Abstract
Red wine polyphenolic compounds (RWPCs) are potent inducers of endothelium-dependent relaxations of coronary arteries, which involve both nitric oxide and endothelium-derived hyperpolarizing factor (EDHF). The EDHF-mediated relaxation to RWPCs is critically dependent on the formation of reactive oxygen species by a flavin-dependent enzyme. The aim of the present study was to determine the role of redox-sensitive protein kinases including p38 MAPK, ERK1/2 and PI3-kinase/Akt in RWPCs-induced EDHF-mediated relaxation.
Porcine coronary artery rings were suspended in organ chambers for measurement of changes in isometric tension. Confluent cultures of porcine coronary artery endothelial cells were used to determine the phosphorylation level of p38 MAPK, ERK1/2 and Akt by Western blot analysis. All experiments were performed in the presence of indomethacin and Nω-nitro-L-arginine.
RWPCs caused pronounced endothelium-dependent relaxations, which were significantly reduced by wortmannin and LY294002, two inhibitors of PI3-kinase, and not affected by PD98059 (an inhibitor of ERK1/2 kinase kinase) and SB203580 (an inhibitor of p38 MAPK). In contrast, wortmannin did not affect relaxations to bradykinin or levcromakalim.
RWPCs elicited within minutes a sustained and concentration-dependent phosphorylation of p38 MAPK, ERK1/2 and Akt in endothelial cells. The phosphorylation of Akt in response to RWPCs was abolished by wortmannin and LY294002, and by the membrane-permeant analogue of superoxide dismutase Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin.
The present findings demonstrate that RWPCs cause EDHF-mediated relaxations of coronary arteries; these responses are critically dependent on the redox-sensitive activation of the PI3-kinase/Akt pathway in endothelial cells.
Keywords: Red wine polyphenolic compounds, endothelium-dependent relaxation, endothelial cells, EDHF, PI3-kinase/Akt, coronary artery
Introduction
Numerous epidemiological studies have indicated that regular consumption of moderate amounts of wine, in particular red wine, is associated with a lower incidence of coronary heart diseases (Renaud & Gueguen, 1998; Gronbaek et al., 2000). The protective effect of red wine has been attributable at least partly to red wine polyphenolic compounds (RWPCs). Although the exact nature of the beneficial effect of red wine polyphenols on the cardiovascular system remains unclear, it might be related to their ability to prevent oxidation of LDL and activation of platelets (Frankel et al., 1993; Demrow et al., 1995; Wang et al., 2002). It may also be due to their direct protective effect on blood vessels. Red wine polyphenols are able to inhibit the migration and proliferation of endothelial cells and smooth muscle cells (Iijima et al., 2000; Brakenhielm et al., 2001; Igura et al., 2001). They also prevent the expression of proatherosclerotic and prothrombotic molecules such as monocyte chemoattractant protein-1, tissue factor and vascular endothelial growth factor in vascular cells (Feng et al., 1999; Hsieh et al., 1999; Pendurthi et al., 1999; Iijima et al., 2000, 2002; Igura et al., 2001; Oak et al., 2003). Moreover, red wine polyphenols are potent endothelium-dependent vasodilators (Ndiaye et al., 2003a). In coronary arteries, red wine polyphenols induce both nitric oxide (NO)- and endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxations (Fitzpatrick et al., 1993; Andriambeloson et al., 1997; Cishek et al., 1997; Flesch et al., 1998; Soares De Moura et al., 2002; Ndiaye et al., 2003a). Although red wine polyphenols have intrinsic antioxidant properties, the EDHF-mediated relaxation in porcine coronary arteries is abolished by membrane-permeant analogues of superoxide dismutase and markedly reduced by diphenylene iodonium, an inhibitor of flavin-dependent enzymes such as the NAD(P)H oxidase (Ndiaye et al., 2003a). In addition, an increased formation of superoxide in cultured endothelial cells has been observed in response to red wine polyphenols (Ndiaye et al., 2003a). Thus, the intracellular formation of superoxide presumably by a flavin-dependent enzyme in endothelial cells plays a key role in the signal transduction of red wine polyphenols, leading to EDHF-mediated relaxations. Since ROS have an important signaling function in vascular cells (Ullrich & Bachschmid, 2000), the aim of the present study was to examine whether redox-sensitive protein kinases including p38 MAPK, ERK1/2 and PI3-kinase are involved in red wine polyphenols-induced EDHF-mediated relaxations.
