Abstract
The voltage-dependent displacement of the scorpion Tityus serrulatus α-toxin Ts3 was investigated in native sodium channels of GH3 cells by examining the removal of its effects in toxin-free solution.
Toxin at saturating concentration was pulsed (∼1 s) directly onto the cell, thus causing an eight-fold increase of the slow component (τs=6 ms) of fast inactivation, and a three-fold increase of the time constant of its fast component.
At 0 mV, maximal conductance was achieved in cells before and after treatment with Ts3, and no displacement of the toxin could be detected.
Toxin displacement occurred if stronger depolarising pulses (>100 mV) were applied. The rate of displacement depended on the amplitude and duration of the pulses, and was not related with outward Na+ flux.
We propose a model in which activation does not require complete movement of segment S4 of domain IV (IVS4) and that a more extensive movement of this segment is needed for normal fast inactivation. A kinetic model is presented that can account for the typical effects of site 3 toxins.
Keywords: Inactivation, sodium channel, sodium current, scorpion toxin, patch clamp, mechanism of action, voltage-dependence, Q matrix
Introduction
Voltage-gated Na+ channels are responsible for the conduction of electrical impulses in most excitable cells, due to their property of opening transiently upon depolarisation. Their 260 kDa α-subunit contains four homologous domains (I–IV), each one with six transmembrane segments (S1–S6). The S4 segment in each domain contains positive charges and acts as voltage sensor, whose movement in response to depolarisation is accounted for the voltage-dependent state transitions of the channel. The fast inactivation gate is formed by the intracellular loop connecting domains III and IV, which blocks the ion-conducting pore few milliseconds after the channel opens (reviewed by Catterall, 2000). The inactivation of the Na+ channel is a voltage-dependent process, and derives most of its voltage dependence from coupling to activation (Armstrong & Bezanilla, 1977). Strong evidence implicates the S4 segment in domain IV (IVS4) in this process (Chen et al., 1996; Sheets et al., 1999; Chanda & Bezanilla, 2002).
Sodium channels are the molecular targets of several groups of neurotoxins that bind to specific receptor sites of the channel protein and modify their function (Cestèle & Catterall, 2000). The α-scorpion toxins comprise a family of structurally and functionally related polypeptide neurotoxins, which bind to site 3 and slow down fast inactivation (Possani et al., 1999). Receptor site 3 is partially formed by amino-acid residues in the extracellular linker between segments IVS3 and IVS4 (Rogers et al., 1996; Benzinger et al., 1998) and its binding affinity is decreased by depolarisation (Catterall, 1977; Couraud et al., 1978). Several evidences suggest that IVS4 has a strong role in voltage-dependent association of these toxins, since part of this segment forms site 3. However, the molecular mechanisms of action of these toxins remain unknown.
The effects of the α-toxin Ts3 (previously named TsIV-5), purified from the Brazilian scorpion Tityus serrulatus, on sodium channel inactivation have been studied by Kirsch et al. (1989). Ts3 contains 62 amino-acid residues, a molecular weight of 7.2 kDa and forms four disulphide bridges (Possani et al., 1999).
The present work investigates the voltage-dependent dissociation of Ts3 from native sodium channels in the absence of free toxin, and correlates it with activation and inactivation of Na+ currents. The data show that Ts3 is displaced from its binding site at potentials higher than those required for full activation. We propose that activation does not require complete movement of all S4 segments, and that a more extensive movement of IVS4 is needed for normal fast inactivation. The proposed model is consistent with data reported on the voltage dependence of inactivation and can quantitatively explain the effect of site 3 toxins. Preliminary results obtained with tityustoxin, a fraction of T. serrulatus venom enriched with Ts3, have been published in abstract form (Beirão et al., 2002).
Methods
Pituitary tumour GH3 cells were obtained from the American Type Culture Collection (ATCC) and grown in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% foetal calf serum (Cultilab, Campinas, Brazil) and 1% penicillin/streptomycin (Sigma Chemical Co., MO, U.S.A.). The cells were incubated in humidified atmosphere with 5% CO2 at 37°C. For electrophysiological experiments, cells were detached with trypsin-EDTA solution and plated in 47-mm sterile dishes, and used 2–7 days thereafter.
Electrophysiological recordings
Inward and outward Na+ currents were recorded with the whole-cell patch-clamp technique, using an Axopatch 200B amplifier operated by PClamp6 software (Axon Instruments). Soft glass patch pipettes had resistances of 1.5–2.5 MΩ that were compensated for by 50%, to minimise voltage errors. Currents were low-pass filtered (cutoff frequency of 5 kHZ) and sampled at 10 kHZ. Leak and capacitive currents were subtracted using a P/6 protocol. The holding potential was −80 mV.
For inward currents the bath solution contained (in mM) NaCl 140, CsCl 5.4, CaCl2 1.8, HEPES 10 and glucose 5, pH 7.4, adjusted with NaOH. The pipette solution contained (in mM) CsF 100, NaCl 5, EGTA 5, tetraethylammonium chloride 40 and HEPES 10, pH 7.2 adjusted with CsOH. For Na+ outward currents, choline chloride substituted for NaCl in the bath solution (pH 7.4 adjusted with CsOH), and the pipette solution contained (in mM) NaCl 130, EGTA 5 and HEPES 10, pH 7.2 adjusted with NaOH. The currents were recorded on cells under continuous perfusion with bath solution at the temperature of 25±1°C, unless otherwise stated. All reagents used were of analytical grade.
