Abstract
7-[2-[4-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine (KMUP-1) produces tracheal relaxation, intracellular accumulation of cyclic nucleotides, inhibition of phosphodiesterases (PDEs) and activation of K+ channels.
KMUP-1 (0.01–100 μM) induced concentration-dependent relaxation responses in guinea-pig epithelium-intact trachea precontracted with carbachol. Relaxation responses were also elicited by the PDE inhibitors theophylline, 3-isobutyl-1-methylxanthine (IBMX), milrinone, rolipram and zaprinast (100 μM), and a KATP channel opener, levcromakalim.
Tracheal relaxation induced by KMUP-1 was attenuated by epithelium removal and by pretreatment with inhibitors of soluble guanylate cyclase (sGC) (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 1 μM), nitric oxide synthase (Nω-nitro-L-arginine methyl ester, 100 μM), K+ channels (tetraethylammonium, 10 mM), KATP channels (glibenclamide, 1 μM), voltage-dependent K+ channels (4-aminopyridine, 100 μM) and Ca2+-dependent K+ channels (charybdotoxin, 0.1 μM or apamin, 1 μM).
Both KMUP-1 (10 μM) and theophylline nonselectively and slightly inhibited the enzyme activity of PDE3, 4 and 5, suggesting that they are able to inhibit the metabolism of adenosine 3′,5′-cyclic monophosphate (cyclic AMP) and guanosine 3′,5′-cyclic monophosphate (cyclic GMP). Likewise, the effects of IBMX were also measured and its IC50 values for PDE3, 4 and 5 were 6.5±1.2, 26.3±3.9 and 31.7±5.3 μM, respectively.
KMUP-1 (0.01–10 μM) augmented intracellular cyclic AMP and cyclic GMP levels in guinea-pig cultured tracheal smooth muscle cells. These increases in cyclic AMP and cyclic GMP were abolished in the presence of an adenylate cyclase inhibitor SQ 22536 (100 μM) and an sGC inhibitor ODQ (10 μM), respectively.
KMUP-1 (10 μM) increased the expression of protein kinase A (PKARI) and protein kinase G (PKG1α1β) in a time-dependent manner, but this was only significant for PKG after 9 h.
Intratracheal administration of tumour necrosis factor-α (TNF-α, 0.01 mg kg−1) induced bronchoconstriction and exhibited a time-dependent increase in lung resistance (RL) and decrease in dynamic lung compliance (Cdyn). KMUP-1 (1.0 mg kg−1), injected intravenously for 10 min before the intratracheal TNF-α, reversed these changes in RL and Cdyn.
These data indicate that KMUP-1 activates sGC, produces relaxation that was partly dependent on an intact epithelium, inhibits PDEs and increases intracellular cyclic AMP and cyclic GMP, which then increases PKA and PKG, leading to the opening of K+ channels and resulting tracheal relaxation.
Keywords: KMUP-1, soluble guanylate cyclase, K+ channels, cyclic GMP, cyclic AMP, tracheal smooth muscle cells
Introduction
Cyclic nucleotides are major intracellular signalling molecules that play a crucial role in the function of mammalian cells. Intracellular concentrations of cyclic nucleotides are regulated by two families of enzymes, adenylate and guanylate cyclases which synthesize adenosine 3′,5′-cyclic monophosphate (cyclic AMP) and guanosine 3′,5′-cyclic monophosphate (cyclic GMP) from their corresponding nucleotide triphosphates, and cyclic nucleotide phosphodiesterase which catalyze the hydrolysis and inactivation of cyclic AMP and/or cyclic GMP. The elevation of cyclic AMP in airway tissues has important implications for cellular responses in an allergic reaction. β2-Adrenoceptor agonists, widely used as bronchodilators in the treatment of asthma, relax airway smooth muscle through a cyclic AMP-dependent mechanism. It is well documented that cyclic GMP, like cyclic AMP, is an important second messenger in the regulation of airway tone. Several nitrogen-containing compounds, such as sodium nitroprusside, and exogenous and endogenous nitric oxide (NO) activate soluble guanylate cyclase (sGC), elevate cyclic GMP and relax airway smooth muscle in vitro (Murad et al., 1978; Gruetter et al., 1989). The relaxations induced by nitrovasodilators in canine and guinea-pig trachea correlate well with the elevation in cyclic GMP, suggesting that cyclic GMP mediates the relaxation (Zhou & Torphy, 1991). Inhibition of cyclic GMP-specific phosphodiesterase (PDE) by xanthine derivatives leads to the relaxation of canine and guinea-pig trachea (Polson et al., 1985; Tanaka et al., 1991). Type 5 PDE inhibitors such as sildenafil and zaprinast can elevate cyclic GMP activity, and they have been shown to induce relaxation in guinea-pig tracheal smooth muscle (Bernareggi et al., 1999). Moreover, YC-1, an NO-independent sGC activator, with nonspecific PDE inhibitory activity, also induces cyclic GMP-mediated relaxation in guinea-pig trachea (Hwang et al., 1999).
The airway epithelium is a physical barrier that protects sensory nerves and smooth muscle from stimulation by inhaled irritants and participates in the regulation of bronchial reactivity. It has been reported that epithelial cells release various smooth muscle inhibitory mediators, such as NO and prostaglandin E2, as well as the so-called epithelium-derived relaxing factor (EpDRF) detected by the coaxial bioassay system (Nijkamp et al., 1993; Folkerts & Nijkamp, 1998).
NO donors have been shown to activate KATP channels via a cyclic GMP-dependent mechanism, presumably involving activation of cyclic GMP-dependent protein kinase, in rat tracheal smooth muscle cells (Kubo et al., 1993) and rabbit mesenteric artery (Murphy & Brayden, 1995), and by a cyclic GMP-independent mechanism in the rat mesenteric artery (Weidelt et al., 1997). In guinea-pig isolated trachea, cromakalim, a KATP channel opener, causes concentration-dependent suppression of spontaneous muscle tone (Arch et al., 1988) and these results were subsequently confirmed by using single tracheal smooth muscle cells under voltage-clamp conditions (Matsushita et al., 2000). This effect is associated with membrane hyperpolarization close to the K+ equilibrium potential. K+ channel-blocking agents, such as tetraethylammonium (TEA), inhibit cromakalim-induced hyperpolarization and relaxation (Allen et al., 1986). It has been reported that the dose of K+ channel openers effective at reducing airway resistance in vivo is lower than that required for the relaxation of airways in vitro (McCaig & De Jonckheere, 1989). A tentative explanation for this phenomenon is that the reactivity of sensory neurones in airway epithelium is reduced by low concentrations of K+ channel openers via activation of KATP channels, which results in hyperpolarization of the neurone.
