μM inhibited response to dopamine in human gastroepiploic vein; Okamura et al., 1991), nor a dopamine D2 receptor antagonist, haloperidol (0.1 or 0.3 μM) (0.3 μM abolished vasodilator response to dopamine in rabbit mesenteric artery; Anwar & Mason, 1980), inhibited the nicotine-induced vasodilation, but rather potentiated the nicotine-induced response.
Figure 6.
Effects of DOPA receptor antagonist (L-DOPA CHE) and dopamine D1 receptor antagonists (SCH23390) and dopamine D2 receptor antagonist (haloperidol) on nicotine-induced vasodilation in rat perfused mesenteric vascular beds without endothelium. L-DOPA CHE, L-DOPA cyclohexyl ester. *P<0.05, **P<0.01, compared with control.
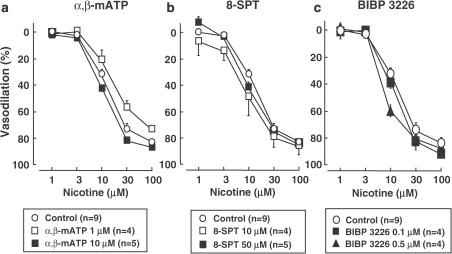
As shown in Figure 7, the nicotine-induced vasodilation was not affected by an ATP P2X receptor-desensitizing agonist, α,β-mATP (1 or 10 μM) (10 μM desensitized response to ATP in rat mesenteric arteries; Ralevic, 2001), an adenosine A2 receptor antagonist, 8-SPT (10 or 50 μM) (1 and 10 μM inhibited response to sciatic nerve stimulation; Meno et al., 2001), or an NPY Y1 receptor antagonist, BIBP3226 (0.1 or 0.5 μM) (0.1 μM inhibited vasoconstrictor response to NPY in forskolin-relaxed rat mesenteric artery; pA2=7.92±0.29; Prieto et al., 2000).
Figure 7.
Effects of ATP P2X receptor-desensitizing agent, α,β-methylene ATP (a; α,β-mATP) and adenosine receptor antagonist (b; 8-(p-sulfophenyl) theophylline; 8-SPT) and NPY Y1 receptor antagonist (c; BIBP3226) on nicotine-induced vasodilation in rat perfused mesenteric vascular beds without endothelium. *P<0.01, compared with control.
Discussion
The present study demonstrated that, in rat mesenteric arteries without endothelium and with active tone produced by methoxamine, perfusion of nicotine (1–100 μM) caused concentration-dependent vasodilation and vanilloid receptors might be involved in this vascular response. The nicotine-induced endothelium-independent vasodilation was not affected by a DOPA receptor antagonist, L-DOPA CHE (Misu et al., 1997; Furukawa et al., 2000). In addition, neither a dopamine D1 selective receptor antagonist, SCH23390, nor a dopamine D2 receptor antagonist, haloperidol, inhibited the nicotine-induced vasodilation, but rather augmented it. Although the reason for the augmentation of the effect is not clear, dopamine receptors on the presynaptic site, probably adrenergic nerves, may have an inhibitory effect on the release of unknown neurotransmitters, which mediate the nicotine-induced vasodilation, since dopamine has an inhibitory effect on adrenergic neurotransmission (Morgadinho et al., 1999; Amenta et al., 2000). In addition, the major findings in this study were that a selective vanilloid receptor-1 antagonist, capsazepine, and a functional capsaicin antagonist, ruthenium red, markedly attenuated the nicotine-induced vasodilation. These findings strongly suggest that vanilloid receptors are involved in the mechanisms underlying the nicotine-induced endothelium-independent vasodilation in rat mesenteric arteries.
We previously reported that the mesenteric resistance arteries of rats are innervated by nonadrenergic, noncholinergic vasodilator nerves, in which CGRP, a potent vasodilator neuropeptide, acts as a vasodilator neurotransmitter (Kawasaki et al., 1988). PNS of the rat mesenteric artery induces neurogenic vasodilation, which is sensitive to the neurotoxic effect of tetrodotoxin and is abolished by capsaicin (Kawasaki et al., 1991). The PNS-induced neurogenic vasodilation in the rat mesenteric arteries is also inhibited by human CGRP[8–37], a C-terminal fragment of CGRP and a CGRP receptor antagonist, suggesting that endogenous CGRP released from CGRPergic nerves produces vasodilation of the rat mesenteric artery (Kawasaki et al., 1991). Capsaicin, a vanilloid receptor agonist, has been shown to release CGRP from primary sensory neurons (Fujimori et al., 1989), which leads to depletion of capsaicin-sensitive primary nerves. Since nicotine-induced vasodilation was sensitive to the effect of capsaicin, it is very likely that the capsaicin-sensitive peptidergic nerves are responsible for the action of nicotine. Furthermore, CGRP[8–37] inhibited nicotine-induced vasodilation (Shiraki et al., 2000). Taken together, CGRPergic vasodilator nerves are likely to be involved in the vasodilation induced by nicotine.