Methods
Preparation of RWPCs
RWPCs dry powder was obtained from French red wine (Corbières A.O.C.) and provided by Dr M. Moutounet (Institut National de la Recherche Agronomique, Montpellier, France) and analyzed by Dr P.-L. Teissedre (Département d'Oenologie, Université de Montpellier, France). The preparation and analysis of RWPCs have been described previously (Andriambeloson et al., 1997; Oak et al., 2003; Ndiaye et al., 2003a).
Chemicals
Wortmannin, LY294002, PD98059, SB203580 and MnTMPyP were obtained from Alexis Chemicals, Lausen, Switzerland. Indomethacin, L-NA and H2O2 were from Sigma, Saint Quentin Fallavier, France. Antibodies directed against phosphorylated p38 MAPK, ERK1/2 and Akt were obtained from Cell Signaling Technology, Beverly, MA, U.S.A.
Vascular reactivity studies
Left anterior descending coronary arteries (obtained from the local slaughterhouse) were cleaned of connective tissue and cut into rings (4–5 mm in length). Rings were suspended in organ baths containing oxygenated (95% O2; 5% CO2) Krebs bicarbonate solution (mM: NaCl 119, KCl 4.7, KH2PO4 1.18, MgSO4 1.18, CaCl2 1.25, NaHCO3 25 and D-glucose 11, pH 7.4, 37°C), the cyclooxygenase inhibitor indomethacin (10 μM) and the NO synthase inhibitor L-NA (100 μM), for the determination of changes in isometric tension. Following equilibration for 90 min under a resting tension of 5 g, rings were twice contracted with KCl (80 mM). Thereafter, the rings were precontracted with the thromboxane mimetic U46619 (1–60 nM) to about 80% of the maximal contraction, and the relaxation to bradykinin (0.3 μM) was determined. After washout and a 30-min equilibration period, rings were again contracted with U46619, before a concentration–relaxation curve to RWPCs, bradykinin or levcromakalim was constructed. In some experiments, rings were exposed to an inhibitor for 30 min before the addition of U46619.
Culture of porcine coronary endothelial cells
Porcine coronary artery segments were flushed with PBS without calcium to remove the remaining blood. Thereafter, endothelial cells were isolated by collagenase treatment (type I, Worthington, 1 mg ml−1 for 12 min at 37°C) and cultured in Petri dishes coated with collagen (type I prepared from rat tail; 60 ng ml−1). The culture medium was RPMI1640/M199 (v v−1) and 15% fetal calf serum supplemented with penicillin (100 U ml−1), streptomycin (100 U ml−1), fungizone (250 μg ml−1) and L-glutamine (2 mM). All experiments were performed with confluent cultures of cells used at the first or second passage in the presence of indomethacin (10 μM) and L-NA (100 μM). Cells were exposed to serum-free culture medium in the presence of 0.1% bovine serum albumin for 6 h prior to treatment.
Western blot analysis
Total proteins (20 μg) were subjected to SDS–PAGE (12%) and blotted on PVDF membrane. Immunodetection was carried out using antibodies directed against phosphorylated Akt, p38 MAPK and ERK1/2. Immunoreactive bands were detected by enhanced chemiluminescence (Amersham). Ponceau staining was performed to verify the quality of the transfer and equal amounts of proteins in each lane.
Statistical analysis
Values are expressed as mean±s.e.m. Statistical evaluation was performed with Student's t-test for paired data or ANOVA, followed by Fischer's protected least significant difference test where appropriate. P<0.05 was considered statistically significant.
