Abstract
Calcitonin gene-related peptide (CGRP) is released into the cranial circulation of humans during acute migraine. To determine whether CGRP is involved in neurotransmission in craniovascular nociceptive pathways, we microiontophoresed onto neurons in the trigeminocervical complex and intravenously administered the CGRP receptor antagonists α-CGRP-(8–37) and BIBN4096BS.
Cats were anaesthetised with α-chloralose, and using halothane during surgical preparation. A craniotomy and C1/C2 laminectomy allowed access to the superior sagittal sinus (SSS) and recording site. Recordings of activity in the trigeminocervical complex evoked by electrical stimulation of the SSS were made. Multibarrelled micropipettes incorporating a recording electrode were used for microiontophoresis of test substances.
Cells recorded received wide dynamic range (WDR) or nociceptive specific (NS) input from cutaneous receptive fields on the face or forepaws. Cell firing was increased to 25–30 Hz by microiontophoresis of L-glutamate (n=43 cells).
Microiontophoresis of α-CGRP excited seven of 17 tested neurons.
BIBN4096BS inhibited the majority of units (26 of 38 cells) activated by L-glutamate, demonstrating a non-presynaptic site of action for CGRP. α-CGRP-(8–37) inhibited a similar proportion of units (five of nine cells).
Intravenous BIBN4096BS resulted in a dose-dependent inhibition of trigeminocervical SSS-evoked activity (ED50 31 μg kg–1). The maximal effect observed within 30 min of administration.
The data suggest that there are non-presynaptic CGRP receptors in the trigeminocervical complex that can be inhibited by CGRP receptor blockade and that a CGRP receptor antagonist would be effective in the acute treatment of migraine and cluster headache.
Keywords: Headache, migraine, cluster headache, CGRP antagonist, trigeminocervical complex
Introduction
The neurobiology of migraine essentially involves three components (Goadsby et al., 2002): first, the inherited migrainous diathesis, currently best characterized in familial hemiplegic migraine by mis-sense mutations in channel genes (Ophoff et al., 1996; De Fusco et al., 2003); secondly, migraine involves activation of brainstem regions (Weiller et al., 1995; Bahra et al., 2001; Matharu et al., 2003) that uniquely mark the condition when compared to other forms of primary headache, such as cluster headache (May et al., 1998); thirdly, the pain component of migraine seems to involve activation (Goadsby et al., 1990), or at least the perception of activation, of the trigeminal innervation of pain-producing intracranial components (Wolff, 1948). The trigeminal innervation of the cranial circulation contains a number of neuropeptides, including calcitonin gene-related peptide (CGRP) (Edvinsson et al., 1987). During acute migraine and cluster headache, CGRP levels are elevated in both adults and adolescents (Edvinsson & Goadsby, 1998), so that the effect of CGRP antagonists on models of trigeminovascular nociception may aid in understanding the potential role of such compounds in these disorders.
Stimulation of the trigeminal ganglion in cat and humans results in elevations in CGRP and substance P levels in the cranial circulation (Goadsby et al., 1988). However, during acute attacks of migraine (Goadsby et al., 1990; Gallai et al., 1995) and cluster headache (Goadsby & Edvinsson, 1994a; Fanciullacci et al., 1995) CGRP is elevated but substance P is not. Triptans, serotonin (5-HT1B/1D) receptor agonists (Goadsby, 2000), which are effective in the treatment of acute migraine (Ferrari et al., 2001) and cluster headache (Ekbom & The Sumatriptan Cluster Headache Study Group, 1991), inhibit release of CGRP into the cranial circulation of experimental animals when it is evoked by trigeminal ganglion activation (Goadsby & Edvinsson, 1993; 1994b). Moreover, successful treatment of acute migraine (Goadsby & Edvinsson, 1993) or cluster headache (Goadsby & Edvinsson, 1994a; Fanciullacci et al., 1995) with sumatriptan normalises cranial CGRP levels.
Stimulation of the superior sagittal sinus (SSS) in humans produces pain that is substantially referred to the first (ophthalmic) division of the trigeminal nerve (Feindel et al., 1960). During stimulation of the superior sagittal sinus (SSS), neurons can be studied using population-based anatomical techniques, such as measurement of Fos with immunohistochemistry (Kaube et al., 1993b), or metabolic activity with 2-deoxyglucose (Goadsby & Zagami, 1991), or single neurons can be more closely tracked using electrophysiological techniques (Hoskin et al., 1996; Cumberbatch et al., 1997). Electrophysiological methods incorporating microiontophoresis facilitate characterization of the pharmacology of neurons of interest by repeated local application of appropriate agonist and antagonist combinations (Bloom, 1974). It has been shown that neurons in the trigeminocervical complex of the cat or rat are inhibited by administration of triptans intravenously or by microiontophoresis, or both (Goadsby, 2000).
In this study, we determined whether the peripheral release of CGRP in the cranial circulation during acute migraine was mirrored in the trigeminocervical complex. The development of a potent specific CGRP receptor antagonist in the form of BIBN4096BS facilitates addressing this issue (Doods et al., 2000). By combining intravenous and microiontophoretic application of CGRP receptor antagonists, we determine here that there is a non-presynaptic CGRP receptor in the trigeminal nucleus that is a potential therapeutic target in migraine and cluster headache.
Methods
All studies reported were conducted and terminated under general anaesthesia in accordance with a project license issued by the Home Office of the United Kingdom under the Animals (Scientific Procedures) Act, 1986. Cats weighing 3.62±0.30 kg (mean±s.d.) were anaesthetised with α-chloralose (60 mg kg–1 i.p.; Sigma, St Louis, MO, U.S.A.) and prepared for physiological monitoring. Halothane (Rhone Merieux, Essex, U.K.) (0.5–3% in a 40% oxygen:air mixture) was administered from an anaesthetic machine during surgical procedures and then discontinued during experimental protocols. For each cat, a catheter was placed in the femoral artery for arterial blood sampling and continuous measurement of blood pressure (DTXplus transducer, Ohmeda, Madison, WI, U.S.A.; PM-1000 amplifier, CWE Instruments, Ardmore, PA, U.S.A.). A second catheter placed in the femoral vein allowed for fluid and drug administration. Supplementary doses of α-chloralose in 2-hydroxypropyl-β-cyclodextrin (RBI, Natick, MA, U.S.A.) were given i.v. at a rate of 5–10 mg kg–1 h−1 (Storer et al., 1997). The cats were intubated after local anaesthesia with lidocaine hydrochloride (Intubeaze, Arnolds, Shrewsbury, U.K.) and fixed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, U.S.A.).