Takenaga et al. (1995) reported that ACh caused endothelium-independent vasodilation of the rat mesenteric artery that was neurogenic in nature and mediated by endogenous CGRP when guanethidine was present to block adrenergic neurotransmission. This vasodilation was inhibited by the muscarinic receptor antagonist atropine and the CGRP receptor antagonist CGRP[8–37], but not by the nicotinic receptor antagonist hexamethonium. Therefore, it is likely that muscarinic receptors, presumably located on CGRPergic nerves, are responsible for the endothelium-independent vasodilation caused by ACh perfusion. On the other hand, in the absence of guanethidine, ACh produced neurogenic and endothelium-independent vasodilation that was resistant to atropine and sensitive to hexamethonium (Shiraki et al., 2001). In addition, the ACh-induced atropine-resistant vasodilation was inhibited not only by the adrenergic neuron blockers guanethidine and bretylium and by selective chemical destruction of the adrenergic neurons by 6-hydroxydopamine, but also by capsaicin and CGRP[8–37]. Therefore, we suggested that ACh acts on presynaptic nicotinic receptors and releases adrenergic neurotransmitters or related substances, which then activate CGRPergic nerves, causing CGRP release and vasodilation (Shiraki et al., 2001).
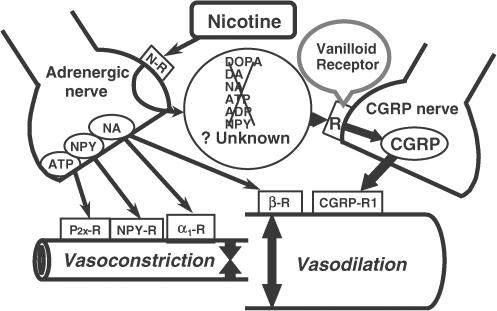
We also reported previously that the nicotine-induced endothelium-independent vasodilation was sensitive to hexamethonium and inhibited by adrenergic neuron blockers (guanethidine and bretylium) and 6-hydroxydopamine (Shiraki et al., 2000). Thus, we suggested that the nicotine-induced vasodilation requires the intact adrenergic nerve function in rat mesenteric arteries and that, in the same manner as the mechanisms of ACh-induced vasodilation, unknown neurotransmitter(s) released from adrenergic nerves by nicotine indirectly elicit vasodilation by stimulating CGRPergic nerves. Adrenergic-dependent vasodilator responses induced by nicotine have been reported in the porcine basilar artery, where the nicotine-induced vasodilation was inhibited by guanethidine or 6-hydroxydopamine-induced chemical destruction of sympathetic adrenergic nerve terminals (Zhang et al., 1998). Nicotine has been shown to stimulate the release of neurotransmitters such as noradrenaline, NPY (Haass et al., 1991) and ATP (Von Kugelgen & Starke, 1991) from adrenergic nerve terminals. Therefore, it is possible that these neurotransmitters released from adrenergic terminals by nicotine stimulate CGRPergic nerves to release CGRP, and induce neurogenic vasodilation. Diana et al. (1990) reported that noradrenaline was released by nicotine stimulation from adrenergic nerves and acted directly on postsynaptic β-adrenoceptors, causing vasodilation to occur. In addition, recent reports by Lee et al. (2000) and Si & Lee (2001) suggested the hypothesis that nicotine acts at nicotinic receptors on presynaptic adrenergic nerve terminals, causing the release of noradrenaline, which then acts on presynaptic β-adrenoceptors located on the nitric oxide (NO)-containing nerve terminals, resulting in the release of NO and dilation of porcine basilar arteries. However, it is unlikely that noradrenaline is involved in the nicotine-induced vasodilation of rat mesenteric arteries, because a β-adrenoceptor antagonist, propranolol, had no effect on the nicotine-induced vasodilation (Shiraki et al., 2000). Since an NO synthase inhibitor, Nω-nitro-L-arginine, did not affect nicotine-induced or PNS-induced vasodilation, NO is also not involved in the mechanisms underling the nicotine-induced vasodilation in rat mesenteric arteries (Shiraki et al., 2000). Noradrenaline and NPY have been reported to inhibit the CGRP release from the CGRPergic nerve terminals of rat mesenteric arteries via presynaptic α2-adrenoceptors and NPY Y2 receptors (Kawasaki et al., 1991). In addition, the present study showed that an ATP P2X receptor-desensitizing agonist, α,β-mATP, and an NPY Y1 receptor antagonist, BIBP3226, had no effect on the action of nicotine. Taken together, it is unlikely that neurally released catecholamines (DOPA, dopamine and noradrenaline), NPY or ATP is involved in the mechanisms underlying the nicotine-induced vasodilation in the rat mesenteric arteries, as shown in the working hypothesis of Figure 8.