Ts3 purification and delivery
The venom from the scorpion Tityus serrulatus was obtained from the Butantan Institute, São Paulo, Brazil. Purification of toxin Ts3 was processed essentially as described by Possani et al. (1981), with a slight modification at the last step of separation. Briefly, the soluble venom was dissolved in 20 mM ammonium acetate buffer, pH 4.7, and gel-filtered in Sephadex G-50. The fraction eluted in position IV was applied into a carboxymethyl-cellulose ion exchanger column in the same buffer, and eluted with a salt gradient from 0 to 0.55 M NaCl. The component number 5 was recovered, concentrated by freeze-drying and applied to an analytical C18 reverse-phase column (Vydac, Hysperia, CA, U.S.A.), and fractionated using a linear gradient made from solution A (0.12% trifluoacetic acid in water) to solution B (0.10% trifluoacetic acid in acetenotrile), run from 20 to 40% solution B, during 20 min. The last chromatographic step separation was performed in a HPLC-Waters 996 with a Photodiode Array Detector (Millipore Co., MA, U.S.A.). The main component eluting at 14.76 min is pure toxin Ts3, and corresponds to 84% of the material applied into the column. The amino-acid sequence was confirmed by Edman degradation of the N-terminal sequence and by mass spectrometry (data not shown).
Ts3 was diluted in the bath solution, containing 50 μg ml−1 cytochrome c (Sigma Chemical Co, MO, U.S.A.), to prevent nonspecific binding. Cytochrome c alone had no effect on the currents.
After recording control currents, a single pulse of Ts3 solution (∼20 μl for 1 s) was applied directly and surrounded the cell under investigation, using a microperfusion system controlled by a solenoid valve. The excess of toxin was washed off by continuous perfusion with the bath solution.
Data analysis
Data analysis and curve fittings were performed using SigmaPlot v.5 (Jandel Scientific). The proportion of channels in the open states, predicted by the model shown in Figure 6, was calculated using the Q-matrix method, as described by Colquhoun & Hawkes (1995), using a routine written by one of us (PSLB) running under Matlab 4.2 (The Mathworks, Inc., MA, U.S.A.). To fit the model and estimate the rate constants, the minimisation procedure fmins was used, assuming that after the peak of the current there is no further significant activation of resting channels, that is, that the decay of the current depends only on inactivation. This is a good approximation at potentials of 0 mV or higher. Four of the rate constants were obtained from records in the presence of saturating concentration of Ts3, and were subsequently used when fitting the control records, in paired experiments, in order to estimate the other rate constants (see Discussion). Data are shown as mean±s.e.m., and Student's t-test was used to compare means (P<0.05).
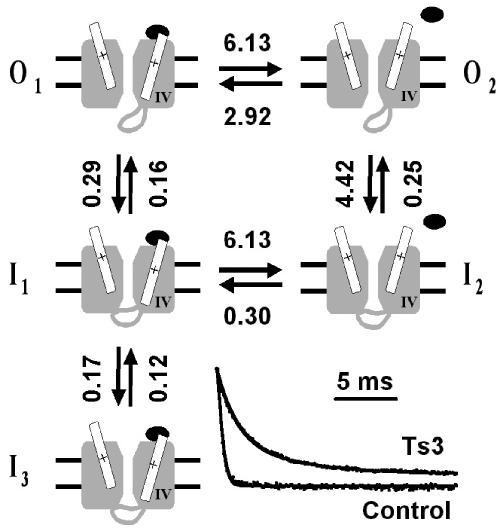
Figure 6.
Model for Ts3 action. A cartoon representing the different conformational states relevant for Ts3 action. For clarity, only two domains of the channel are represented, the one on the right being domain IV. The movement of the S4 segments is largely exaggerated, for better visualisation, and does not intend to represent the actual movement. Transition from closed to open state is not represented in the cartoon, because it is assumed that immediately after the peak of the current, at 0 mV, the fraction of channels that remain in closed states is negligible. When Ts3 (closed ellipse) is bound, only states O1, I1 and I3 can exist. To reach states O2 or I2, IVS4 must move further. The inset shows representative records before and after Ts3 treatment, normalised and superimposed with theoretical curves calculated from the rate constants obtained by fitting the model. The rate constants assigned in the figure (in m s−1) are averages of seven paired experiments. The rates between I1 and I2 are presumed (see Discussion).