Results
Role of the p38 MAPK, ERK1/2 and the PI3-kinase/Akt pathway in the RWPCs-induced EDHF-mediated relaxation
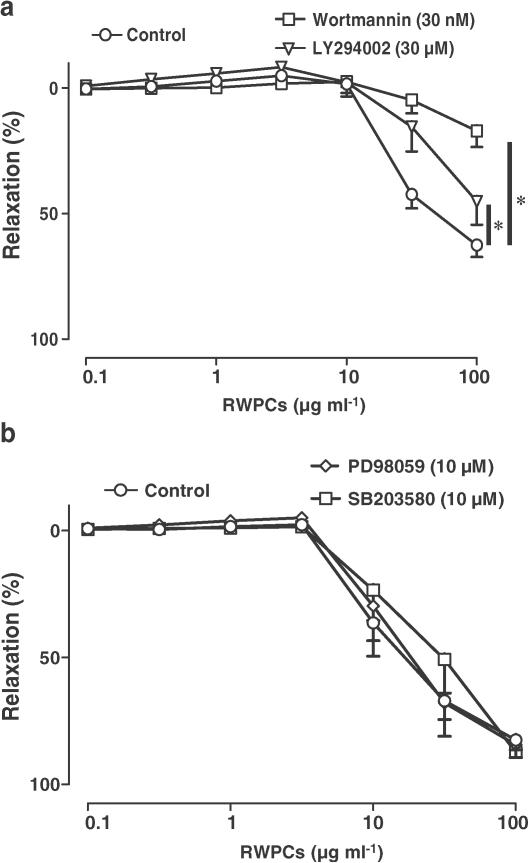
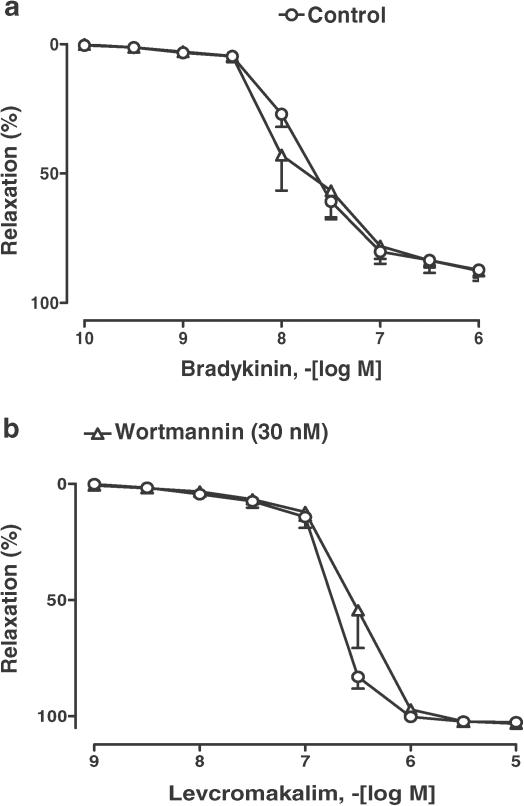
Previous findings have indicated that the RWPCs induce EDHF-mediated relaxations in coronary arteries, which are critically dependent on an intracellular redox-sensitive mechanism involving predominantly superoxide (Ndiaye et al., 2003a). Therefore, the role of redox-sensitive protein kinases in EDHF-mediated relaxations to RWPCs was assessed. In the presence of indomethacin and L-NA, RWPCs caused concentration-dependent relaxations of coronary arteries with endothelium (Figure 1). Relaxations to RWPCs were significantly inhibited by the PI3-kinase inhibitors wortmannin and LY294002, and not affected by inhibitors of p38 MAPK (SB203580) and neither ERK1/2 kinase kinase (PD98059, Figures 1a and b). In contrast, wortmannin did affect neither EDHF-mediated relaxations to bradykinin nor those to levcromakalim (an activator of ATP-sensitive potassium channels, Figures 2a and b). Altogether, these findings indicate that EDHF-mediated relaxations to RWPCs involve a PI3-kinase-dependent mechanism.
Figure 1.
Effect of PI3-kinase inhibitors (a) and inhibitors of ERK1/2 kinase kinase (PD98059, b) and p38 MAPK (SB203580, b) on RWPC-induced EDHF-mediated relaxations in porcine coronary artery rings with endothelium. All experiments were performed in the presence of indomethacin (10 μM) and L-NA (100 μM). Results are shown as the mean±s.e.m. of six to seven different experiments. * indicates a significant inhibitory effect.
Figure 2.
Effect of wortmannin on bradykinin (a) and levcromakalim (b)-induced relaxations in porcine coronary artery rings with endothelium. All experiments were performed in the presence of indomethacin (10 μM) and L-NA (100 μM). Results are shown as the mean±s.e.m. of six different experiments.