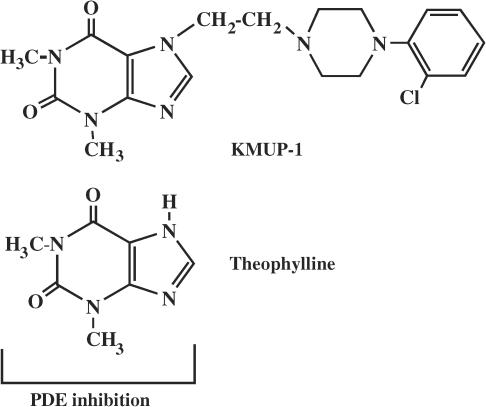
7-[2-[4-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine (KMUP-1), Figure 1, a chemically synthetic xanthine-based derivative, has been demonstrated to inhibit PDE and increase cyclic GMP in rat aortic smooth muscle (Wu et al., 2001). Further, we have shown that KMUP-1 relaxes the rabbit corpus cavernosum smooth muscle and that stimulation of cyclic GMP and opening of K+ channels is involved in this response (Lin et al., 2002). These observations indicate that the relaxing activity of KMUP-1 in trachea might also be attributed to accumulation of cyclic nucleotides and the resulting opening of K+ channels. It is generally accepted that inhibition of type 4 PDE by xanthine derivatives, including methylxanthine, increases intracellular formation of cyclic AMP in various smooth muscle cells. Theophylline has been shown to exert nonspecific inhibition of PDE and to increase cyclic AMP, leading to long-term relaxation in smooth muscles (Beavo, 1995).
Figure 1.
Chemical structures of KMUP-1 and theophylline.
The purpose of this study was to elucidate the mechanism by which KMUP-1 activates the cyclic AMP/GMP-dependent protein kinase pathway, and thus the opening of K+ channels and resulting relaxation of guinea-pig tracheal smooth muscle.
Methods
Animals
Male Dunkin Hartley guinea-pigs (300–400 g) were provided from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). They were housed under conditions of constant temperature and controlled illumination. Food and water were available ad libitum. The study was approved by the Animal Care and Use Committee of Kaohsiung Medical University.
Isolated tracheal preparation and measurement of tension
Guinea-pigs were killed after being anaesthetized with ether and their tracheas were quickly excised. Tracheas were dissected free of adhering fat and connective tissue, and cut into spiral strips as previously described in this laboratory (Wu et al., 1994). These spiral strips were then mounted in organ baths filled with 20 ml of Krebs solution of the following composition (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24 and glucose 11. The solution was maintained at 37°C and aerated with 95% O2 plus 5% CO2. To achieve a steady spontaneous tone level, an initial tension of 2 g was applied for 1 h. All experiments were carried out in the presence of indomethacin (3 μM) and propranolol (1 μM) to prevent the formation of prostanoids and to inhibit β-adrenoceptor-mediated responses, respectively. Contractions were measured isometrically with a force–displacement transducer (Model FT03, Grass, Quincy, MA, U.S.A.) and recorded on a high-speed videograph (Model L1969, Coulbourn, Lehigh Valley, PA, U.S.A.).
In some experiments, the epithelial cells were removed mechanically by rubbing the internal surface of trachea with a fine silver wire (200 μm diameter) as previously described for removal of endothelial cells in vascular tissues (Bolton et al., 1984). The removal of the epithelial layer was confirmed by histological examination. Briefly, segments of trachea were fixed at in situ length in 10% phosphate-buffered formalin and embedded with Paraplast-Plus paraffin. Sections (5 μm) were stained with a routine Harris H & E procedure. Isolated tracheal strips were contracted with carbachol (10 μM) and, once the contractions had reached a plateau, various concentrations of KMUP-1 (0.01–100 μM) were added. All relaxations are expressed as a percentage of carbachol-induced maximal contractile responses.
To examine the possible mechanisms of tracheal relaxant effects of KMUP-1, the tracheal strips were pretreated with a sGC inhibitor ODQ (1 μM), a NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 100 μM), a K+ channel blocker TEA (10 mM), a KATP channel blocker glibenclamide (1 μM), a voltage-dependent K+ channel blocker 4-AP (100 μM) and Ca2+-dependent K+ channel blockers apamin (1 μM) and ChTX (0.1 μM) for 30 min prior to the addition of KMUP-1.
Phosphodiesterase assay
PDE activity was determined by the method of Hidaka & Asano (1976). Washed human platelets were used for both PDE3 and 5 analyses and human U937 cells for PDE4. Purified protein containing PDE3, 4 or 5 enzyme was resuspended in 50 mM Tris–HCl containing 5 mM MgCl2 (pH 7.5). Subsequently, the enzyme (11.5 mg ml−1, 10 μl) was incubated with Tris–HCl (80 μl) and 10 μM cyclic GMP or cyclic AMP substrate (final concentration 1 μM containing 0.1 μCi [3H]-cyclic GMP or [3H]-cyclic AMP) was added. After 20 min at 37°C, the samples were heated to 100°C for 2 min. Ophiophagus hannah snake venom (10 mg ml−1, 10 μl) was then added and incubated at 37°C for 10 min to convert the 5′-GMP and 5′-AMP to the uncharged nucleosides, guanosine and adenosine, respectively. An ion-exchange resin (200 μl) was added to bind all unconverted cyclic GMP or cyclic AMP. After centrifugation, the supernatant was removed for determination of radiolabelled guanosine or adenosine by a liquid scintillation counter.
Culture of guinea-pig tracheal smooth muscle cells
Guinea-pig tracheal smooth muscle (TSM) strips were obtained as sterile surgical specimens under dissection microscopy. The tissue was washed and cut into 1–2 mm pieces and placed into culture dishes with Dulbecco's modified Eagle's medium (DMEM) containing 20% foetal bovine serum, 100 units ml−1 penicillin G, 100 μg ml−1 streptomycin and 2 mM glutamine. After these explants attached to the culture dish, in usually 1–2 days, DMEM supplement with 10% FBS, penicillin, streptomycin and glutamine was added. Tracheal smooth muscle cells (TSMCs) migrated out from the explants in 3–5 days. At this time, the explants were removed, and cells were allowed to achieve confluence. Cells were detached using 0.05% trypsin and 0.02% EDTA at 37°C for 5 min to establish secondary cultures. Cultures were maintained for no more than four passages. To exclude contamination by epithelial cells and fibroblasts, the cell homogeneity was further confirmed by the presence of smooth muscle-specific α-actin and α-myosin. Indirect immunofluorescence staining for a variety of antigens was carried out by first plating the cells on chamber slides, fixing the cells in 3.7% formaldehyde-phosphate buffered saline for 10 min and then permeabilizing the cells with phosphate-buffered saline, 0.1% Triton X-100. Cells were stained with a mouse monoclonal antibody directed against the amino-terminal 10 amino acids of α-smooth muscle actin and α-myosin (Boehringer Mannheim, Indianapolis, IN, U.S.A.). All were stained with fluorescein-labelled goat anti-mouse IgG antibody. Over 95% of the cell preparation was found to be composed of smooth muscle cells.
Measurement of cyclic AMP and cyclic GMP
Intracellular cyclic GMP and cyclic AMP concentrations in guinea-pig TSMCs were assayed as previously described (Wu et al., 2001). In brief, cells were grown in 24-well plates 105 cells per well. At confluence, monolayer cells were washed with phosphate buffer solution (PBS) and then incubated with KMUP-1 (0.1–100 μM) in the presence of 100 μM IBMX for 20 min. Incubation was terminated by the addition of 10% trichloroacetic acid (TCA). Cell suspensions were sonicated and then centrifuged at 2500 × g for 15 min at 4°C. To remove TCA, the supernatants were extracted three times with 5 volumes of water-saturated diethyl ether. Then, the supernatants were lyophilized and the cyclic GMP or AMP of each sample was determined by using commercially available radioimmunoassay kits (Amersham Pharmacia Biotech, Buckinghamshire, England).