Figure 8.
Possible mechanisms underlying adrenergic nerve-mediated and endothelium-independent vasodilation induced by nicotine in the rat mesenteric artery. N-R, α1-R and β-R indicate nicotinic receptor, α1- and β-adrenoceptor, respectively. P2x shows the ATP P2x receptor. ATP, adenosine triphosphate; ADP, adenosine diphosphate; CGRP, calcitonin gene-related peptide; DA, dopamine; DOPA, 3,4-dihydroxyphenylalanine; NA, noradrenaline; NPY, neuropeptide Y; R, receptor.
An endogenous vanilloid receptor agonist, anandamide, induced vasodilation by activating vanilloid receptor-1 on the perivascular sensory nerves, causing the release of CGRP (Zygmunt et al., 1999). Additionally, a competitive vanilloid receptor-1 antagonist, capsazepine, suppressed the anandamide-induced release of CGRP from capsaicin-sensitive nerves. In the present study, the nicotine-induced vasodilation was markedly inhibited by capsazepine in rat mesenteric arteries. In addition, ruthenium red, an inhibitor of vanilloid response (Amann & Maggi, 1991), abolished the nicotine-induced vasodilation. Since both antagonists blocked vasodilation induced by capsaicin, an agonist of vanilloid receptors, it is very likely that the nicotine-induced vasodilation is mediated by vanilloid receptor-1. In the present immunohistochemical study, CGRP-LI-positive nerves were overlaid with vanilloid receptor-1-LI-positive nerves, suggesting that vanilloid receptor-1 is present on CGRPergic nerves. In addition, the present immunohistochemical study showed that CGRP-LI-positive nerves closely contacted with NPY-LI-positive adrenergic nerves. The vanilloid receptor antagonists did not affect the vasodilation induced by exogenously applied CGRP. Therefore, it is unlikely that the blocking effect of the vanilloid receptor antagonists on the action of nicotine resulted from the inhibition of postsynaptic CGRP receptors. The vanilloid receptor-1 has been shown to be expressed exclusively in primary sensory nerves and to be activated by an endogenous substance (anandamide) and chemical (protons) and physical stimuli (low pH and heat) (Szallasi & Blumberg, 1999). Therefore, it is possible that the substance(s), as neurotransmitter(s) released from adrenergic nerves, mediates the nicotine-induced vasodilation (Figure 7). Vanilloid receptor antagonists, capsazepine and ruthenium red, concentration-dependently inhibited the PNS-induced vasodilation, which is mediated by activation of CGRPergic nerves. Since the PNS of the mesenteric artery could stimulate not only perivascular CGRPergic nerves but also adrenergic nerves, attenuation of the PNS response by vanilloid receptor antagonists may result from inhibition of the action of transmitter(s) released by adrenergic nerve stimulation. This finding suggests that adrenergic nerve stimulation releases transmitter substance(s), which activate vanilloid receptor-1 located on CGRPergic nerves. However, capsazepine has been shown to block voltage-activated calcium channels with lower concentrations than 10 μM in rat dorsal root ganglion neurons in culture (Docherty et al., 1997), suggesting that capsazepine has an ability to inhibit the PNS-induced vasodilator response. The nicotine-induced vasodilation has been shown to be insensitive to tetrodotoxin, but the field-stimulation-induced vasodilation was abolished by tetrodotoxin (Zhang et al., 1998). Therefore, it is unlikely that voltage-activated calcium channels are involved in the nicotine-induced vasodilation. This implies that the inhibitory effect of capsazepine on the nicotine-induced vasodilation is mainly due to blocking action of vanilloid receptors on CGRPergic nerves. However, further studies will be required to identify the transmitter(s) involved.