Results
Effects of Ts3 on inward sodium currents
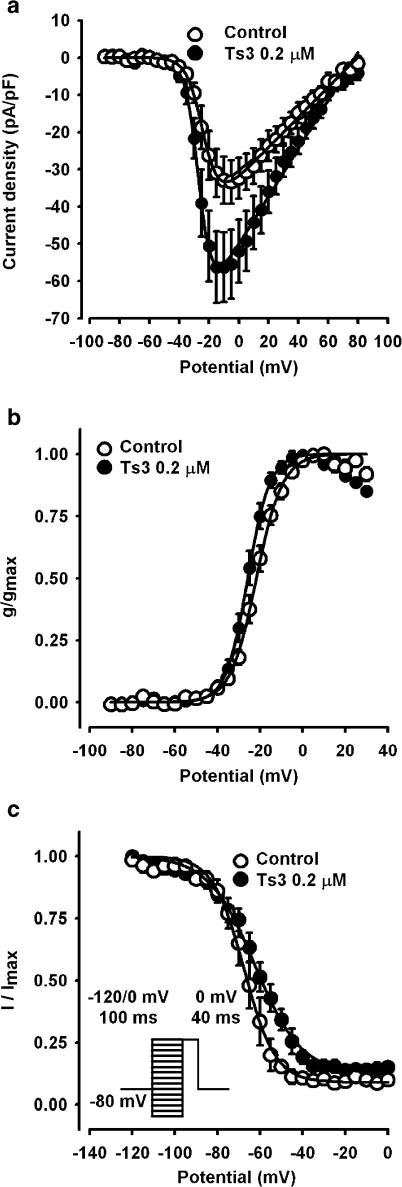
Figure 1 shows that pre-treatment of GH3 cells with saturating concentration (0.2 μM) of Ts3 increases the peak Na+ current and slows down its subsequent decay. The concentration dependence of this effect follows a quadratic hyperbole relationship with half-maximal effect at 10 nM (not shown). Current–voltage (I × V) relationships show that the currents are activated at −30 mV and have maximal amplitude at −10 mV. The I × V curves of 10 experiments were fitted with a modified Boltzmann function and the parameters obtained showed a −4.2 mV shift of the activation and a slight decrease of its slope factor, as shown in Figure 2b. In five paired experiments, the maximal conductance increased 39% by the pre-treatment with Ts3. Both the voltage shift and the increased conductance may result from the inhibition of the inactivation step (Gonoi & Hille, 1987). The calculated voltage dependence of steady-state fast inactivation was shifted by +5.0 mV. Ts3 decreases the steepness of the voltage dependence of inactivation, and increases to 10.7% the percentage of non-inactivating channels (Figure 2c). Nevertheless, these changes were not statistically significant.
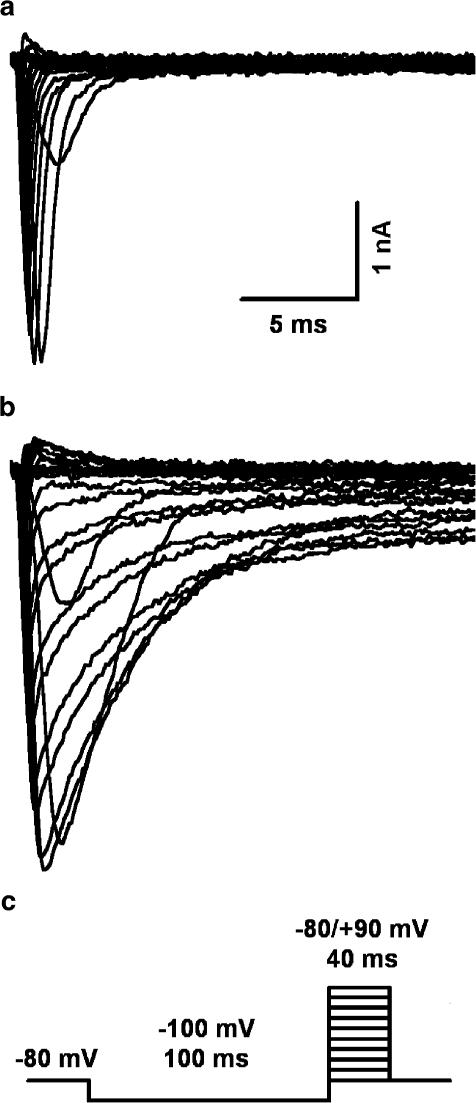
Figure 1.
Effect of Ts3 on Na+ currents. Family of Na+ currents measured in the same cell before (a) and after the treatment with saturating (0.2 μM) concentration of Ts3 (b). The protocol is shown in (c). Maximal currents were obtained at 0 mV.
Figure 2.
Effects of Ts3 on inward Na+ current kinetics and voltage-dependent parameters. (a) I × V curves obtained with the protocol shown in Figure 1c, before and after treatment with Ts3 0.2 μM. Continuous curves were obtained by fitting the curves to the function: I=g(max)·(Vm–Vrev)/{1+exp[(Vg–Vm)/kg]}, where I is the current density, g(max) is the maximal conductance for Na+, Vm is the membrane potential, Vrev is the reversal potential, Vg is the potential of half-maximal conductance and kg is the slope factor. The parameters obtained were: g(max)=0.38±0.06 and 0.58±0.09; Vrev=82.2±4.5 and 83.6±3.3; Vg=−22.1±1.0 and −26.3±1.2; kg=5.4±0.4 and 4.2±0.4, for control (n=10) and experimental (n=10), respectively. (b) G × V curves obtained from individual I × V curves, before and after Ts3 0.2 μM. Continuous curves were obtained by fitting the curves to the Boltzmann function: g/g(max)=1/{1+exp[(Vg−Vm)/kg]}. The parameters obtained were: Vg=−22.1±3.3 and −26.3±3.8; kg=5.3±1.3 and 4.1±1.2, for control (n=10) and experimental (n=10), respectively. (c) Steady-state inactivation before and after Ts3 0.2 μM, obtained with the protocol described on insert. Continuous curves were obtained by fitting the data to the function: I/I(max)=(1−a)/{1+exp[(V−Vh)/kh]}+a, where a is the offset (fraction of channels that do not inactivate), V is the potential during the prepulse, Vh is the potential of half-inactivation and kh is the slope factor. The parameters obtained were: Vh=−67.2±2.7 and −62.3±3.4; kh=6.9±0.8 and 10.7±1.2, for control (n=3) and experimental (n=3), respectively.