Western blot analysis of protein kinase A and protein kinase G
The expression of protein kinases A (PKA) and G (PKG) were determined by Western blot as previously described by Murthy et al. (2002). KMUP-1 was incubated with guinea-pig TSMCs for 3, 6, 9, 12, 18 and 24 h. Each of the cell lysates containing 1 μg of cellular protein was electrophoresed in 7.5% SDS–PAGE and then transferred to PVDF membrane (Millipore Corp., Bedford, MA, U.S.A.). The membrane was stained with Ponceau S to verify the integrity of the transferred proteins and to monitor the unbiased transfer of all protein samples. The membrane was then washed with 25 ml of TBS (100 mM NaCl, 0.1% and 10 mM Tris–HCl, pH 7.5) for 5 min at room temperature and incubated in 25 ml of blocking buffer (TBS plus 0.1% Tween 20 and 5% nonfat milk) overnight at 4°C. To detect the protein of interest, the membrane was incubated with appropriate PKARI or PKG1α1β primary antibody (diluted 1 : 250) in 2 ml of blocking buffer for 1 h at room temperature. It was washed three times for 5 min each with 15 ml of TBST (TBS plus 0.1% Tween 20), incubated with HRP-conjugated anti-mouse and anti-rabbit IgG secondary antibody (1 : 10,000) for PKA and PKG, respectively, in 15 ml of blocking buffer with gentle agitation for 1 h at room temperature. Finally, the membrane was washed three times for 5 min each with 15 ml of TBST and then subject to ECL (Amersham Life Sciences Inc., Arlington Heights, IL, U.S.A.) for the detection of specific antigen.
Airway obstruction and lung function
Guinea-pigs were anaesthetized with pentobarbitone (40 mg kg−1, i.p. initially and maintained with further doses of 2–5 mg kg−1, i.v. when required). The animal was tethered in a supine position and the trachea was cannulated below the larynx with a short cannula via a tracheotomy. Tracheal pressure was measured by a catheter connected to the side arm of the tracheal cannula. The carotid artery and jugular vein were cannulated for monitoring blood pressure and for drug administration, respectively. Throughout the experiment, the body temperature of the guinea-pig was maintained at ∼37°C by means of a servo heating blanket. Intrapleural pressure was measured by a catheter inserted into the right intrapleural cavity via a surgical incision between the fifth and sixth ribs. The incision was subsequently sutured and further sealed with silicone jelly. Transpulmonary pressure was measured as the difference between tracheal pressure and intrapleural pressure, using a differential pressure transducer (P300D, Validyne, Northridge, CA, U.S.A.). Respiratory flow was measured with a pneumotachograph (No. 4/0, Fleisch, Richmond, VA, U.S.A.) coupled to a differential pressure transducer (MP45-14, Validyne), and was integrated to give tidal volume. Measurements were carried out as previously described (Hsu et al., 1998). Total lung resistance (RL) and dynamic lung compliance (Cdyn) were measured on a breath-by-breath basis with a computer equipped with an A/D interface (DAS 1600, Buxco) and software (version 1.5.7, Buxco Electronics, Inc, U.S.A.). Results obtained from the computer analysis were routinely checked for accuracy with those calculated manually.
Animals were allowed to stabilize for 10 min following surgical manipulations before the test agent was administered. Intratracheal instillation was performed using a catheter placed through the tracheal tube into the bronchial system. The animals were pretreated with KMUP-1 (1.0 mg kg−1, i.v.) or an equivalent volume of vehicle for 10 min before the intratracheal TNF-α (0.01 mg kg−1 in 300 μl of 0.9% saline), was used to induce acute bronchoconstriction.
Drugs and chemicals
Levcromakalim was generously supplied by the GlaxoSmithKline Pharmaceuticals and dissolved in ethanol at 10 mM. 4-aminopyridine (4-AP), apamin, carbachol, charybdotoxin (ChTX), glibenclamide, 3-isobutyl-1-methylxanthine (IBMX), indomethacin, Nω-nitro-L-arginine methyl ester (L-NAME), methylene blue, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), propranolol, 9-(terahydro-2-furanyl)-9H-purin-6-amine (SQ 22536), TEA and tumour necrosis factor-α (TNF-α) were all obtained from Sigma-Aldrich Chemical Co. (St Louis, MO, U.S.A.). PKARI and PKG1α1β antibodies were purchased from Calbiochem Co. (San Diego, CA, U.S.A.). All drugs and reagents were dissolved in distilled water, unless noted otherwise. Apamin was dissolved in 0.05 M acetic acid; indomethacin was dissolved in 100 mM sodium carbonate; ChTX, glibenclamide, IBMX and ODQ were dissolved in DMSO at 10 mM; KMUP-1 was dissolved in 10% absolute alcohol, 10% propylene glycol and 2% 1 N HCl at 10 mM. Serial dilutions were made in distilled water.
Statistical evaluation of data
The results are expressed as mean±s.e.m. Statistical differences were determined by independent and paired Student's t-test in unpaired and paired samples, respectively. Whenever a control group was compared with more than one treated group, the one-way ANOVA or two-way repeated-measures ANOVA was used. When the ANOVA manifested a statistical difference, Dunnett's or Student–Newman–Keuls test was applied. A P-value less than 0.05 was considered to be significant in all experiments. Analysis of the data and plotting of the figures were done with the aid of software (SigmaPlot Version 8.0 and SigmaStat Version 2.03, Chicago, IL, U.S.A.) run on an IBM compatible computer.
Results
Tracheal smooth muscle relaxant effects
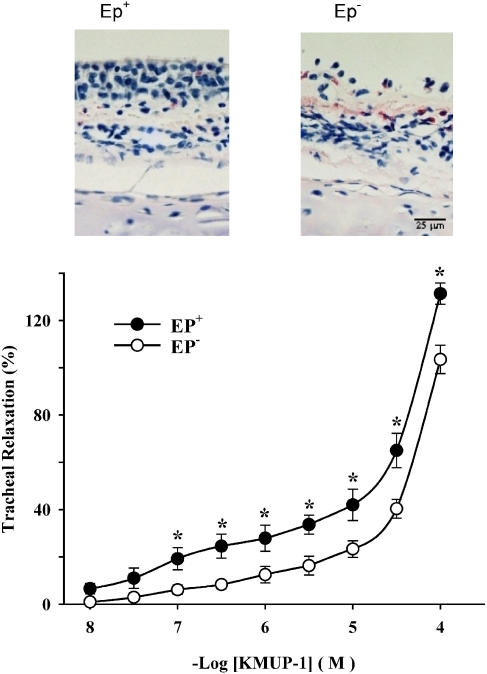
KMUP-1 (0.01–100 μM) caused concentration-dependent relaxations in epithelium-intact (Ep+) tracheal strips pre-contracted by carbachol. The concentration–response curve for KMUP-1 appeared to be biphasic, characterized by a shallow foot and, at concentrations greater than 10 μM, a steeper component (Figure 2). No plateau phase was obtained, even at quite high concentrations. Consequently, it was not possible to determine EC50 values for this agent. Following removal of the epithelium, a downward shift in the concentration–response curve for KMUP-1-induced tracheal relaxation was obtained. The magnitude of relaxations in denuded tissues was significantly smaller than in intact preparation at all concentrations between 0.1 and 100 μM (Figure 2). Thus, KMUP-1-induced relaxation of guinea-pig trachea is partly dependent on an intact epithelium. We also observed that relaxation in response to a nonselective PDE inhibitor, theophylline, and more selective inhibitors of PDE3, 4 and 5, respectively, milrinone, rolipram and zaprinast, were not significantly affected by removal of the epithelium (data not shown).