A Jackson/Foley urethral catheter was inserted to drain the bladder, providing more even temperature regulation, more stable control of blood pressure through control of bladder distension, and monitoring of urine output. Core temperature was monitored and maintained between 37 and 39°C using a rectal thermistor probe and a low-electromagnetic-noise-emitting homeothermic heater blanket system (Harvard Apparatus, Holliston, MA, U.S.A.). The cat was ventilated with a 40% oxygen:air mixture (Harvard Apparatus), end-tidal CO2 was maintained between 2 and 4%, and expired oxygen continuously monitored (Datex-Ohmeda, Helsinki, Finland). Heart rate was monitored by electrocardiogram (CT-1000; CWE Instruments). The depth of anaesthesia was monitored periodically throughout the experiment by testing for sympathetic (pupillary and cardiovascular) responses to noxious stimulation and withdrawal reflexes in the absence of neuromuscular blockade.
Surgery
A midline craniotomy (20-mm diameter) and C1–C2 laminectomy were performed, allowing access to the superior sagittal sinus and the recording site in the spinal cord. The sinus was isolated by dissecting the dura and falx cerebri adjacent to the sinus over approximately 15 mm. A small polyethylene sheet was inserted under the isolated sinus and laid over the outlying dura mater, then tucked under the edges of the craniotomy to hold it in position. To prevent dehydration and to provide electrical insulation to the cortex, a cylindrical polypropylene dam was sealed to the bone around the craniotomy with dental acrylic (Vertex, Zeist, Netherlands) and filled with liquid paraffin (BDH Laboratory Supplies, Poole, England). Possible artefacts from arterial pulsation and respiratory movement were reduced by: bilateral pneumothoraces, held patent with polypropylene tubes; immobilization of the spine by clamping a thoracic spinous process to the stereotaxic frame; clamping the C1 transverse processes to auxiliary ear bar holders on the frame, and clamping the remaining caudal portion of the dorsal C2 spinous process to the frame.
Stimulation and recording
The isolated SSS was gently lifted onto a pair of bipolar platinum hook electrodes connected to a stimulus isolation unit (SIU5A; Grass Instruments, West Warwick, RI, U.S.A.). To activate primary trigeminal afferents, the SSS was supramaximally stimulated with stimulus-isolated (Grass SIU) square wave pulses from a Grass S88 stimulator (250 μs, 110–150 V, 0.3 – 0.5 Hz) after neuromuscular blockade with gallamine triethiodide (Concord, Essex, U.K.), initially 10–15 mg kg−1 i.v. and maintained at 5–10 mg kg−1 h−1. Extracellular recordings were made using microiontophoretic combination electrodes consisting of seven- or nine-barrelled glass pipettes, incorporating a central tungsten recording electrode with an exposed recording tip length of approximately 12 μm (Hellier et al., 1990). Recording electrode impedances were typically 400 kΩ when measured at 1 kHz in 0.9% saline. The dura mater above the recording regions on the surface of the spinal cord was reflected and held to the edges of the laminectomy with N-butyl-cyanoacrylate. After local removal of the pia mater, the electrode was lowered into the cord substance caudal to the C2 roots in the area of the dorsal root entry zone. The electrode was advanced or retracted in the cord substance in 5 μm steps using a microelectrode positioner consisting of a piezoelectric motor (IW-711, Burleigh Instruments, Harpenden, U.K.) and ultra-low-noise controller (6000ULN) attached to a micromanipulator (Kopf 1760-61). Tissue culture grade agar (3% (w v−1) in saline; Sigma, St Louis, MO, U.S.A.) was set over the exposed cord after electrode insertion to further reduce cardiovascularly related movements. Signal from the recording electrode attached to a high-impedance headstage preamplifier (NL100AK; Neurolog, Digitimer, Herts, U.K.) was fed via an AC preamplifier (Neurolog NL104, gain × 1000) through filters (Neurolog NL125; bandwidth approximately 300 Hz to 20 kHz) and a 50 Hz noise eliminator (Humbug, Quest Scientific, North Vancouver, BC, Canada) to a second-stage amplifier (Neurolog NL106) providing variable gain (× 20 – × 90). This signal (total gain approximately × 20,000 – × 90,000) was fed to a gated amplitude discriminator (Neurolog NL201) and analogue-to-digital converter (Labmaster DMA, Scientific Solutions, Mentor, OH, U.S.A.) in a personal computer, where the signal was processed and stored. Filtered and amplified action potentials were fed to loudspeaker via a power amplifier (Neurolog NL120) for audio monitoring and displayed on an oscilloscope to assist the isolation of single-unit activity from adjacent cell activity and noise.
In order to record the response of single units to stimulation, post-stimulus histograms were constructed on-line and saved to disk. Free-running neuronal activity, such as stimulated by local L-glutamate microiontophoresis, was analysed as cumulative rate histograms, where activity gated through the amplitude discriminator was collected into successive 1-s bins. Averaged action potentials were constructed using an averaging routine and an analogue signal delay unit (NL202), to assist the discrimination between somatic and axonal recordings, setting the NL125 filter bandwidth from d.c. to approximately 30 kHz. During experiments electrophysiological data, blood pressure, heart rate and end-tidal CO2 were processed and recorded on a video home system (VHS) magnetic tape (Pulse Code Modulator; Vetter, Rebersburgh, PA, U.S.A.) for documentation and latter review.
The position of the recording electrodes was controlled by use of a stereotaxic micropositioner (Kopf 1760–61) with reference to the mid-point of the C2 dorsal roots. Together with the depth of the recording electrode tip with respect to the surface of the spinal cord at the dorsal root entry zone, as determined by the distance travelled display on the ULN6000 pizoelectric motor controller (Burleigh Instruments), this provided the coordinates of the recording sites. The location of selected recording sites were marked with Pontamine sky blue dye (‘Gurr' 6BX dye (C.I.24410), BDH Laboratory Supplies, Poole, U.K.; 2.5% in 100 mM sodium acetate) using a −2.00 μA current for 5–10 min. Animals were euthanased with sodium pentobarbital (400 mg), followed by KCl (10% w v−1; 5 ml). After termination of experiments, the sections of spinal cord containing the recording sites were resected, fixed with neutral buffered 10% formalin, and sectioned (40 μm). Pontamine sky blue marks were counterstained with neutral red, a Nissl procedure that allowed identification of the laminae of the grey matter. The position of the recording sites within the cord were determined from histologically identified dye marks, and unmarked recording sites were located by reference to other dye marks, for example marked at the end of recording tracks, and the stereotaxic coordinates of the recording electrode tip.
Receptive fields
Cells responding to superior sagittal sinus (SSS) stimulation were characterised as receiving low threshold mechanoreceptor (LTM) input if they responded to non-noxious input such as brush or stroke on cutaneous receptive fields on the face or forepaws. They were characterized as nociceptive specific (NS) if they responded to noxious mechanical stimuli, such as pinch or pricking with a needle, or wide dynamic range (WDR) if they responded to both. These cells usually had increased firing in response to noxious stimuli (Hu et al., 1981).