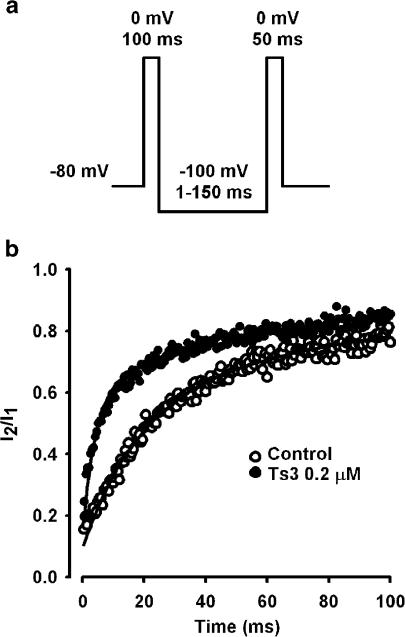
Effect of Ts3 on recovery from fast inactivation
Figure 3 shows the effect of Ts3 on recovery from fast inactivation, obtained with the protocol described in Figure 3a. In cells pre-treated with Ts3 (0.2 μM) the recovery was significantly faster.
Figure 3.
Effect of Ts3 on recovery from inactivation. (a) Pulse protocol used to measure the recovery from inactivation. (b) Comparison of the recovery before (n=6) and after Ts3 treatment (n=7). The fraction of current recovered was plotted as a function of the time between the two pulses applied. Continuous curves show the best fit obtained for each set of data with the function: I2/I1=1−[a exp(−t/τf)+b exp(−t/τs)], where a and b are the amplitudes of the fast and slow components of the recovery, respectively, τf is the fast time constant and τs is the slow time constant. The parameters obtained were: a=0.6±0.03 and 0.6±0.01; b=0.4±0.03 and 0.4±0.01; τf=12.4±0.9 and 3.1±0.4; τs=143.1±29.3 and 85.9±10.0, for control (n=11) and experimental (n=11), respectively. These experiments were carried out at 15°C.
Effects of Ts3 on the inactivation kinetics of the sodium current
In order to quantify Ts3 effect, the decay of each sodium current was fitted to single and double exponential functions (Figure 4b). In most experiments, at 0 mV membrane potential, control records were best fitted with double exponential, with a fast component and a slow component (τf=0.44±0.04 ms, τs=5.9±1.3 ms; n=9). In this case the contribution of the fast component (a) was greater than the contribution of the slow component (b) (a(%)=92.6±1.9, b(%)=7.4±1.9). The decay of the current, at 0 mV, in cells pre-treated with Ts3 was best fitted with double exponential (τf=1.47±0.09 ms, τs=6.13±0.44 ms; n=9), but in this case the contribution of the slow component was greater (b(%)=61.7±5.9), as shown in Figure 4b. Note the significant increase in the fast time constant of decay, in cells pre-treated with Ts3.
Figure 4.
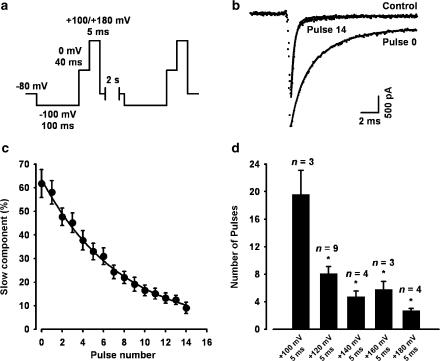
Effect of depolarisation on Ts3 displacement. (a) Pulse protocol used, repeated 15 times every 2 s. (b) Na+ current record in control condition (dotted lines) superimposed with two records obtained from a cell pre-treated with Ts3, before (pulse 0) and after (pulse 14) been subjected to the protocol shown in (a). The depolarising pulse was +120 mV. Superimposed continuous curves were obtained by fitting the current decay to the double exponential function: y=a exp(−t/τf)+b exp(−t/τs)+c, where a, b and c are amplitudes of the fast, slow and non-inactivating components, respectively; t is the time after the peak and τf and τs are the time constants of decay of the fast and slow components, respectively. For single exponential function, b=0. (c) Effect of consecutive depolarising pulses to +120 mV on the percentage contribution of the slow component. Exponential fit to the decay (continuous curve) gives the number of pulses needed for an e-fold displacement of Ts3. (d) Effect of the amplitude of the depolarising pulses on the number of pulses needed for an e-fold displacement of Ts3. *, significantly different from +100 mV data.
Effects of depolarisation on the removal of Ts3 effect
To check the effect of strong depolarising pulses, Na+ currents were recorded during test pulses to 0 mV, which was immediately followed by a stronger depolarising pulse to the potential under investigation. This protocol was repeated 15 times, and the effect of each strong depolarising pulse was probed by the succeeding test pulse (Figure 4a). Note that during the whole protocol the cell is kept under continuous perfusion with toxin-free solution. Therefore, if Ts3 is displaced by depolarisation, at each test pulse its effect should be smaller than in the previous one. In the absence of the strong depolarising pulse (up to 50 pulses) there is no decrease in the effect of Ts3, meaning that it remains bound to its site (not shown). Figure 4b compares representative records obtained with the pulses number 0 (before the first +120 mV strong depolarisation) and number 14 of the series, with a control record (without Ts3). The decay of all pulses no. 14 were also best fitted with double exponential (τf=0.53±0.03 ms, τs=5.3±1.0 ms; n=9), but in this case the contribution of the fast component was greater, and the fast time constant decreased. After successive depolarising pulses the current decay fits were similar to control, suggesting that all Ts3 were displaced from the channels. If another flush of Ts3 is applied, the toxin effect reappears, demonstrating that the channels remain susceptible to it (not shown). Figure 4c shows the progressive decrease of the contribution of the slow component. A single exponential fit provides the number of pulses needed for an e-fold displacement of Ts3. This parameter was used to analyse the voltage dependence of the toxin displacement (Figure 4d), and the number of pulses required for the displacement was significantly voltage-dependent, saturating at +160 mV.