Figure 2.
H & E-stained histological sections of guinea-pig trachea displayed before (EP+) and after (EP−) removal of the epithelial layer (upper). Tracheal relaxations induced by cumulative concentrations of KMUP-1 in EP+ and EP− guinea-pig trachea preconstricted with carbachol (10 μM). KMUP-1-induced relaxant responses were significantly different in EP+ and EP− tracheal strips at the indicated KMUP-1 concentrations (lower). Each point represents the mean±s.e.m. of eight experiments. *Indicates P<0.05, repeated-measures ANOVA followed by Student–Newman–Keuls test.
KMUP-1 elicited maximal relaxations that exceeded 100% of the magnitude of carbachol-induced contraction from baseline tension. Since all of our experiments were conducted in the presence of indomethacin, which was added to abolish the eicosanoid-dependent basal tension that is characteristic of guinea-pig trachea, this is a surprising observation for which we currently have no explanation. However, since we could not ascertain the maximum relaxation in these experiments, and since no plateau in the concentration–response curve was obtained, EC50 values for KMUP-1 could not be accurately determined.
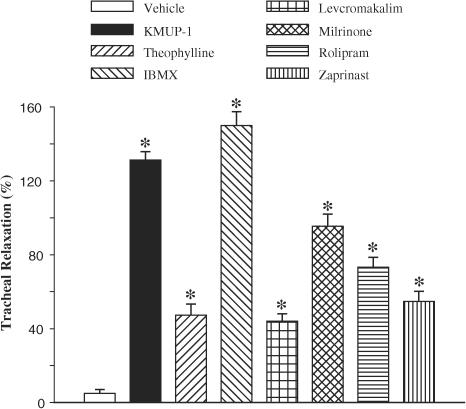
Next, we also compared the relaxant effects of KMUP-1 with those to the PDE inhibitors theophylline, IBMX, milrinone, rolipram and zaprinast, and a KATP channel opener, levcromakalim, at 100 μM (Figure 3). At this high concentration, we observed that, of these agents, KMUP-1 and IBMX were the most effective at inducing tracheal relaxation; the magnitude of the relaxation responses induced by KMUP-1 and IBMX were not significantly different.
Figure 3.
Effects of KMUP-1, theophylline, milrinone, rolipram, zaprinast and levcromakalim (100 μM) on carbachol (10 μM)-precontracted guinea-pig trachea. Each point represents the mean±s.e.m. of 6–8 experiments. *Indicates P<0.05 as compared with the control, ANOVA followed by Dunnett's test.
Effects on K+ channels
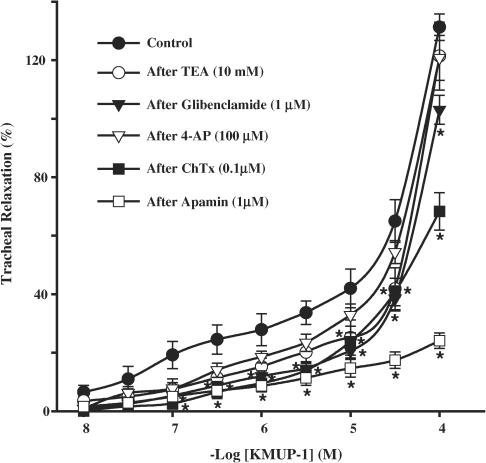
Figure 4 shows the cumulative concentration–response curves to KMUP-1 against carbachol-induced contractions in Ep+ guinea-pig tracheal strips. KMUP-1-induced tracheal relaxations were reduced by a K+ channel blocker, TEA, a KATP channel blocker, glibenclamide, and large- and small-conductance Ca2+-dependent K+ channel blockers, ChTX and apamin, respectively. However, the responses did not appear to be reduced significantly by the voltage-dependent K+ channel blocker, 4-AP.
Figure 4.
Effects of KMUP-1 on guinea-pig tracheal strips precontracted by carbachol in the absence and presence of various K+ channel blockers. Each point represents the mean±s.e.m. of 6–8 experiments. *Indicates P<0.05, repeated–measures ANOVA followed by Student–Newman–Keuls test.
Involvement of NOS, sGC and adenylyl cyclase
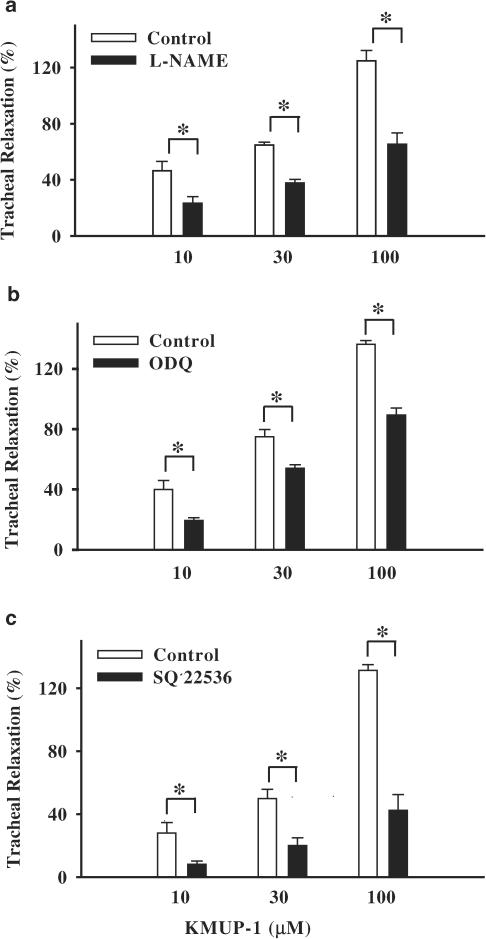
After pretreatment with a NOS inhibitor L-NAME and an sGC inhibitor ODQ, the relaxations elicited by KMUP-1 were significantly reduced. In addition, pretreatment with an adenylyl cyclase inhibitor SQ 22536 also attenuated the relaxant effects induced by KMUP-1 (Figure 5).
Figure 5.
Tracheal relaxant effects of KMUP-1 on guinea-pig tracheal strips precontracted by carbachol in the absence and presence of L-NAME (100 μM), ODQ (10 μM) and SQ 22536 (100 μM). Each value represents the mean±s.e.m. of eight experiments. *Indicates P<0.05, paired Student's t-test.
Inhibition of phosphodiesterase
The inhibitory effects of KMUP-1 (10 μM) on PDE3, 4 and 5 enzyme activities were 23±2.7, 18±2.5 and 29±3.1% (n=3), respectively. Similarly, the effects of theophylline (10 μM) on PDE3, 4 and 5 were 8±1.0, 8±1.2 and 12±2.1% (n=3). The IC50 values of both agents were not estimated because 50% inhibition was not achieved at concentrations up to 10 μM. Under these conditions, IBMX was used as a reference agent and its IC50 values for PDE3, 4 and 5 were 6.5±1.2, 26.3±3.9 and 31.7±5.3 μM (n=3), respectively.