RWPCs induce Akt, p38 MAPK and ERK1/2 phosphorylation in endothelial cells
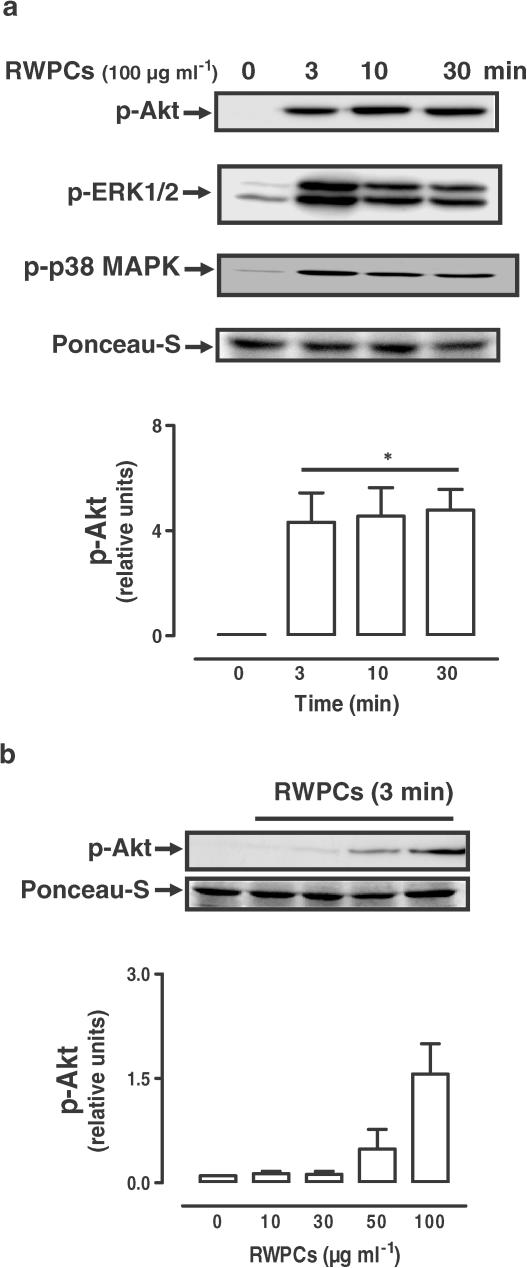
To obtain further evidence that RWPCs can activate the PI3-kinase/Akt pathway in endothelial cells, the phosphorylation level of Akt was assessed in confluent cultures of porcine coronary artery endothelial cells by Western blot analysis. Exposure of endothelial cells to RWPCs caused the appearance of a marked immunoreactive band for p-Akt within 3 min (Figure 3a). Increased levels of p-Akt persisted for at least 30 min (Figure 3a). In addition, RWPCs also caused the phosphorylation of ERK1/2 and p38 MAPK with a similar time course as that of Akt (Figure 3a). The stimulatory effect of RWPCs on the phosphorylation of Akt was concentration-dependent (Figure 3b).
Figure 3.
RWPCs cause a time- (a) and concentration (b)-dependent phosphorylation of Akt, ERK1/2 and p38 MAPK in cultured porcine coronary artery endothelial cells. Endothelial cells were treated with RWPCs for the indicated times at 37°C. Thereafter, the level of p-Akt, p-ERK1/2 and p-p38 MAPK was determined by Western blot analysis. Upper panels show representative immunoblots and lower panels the corresponding cumulative data. All experiments were performed in the presence of indomethacin (10 μM) and L-NA (100 μM). Results are shown as the mean±s.e.m. of four to seven different experiments. * indicates a significant stimulatory effect.
RWPCs cause the redox-sensitive activation of the PI3-kinase/Akt pathway in endothelial cells
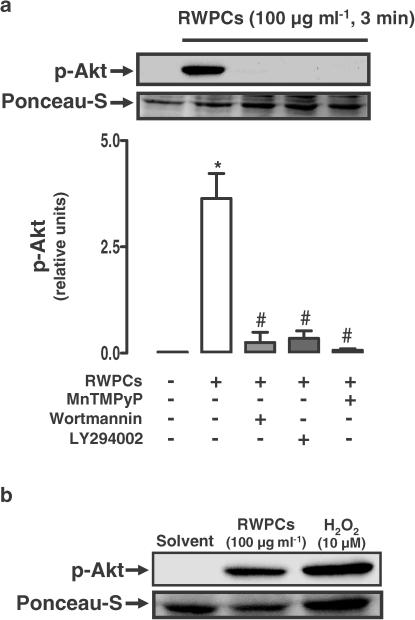
Previous studies have indicated that RWPCs stimulate the formation of superoxide in endothelial cells (Ndiaye et al., 2003a) and that ROS are potent activators of the PI3-kinase/Akt pathway in endothelial cells (Thomas et al., 2002; Cai et al., 2003). Therefore, the role of superoxide in the RWPCs-induced phosphorylation of Akt was assessed using the membrane-permeant analogue of superoxide dismutase MnTMPyP. Exposure of endothelial cells to MnTMPyP for 30 min before addition of RWPCs (100 μg ml−1) abolished the phosphorylation of Akt (Figure 4a). Similar inhibitory effects were also obtained with the PI3-kinase inhibitors (wortmannin, LY294002, Figure 4a). In addition, exposure of endothelial cells to exogenous H2O2 strongly induced the phosphorylation of Akt (Figure 4b). Altogether, these findings suggest that RWPCs cause activation of the PI3-kinase pathway leading to phosphorylation of Akt, and that this event is dependent on the formation of superoxide.