Test compounds
Micropipette barrels used for microiontophoresis were filled with 1.0 M L-glutamate, monosodium, pH 8.0 (Sigma); saline (for controls); 20 mM BIBN4096BS in helium sparged water for injection and titrated with an equimolar amount of 0.1 M HCl, pH 5.4–5.8; 1.0 mM α-CGRP in helium sparged saline (150 mM NaCl), pH 4.0–6.5; and 1.0 mM α-CGRP-(8–37) in helium sparged 160 mM NaCl, pH 4.0–6.5; 2.5% Pontamine sky blue in 100 mM sodium acetate. L-Glutamate and Pontamine sky blue were ionized as anions and retained with small positive currents (∼3–5 nA). BIBN4096BS (Boehringer Ingelheim GmbH, Biberach, Germany), human α-CGRP (Sigma) and human α-CGRP-(8–37) (Sigma) were ionized as cations and were retained in the pipette barrels with small negative retaining currents (∼−3 to −5 nA). Ejection currents (2–200 nA) in directions opposite to the retaining currents were used. Chloride or sodium ions ejected from the barrel containing saline were used as controls. Current balancing was provided through a barrel containing 1 M NaCl. For intravenous injections BIBN4096BS was made up to 17.4 mg ml−1 and diluted in saline for injection (BP).
Microiontophoresis
After filling, the iontophoretic electrode micropipette barrels had resistances 60–150 MΩ. A microiontophoresis current generator (Dagan 6400, Dagan Corporation, Minneapolis, MN, U.S.A.) provided the current for ejecting test substances from the barrels. Retaining and balancing currents were used routinely (Bloom, 1974). The L-Glu ejection current was adjusted so cells had free running activity at a rate of around 20 Hz, such that inhibition of the cell activity could be distinguished from random firing. Where L-Glu was applied in pulses the evoked firing rate was often higher. When examining its effect on SSS-evoked firing control, post-stimulus histograms were constructed from the response to 50 supramaximal electrical stimuli to the SSS. BIBN4096BS was applied microiontophoretically for 300 s and the response of units to 50 supramaximal electrical stimuli to the SSS immediately recorded.
Intravenous BIBN4096BS administration
BIBN4096BS was administered intravenously through a catheterized femoral vein in saline as a vehicle after recording control post-stimulus histograms during 50 supramaximal electrical stimuli to the SSS. Post-stimulus histograms were again recorded at 5 min intervals for up to 45 min after BIBN4096BS administration.
Statistical analysis
Neuronal firing was distinguished from noise using an amplitude discriminator. Statistical evaluations were made using the average rate of firing in Hz evoked during each epoch of microiontophoretic application of L-glutamate. The background neuronal discharge was calculated by averaging the period of ongoing activity immediately preceding each epoch of excitation and subtracting this value from the evoked responses. For each unit a minimum of five-paired baseline-response data were collected. For the control responses to L-glutamate, an analysis of variance (ANOVA) with repeated measures was carried out. When this was not significant, the reliability of the measurements was tested using Cronbach's alpha. The baseline data were then pooled and used to further analyse the responses to drugs using either again pooling if the drug effect was reliable (Cronbach's alpha) and doing a t-test for independent samples, or an ANOVA with repeated-measures design, if not. Mauchly's test of sphericity was evaluated and when appropriate the Greenhouse–Geisser correction applied. The P-values were assessed at the 0.05 level and the Bonferroni test applied to comparisons between drugs (SPSS v.10, Chicago, IL, U.S.A.). Where we considered individual cells, we applied the critical ratio test (Nagler et al., 1973; Mandelbrod et al., 1974; 1983) that is based on the count data having a Poisson distribution, and employs the standard normal deviate (Armitage & Berry, 1994) to determine at the 5% level whether two firing rates differ. In practice, this very closely approximates a 30% change from baseline, which we considered significant change. We classified change between 10 and 30% as unclear. Summary data are presented as the mean ± standard error of the mean.
Results
Animals (n=23), whose data contributed to these results had cardio-respiratory parameters that were normal for the anaesthetised cat. Blood gas parameters were measured at intervals throughout the experiment and were within normal limits: arterial blood pH 7.39±0.04 and pCO2 3.39±0.41 kPa.
Localisation and neuronal characteristics
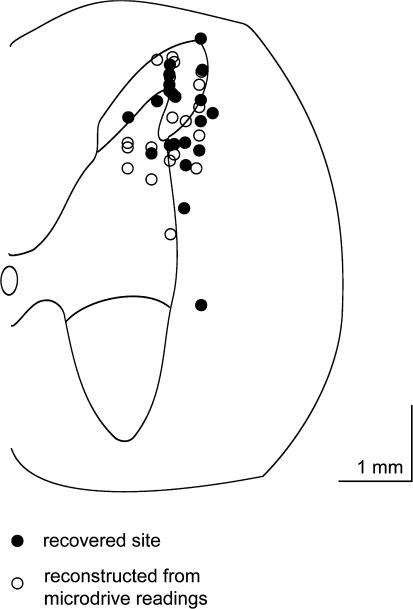
Extracellular recordings were made and data collected from 43 neurons in the trigeminocervical complex (Kaube et al., 1993b). Cells were located +4 mm rostral to −4 mm caudal to the midpoint of the C2 rootlets, 0–150 μm lateral to the dorsal root entry zone at a depth of approximately −700 μm to around −2000 μm below the (dorsal) cord surface (Figure 1). Cells responded to electrical sagittal sinus stimulation with latencies consistent with A-δ fibres (typically 8–10 ms). Recordings were made from cell bodies and were characterized by their unfiltered biphasic action potential morphology (Fussey et al., 1970) and the reversible excitatory effect of L-glutamate on cell firing. Cells received wide dynamic range or nociceptive specific mechanoreceptor input from cutaneous receptive fields on the face and forepaws. The receptive fields were all ipsilateral and at least involved the ophthalmic (first) division of the trigeminal nerve.
Figure 1.
Localisation of recording sites: A transverse section through the spinal cord at the level of C2 is represented by this schematic diagram. Microiontophoretically ejected Pontamine sky blue dye (6BX (C.I.24410)) was used to mark recording sites. Solid circles indicate sites where recording sites were identified histologically. The positions of unmarked recording sites, or sites where marks could not be recovered, were identified by reference to the position of dye marks at other recording sites and, or at the end of electrode tracts, and electrode tip coordinates, and are indicated by open circles. Although the recorded units are only mapped to one side of the cord in the schematic, they represent the data obtained from both the left-hand side and right-hand side of the spinal cord. The scale bar represents a distance of 1 mm in both directions.
Control ejections
Cell firing in response to control ejection of sodium ions from a barrel containing saline at similar currents (−30 to −80 nA; n=13) to those used for ejection of active compounds produced similar negligible effects across five applications of L-glutamate (F2.4,28=0.57, P=0.69).
Baseline L-glutamate firing
Neurons identified as linked to stimulation of the superior sagittal sinus were tested for the stability of their baseline response to L-glutamate application. All SSS-linked neurons responded to L-glutamate. The firing rate for trigeminal nucleus caudalis neurons was 30.5±3.0, 30.0±3.0, 26.9±2.6, 24.7±2.2, and 25.1±2.2 Hz for each of the five consecutive applications of L-glutamate (−30 to −80 nA; n=22). There was no difference across these responses (F4,84=2.03, P=0.098). The reliability of these responses across time was excellent, with an alpha of 0.98. We thus pooled these data for further comparison with other drug treatments. Higher rates of firing could be achieved by greater ejection currents up to −120 nA.