Measurement of cyclic AMP and GMP in cultured TSMCs
We examined the effect of KMUP-1 on cyclic AMP and GMP levels in the presence of the nonselective PDE inhibitor IBMX (100 μM) in primary cultures of guinea-pig TSMCs. The basal release of cyclic AMP and cyclic GMP was 63.1±5.6 and 1.12±0.08 pmol mg−1 protein, respectively (n=3). KMUP-1 (0.01, 0.1, 1, 10 μM) significantly increased both cyclic AMP (90.2±5.3, 96.1±5.8, 113.3±7.1, 110.8±7.9 pmol mg−1 protein) and GMP (1.45±0.07, 1.60±0.07, 1.69±0.05, 1.76±0.12 pmol mg−1 protein) levels as compared with each basal value. In addition, we observed the effects of KMUP-1 alone, in the absence of IBMX, on both cyclic AMP and cyclic GMP levels. The effects of KMUP-1 in the absence of IBMX on cyclic AMP and cyclic GMP levels were not significantly different from those in the presence of IBMX. We propose that the PDE inhibitory activity of KMUP-1 per se is enough to prevent the cyclic nucleotides breakdown.
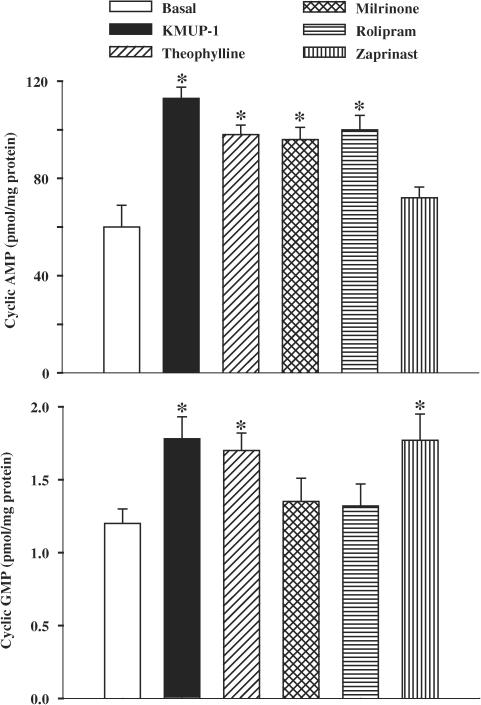
Moreover, we compared the cyclic AMP and GMP levels obtained in the presence of KMUP-1 to those with theophylline, milrinone, rolipram and zaprinast at 100 μM. KMUP-1, theophylline, milrinone and rolipram all elicited significant elevations of cyclic AMP accumulation, but this was not so for the selective PDE5 inhibitor zaprinast. Although KMUP-1, theophylline and zaprinast significantly enhanced the amounts of cyclic GMP, this was not observed with milrinone and rolipram, selective PDE3 and 4 inhibitors, respectively (Figure 6). Furthermore, the raised cyclic AMP levels induced by KMUP-1 and forskolin, an adenylyl cyclase activator, were almost eliminated by pretreatment with an adenylyl cyclase inhibitor SQ 22536 (100 μM) (Table 1). Likewise, the increased accumulation of cyclic GMP induced by KMUP-1 was abolished by pretreatment with the NOS inhibitor L-NAME or the sGC inhibitor ODQ (Table 2).
Figure 6.
Effects of KMUP-1, theophylline, milrinone, rolipram and zaprinast (100 μM) on cyclic AMP and GMP levels in guinea-pig cultured tracheal smooth muscle cells. Each value represents the mean±s.e.m. of three independent experiments. *P<0.05 as compared with the basal value, ANOVA followed by Dunnett's test.
Table 1.
Effects of KMUP-1 and forskolin (100 μM) on cyclic AMP levels in cultured guinea-pig tracheal smooth muscle cells, and in the absence and presence of SQ 22536 (100 μM)
| Treatment | Cyclic AMP (pmol mg protein−1) |
|---|---|
| Control | 70.5±4.6 |
| SQ 22536 | 75.2±6.0 |
| Forskolin | 105.3±4.9* |
| SQ 22536 plus Forskolin | 76.1±3.7a |
| KMUP-1 | 118.6±5.8* |
| SQ 22536 plus KMUP-1 | 84.0±5.2b |
Each value represents the mean±s.e.m. of three independent experiments.
Indicates P<0.05 as compared with the control.
Indicates P<0.05 as compared with forskolin.
Indicates P<0.05 as compared with KMUP-1.
Table 2.
Effects of KMUP-1 (100 μM) on cyclic GMP levels in cultured guinea-pig tracheal smooth muscle cells in the absence and presence of L-NAME (100 μM) and ODQ (10 μM)
| Treatment | Cyclic GMP (pmol mg protein−1) |
|---|---|
| Control | 1.10±0.11 |
| L-NAME | 1.15±0.12 |
| ODQ | 0.98±0.14 |
| KMUP-1 | 1.72±0.08* |
| L-NAME plus KMUP-1 | 1.16±0.10* |
| ODQ plus KMUP-1 | 1.03±0.09a |
Each value represents the mean±s.e.m. of three independent experiments.
Indicates P<0.05 as compared with the control.
Indicates P<0.05 as compared with KMUP-1.
Expression of protein kinases A and G in cultured TSMCs
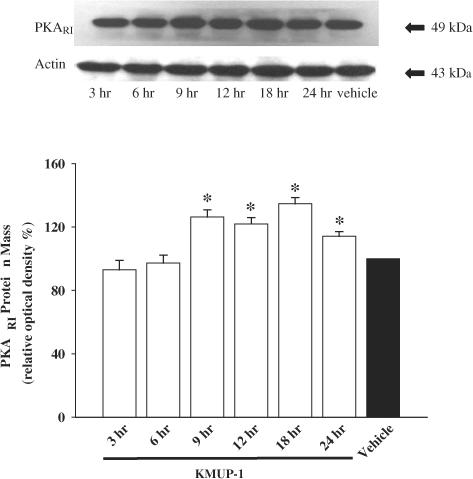
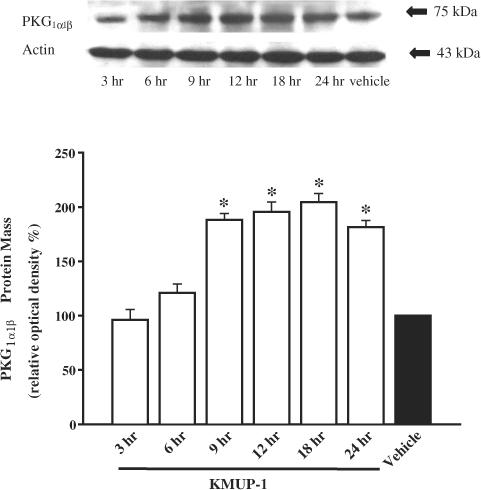
The effect of KMUP-1 on the expression of PKA and PKG was evaluated using the Western blot technique. As shown in Figure 7, KMUP-1 (10 μM) stimulated the expression of immunoreactive PKARI in a time-dependent manner. The monoclonal antibody of PKARI recognizes a band at 49 kDa in guinea-pig TSMCs. In addition, KMUP-1 also stimulated the expression of immunoreactive PKG1α1β in a time-dependent manner (Figure 8). The polyclonal antibody of PKG1α1β recognizes a band at 75 kDa. KMUP-1 increased the level of expression of both PKA and PKG proteins, although this did not achieve statistical significance until 9 h of exposure to the drug.