In conclusion, the present study demonstrated for the first time that vanilloid receptors, probably vanilloid receptor-1, mediate the nicotine-induced endothelium-independent vasodilation in rat mesenteric arteries. It is suggested that nicotine acts on presynaptic nicotinic receptors, resulting in the release of adrenergic neurotransmitters or related substances, which possibly activate vanilloid receptor-1 located on CGRPergic nerves and cause CGRP release and vasodilation (Figure 8).
Acknowledgments
This work was supported by a Grant from the Smoking Research Foundation and in part by a Grant-in-Aid for Scientific Research (KAKENHI) (No 13672389) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- α,β-mATP
α,β-methylene ATP
- ACh
acetylcholine
- CGRP
calcitonin gene-related peptide
- CGRP-LI
CGRP-like immunoreactivities
- FITC
fluorescein-5-isothiocyanate
- L-DOPA CHE
L-3,4-dihydroxyphenylalanine cyclohexyl ester
- NO
nitric oxide
- NPY
neuropeptide Y
- PBS
phosphate-buffered saline
- PNS
periarterial nerve stimulation
- SD
sodium deoxycholate
- 8-SPT
8(p-sulfophenyl)theophylline
References
- AMANN R., MAGGI C.A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–859. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- AMENTA F., BARILI P., BRONZETTI E., FELICI L., MIGNINI F., RICCI A. Localization of dopamine receptor subtypes in systemic arteries. Clin. Exper. Hypertens. 2000;22:277–288. doi: 10.1081/ceh-100100077. [DOI] [PubMed] [Google Scholar]
- ANWAR N, MASON D.F.J. Actions of dopamine and apomorphine on the vasoconstrictor responses of perfused mesenteric arteries of mouse, rat and rabbit. J Pharm Pharmacol. 1980;33:150–154. doi: 10.1111/j.2042-7158.1981.tb13738.x. [DOI] [PubMed] [Google Scholar]
- BEVAN J.A., BRAYDEN J.E. Nonadrenergic neural vasodilator mechanisms. Circ. Res. 1987;60:309–326. doi: 10.1161/01.res.60.3.309. [DOI] [PubMed] [Google Scholar]
- DIANA J.N., QIAN S.F., HEESCH C.M., BARRON K.W., CHIEN C.Y. Nicotine-induced skeletal muscle vasodilation is mediated by release of epinephrine from nerve terminals. Am. J. Physiol. 1990;259:H1718–H1729. doi: 10.1152/ajpheart.1990.259.6.H1718. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIMORI A., SAITO A., MIMURA S., WATANABE T., UCHIYAMA Y., KAWASAKI H., GOTO K. Neurogenic vasodilator and release of calcitonin gene-related peptide (CGRP) from perivascular nerve from the rat mesenteric artery. Biochem. Biophs. Res. Commun. 1989;165:1391–1398. doi: 10.1016/0006-291x(89)92758-7. [DOI] [PubMed] [Google Scholar]
- FURUKAWA N., GOSIMA Y., MIYAMAE T., SUGIYAMA Y., SHIMIZU M., OHSHIMA E., SUZUKI F., ARAI N., FUJITA K., MISU Y. L-DOPA cyclohexyl ester is a novel potent and relatively stable competitive antagonist against L-DOPA among several L-DOPA ester compounds. JPN. J. Pharmacol. 2000;82:40–47. doi: 10.1254/jjp.82.40. [DOI] [PubMed] [Google Scholar]
- HUA X.-Y., JINNO S., BACK M., TAM E.K., YAKSH T.L. Multiple mechanisms for the effects of capsaicin, bradykinin and nicotine on CGRP release from tracheal afferent nerves: Role of prostaglandins, sympathetic nerves and mast cells. Neuropharmacology. 1994;33:1147–1154. doi: 10.1016/s0028-3908(05)80004-8. [DOI] [PubMed] [Google Scholar]
- LEE T.J.-F., ZHANG W., SARWINSKI S. Presynaptic β2-adrenoceptors mediate nicotine-induced NOergic neurogenic dilation in porcine basilar arteries. Am. J. Physiol. 2000;279:H808–H816. doi: 10.1152/ajpheart.2000.279.2.H808. [DOI] [PubMed] [Google Scholar]