In average, eight pulses to +120 mV with 5 ms duration were needed for an e-fold decrease of the contribution of the slow component, while four pulses of the same amplitude and 20 ms duration produced the same effect (see Figure 5c). Thus, the amplitude as well as duration of the pulse influences the displacement of Ts3 effect.
Figure 5.
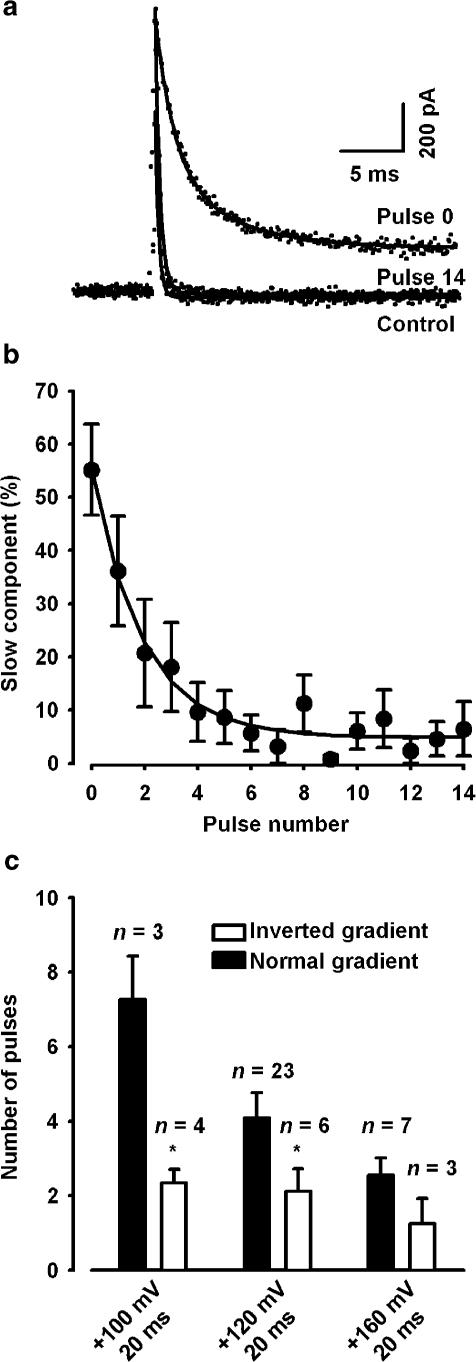
Effect of depolarising pulses on Ts3 displacement with inverted Na+ gradient. (a) Outward Na+ currents (dotted lines) in control condition superimposed with two records obtained from a cell pre-treated with saturating concentration of Ts3, before (pulse 0) and after (pulse 14) been subjected to the protocol shown in Figure 4a (depolarising pulse to +120 mV). Superimposed continuous lines show the best fit to the decay of the currents. Control currents and current generated by pulse 14 were best fitted with single exponential, whereas double exponential was needed after treating with Ts3. (b) Effect of consecutive depolarising pulses to +120 mV on the contribution of the slow component. The exponential curve gives the number of pulses needed for an e-fold displacement of Ts3. (c) Comparison of the number of pulses needed for an e-fold displacement of Ts3 in normal and inverted gradient. *, significantly different from normal gradient at each potential.
Effects of outward sodium currents on Ts3 displacement
The results displayed above suggest that large depolarisation directly affects Ts3 binding. However, one should consider that during very strong depolarisation the Na+ gradient is reversed, and outward currents occur. It is conceivable that the outward movement of Na+ may directly affect the toxin binding. To test this possibility, choline+ was substituted for all external Na+ and NaCl was substituted for CsF in the pipette solution. In that case, outward sodium current is observed in all potentials that activate sodium channels. Consequently, if the sodium efflux was responsible for Ts3 displacement, this phenomenon should be also observed at 0 mV.
Figure 5a shows superimposed outward Na+ currents recorded at 0 mV before and after exposure to saturating concentration of Ts3. The third record was obtained after subsequent application of 14 depolarising pulses, using the same protocol described in Figure 4a, but with a longer (20 ms) depolarising pulse. We can observe that Ts3 slows down the current decay even in those conditions. In this case, the best fits to current decay in control conditions were obtained with a single exponential, with a fast time constant (τf=0.51±0.07 ms, n=7). In cells pre-treated with Ts3 the best fits were obtained with double exponential (τf=1.35±0.12 ms, τs=6.0±1.5 ms), whose slow component gave conspicuous contribution (b(%)=55.0±10.8). Record number 14 shows that in this condition the toxin effect is also removed. Record number 14 was best fitted with a single exponential, similar to control (without Ts3). The contribution of the slow component progressively decreased (Figure 5b) and this decay was fitted with a single exponential. The number of pulses needed for an e-fold decrease was 1.9. Meaningfully, the contribution of the slow component was not altered after 15 pulses to 0 mV without the strong depolarising pulses (not shown), which means that outward Na+ flux is not the cause of Ts3 displacement.