Effect of α-CGRP
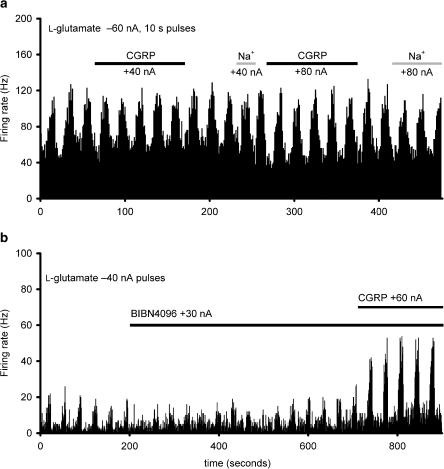
The firing rate for trigeminal nucleus caudalis neurons during ejection of α-CGRP was 16.2±8.5, 15.5±7.0, 19.1±7.6, 15.8±7.2, and 17.9±7.8 Hz for each of the five consecutive applications of α-CGRP (60 nA; n=6). There was no difference across these responses (F4,16=2.2, P=0.12). The reliability of these responses across time was excellent, with an alpha of 0.99. When α-CGRP was iontophoresed in the presence of L-glutamate (−30 to −80 nA), there was no significant added effect (n=17; t16=0.55; Figure 2), for the group as a whole. Seven of the 17 neurons tested increased their firing rate by more than 30%.
Figure 2.
Effect of α-CGRP and L-glutamate: L-glutamate (1.0 M, pH 8.0) was applied microiontophoretically (−60 nA) and increased the firing of trigeminocervical neurons linked to stimulation of the superior sagittal sinus. When the firing had reached a steady state over five epochs, α-CGRP (1 mM in 150 mM NaCl, pH 4.5) was co-microiontophoresed (solid line) with no added effect from sodium (+40 and +80 nA) in 10/17 units (panel a). In 7/17 units, trigeminal activity was increased by CGRP, albeit in the presence of BIBN4096BS (panel b). There was no significant effect of microiontophoresis of sodium. The histogram indicates the rate of firing seen in one second bins.
Effect of BIBN4096BS
Microiontophoresis
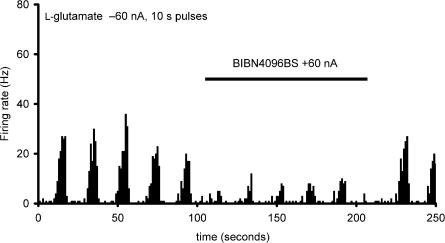
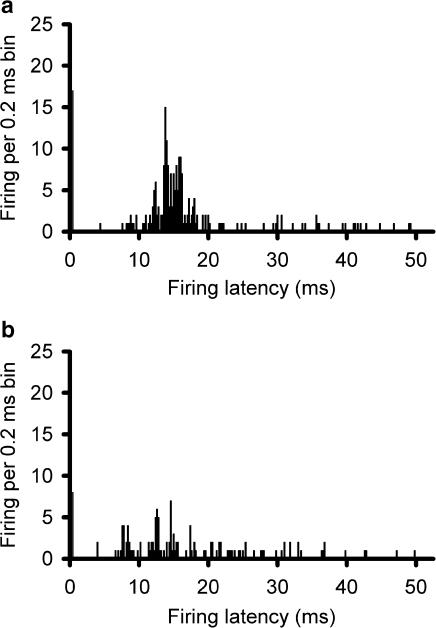
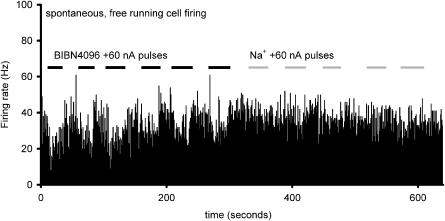
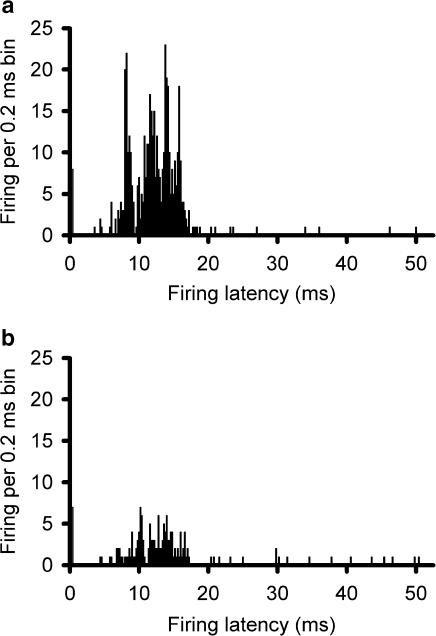
The firing rate for trigeminal nucleus caudalis neurons during ejection of L-glutamate across five consecutive applications was 8.4±1.5, 7.4±1.9, 7.4±1.8, 8.3±1.8, and 7.9±1.7 Hz during BIBN4096BS administration (60 nA; n=16). There was no difference across these responses (F2.0,29.6=0.51, P=0.61). The reliability of these responses across time was excellent, with an alpha of 0.95. When compared with the effect of L-glutamate alone, there was a significant inhibition of trigeminal neuronal firing (t37=2.3, P< 0.03; Figure 3). The effect of BIBN4096BS on evoked trigeminal neuronal activity was dose-dependent across the range of currents from 10 to 120 nA. BIBN4096BS inhibited trigeminocervical neuronal activity evoked by stimulation of the superior sagittal sinus after 300 s application (Figure 4). BIBN4096BS also inhibited the spontaneous firing of neurons linked to SSS stimulation (Figure 5).
Figure 3.
Effect of BIBN4096BS: Microiontophoresis of BIBN4096BS (20 mM, pH 5.7; +60 nA, solid bar) produces a robust reduction in L-glutamate-evoked (1.0 M, pH 8.0; 60 nA) firing in the trigeminocervical complex. The histogram indicates the rate of firing seen in one second bins.
Figure 4.
Effect of BIBN4096BS microiontophoresis on SSS-evoked firing: Post-stimulus histograms showing that supramaximal electrical stimulation (50 × 250 μs) of the SSS via bipolar platinum hook electrodes recruits units in the trigeminocervical complex responding to the stimulus with a latency peak of 12 ms (panel a) that is inhibited immediately after microiontophoresis of BIBN4096BS (20 mM, pH 5.8; +60 nA for 300 s) (panel b). The apparent response within the first 0.2 ms is part of the stimulus artefact.
Figure 5.