- LUNDBERG M.J., TERENIUS L., HOKFELT T., MARTLING R.C., TATEMOTO K., MUTT V., POLAK J., BLOOM S., GOKDSTEIN M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol. Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- HAASS M., RICHARDT G., BRENN T., SCHOMIG E., SCHOMIG A. Nicotine-induced release of noradrenaline and neuropeptide Y in guinea-pig heart: role of calcium channels and protein kinase C. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:527–531. doi: 10.1007/BF00170647. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., NUKI C., SAITO A., TAKASAKI K. NPY modulates neurotransmission of CGRP-containing vasodilator nerves in rat mesenteric arteries. Am. J. Physiol. 1991;261:H683–H690. doi: 10.1152/ajpheart.1991.261.3.H683. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., TAKASAKI K., SAITO S., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:54–56. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- MENO J.R., CRUM A.V., WINN H.R. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am. J. Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- MISU Y., GOSHIMA Y., MIYAMAE T., FURUKAWA N., SUGIYAMA Y., OKAMURA Y., SHIMIZU M., OHSHIMA E., SUZUKI F. L-DOPA cyclohexyl ester is a novel stable and potent competitive antagonist against L-DOPA, as compared to L-DOPA methyl ester. JPN. J. Pharmacol. 1997;75:307–309. [PubMed] [Google Scholar]
- MORGADINHO M.T., RIBERO C.A.F., MACEDO T.R.A. Presynaptic dopamine receptors involved in the inhibition of noradrenaline and dopamine release in the human gastric and uterine arteries. Fundam. Clin. Pharmacol. 1999;13:662–670. doi: 10.1111/j.1472-8206.1999.tb00378.x. [DOI] [PubMed] [Google Scholar]
- NAWA H., KAWASAKI H., ISOBE S., KUROSAKI Y. Triphasic vascular responses to bradykinin in the mesenteric resistance artery of the rat. Eur. J. Pharmacol. 2001;433:105–113. doi: 10.1016/s0014-2999(01)01513-8. [DOI] [PubMed] [Google Scholar]
- OKAMURA T., YAMAZAKI M., TODA N. Responses to dopamine of isolated human and monkey veins compared with those of the arteries. J. Pharmacol. Exp. Ther. 1991;258:275–279. [PubMed] [Google Scholar]
- PRIETO D., BUUS C.L., MULVANY M.J., NILSSON H. Neuropeptide Y regulates intracellular calcium through different signalling pathways linked to a Y(1)-receptor in rat mesenteric small arteries. Br. J. Pharmacol. 2000;129:1689–1699. doi: 10.1038/sj.bjp.0703256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAKI H., KAWASAKI H., TEZUKA S., NAKATSUMA A., KUROSAKI U. Endogenous calcitonin gene-related peptide (CGRP) mediates adrenergic dependent vasodilation induced by nicotine in mesenteric resistance arteries of the rat. Br. J. Pharmacol. 2000;130:1083–1091. doi: 10.1038/sj.bjp.0703376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAKI H., KAWASAKI H., TEZUKA S., NAKATSUMA A., KUROSAKI U. Adrenergic nerves mediate acetylcholine-induced endothelium-independent vasodilation in the rat mesenteric resistance artery. Eur. J. Pharmacol. 2001;419:231–242. doi: 10.1016/s0014-2999(01)00981-5. [DOI] [PubMed] [Google Scholar]
- SI L.M., Lee T.F.-J. Presynaptic a7-nicotinic acetylcholine receptors mediate nicotine-induced nitric oxidergic neurogenic vasodilation in porcine basilar arteries. J. Pharmacol. Exp. Ther. 2001;298:122–128. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- TAKENAGA M., KAWASAKI H., WADA A., ETO T. Calcitonin gene-related peptide mediates acetylcholine-induced endothelium-independent vasodilation in mesenteric resistance blood vessels of the rat. Circ. Res. 1995;76:935–941. doi: 10.1161/01.res.76.6.935. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I.V., STARKE K. Release of noradrenaline and ATP by electrical stimulation and nicotine in guinea-pig vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:419–429. doi: 10.1007/BF00172581. [DOI] [PubMed] [Google Scholar]
- ZHANG W., EDVINSSON L., Lee T.J.-F. Mechanism of nicotine-induced relaxation in the porcine basilar artery. J. Pharmacol. Exp. Ther. 1998;284:790–797. [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., HUAI-HU C., SORGARD M., MARZO V.D., JULIUS D., HOGESTATT E. Vanilloid receptors on sensory nerves mediate the vasodilator action on anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]