The number of pulses needed to an e-fold decrease of the fast component was used to compare the effects of depolarisation on Ts3 displacement in normal and inverted gradient (Figure 5c). It is noteworthy that the time constants of inactivation, both fast and slow, were the same, regardless of the direction of the gradient. Interestingly, in all potentials studied Ts3 displacement was faster in the inverted gradient. One possible explanation is that the binding of Ts3 is weakened in the absence of extracellular Na+. It has been reported that a small concentration of Na+ is required for the full effect of tityustoxin on the frog nerve (Cruz et al., 2000). Further experiments are required to clarify this point.
Discussion
The molecular mechanism involved in the control of sodium channel inactivation is one of the most exciting problems of the present. There is a consensus that the movement of the S4 segments determine the voltage dependence of the channel transitions (Catterall, 2000). However, growing evidences show that the contribution of the S4 segment of each domain is different. Previous reports show that the movements of IS4, IIS4 and IIIS4 are closely related to activation of the channel (Stühmer et al., 1989; Horn et al., 2000; Chanda & Bezanilla, 2002). On the other hand, the S4 voltage sensors in domains III and IV, but not in I and II, are immobilised in the ‘on' position by fast inactivation, thus providing the molecular basis for the coupling of both phenomena (Cha et al., 1999). Whereas the movement of IIIS4 seems also to be required for activation, the importance of the outward movement of IVS4 is still a matter of dispute. However, the movement of IVS4 seems to be determinant on inactivation (Chahine et al., 1994; Kühn & Greeff, 1999; Horn et al., 2000; Chanda & Bezanilla, 2002).
It has been long recognised that Ts3, as well as other α-scorpion toxins, decreases the rate of Na+ channel inactivation, but a quantitative model that can account for this effect has not been proposed so far. An important clue to their mechanism of action is provided by their decreased affinity at depolarising membrane potentials. We addressed this issue by measuring the decay of the toxin effect upon imposition of depolarising pulses, in the absence of the toxin, to cells previously treated with saturating concentration of Ts3.
We attribute the observed decay of the Ts3 effect to a voltage dependent displacement of the toxin from its binding site, followed by its washing, for two reasons: (1) the current record that follows the sequence of depolarising pulses is very similar to the control, and (2) subsequent addition of Ts3 restores its typical effect, meaning that its binding site was unoccupied. This is consistent with previous demonstrations that the dissociation rate of α-scorpion toxins is voltage-dependent (Catterall et al., 1976; Catterall, 1977; Mozhayeva et al., 1980; Strichartz & Wang, 1986; Rogers et al., 1996; Chen et al., 2000).
The dissociation of Leiurus quinquestriatus toxins from cardiac sodium channels was shown to be voltage-dependent. Since its voltage range extended to higher values, where both activation and inactivation voltage dependence were saturated, a coupling to either phenomena was excluded (Chen & Heinemann, 2001).
We can exclude that the displacement of Ts3 is induced by the outward flux of Na+, because no displacement could be seen at 0 mV with reversed Na+ gradient. In this condition, the outward current was about the same as at +180 mV with normal Na+ gradient. Our results show that Ts3 markedly inhibits inactivation without harming activation, and that at 0 mV normal activation takes place without any measurable displacement of Ts3. Therefore, we can conclude that the binding of Ts3 is compatible with normal activation. The available evidences converge to the idea that the effect of Ts3 results from the hindrance of the complete movement of IVS4: (1) scorpion α-toxins bind to the IVS3-IVS4 linker (Rogers et al., 1996; Cestèle & Catterall, 2000); (2) it has been shown that anthopleurin A, a site 3 toxin, decreases the number of gating charges on the onset of the pulse (Sheets & Hank, 1995) and that the affected charges are in IVS4 (Sheets et al., 1999); (3) the full outward movement of IVS4 seems not to be required for activation (Chanda & Bezanilla, 2002). This set of data strongly suggests that scorpion α-toxins (as other site 3 toxins) prevent the full outward movement of charges and offers a simple explanation for the voltage dependence of Ts3 displacement: when stronger depolarising pulses are applied, the electrical field on IVS4 tends to push the toxin off, thereby increasing the probability of its dissociation.
An alternative explanation, that the toxin is removed by the membrane electrical field acting directly on it, is difficult to sustain. It would require the toxin to immerse somehow into the membrane or channel structure, into a position where it could sense the electrical field across the membrane. Ts3 is polar and highly charged, with nine acidic and nine basic amino acids (one of which is histidine, thus conferring a partial negative charge at neutral pH). Known scorpion toxins adopt a conserved compact folding, and Ts3 is stabilised by four disulphide bridges (Possani et al., 1999). Furthermore, its net negative charge would be attracted, and not repelled, by strong depolarisation.