Effect of BIBN4096BS on spontaneous trigeminal neurons: Neurons firing spontaneously at a rate indicated in the histogram as firing per 1 s bin have a reduced firing frequency when BIBN4096BS is microiontophoretically ejected (20 mM, pH 5.7; +60 nA; black bars), while sodium (150 mM, pH 7.0) has no apparent effect at similar currents (grey bars).
Intravenous administration
Intravenous administration of BIBN4096BS at cumulative doses of 1, 3, 10, 30, 100 μg kg–1 resulted in a dose-dependent inhibition of superior sagittal sinus evoked trigeminocervical nucleus activity (n=4; Figure 6). Maximal effects were seen within 30 min of drug administration with a calculated ED50 of 31 μg kg–1 (Goadsby & Lambert, 1986).
Figure 6.
Effect of intravenously administrated BIBN4096BS on SSS-evoked firing: Post-stimulus histograms showing that supramaximal electrical stimulation (50 × 250 μs) of the SSS via bipolar platinum hook electrodes recruits units in the trigeminocervical complex (panel a) that are substantially inhibited within 30 min of intravenous administration of BIBN4096BS (30 μg kg–1; panel b). The apparent response within the first 0.2 ms is part of the stimulus artefact.
Neuronal characteristics and BIBN4096BS
Four cells were characterized as nociceptive specific and had receptive fields on ipsilateral forepaws, face, or bridge of the nose. One was inhibited by microiontophoretically applied BIBN4096BS, there was no effect on one cell and the effect on two cells was not clear. In all, 13 cells were characterized as wide dynamic range, with receptive fields on ipsilateral forearms, forepaws, or face. Eight cells were inhibited by BIBN4096BS, and the effect of BIBN4096BS was unclear in three, two cells were not tested with BIBN4096BS. Three cells were classified as LTM and had receptive fields on the ipsilateral face, two of these were inhibited by BIBN4096BS and the effect on the other was not clear. Cutaneous receptive fields were not found for seven tested cells, five were inhibited by BIBN4096BS and two were not tested. In all, 18 cells were not characterized, eight of these were inhibited by BIBN4096BS, four were not inhibited by BIBN4096BS and the effect of BIBN4096BS on four cells was not clear.
Effect of α-CGRP-(8–37)
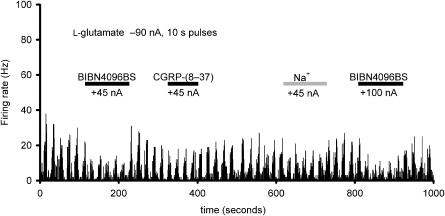
The firing rate for trigeminal nucleus caudalis neurons during ejection of L-glutamate was 9.1±1.8, 9.6±2.1, 9.3±1.8, 9.5±1.6, and 9.6±1.9 Hz for each of the five consecutive applications of α-CGRP-(8–37) (50 nA; n=6). There was no difference across these responses (F4,20=0.24, P=0.91). The reliability of these responses across time was excellent with an alpha of 0.99. When compared with the effect of L-glutamate alone, there was a significant inhibition of trigeminal neuronal firing (t17=2.5, P=0.03). The effect of α-CGRP-(8–37) on evoked trigeminal neuronal activity was dose-dependent across a range of currents from 40 to 120 nA, but apparently not as potent as that of BIBN4096BS under these experimental conditions (Figure 7).
Figure 7.
Comparison of the effect of BIBN4096BS (20 mM, pH 5.8; +45 and +100 nA) and α-CGRP-(8 – 37) (1 mM in 160 mM NaCl, pH 4.5; +45 nA) in inhibiting the firing evoked by 10 s pulses of microiontophoretically applied L-glutamate (1.0 M, pH 8.0; −90 nA) onto a trigeminocervical complex unit linked to stimulation of the SSS, where sodium (150 mM; pH 7.0) has no apparent effect (+45 nA, grey bar). The firing rate indicated on the ordinate is indicated for 1 s bins by the rate histogram.
Discussion
These studies demonstrate that the CGRP receptor antagonists BIBN4096BS and CGRP-(8–37), can inhibit neurons in the trigeminocervical complex that are linked to stimulation of the superior sagittal sinus. That microiontophoresis of BIBN4096BS and CGRP-(8–37) is effective demonstrates that one group of trigeminovascular CGRP receptors involved must be local to the trigeminocervical complex. Furthermore, since local application of the CGRP antagonists blocks the activation seen with iontophoretic application of L-glutamate, this CGRP receptor must be non-presynaptic, probably post-synaptic, in location (Goadsby et al., 2001). Moreover, at least in this model, BIBN4096BS was capable of reducing resting spontaneous firing, suggesting some ongoing trigeminovascular activity independent of SSS stimulation, perhaps due to surgical trauma (Hoskin & Goadsby, 1999). The data are consistent with the recent preliminary report that BIBN4096BS is an effective treatment of acute migraine (Olesen et al., 2003).
Why study trigeminocervical neurons? The trigeminocervical complex is pivotal in the transmission of nociceptive input from pain-producing intracranial structures (De Vries et al., 1999). Stimulation of both intracranial structures, such as dural afferents by chemical irritation (Cutrer et al., 1995), superior sagittal sinus (Kaube et al., 1993b; Goadsby & Hoskin, 1997) or middle meningeal artery (Hoskin et al., 1999) by electrical stimulation, or cutaneous structures, such as cornea (Panneton & Burton, 1981), result in activation of neurons in the trigeminal nucleus caudalis and for intracranial structures its extension into the dorsal horn of C1 and C2. It is possible to study trigeminocervical complex neurons using electrophysiological methods, and these experiments produce comparable pharmacological results to population-based methods, such as Fos immunohistochemistry (Goadsby & Hoskin, 1996; Hoskin et al., 1996; Hoskin & Goadsby, 1998). We have chosen electrophysiological methods here so that we could determine in the same animal, and recording from the same unit in the trigeminocervical complex, whether CGRP-mediated transmission made a significant contribution at this pivotal synapse. This method has the advantage of temporal resolution, although it cannot provide an assessment of the population implications of blocking CGRP-mediated transmission, that question would require a method such a Fos immunohistochemistry.