Figure 7.
Time dependence of the representative Western blot and corresponding data depicting PKARI protein abundance in guinea-pig cultured tracheal smooth muscle cells incubated for 3–24 h in the presence of vehicle and KMUP-1 (10 μM). Each value represents the mean±s.e.m. of three independent experiments. *P<0.05 as compared with the vehicle, ANOVA followed by Dunnett's test.
Figure 8.
Time dependence of the representative Western blot and corresponding data depicting PKG1α1β protein abundance in guinea-pig cultured tracheal smooth muscle cells incubated for 3–24 h in the presence of vehicle and KMUP-1 (10 μM). Each value represents the mean±s.e.m. of three independent experiments. *P<0.05 as compared with the vehicle, ANOVA followed by Dunnett's test.
Airway obstruction and lung function
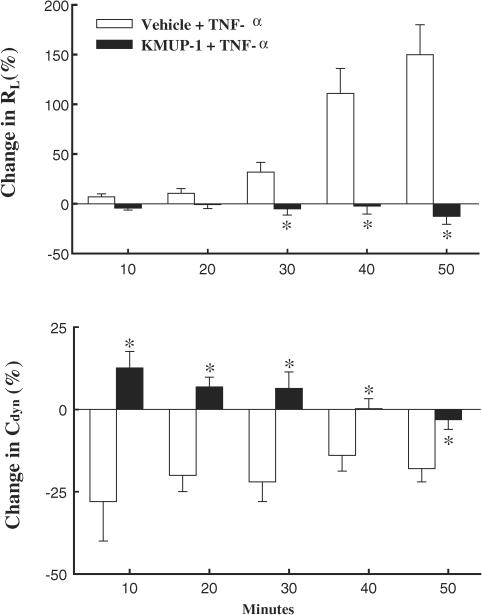
The baseline values of total lung resistance and dynamic lung compliance were 0.26±0.01 cmH2O ml−1 s−1 and 0.32±0.01 ml cmH2O−1, respectively. The intratracheal administration of TNF-α to guinea-pig induced bronchoconstriction and probably oedema, and a marked increase in lung resistance and decrease in dynamic compliance were observed at 10, 20, 30, 40 and 50 min. Intravenous administration of KMUP-1 (1.0 mg kg−1) 10 min before the intratracheal instillation of TNF-α reversed these changes in RL and Cdyn, with the exception of the Cdyn at 50 min (Figure 9).
Figure 9.
Effects of pretreatment with KMUP-1 (1.0 mg kg−1) on total lung resistance (RL) and dynamic lung compliance (Cdyn) during intratracheal TNF-α-induced (0.01 mg kg−1) bronchoconstriction. Each value represents the mean±s.e.m. from six independent experiments. *P<0.05 as compared with the vehicle+TNF-α, ANOVA followed by Student–Newman–Keuls test.
Discussion
It was established previously that KMUP-1, derived from the xanthine-based theophylline, induces vascular and corpus cavernosum smooth muscle relaxations via inhibition of PDEs, opening of K+ channels and activation of the NO/sGC/cyclic GMP pathway (Wu et al., 2001; Lin et al., 2002). In this study, we further observed that KMUP-1 stimulates the accumulation of cyclic AMP and GMP and consequently activates the expression of PKG1α1β and PKARI in guinea-pig TSMs. We know that increased PKA and PKG can stimulate K+ outflow, leading to the blockade of Ca2+ influx-associated contractility. Thus, KMUP-1-induced relaxation of guinea-pig trachea appears to be the result of multiple intracellular actions.
The role of phosphodiesterases in regulating smooth muscle relaxation has received much interest in recent years. Several PDE isoenzymes have been identified in airway smooth muscle, although the proportion of these enzymes varies between species and at different airway levels (Barnes, 1995; Rabe et al., 1995). PDE3 and PDE4 are important in the breakdown of cyclic AMP; PDE3 is involved in the regulation of airway smooth muscle tone, whereas PDE4 is more important in inflammatory cells, such as mast cells, macrophages, eosinophils, T-lymphocytes and epithelial cells. PDE5 is involved in the breakdown of cyclic GMP in airway and vascular smooth muscle (Barnes, 1995). Nonselective inhibitors of cyclic nucleotide phosphodiesterase, such as theophylline, have been used for the treatment of obstructive airway diseases for several decades (Serafin, 1996). In our guinea-pig isolated TSM experiments, we observed that KMUP-1 was more potent at inducing relaxant effects than the nonselective and selective PDE inhibitors, theophylline, milrinone, rolipram and zaprinast, as well as the KATP channel opener levcromakalim. Notably, the response to KMUP-1 was significantly attenuated by pretreatment with the adenylyl cyclase inhibitor SQ 22536. These results encouraged us to investigate further the underlying mechanism of the effect of KMUP-1 on guinea-pig trachea. In biological enzyme experiments, we demonstrated that KMUP-1 affected cyclic nucleotide breakdown at 10 μM. Although it was not particularly potent at producing this effect, it did inhibit the enzyme activities of PDE3, 4 and 5. Furthermore, KMUP-1 and the PDE inhibitors theophylline, milrinone and rolipram markedly elevated the intracellular cyclic AMP levels. However, the selective PDE5 inhibitor zaprinast did not increase cyclic AMP and was treated as a negative control. SQ 22536 completely abolished the raised cyclic AMP levels induced by KMUP-1, as did the adenylyl cyclase activator forskolin. Hence, KMUP-1-induced TSM relaxation could be due to activation of the adenylyl cyclase pathway and also to inhibition of the PDE3 and 4 enzymes. Previous studies have indicated that the combination of PDE3 and 4 inhibitors is most effective in relaxing airway smooth muscle, suggesting that co-operation of the two isoenzymes PDE3 and 4 is needed for optimal bronchodilator effects (de Boer et al., 1992).
NO is generally accepted to play an important role in the regulation of the airway function. Several NO-related compounds, such as SNP, and endogenous NO activate sGC, elevate cyclic GMP and relax airway smooth muscles (Katsuki & Murad, 1977). NO has been shown to be a relaxant transmitter between epithelium and smooth muscle in guinea-pig tracheas and in human airways due to its ability to activate sGC (Tucker et al., 1990). Since KMUP-1 was still able to evoke a trachea relaxant effect in epithelium-denuded tracheal strips or during inhibition of NOS, it is likely to have a direct action on TSM that does not involve the epithelium. However, at least part of the response to KMUP-1 is epithelium-dependent because a significant downward shift in the dose–response curve was observed after epithelium denudation. In addition, we demonstrated that the tracheal relaxant effect of KMUP-1 was significantly attenuated, but not completely inhibited, by pretreatment with the sGC inhibitor ODQ. KMUP-1 markedly increased the intracellular accumulation of cyclic GMP and this response was completely eliminated by the NOS inhibitor L-NAME and the sGC inhibitor ODQ. This suggests that under these conditions the KMUP-1-induced TSM relaxations are mediated by activation of the NO/sGC pathway, inhibition of PDE5, and the resulting accumulation of cyclic GMP. Indeed, these results accord with those previously observed in rat aortic (Wu et al., 2001) and rabbit corpus cavernosum smooth muscles (Lin et al., 2002).