We propose a model to explain the action of Ts3 and the voltage dependence of its displacement. The toxin can easily bind to channels in the resting states. With depolarising pulses to 0 mV the channel activates, but the bound toxin impairs the full movement of IVS4. At that conformation the channel is open and inactivation can take place, but at a slower rate. This conformation is referred to as O1. Figure 6 shows schematically the proposed model. There are at least two open states: in O1 the channel is activated, but IVS4 is not completely in the outward position. In that situation the channel is open, but inactivation from this state is slow. If there is no hindrance to the complete movement of IVS4, the channel can rapidly proceed to O2, from which inactivation is fast. Therefore, in normal conditions, the predominant sequence of transitions is: O1 → O2 → I2. In the presence of a site 3 toxin, the transition from O1 to O2 is blocked, due to the hindrance of the complete movement of IVS4. Therefore, the channel must follow the much slower sequence: O1 → I1. This transition is likely to show little or no voltage dependence. In fact, preliminary analysis of the time constants of inactivation in Ts3-treated cells, at potentials of 0 mV or higher, shows no significant voltage dependence (not shown). A detailed kinetic analysis of the voltage-dependence of the rate constants is currently under examination. The fitting of the data predicts the presence of a third inactivated state (I3) that is reached at a slower rate. The inclusion of state I3 consistently improves the fitting in all records with Ts3, but its molecular nature cannot be determined at present. Furthermore, its existence is in accord with the two time constants obtained on the analysis of the recovery from inactivation (Figure 3).
Since the proposed model states that Ts3 only blocks the complete outward movement of IVS4, the rate constants between O1, I1 and I3 (back and forth) should be the same, regardless of the presence of Ts3. Therefore, these rates were estimated in the presence of Ts3 and used as fixed parameters when fitting control records. Figure 6 shows representative current records of the same cell before and after treatment with Ts3. Theoretical curves generated by the model are superimposed with the experimental records. The rate constants, calculated from currents generated at 0 mV in seven paired experiments, are shown in the figure. In a normal channel, our model predicts a very short dwell time of state O1 (<0.16 ms), and a low probability of the transition O1 → I1. The maximal fraction of channels in state I1 is about 4% in the control. This means that only a very small fraction of the channels will transit from I1 to I2, and therefore this transition rate cannot be estimated by fitting our data. For the sake of calculation, this transition rate was arbitrarily set as equal to the rate between O1 and O2. It must be emphasised that this value gives minor contribution to the overall current. In fact, when we simulated currents using rates 10 times faster and 10 times slower than the chosen value, the maximal difference between these two currents was less than 0.5% (not shown). In all simulations the rate constant from I2 to I1 was calculated to comply with the microscopic reversibility principle.
Reduction of the rate constant between O1 and O2 will decrease the rate of inactivation. However, the proposition that state O2 and I2 cannot exist when Ts3 is bound provides a simple explanation for all features reported in the present paper, mainly the voltage-dependent removal of the toxin. It can also explain available data in the literature reporting effects of other site 3 toxins, and can explain the inhibition of the inactivation, as has been suggested by Rogers et al. (1996).
It has been proposed recently by Lecar et al. (2003) that the movement of S4 segments of Shaker channels passes through a series of deep energy minima corresponding to the transition states of the gating process, lurching from well to well via thermal transitions. It is conceivable that a similar model applies to the movements of IVS4, and that a few intermediate states exist between the innermost and the outermost positions of IVS4, but with too short dwell times to be resolved. State O1 in our model may correspond to one of these minima, whose subsequent energy barrier would be greatly increased by the bound toxin, thus ceasing its progression. To overcome this minimum, a greater energy (necessary for displacing the toxin) would be required, and it was derived from the increased electrical field in our experiments.
The present model predicts a partial or complete absence of charge immobilisation of the IVS4 segment by inactivation when Ts3 is bound. This prediction is under investigation. Furthermore, it can explain the faster recovery from inactivation seen in cells treated with Ts3, and other site 3 toxins (Benzinger et al., 1999; Chen et al., 2000), because IVS4 is already near its resting state, that is, the channel can proceed from I1 to closed states. It has been shown that the recovery from inactivation and the recovery of immobilised charges have the same time course (Armstrong & Bezanilla, 1977; Kühn & Greeff, 1999), suggesting that this is the limiting step for recovery from inactivation. When IVS4 is free to move, the correlation between its movement and inactivation may be strict, as reported by Kühn and Greef (1999).
Acknowledgments
We acknowledge Dr Lourival D. Possani of the Institute of Biotechnology, UNAM, Mexico, for providing us with a sample of highly purified toxin (Ts3) of the scorpion T. serrulatus. This work was supported by the Brazilian Research Council - CNPq and by FAPEMIG EDT 24000/01. FVC and PSLB are recipient of research fellowships from CNPq. The contributions of Dr Tasso Moraes-Santos and Dr Jader S. Cruz on previous experiments with tityustoxin are acknowledged.