Figure 4.
Effect of PI3-kinase inhibitors and the membrane-permeant superoxide dismutase mimetic MnTMPyP on the phosphorylation of Akt induced by RWPCs. (a) Endothelial cells were incubated for 30 min with either solvent, MnTMPyP (100 μM), LY294002 (30 μM) or wortmannin (30 nM) for 30 min prior to addition of RWPCs (100 μg ml−1) for 3 min. (b) In addition to RWPCs, H2O2 also caused phosphorylation of Akt in endothelial cells. Endothelial cells were exposed to either RWPCs (100 μg ml−1) or H2O2 (10 μM) for 3 min. Thereafter, the level of p-Akt was determined by Western blot analysis. Upper panels show representative immunoblots, and the lower panel cumulative data. All experiments were performed in the presence of indomethacin (10 μM) and L-NA (100 μM). (a) Results are shown as the mean±s.e.m. of three different experiments. (b) Similar observations were made in two additional experiments. * indicates a significant stimulatory effect and # a significant inhibitory effect.
Discussion and conclusions
Numerous investigations have indicated that red wines, grape juices, red wine polyphenolic extracts and grape skin extracts are potent endothelium-dependent vasodilators of isolated arteries such as the rat and rabbit aortas, the porcine and human coronary arteries and also the perfused rat mesenteric bed (Fitzpatrick et al., 1993; Andriambeloson et al., 1997; Flesch et al., 1998; Soares De Moura et al., 2002; Ndiaye et al., 2003a). Endothelium-dependent relaxations in isolated aortas were associated with an increased tissue content of cyclic GMP and both responses were abolished by inhibitors of NO synthase, indicating that they are mediated by NO (Fitzpatrick et al., 1993; Andriambeloson et al., 1997; Cishek et al., 1997; Flesch et al., 1998). However, inhibition of NO synthase reduced red wine polyphenol-induced relaxations in porcine coronary artery only to some extent (Ndiaye et al., 2003a). Since these L-NA-resistant relaxations were associated with hyperpolarizations and both responses were inhibited by the combination of charybdotoxin plus apamin (two inhibitors of EDHF-mediated responses), they have been attributed to EDHF (Ndiaye et al., 2003a). The characterization of the EDHF-mediated relaxation to red wine polyphenols has indicated the involvement of an intracellular redox-sensitive mechanism, since the relaxation was abolished by membrane-permeant forms of superoxide dismutase but not by superoxide dismutase and also not by a membrane-permeant form of catalase or catalase (Ndiaye et al., 2003a). In contrast, reactive oxygen species (ROS) were not involved in bradykinin-induced EDHF-mediated relaxation (Pomposiello et al., 1999; Ndiaye et al., 2003a). Further evidence for the intracellular formation of superoxide in response to red wine polyphenols was obtained with cultured coronary artery endothelial cells using the oxidative fluorescent dye hydroethidine (Ndiaye et al., 2003a). Altogether, these findings have indicated that red wine polyphenols activate a novel intracellular redox-sensitive pathway involving superoxide, leading to EDHF-mediated relaxation.
Recent findings have indicated that ROS have an important signaling function in vascular cells (Ullrich & Bachschmid, 2000). Therefore, the potential role of redox-sensitive protein kinases including PI3-kinase/Akt (Thomas et al., 2002; Cai et al., 2003), p38 MAPK (Viedt et al., 2000) and ERK1/2 (Baas & Berk, 1995) in the signal transduction pathway leading to EDHF-mediated relaxation in response to red wine polyphenols was investigated using pharmacological inhibitors. Inhibition of the PI3-kinase/Akt pathway by wortmannin or LY294002 significantly reduced the relaxation to red wine polyphenols, whereas no such effect was obtained by inhibition of the p38 MAPK pathway or the ERK1/2 pathway. These findings, in conjunction with those showing that wortmannin did not affect EDHF-mediated relaxation to bradykinin or those to levcromakalim, indicate a key role of the PI3-kinase pathway in the red wine polyphenol-induced EDHF-mediated relaxation.