There is evidence suggesting that NO is capable of directly stimulating Ca+2-dependent K+ channels in smooth muscle cells (Bolotina et al., 1994), including airway smooth muscle (Abderrahmane et al., 1998), which leads to hyperpolarization and decreases in Ca2+ influx and subsequently causes relaxation (Lincoln & Cornwell, 1991). To investigate whether these K+ channels are also involved in guinea-pig TSM relaxation responses to KMUP-1, the preparation was pretreated with various K+ channel blockers. We observed that the ability of these K+ channel blockers to reduce the responses to KMUP-1 was relatively weak, with the exception of apamin. Thus, the relaxant responses to KMUP-1 appear to be due to a more selective effect on the small-conductance Ca2+-dependent K+ channels in guinea-pig TSMs. This result also confirms our hypothesis that KMUP-1 stimulates K+ efflux, which in turn causes hyperpolarization and relaxation of guinea-pig TSMs.
In our isolated tissue experiments, the tracheal relaxation response to KMUP-1 occurs within a few minutes and its concentration–response curve appears to be biphasic. From these results, we propose that at least two distinct signalling pathways are involved in the KMPU-1–induced tracheal relaxations: the fast response is probably caused by the activation of K+ channels, whereas the slow response is mediated via the accumulation of intracellular cyclic nucleotide accumulation due to an effect on protein kinases. This would account for the rapid tracheal relaxation response to KMUP-1 and the significant change in the protein expression of either PKA or PKG not being observed until after 9 h in cultured TSMCs. In this study, we also demonstrated that KMUP-1 exhibits a time-dependent increase in the expression of proteins PKARI and PKG1α1β in cultured TSMCs. Taken together, these results indicate that the TSM relaxation responses to KMUP-1 are mediated via two major pathways: one involves activation of K+ channels and is independent of cellular cyclic nucleotides; the other is probably mediated by increases in both cyclic AMP and cyclic GMP, followed by stimulation of PKA and PKG cascades. Increased PKA and PKG appear to activate K+ channels, resulting in the lowering of cellular Ca2+ levels (Figure 10). The precise mechanism by which KMUP-1 activates the K+ channels remains to be elucidated by use of conventional ruptured patch-recording techniques. However, KMUP-1 has been confirmed to open Ca2+-dependent K+ channels directly in rat cerebral vascular smooth muscle cells (data not shown).
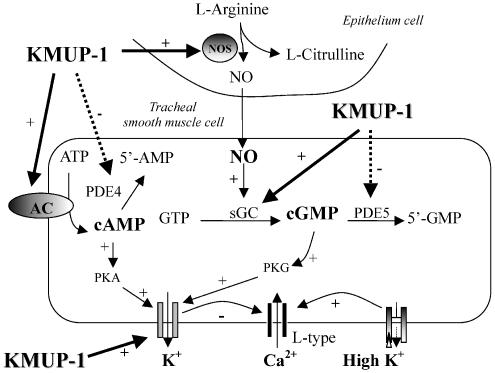
Figure 10.
Proposed mechanisms of action of KMUP-1 on guinea-pig tracheal epithelium and smooth muscle cells. KMUP-1 activates the NO/sGC/cyclic GMP and AC/cyclic AMP pathways and subsequently increases the intracellular levels of cyclic AMP and cyclic GMP. Additionally, KMUP-1 inhibits PDE4 and 5-specific enzymes for cyclic AMP and cyclic GMP, respectively. Increased cyclic AMP and cyclic GMP then activate the protein kinases PKA and PKG cascades, and thus enhance the K+ efflux, leading to attenuation of Ca2+ influx-associated contractility in smooth muscle cells. AC indicates adenylate cyclase.
TNF-α, a cytokine which can attract and activate inflammatory macrophages and neutrophils during type IV hypersensitivity reactions, is usually synthesized in response to infection, including those found in the trachea or airway system (Hakonarson et al., 1996). Since KMUP-1 enhances cyclic nucleotide levels and inhibits the activity of PDEs, we decided to investigate the ability of KMUP-1 to protect against intratracheal TNF-α-induced airway obstruction, which probably results from both bronchoconstriction and oedema. The acute TNF-α-induced bronchoconstriction was manifested as a prominent increase in RL with a concomitant decrease in Cdyn. Our results showed that TNF-α-induced increases in RL and reduction in Cdyn could be reversed by KMUP-1. This indicates that KMUP-1 inhibits the onset of TNF-α-mediated bronchoconstriction. Indeed, we also found that KMUP-1 attenuated the TNF-α-induced increased expression of both inducible NOS (iNOS) and cyclooxygenase II (COX-II) associated with inflammatory responses (data not shown).
In summary, we have provided evidence that the relaxation of guinea-pig isolated trachea induced by the xanthine derivative KMUP-1 is a result of several intracellular actions of this drug. These include sGC activation, inhibition of PDEs, an additional epithelium-derived effect, elevation of intracellular cyclic AMP and GMP levels, increased expression of cyclic nucleotide-dependent PKA and PKG and associated K+ channel openings.
Acknowledgments
We thank B. William Cypaers, MS for his helpful discussions and reading of this manuscript. This study was supported by grants NSC-89-2320-B-037-056-M59 to Dr Ing-Jun Chen, NSC-92-2320-B-037-025 to Dr Bin-Nan Wu from the National Science Council, Taiwan and Vital Pharm Co, Ltd (Kaohsiung, Taiwan).