In this study, we employed both peripheral activation with superior sagittal sinus stimulation and direct activation of trigeminal neurons using L-glutamate. Glutamate is an important transmitter in the trigeminal nucleus. N-Methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA), kainate and metabotropic glutamate receptors have been identified in the superficial laminae of the trigeminal nucleus caudalis of the rat (Tallaksen-Greene et al., 1992). It has been shown that microiontophoretically applied L-glutamate excites neurons in the trigeminal nucleus caudalis (Hill & Salt, 1982; Salt & Hill, 1982). It can be shown that blockade of NMDA and AMPA receptors attenuates trigeminovascular nociceptive transmission (Mitsikostas et al., 1998; 1999; Storer & Goadsby, 1999). Similarly, in the cornea, a substantially first (ophthalmic) division of trigeminal innervated structure, there is a significant glutamatergic component to transmission (Bereiter et al., 1996). Microiontophoresis of D,L-homocysteic acid (Storer & Goadsby, 1997) or L-glutamate (Storer et al., 2001a) produces a rapid and tightly stimulus-linked increase in trigeminal neuronal activity well suited to pharmacological exploration, while co-microiontophoresis of other agonists and antagonists offers clear characterization of trigeminal synaptic pharmacology (Storer et al., 2001b). We have used a minimal repetition of five epochs to ensure stable baseline measurements, and saline controls to test whether there is an ordering effect of activation prior to antagonist administration. No such effect was seen. In some experiments (data not shown), more than five epochs were used and neurons continued to respond. In addition, we observed that local microiontophoresis of α-CGRP following L-glutamate still produced significant neuronal activation. Taken together, tachyphylaxis is an unlikely explanation for the inhibitory effects we have seen. Moreover, although presynaptic glutamate receptors have been identified in dorsal horn (Carlton et al., 2001), even if they are activated and involved in the release of CGRP, it remains likely that the major action of CGRP itself is post-synaptic, and thus the effect we observed is probably post-synaptic. The question of localization will be best finalised with electron microscopy. Although excellent in terms of localization of effect, microiontophoresis cannot precisely predict dosing of compounds. While the ejection current is indicative of the amount of substance delivered, physicochemical issues, such as compound valency, molecular weight, and solubility, make absolute comparisons based on current inappropriate. The relative molecular mass of BIBN4096BS is approximately one-quarter that of the peptides, so its flux, electrophoretic mobility, and diffusional properties will be greater than for the peptides. BIBN4096BS's valency is estimated at +3 which is the same as that expected at the pH range used for the CGRP fragment 8–37, but higher than that expected for CGRP (+2). Thus, while the effect and localization of the effect of BIBN4096BS is clear, its relative potency cannot be determined from these studies.
CGRP receptors have been difficult to characterize pharmacologically, probably due to the recently described presence of the receptor activity-modifying proteins (RAMPs) (McLatchie et al., 1998). There are two classes of CGRP receptor, CGRP1 and CGRP2, broadly defined by the ability of CGRP-(8–37) to inhibit the actions of CGRP at one receptor (Dennis et al., 1990). These receptors are made up of combinations of two distinct seven transmembrane receptors, the calcitonin receptor and the calcitonin receptor-like receptor (CRLR) (Choksi et al., 2002), along with the appropriate RAMP. CGRP release into the cranial circulation during acute headache has been described in both migraine and cluster headache (Edvinsson & Goadsby, 1998). CGRP has a pivotal role in vasodilation due to trigeminovascular activation in the cerebral (Goadsby, 1993) and meningeal circulation (Williamson et al., 1997), and nitric oxide generation mediates some component of that effect in some settings (Wahl et al., 1994; Akerman et al., 2002). CGRP release is under opioid influence in the spinal cord (Collin et al., 1993), has been implicated in some pain mechanisms (Yu et al., 1996), and may be involved in morphine tolerance (Powell et al., 2000). CGRP immuno-reactivity is found near trigeminal neurons that have 5HT1B and 5HT1D receptors in neurons in rat (Ma et al., 2001) and human (Hou et al., 2001) trigeminal ganglia. Given that 5HT1B/1D receptor agonists are effective in the treatment (Ferrari et al., 2001), the role of CGRP receptors in the trigeminovascular system is an important question for understanding the pathophysiology of these disorders.
The new data are consistent with an important role for CGRP in the transmission of trigeminovascular nociceptive information, and thus for a pivotal role of CGRP in acute attacks of migraine and cluster headache. The emerging data, buttressed by the clinical evidence that BIBN4096BS is effective in acute migraine (Olesen et al., 2003), invite some consideration of the role of CGRP in migraine and how and where blockade of its effect alters the acute attack. BIBN4096BS is apparently devoid of direct vascular actions in animals (Moreno et al., 2002) or humans (Petersen et al., 2003b). It also has no apparent effect on brain blood flow (Petersen et al., 2003a), which is consistent with the lack of effect of triptans on cerebral blood flow (Weiller et al., 1995). Given that there are no consistent changes in brain blood flow in migraine without aura (Olesen, 1991), and no relationship to the pain in terms of timing (Olesen et al., 1990), or effects of acute anti-migraine compounds (Friberg et al., 1991; Limmroth et al., 1996), evidence has been accumulating that a vascular effect may be unnecessary for acute anti-migraine compounds. It seems more likely that acute anti-migraine compounds work by blocking trigeminal nociceptive traffic either at the vessel/nerve interface (Moskowitz & Cutrer, 1993), or in the trigeminocervical complex (Kaube et al., 1993a), or both. The new data establish that for CGRP antagonists the trigeminocervical complex is one possible target, and further suggest that newer compounds should actively target central structures.
This study has shown that local microiontophoretic application of the CGRP receptor antagonists BIBN4096BS and CGRP-(8–37) blocks nociceptive trigeminovascular transmission in the trigeminocervical complex of the cat. This effect was seen with both peripheral activation of the pathway by stimulation of the superior sagittal sinus and by local trigeminal nucleus activation with microiontophoresis of L-glutamate. The effect was present during L-glutamate and BIBN4096BS co-microiontophoresis, where pre-synaptic mechanisms are by-passed, suggesting a non-presynaptic, probably post-synaptic effect. The data establish a role for CGRP-mediated transmission of trigeminovascular nociceptive inputs in the trigeminocervical complex, and argue strongly for further clinical development of CGRP antagonists for the acute treatment of both migraine and cluster headache.
Acknowledgments
We thank Paul Hammond and Michele Lasalandra for their excellent technical assistance. This work was supported by Boehringer-Ingelheim and the Wellcome Trust. P.J.G. is a Welcome Trust Senior Research Fellow. This work has been presented in preliminary form at the XIth Congress of the International Headache Society, Rome, 13–16 September 2003 (Storer et al., Abstract P5O5).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid
- ANOVA
analysis of variance
- BIBN4096BS
1-piperidinecarboxamide, N-[2-[[5amino-1-[[4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)
- CGRP
calcitonin gene-related peptide
- CRLR
calcitonin receptor-like receptor
- 5-HT
serotonin
- i.v.