Activated PI3-kinase converts the plasma membrane lipid phosphatidylinositol-4,5-biphosphate to phosphatidylinositol-3,4,5-triphosphate (Cantley, 2002). Thereafter, signaling molecules with pleckstrin-homology domains, such as the serine/threonine protein kinases, Akt and phosphoinositide-dependent kinase-1 (PDK-1), accumulate at sites of PI3-kinase activation. Association with phosphatidylinositol-3,4,5-triphosphate at the membrane brings these proteins into proximity and facilitates phosphorylation of Akt by PDK-1. Therefore, the possibility that red wine polyphenols cause the PI3-kinase-dependent activation of Akt was assessed in cultured coronary artery endothelial cells. Western blot analysis indicated that the level of phosphorylated Akt was either low or below the detection level in control endothelial cells. Exposure of endothelial cells to red wine polyphenols rapidly caused a time- and concentration-dependent phosphorylation of Akt. This response was abolished by inhibitors of PI3-kinase. In addition to Akt, red wine polyphenols also caused a time-dependent phosphorylation of p38 MAPK and ERK1/2. Since inhibition of p38 MAPK and ERK1/2 pathways did not affect EDHF-mediated relaxations to red wine polyphenols, the functional role of these two signaling pathways in response to red wine polyphenols remains to be clarified.
In endothelial cells, Akt can be activated by a wide variety of growth stimuli, including vascular endothelial growth factor (Gerber et al., 1998), insulin-like growth factor-I (Michell et al., 1999), hepatocye growth factor (Nakagami et al., 2001), fluid shear stress (Dimmeler et al., 1998), estrogen (Simoncini et al., 2000) and corticosteroids (Hafezi-Moghadam et al., 2002). Recent findings have also indicated that ROS and, in particular, H2O2 can cause the PI3-kinase-dependent activation of Akt in cultured endothelial cells within minutes (Thomas et al., 2002; Cai et al., 2003, present findings). These findings in conjunction with those showing that red wine polyphenols are able to stimulate the formation of ROS in endothelial cells (Ndiaye et al., 2003a) prompt investigations to clarify whether ROS act upstream of the PI3-kinase/Akt pathway. This concept is supported by the fact that exposure of endothelial cells to the membrane-permeant analogue of SOD MnTMPyP abolished the phosphorylation of Akt in response to red wine polyphenols. Altogether, the present findings indicate that red wine polyphenols cause the ROS-sensitive activation of the PI3-kinase/Akt pathway in endothelial cells, and that this response plays a determinant role in EDHF-mediated relaxation. They further indicate that the signaling pathway via the PI3-kinase/Akt pathway leading to EDHF-mediated relaxation is specific to red wine polyphenols, but not other inducers of EDHF-mediated relaxation such as bradykinin.
Currently, relatively little is known about the downstream mediators of the PI3-kinase/Akt-dependent EDHF-mediated relaxation. The possibility that this pathway directly affects the activity of potassium channels in endothelial cells is an attractive hypothesis, which however has not yet been addressed. Alternatively, the PI3-kinase/Akt pathway may contribute to increase intracellular Ca2+ levels in endothelial cells, which in turn increases the activity of Ca2+-dependent potassium channels involved in EDHF-mediated hyperpolarization (Franceschi et al., 1990; Martin et al., 2002). The PI3-kinase pathway has been involved in H2O2-induced increases in intracellular calcium level in vascular smooth muscle cells (Yang et al., 1999) and also in endothelin-1-induced activation of Ca2+-permeable nonselective cation channels (Kawanabe et al., 2003).
Finally, the present study also prompts investigations on the potential role of the PI3-kinase/Akt pathway in the endothelial formation of NO in response to red wine polyphenols. Indeed, the activation of this signaling pathway has been associated with the phosphorylation of endothelial NO synthase, with subsequent increased formation of NO in response to a variety of stimuli including vascular endothelial growth factor, estrogens, shear stress and corticosteroids (Dimmeler et al., 1998, 1999; Fulton et al., 1999; Simoncini et al., 2000; Hafezi-Moghadam et al., 2002). Our recent findings support such a concept since the red wine polyphenol-induced endothelium-dependent NO-mediated relaxation was prevented by inhibitors of PI3-kinase and associated with the phosphorylation of both Akt and endothelial NO synthase (Ndiaye et al., 2003b).