Abbreviations
- AC
adenylate cyclase
- 4-AP
4-aminopyridine
- cyclic AMP
adenosine 3′,5′-cyclic monophosphate
- cyclic GMP
guanosine 3′,5′-cyclic monophosphate
- IBMX
3-isobutyl-1-methylxanthine
- KATP channels
ATP-sensitive potassium channels
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PDE
phosphodiesterase
- PKA
protein kinase A
- PKG
protein kinase G
- sGC
soluble guanylate cyclase
- SQ 22536
9-(terahydro-2-furanyl)-9H-purin-6-amine
- SNP
sodium nitroprusside
- TEA
tetraethylammonium
- TNF-α
tumour necrosis factor-α
- TSMC
tracheal smooth muscle cell
References
- ABDERRAHMANE A., SALVAIL D., DUMOULIN M., GARON J., CADIEUX A., ROUSSEAU E. Direct activation of K(Ca) channel in airway smooth muscle by nitric oxide: involvement of a nitrothiosylation mechanism. Am. J. Respir. Cell. Mol. Biol. 1998;19:485–497. doi: 10.1165/ajrcmb.19.3.2996. [DOI] [PubMed] [Google Scholar]
- ALLEN S.L., BOYLE J.P., CORTIJO J., FOSTER R.W., MORGAN G.P., SMALL RC. Electrical and mechanical effects of BRL34915 in guinea-pig isolated trachealis. Br. J. Pharmacol. 1986;89:395–405. doi: 10.1111/j.1476-5381.1986.tb10273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCH J.R.S., BUCKLE D.R., BUMSTEAD J., CLARKE G.D., TAYLOR J.F., TAYLOR S.G. Evaluation of the potassium channel activator cromakalim (BRL34915) as a bronchodilator in the guinea-pig: comparison with nifedipine. Br. J. Pharmacol. 1988;95:763–770. doi: 10.1111/j.1476-5381.1988.tb11702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J. Cyclic nucleotides and phosphodiesterases and airway function. Eur. Respir. J. 1995;8:457–462. doi: 10.1183/09031936.95.08030457. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BERNAREGGI M.M., BELVISI M.G., PATEL H., BARNES P.J., GIEMBYCZ M.A. Anti-spasmogenic activity of isoenzyme-selective phosphodiesterase inhibitors in guinea-pig trachealis. Br. J. Pharmacol. 1999;128:327–336. doi: 10.1038/sj.bjp.0702779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BOLTON T., LANG R., TAKEWAKI T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J. Physiol. 1984;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER J., PHILPOTT K.J., VAN AMSTERDAM R.G.M., SHAHID M., ZAAGSMA H., NICHOLSON CD. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br. J. Pharmacol. 1992;106:1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKERTS G., NIJKAMP F.P. Airway epithelium: more than just a barrier! Trends Pharmacol. Sci. 1998;19:334–341. doi: 10.1016/s0165-6147(98)01232-2. [DOI] [PubMed] [Google Scholar]
- GRUETTER C.A., CHILDERS C.E., BOSSERMAN M.K., LEMKE S.M., BALL J.G., VALENTOVIC M.A. Comparison of relaxation induced by glyceryl trinitrate, isosorbide dinitrate, and sodium nitroprusside in bovine airways. Am. Rev. Respir. Dis. 1989;139:1192–1197. doi: 10.1164/ajrccm/139.5.1192. [DOI] [PubMed] [Google Scholar]
- HAKONARSON H., HERRICK D.J., SERRANO P.G., GRUNSTEIN M.M. Mechanism of cytokine-induced modulation of β-adrenoceptor responsiveness in airway smooth muscle. J. Clin. Invest. 1996;97:2593–2600. doi: 10.1172/JCI118708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIDAKA H., ASANO T. Human blood platelet 3′:5′-cyclic nucleotide phosphodiesterase: isolation of low-Km and high-Km phosphodiesterase. Biochim. Biophys. Acta. 1976;429:485–497. doi: 10.1016/0005-2744(76)90296-5. [DOI] [PubMed] [Google Scholar]
- HSU T.H., LAI Y.L., KOU Y.R. Acetylcholine and tachykinin receptor antagonists attenuate wood smoke-induced bronchoconstriction in guinea pigs. Eur. J. Pharmacol. 1998;360:175–183. doi: 10.1016/s0014-2999(98)00690-6. [DOI] [PubMed] [Google Scholar]
- HWANG T.L., WU C.C., TENG C.M. YC-1 potentiates nitric oxide-induced relaxation in guinea-pig trachea. Br. J. Pharmacol. 1999;128:577–584. doi: 10.1038/sj.bjp.0702830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATSUKI S., MURAD F. Regulation of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol. Pharmacol. 1977;13:330–341. [PubMed] [Google Scholar]
- KUBO M., NAKAYA Y., MATSUOKA S., SAITO K., KURODA Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle. Circ. Res. 1993;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- Lin R.J., WU B.N., LO Y.C., SHEN K.P., LIN Y.T., HUANG C.H., CHEN I.J. KMUP-1 relaxes rabbit corpus cavernosum smooth muscle in vitro and in vivo: involvement of cyclic GMP and K+ Channels. Br. J. Pharmacol. 2002;135:1159–1166. doi: 10.1038/sj.bjp.0704554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINCOLN T.M., CORNWELL T.L. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28:129–137. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- MATSUSHITA Y., HENMI S., MURAKI K., IMAIZUMI Y., WATANABE M. Cromakalim-induced membrane current in guinea-pig tracheal smooth muscle cells. Eur. J. Pharmacol. 2000;389:51–58. doi: 10.1016/s0014-2999(99)00872-9. [DOI] [PubMed] [Google Scholar]
- MCCAIG D.J., DE JONCKHEERE B. Effect of cromakalim on bronchoconstriction evoked by cholinergic nerve stimulation in guinea-pig isolated trachea. Br. J. Pharmacol. 1989;98:662–668. doi: 10.1111/j.1476-5381.1989.tb12641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAD F., MITTAL C.K., ARNOLD W.P., KATSUKI S., KIMURA H. Guanylate cyclase: activation by azide, nitrocompounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv. Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitivity potassium channels. J. Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURTHY K.S., ZHOU H., MAKHLOUF G.M. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am. J. Physiol. Cell Physiol. 2002;282:C508–C517. doi: 10.1152/ajpcell.00373.2001. [DOI] [PubMed] [Google Scholar]
- NIJKAMP F.P., VAN DER LINDE H.J., FOLKERTS G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro: role of the epithelium. Am. Rev. Respir. Dis. 1993;148:727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- POLSON J.B., KRZANOWSKI J.J., SZENTIVANYI A. Correlation between inhibition of a cyclic GMP phosphodiesterase and relaxation of canine tracheal smooth muscle. Biochem. Pharmacol. 1985;34:1875–1879. doi: 10.1016/0006-2952(85)90301-6. [DOI] [PubMed] [Google Scholar]
- RABE K.F., MAGNUSSEN H., DENT G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur. Respir. J. 1995;8:637–642. [PubMed] [Google Scholar]
- SERAFIN W.E.Drugs used in the treatment of asthma Goodman and Gilman's The Pharmacological Basis of Therapeutics 1996New York: McGraw-Hill; 659–682.In: ed. HARDMAN, J.G., LIMBIRD, L.E., MOLINOFF, P.B., RUDDON, R.W. & GILMAN A.G. [Google Scholar]
- TANAKA H., OGAWA K., TAKAGI K., SATAKE T., HIDAKA H. Inhibition of cyclic GMP phosphodiesterase by xanthine derivatives relaxes guinea-pig trachealis smooth muscle. Clin. Exp. Pharmacol. Physiol. 1991;18:163–168. doi: 10.1111/j.1440-1681.1991.tb01427.x. [DOI] [PubMed] [Google Scholar]
- TUCKER J.F., BRAVE S.R., CHARALAMBOUS L., HOBBS A.J., GIBSON A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br. J. Pharmacol. 1990;100:663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDELT T., BOLDT W., MARKWARDT F. Acetylcholine-induced K+ currents in smooth muscle cells of intact rat small arteries. J. Physiol. 1997;500:617–630. doi: 10.1113/jphysiol.1997.sp022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B.N., HWANG T.L., LIAO C.F., CHEN I.J. Vaninolol: a new selective β1-adrenoceptor antagonist derived from vanillin. Biochem. Pharmacol. 1994;48:101–109. [PubMed] [Google Scholar]
- WU B.N., LIN R.J., LIN C.Y., SHEN K.P., CHIANG L.C., CHEN I.J. A xanthine-based KMUP-1 with cyclic GMP enhancing and K+ channels opening activities in rat aortic smooth muscle. Br. J. Pharmacol. 2001;134:265–274. doi: 10.1038/sj.bjp.0704231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU H.L., TORPHY T.J. Relationship between cyclic guanosine monophosphate accumulation and relaxation of canine trachealis induced by nitrovasodilators. J. Pharmacol. Exp. Ther. 1991;258:972–978. [PubMed] [Google Scholar]