intravenous
- LTM
low threshold mechanoreceptor
- NMDA
N-methyl-D-aspartate
- NS
nociceptive specific
- RAMP
receptor activity-modifying protein
- SSS
superior sagittal sinus
- WDR
wide dynamic range
References
- AKERMAN S., WILLIAMSON D.J., KAUBE H., GOADSBY P.J. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMITAGE P, BERRY G. Statistical Methods in Medical Research 1994Oxford: Blackwell Science; 3rd edn [Google Scholar]
- BAHRA A., MATHARU M.S., BUCHEL C., FRACKOWIAK R.S.J., GOADSBY P.J. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- BEREITER D.A., BEREITER D.F., HATHAWAY C.B. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity in central trigeminal neurons and blocks select endocrine and autonomic responses to corneal stimulation in the rat. Pain. 1996;64:179–189. doi: 10.1016/0304-3959(95)00095-X. [DOI] [PubMed] [Google Scholar]
- BLOOM F.E. To spritz or not to spritz: the doubtful value of aimless iontophoresis. Life Sci. 1974;14:1819–1834. doi: 10.1016/0024-3205(74)90400-7. [DOI] [PubMed] [Google Scholar]
- CARLTON S.M., HARGETT G.L., COGGESHALL R.E. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- CHOKSI T., HAY D.L., LEGON S., POYNER D.R., HAGER S., BLOOM S.R., SMITH D.M. Comparison of the expression of calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br. J. Pharmacol. 2002;136:784–792. doi: 10.1038/sj.bjp.0704761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIN E., FRECHILLA D., POHL M., BOURGOIN S., LE BARS D., HAMON M., CESSELIN F. Opioid control of the release of calcitonin gene-related peptide-like material from the rat spinal cord in vivo. Brain Res. 1993;609:211–222. doi: 10.1016/0006-8993(93)90875-n. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Rizatriptan has central antinociceptive effects against durally evoked responses. Eur. J. Pharmacol. 1997;328:37–40. doi: 10.1016/s0014-2999(97)83024-5. [DOI] [PubMed] [Google Scholar]
- CUTRER F.M., LIMMROTH V., AYATA G., MOSKOWITZ M.A. Attenuation by valproate of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br. J. Pharmacol. 1995;116:3199–3204. doi: 10.1111/j.1476-5381.1995.tb15124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FUSCO M., MARCONI R., SILVESTRI L., ATORINO L., RAMPOLDI L., MORGANTE L., BALLABIO A., ARIDON P., CASARI G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- DE VRIES P., VILLALON C.M., SAXENA P.R. Pharmacological aspects of experimental headache models in relation to acute antimigraine therapy. Eur. J. Pharmacol. 1999;375:61–74. doi: 10.1016/s0014-2999(99)00197-1. [DOI] [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., CADIEUX A., POMERLEAU F., JOLICOEUR F.B., ST PIERRE S., QUIRION R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J. Pharmacol. Exp. Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVINSSON L., EKMAN R., JANSEN I., McCULLOCH J., UDDMAN R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J. Cereb. Blood Flow Metabol. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GOADSBY P.J. Neuropeptides in headache. Eur. J. Neurol. 1998;5:329–341. [Google Scholar]
- EKBOM K., THE SUMATRIPTAN CLUSTER HEADACHE STUDY GROUP Treatment of acute cluster headache with sumatriptan. N. Engl. J. Med. 1991;325:322–326. doi: 10.1056/NEJM199108013250505. [DOI] [PubMed] [Google Scholar]
- FANCIULLACCI M., ALESSANDRI M., FIGINI M., GEPPETTI P., MICHELACCI S. Increase in plasma calcitonin gene-related peptide from extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- FEINDEL W., PENFIELD W., McNAUGHTON F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555–563. doi: 10.1212/wnl.10.6.555. [DOI] [PubMed] [Google Scholar]
- FERRARI M.D., ROON K.I., LIPTON R.B., GOADSBY P.J. Oral triptans (serotonin 5-HT1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- FRIBERG L., OLESEN J., IVERSEN H.K., SPERLING B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- FUSSEY I.F., KIDD C., WHITWAM J.G. The differentiation of axonal and soma-dendritic spike activity. Pflugers Arch. 1970;321:283–292. doi: 10.1007/BF00588643. [DOI] [PubMed] [Google Scholar]
- GALLAI V., SARCHIELLI P., FLORIDI A., FRANCESCHINI M., CODINI M., TREQUATTRINI A., PALUMBO R. Vasoactive peptides levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15:384–390. doi: 10.1046/j.1468-2982.1995.1505384.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. Inhibition of calcitonin gene-related peptide by h-CGRP(8–37) antagonizes the cerebral dilator response from nasociliary nerve stimulation in the cat. Neurosci. Lett. 1993;151:13–16. doi: 10.1016/0304-3940(93)90033-h. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. The pharmacology of headache. Prog. Neurobiol. 2000;62:509–525. doi: 10.1016/s0301-0082(00)00010-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., AKERMAN S., STORER R.J. Evidence for postjunctional serotonin (5-HT1) receptors in the trigeminocervical complex. Ann. Neurol. 2001;50:804–807. doi: 10.1002/ana.10066. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Human in vivo evidence for trigeminovascular activation in cluster headache. Brain. 1994a;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994b;34:394–399. doi: 10.1111/j.1526-4610.1994.hed3407394.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites a therapeutic target in migraine. Pain. 1996;67:355–359. doi: 10.1016/0304-3959(96)03118-1. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: a c-fos immunocytochemical study. J. Anat. 1997;190:367–375. doi: 10.1046/j.1469-7580.1997.19030367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., LAMBERT G.A. An interactive, readily transportable program using a log/logit transformation for the analysis of radioimmunoassay data. Comput. Methods Programs Biomed. 1986;23:263–268. doi: 10.1016/0169-2607(86)90060-x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine—current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., ZAGAMI A.S. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991;114:1001–1011. doi: 10.1093/brain/114.2.1001. [DOI] [PubMed] [Google Scholar]
- HELLIER M., BOERS P., LAMBERT G.A. Fabrication of a metal-cored multi-barrelled microiontophoresis assembly. J. Neurosci. Meth. 1990;32:55–61. doi: 10.1016/0165-0270(90)90071-m. [DOI] [PubMed] [Google Scholar]