In conclusion, red wine polyphenols are potent endothelium-dependent relaxing agonists by increasing the formation of both EDHF and NO. The EDHF-mediated relaxation is critically dependent on the redox-sensitive activation of the PI3-kinase/Akt pathway in endothelial cells.
Acknowledgments
This study was supported in part by the Office National Interprofessionnel des Vins (ONIVINS, Ministry of Agriculture, France) and by Institut Européen Vin et Santé des Régions Viticoles (France). Dr Ndiaye is supported by the Government of Senegal.
Abbreviations
- EDHF
endothelium-derived hyperpolarizing factor
- ERK1/2
extracellular signal-regulated kinase 1/2
- L-NA
Nω-nitro-L-arginine
- MnTMPyP
mimetic Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin
- p38 MAPK
p38 mitogen-activated protein kinase
- PI3-kinase
phosphoinositide-3-kinase
- RWPCs
red wine polyphenolic compounds
References
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAAS A.S., BERK B.C. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ. Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- BRAKENHIELM E., CAO R., CAO Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- CAI H., LI Z., DAVIS M.E., KANNER W., HARRISON D.G., DUDLEY S.C., JR Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol. Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- CANTLEY L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- CISHEK M.B., GALLOWAY M.T., KARIM M., GERMAN J.B., KAPPAGODA C.T. Effect of red wine on endothelium-dependent relaxation in rabbits. Clin. Sci. (Lond.) 1997;93:507–511. doi: 10.1042/cs0930507. [DOI] [PubMed] [Google Scholar]
- DEMROW H.S., SLANE P.R., FOLTS J.D. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., ASSMUS B., HERMANN C., HAENDELER J., ZEIHER A.M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., FLEMING I., FISSLTHALER B., HERMANN C., BUSSE R., ZEIHER A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- FENG A.N., CHEN Y.L., CHEN Y.T., DING Y.Z., LIN S.J. Red wine inhibits monocyte chemotactic protein-1 expression and modestly reduces neointimal hyperplasia after balloon injury in cholesterol-fed rabbits. Circulation. 1999;100:2254–2259. doi: 10.1161/01.cir.100.22.2254. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK D.F., HIRSCHFIELD S.L., COFFEY R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- FLESCH M., SCHWARZ A., BOHM M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am. J. Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- FRANCESCHI D., GRAHAM D., SARASUA M., ZOLLINGER R.M., JR Mechanisms of oxygen free radical-induced calcium overload in endothelial cells. Surgery. 1990;108:292–297. [PubMed] [Google Scholar]
- FRANKEL E.N., KANNER J., GERMAN J.B., PARKS E., KINSELLA J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- FULTON D., GRATTON J.P., MCCABE T.J., FONTANA J., FUJIO Y., WALSH K., FRANKE T.F., PAPAPETROPOULOS A., SESSA W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER H.P., MCMURTREY A., KOWALSKI J., YAN M., KEYT B.A., DIXIT V., FERRARA N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- GRONBAEK M., BECKER U., JOHANSEN D., GOTTSCHAU A., SCHNOHR P., HEIN H.O., JENSEN G., SORENSEN T.I. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- HAFEZI-MOGHADAM A., SIMONCINI T., YANG E., LIMBOURG F.P., PLUMIER J.C., REBSAMEN M.C., HSIEH C.M., CHUI D.S., THOMAS K.L., PROROCK A.J., LAUBACH V.E., MOSKOWITZ M.A., FRENCH B.A., LEY K., LIAO J.K. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIEH T.C., JUAN G., DARZYNKIEWICZ Z., WU J.M. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. 1999;59:2596–2601. [PubMed] [Google Scholar]
- IGURA K., OHTA T., KURODA Y., KAJI K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- IIJIMA K., YOSHIZUMI M., OUCHI Y. Effect of red wine polyphenols on vascular smooth muscle cell function – molecular mechanism of the ‘French paradox'. Mech. Ageing Dev. 2002;123:1033–1039. doi: 10.1016/s0047-6374(01)00386-4. [DOI] [PubMed] [Google Scholar]