Abbreviations
- Ts3
Tityus serrulatus toxin Ts3
References
- ARMSTRONG C.M., BEZANILLA F. Inactivation of the sodium channel II gating current experiments. J. Gen. Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIRÃO P.S.L., CAMPOS F.V., CRUZ J.S., MORAES-SANTOS T. Voltage-dependent dissociation of tityustoxin from sodium channels. Biophys. J. 2002;82:85a. [Google Scholar]
- BENZINGER G.R., KYLE J.W., BLUMENTHAL K.M., HANCK D.A. A specific interaction between the cardiac sodium channel and site-3 toxin anthopleurin B. J. Biol. Chem. 1998;273:80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- BENZINGER G.R., TONKOVICH G.S., HANCK D.A. Augmentation of recovery from inactivation by site-3 Na channel toxins. A single-channel and whole-cell study of persistent currents. J. Gen. Physiol. 1999;113:333–346. doi: 10.1085/jgp.113.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATTERALL W.A. Membrane potential-dependent binding of scorpion toxin to the action potential Na+ ionophore. Studies with a toxin derivative prepared by lactoperoxidase-catalyzed iodination. J. Biol. Chem. 1977;252:8660–8668. [PubMed] [Google Scholar]
- CATTERALL W.A. From ionic currents to molecular mechanisms: the structure and function of voltage gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A., RAY R., MORROW C.S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc. Natl. Acad. Sci. U.S.A. 1976;73:2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CESTÈLE S., CATTERALL W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- CHA A., REUBEN P.C., GEORGE A.L., JR, FUJIMOTO E., BEZANILLA F. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 1999;22:73–87. doi: 10.1016/s0896-6273(00)80680-7. [DOI] [PubMed] [Google Scholar]
- CHAHINE M., GEORGE A.L., JR, ZHOU M., JI S., SUN W., BARCHI R.L., HORN R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994;12:281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- CHANDA B., BEZANILLA F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J. Gen. Physiol. 2002;120:629–645. doi: 10.1085/jgp.20028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H., GORDON D., HEINEMANN S.H. Modulation of cloned skeletal muscle sodium channels by the scorpion toxins Lqh II, Lqh III and Lqh alphaIT. Pflugers Arch. 2000;439:423–432. doi: 10.1007/s004249900181. [DOI] [PubMed] [Google Scholar]
- CHEN H., HEINEMANN S.H. Interaction of scorpion alpha toxins with cardiac sodium channels: binding properties and enhancement of slow inactivation. J. Gen. Physiol. 2001;117:505–518. doi: 10.1085/jgp.117.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN L.Q., SANTARELLI V., HORN R., KALLEN R.G. A unique role for the S4 segment of domain 4 in the inactivation of sodium channels. J. Gen. Physiol. 1996;108:549–556. doi: 10.1085/jgp.108.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D., HAWKES A.G.A Q-matrix cookbook Single Channel Recording 1995New York: Plenum Press; 589–633.ed. Sakmann, B. & Neher, E. pp [Google Scholar]
- COURAUD F., ROCHAT H., LISSITZKY S. Binding of scorpion and sea anemone neurotoxins to a common site related to the action potential Na+ ionophore in neuroblastoma cells. Biochem. Biophys. Res. Commun. 1978;83:1525–1530. doi: 10.1016/0006-291x(78)91394-3. [DOI] [PubMed] [Google Scholar]
- CRUZ J.S., MATAVEL A.C.S., LEÃO-FILHO H.M., MORAES-SANTOS T., BEIRÃO P.S.L. Tityustoxin effect on nerve compound action potentials requires extracellular sodium. Neurosci. Lett. 2000;282:25–28. doi: 10.1016/s0304-3940(00)00862-4. [DOI] [PubMed] [Google Scholar]
- GONOI T., HILLE B. Gating of Na+ channels. Inactivation modifiers discriminate among models. J. Gen. Physiol. 1987;89:253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORN R., DING S., GRUBER H.J. Immobilizing the moving parts of voltage-gated ion channels. J. Gen. Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCH G.E., SKATTEBOL A., POSSANI L.D., BROWN A.M. Modification of Na channel gating by an alpha scorpion toxin from Tityus serrulatus. J. Gen. Physiol. 1989;93:67–83. doi: 10.1085/jgp.93.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KÜHN F.J., GREEFF N.G. Movement of voltage sensor S4 in domain 4 is tightly coupled to sodium channel fast inactivation and gating charge immobilization. J. Gen. Physiol. 1999;114:167–183. doi: 10.1085/jgp.114.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECAR H., LARSSON H.P., GRABE M. Electrostatic model of S4 motion in voltage-gated ion channels. Biophys. J. 2003;85:2854–2864. doi: 10.1016/S0006-3495(03)74708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOZHAYEVA G.N., NAUMOV A.P., NOSYREVA E.D., GRISHIN E.V. Potential-dependent interaction of toxin from venom of the scorpion Buthus eupeus with sodium channels in myelinated fibre: voltage clamp experiments. Biochim. Biophys. Acta. 1980;597:587–602. doi: 10.1016/0005-2736(80)90230-8. [DOI] [PubMed] [Google Scholar]
- POSSANI L.D., BECERRIL B., DELEPIERRE M., TYTGAT J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- POSSANI L.D., MARTIN B.M., MOCHCA-MORALES J., SVENDSEN I. Purification and chemical characterization of the major toxins from the venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Carlsberg Res. Com. 1981;46:195–205. [Google Scholar]
- ROGERS J.C., QU Y., TANADA T.N., SCHEUER T., CATTERALL W.A. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J. Biol. Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- SHEETS M.F., HANCK D.A. Voltage-dependent open-state inactivation of cardiac sodium channels: gating current studies with Anthopleurin-A toxin. J. Gen. Physiol. 1995;106:617–640. doi: 10.1085/jgp.106.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEETS M.F., KYLE J.W., KALLEN R.G., HANCK D.A. The Na+ channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys. J. 1999;77:747–757. doi: 10.1016/S0006-3495(99)76929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRICHARTZ G.R., WANG G.K. Rapid voltage-dependent dissociation of scorpion alpha-toxins coupled to Na channel inactivation in amphibian myelinated nerves. J. Gen. Physiol. 1986;88:413–435. doi: 10.1085/jgp.88.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STÜHMER W., CONTI F., SUZUKI H., WANG X., NODA M., YAHAGI N., KUBO H., NUMA S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]