- IIJIMA K., YOSHIZUMI M., HASHIMOTO M., KIM S., ETO M., AKO J., LIANG Y.Q., SUDOH N., HOSODA K., NAKAHARA K., TOBA K., OUCHI Y. Red wine polyphenols inhibit proliferation of vascular smooth muscle cells and downregulate expression of cyclin A gene. Circulation. 2000;101:805–811. doi: 10.1161/01.cir.101.7.805. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., HASHIMOTO N., MASAKI T. Role of phosphoinositide 3-kinase in the nonselective cation channel activation by endothelin-1/endothelin B receptor. Am. J. Physiol. Cell. Physiol. 2003;284:C506–C510. doi: 10.1152/ajpcell.00384.2002. [DOI] [PubMed] [Google Scholar]
- MARTIN S., ANDRIAMBELOSON E., TAKEDA K., ANDRIANTSITOHAINA R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHELL B.J., GRIFFITHS J.E., MITCHELHILL K.I., RODRIGUEZ-CRESPO I., TIGANIS T., BOZINOVSKI S., DE MONTELLANO P.R., KEMP B.E., PEARSON R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- NAKAGAMI H., MORISHITA R., YAMAMOTO K., TANIYAMA Y., AOKI M., MATSUMOTO K., NAKAMURA T., KANEDA Y., HORIUCHI M., OGIHARA T. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–586. doi: 10.1161/01.hyp.37.2.581. [DOI] [PubMed] [Google Scholar]
- NDIAYE M., CHATAIGNEAU T., ANDRIANTSITOHAINA R., STOCLET J.C., SCHINI-KERTH V.B. Red wine polyphenols cause endothelium-dependent EDHF-mediated relaxations in porcine coronary arteries via a redox-sensitive mechanism. Biochem. Biophys. Res. Commun. 2003a;310:371–377. doi: 10.1016/j.bbrc.2003.09.028. [DOI] [PubMed] [Google Scholar]
- NDIAYE M., CHATAIGNEAU T., CHATAIGNEAU M., SCHINI-KERTH V.B.Red wine polyphenol-induced endothelium-dependent NO-mediated relaxation is due to the PI3kinase/Akt-dependent phosphorylation of endothelial NO synthase in the isolated porcine coronary artery Circulation 2003b108IV-101(abstract) [DOI] [PubMed] [Google Scholar]
- OAK M.H., CHATAIGNEAU M., KERAVIS T., CHATAIGNEAU T., BERETZ A., ANDRIANTSITOHAINA R., STOCLET J.C., CHANG S.J., SCHINI-KERTH V.B. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2003;23:1001–1007. doi: 10.1161/01.ATV.0000070101.70534.38. [DOI] [PubMed] [Google Scholar]
- PENDURTHI U.R., WILLIAMS J.T., RAO L.V. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells: a possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler. Thromb. Vasc. Biol. 1999;19:419–426. doi: 10.1161/01.atv.19.2.419. [DOI] [PubMed] [Google Scholar]
- POMPOSIELLO S., RHALEB N.E., ALVA M., CARRETERO O.A. Reactive oxygen species: role in the relaxation induced by bradykinin or arachidonic acid via EDHF in isolated porcine coronary arteries. J. Cardiovasc. Pharmacol. 1999;34:567–574. doi: 10.1097/00005344-199910000-00014. [DOI] [PubMed] [Google Scholar]
- RENAUD S., GUEGUEN R.The French paradox and wine drinking Novartis. Found. Symp. 1998216208–217.discussion 217–222, 152–158 [DOI] [PubMed] [Google Scholar]
- SIMONCINI T., HAFEZI-MOGHADAM A., BRAZIL D.P., LEY K., CHIN W.W., LIAO J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOARES DE MOURA R., COSTA VIANA F.S., SOUZA M.A., KOVARY K., GUEDES D.C., OLIVEIRA E.P., RUBENICH L.M., CARVALHO L.C., OLIVEIRA R.M., TANO T., GUSMAO CORREIA M.L. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J. Pharm. Pharmacol. 2002;54:1515–1520. doi: 10.1211/002235702153. [DOI] [PubMed] [Google Scholar]
- THOMAS S.R., CHEN K., KEANEY J.F., JR Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- ULLRICH V., BACHSCHMID M. Superoxide as a messenger of endothelial function. Biochem. Biophys. Res. Commun. 2000;278:1–8. doi: 10.1006/bbrc.2000.3733. [DOI] [PubMed] [Google Scholar]
- VIEDT C., SOTO U., KRIEGER-BRAUER H.I., FEI J., ELSING C., KUBLER W., KREUZER J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- WANG Z., HUANG Y., ZOU J., CAO K., XU Y., WU J.M. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int. J. Mol. Med. 2002;9:77–79. [PubMed] [Google Scholar]
- YANG Z.W., ZHENG T., WANG J., ZHANG A., ALTURA B.T., ALTURA B.M. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signaling pathways. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:646–653. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]