- HILL R.G., SALT T.E. An iontophoretic study of the responses of rat caudal trigeminal nucleus neurones to non-noxious mechanical sensory stimuli. J. Physiol. 1982;327:65–78. doi: 10.1113/jphysiol.1982.sp014220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSKIN K.L., GOADSBY P.J. Comparison of a more and less lipophilic serotonin (5HT1B/1D) agonists in a model of trigeminovascular nociception in cat. Exp. Neurol. 1998;150:45–51. doi: 10.1006/exnr.1997.6749. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., GOADSBY P.J. Exposure and isolation of the superior sagittal sinus elicits Fos in the trigeminal nucleus caudalis and dorsal horn of the cervical spinal cord: how long should you wait. Brain Res. 1999;824:133–135. doi: 10.1016/s0006-8993(99)01135-x. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiology study. Brain. 1996;119:249–256. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., ZAGAMI A., GOADSBY P.J. Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: a comparative study of monkey and cat. J. Anat. 1999;194:579–588. doi: 10.1046/j.1469-7580.1999.19440579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOU M., KANJE M., LONGMORE J., TAJTI J., UDDMAN R., EDVINSSON L. 5-HT1B and 5-HT1D receptors in the human trigeminal ganglion: co-localization with calitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001;909:112–120. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- HU J.W., DOSTROVSKY J.O., SESSLE B.J. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral–facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J. Neurophysiol. 1981;45:173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- KAUBE H., HOSKIN K.L., GOADSBY P.J. Inhibition by sumatriptan of central trigeminal neurones only after blood–brain barrier disruption. Br. J. Pharmacol. 1993a;109:788–792. doi: 10.1111/j.1476-5381.1993.tb13643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUBE H., KEAY K.A., HOSKIN K.L., BANDLER R., GOADSBY P.J. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993b;629:95–102. doi: 10.1016/0006-8993(93)90486-7. [DOI] [PubMed] [Google Scholar]
- LIMMROTH V., MAY A., AUERBACH P., WOSNITZA G., EPPE T., DIENER H.C. Changes in cerebral blood flow velocity after treatment with sumatriptan or placebo and implications for the pathophysiology of migraine. J. Neurol. Sci. 1996;138:60–65. doi: 10.1016/0022-510x(95)00344-2. [DOI] [PubMed] [Google Scholar]
- MA Q.-P., HILL R., SIRINATHSINGHJI D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur. J. Neurosci. 2001;13:2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- MANDELBROD I., FELDMAN S., WERMAN R. Inhibition of firing is the primary effect of microelectrophoresis of cortisol to units in the rat tuberal hypothalamus. Brain Res. 1974;80:303–315. doi: 10.1016/0006-8993(74)90693-3. [DOI] [PubMed] [Google Scholar]
- MANDELBROD I., FELDMAN S., WERMAN R. Mediobasal hypothalamic neurons are excited by the iontophoretic application of sodium. Brain Res. 1983;273:35–44. doi: 10.1016/0006-8993(83)91091-0. [DOI] [PubMed] [Google Scholar]
- MATHARU M., BARTSCH T., WARD N., FRACKOWIAK R., WEINER R., GOADSBY P. Central neuromodulation with implanted suboccipital stimulators in patients with chronic migraine. Cephalalgia. 2003;23:655. doi: 10.1093/brain/awh022. [DOI] [PubMed] [Google Scholar]
- MAY A., BAHRA A., BÜCHEL C., FRACKOWIAK R.S.J., GOADSBY P.J. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- McLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ del RIO M., WAEBER C., HUANG Z., CUTRER F.M., MOSKOWITZ M.A. Non-NMDA glutamate receptors modulate capsaicin induced c-fos expression within trigeminal nucleus caudalis. Br. J. Pharmacol. 1999;127:623–630. doi: 10.1038/sj.bjp.0702584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., SANCHEZ del RIO M., WAEBER C., MOSKOWITZ M.A., CUTRER F.M. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- MORENO M.J., ABOUNADER R., HÉBERT E., DOODS H., HAMEL E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: potential implications in acute migraine treatment. Neuropharmacology. 2002;42:568–576. doi: 10.1016/s0028-3908(02)00008-4. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A., CUTRER F.M. SUMATRIPTAN: a receptor-targeted treatment for migraine. Ann. Rev. Med. 1993;44:145–154. doi: 10.1146/annurev.me.44.020193.001045. [DOI] [PubMed] [Google Scholar]
- NAGLER J., CONFORTI N., FELDMAN S. Alterations produced by cortisol in the spontaneous activity and responsiveness to sensory stimuli of single cells in the tuberal hypothalamus of the rat. Neuroendocrinology. 1973;12:52–66. doi: 10.1159/000122154. [DOI] [PubMed] [Google Scholar]
- OLESEN J. Cerebral and extracranial circulatory disturbances in migraine: pathophysiological implications. Cerebrovasc. Brain Metab. Rev. 1991;3:1–28. [PubMed] [Google Scholar]
- OLESEN J., DIENER H.-C., HUSSTEDT I.W., GOADSBY P.J., HALL D., MEIER U., POLLENTIER S., LESKO L.M. Calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS is effective in the treatment of migraine attacks. Cephalalgia. 2003;23:579. [Google Scholar]
- OLESEN J., FRIBERG L., SKYHOJ-OLSEN T., IVERSEN H.K., LASSEN N.A., ANDERSEN A.R., KARLE A. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann. Neurol. 1990;28:791–798. doi: 10.1002/ana.410280610. [DOI] [PubMed] [Google Scholar]
- OPHOFF R.A., TERWINDT G.M., VERGOUWE M.N., van EIJK R., OEFNER P.J., HOFFMAN S.M.G., LAMERDIN J.E., MOHRENWEISER H.W., BULMAN D.E., FERRARI M., HAAN J., LINDHOUT D., van OMMEN G.-J.B., HOFKER M.H., FERRARI M.D., FRANTS R.R. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- PANNETON W.M., BURTON H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J. Comp. Neurol. 1981;199:327–344. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- PETERSEN K.A., BIRK S., LASSEN L.H., KRUUSE C., JONASSEN O., OLESEN J. The novel CGRP-antagonist, BIBN4096BS does not affect the cerebral hemodynamics in healthy volunteers. Cephalalgia. 2003a;23:729. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- PETERSEN K.A., LASSEN L.H., BIRK S., OLESEN J. The effect of the nonpeptide CGRP-antagonist, BIBN406BS on human-alphaCGRP induced headache and hemodynamics in healthy volunteers. Cephalalgia. 2003b;23:725. [Google Scholar]
- POWELL K.J., MA W., SUTAK M., DOODS H., QUIRION R., JHAMANDAS K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br. J. Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALT T.E., HILL R.G. Differentiation of excitatory amino acid receptors in the rat caudal trigeminal nucleus: a microiontophoretic study. Neuropharmacology. 1982;21:385–390. doi: 10.1016/0028-3908(82)90020-x. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., CONNOR H.E., GOADSBY P.J. 4991W93, a potent blocker of neurogenic plasma protein extravasation, inhibits trigeminal neurons at 5-hydroxytryptamine (5-HT1B/1D) agonist doses. Neuropharmacology. 2001a;40:911–917. doi: 10.1016/s0028-3908(01)00014-4. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., GOADSBY P.J. GABA receptors modulate trigeminovascular nociceptive neurotransmission in the trigeminocervical complex. Br. J. Pharmacol. 2001b;134:896–904. doi: 10.1038/sj.bjp.0704325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORER R.J., BUTLER P., HOSKIN K.L., GOADSBY P.J. A simple method, using 2-hydroxypropyl-β-cyclodextrin, of administering α-chloralose at room temperature. J. Neurosci. Meth. 1997;77:49–53. doi: 10.1016/s0165-0270(97)00110-6. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120:2171–2177. doi: 10.1093/brain/120.12.2171. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience. 1999;90:1371–1376. doi: 10.1016/s0306-4522(98)00536-3. [DOI] [PubMed] [Google Scholar]
- TALLAKSEN-GREENE S.J., YOUNG A.B., PENNEY J.B., BEITZ A.J. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992;141:79–83. doi: 10.1016/0304-3940(92)90339-9. [DOI] [PubMed] [Google Scholar]
- WAHL M., SCHILLING L., PARSONS A.A., KAUFMANN A. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res. 1994;637:204–210. doi: 10.1016/0006-8993(94)91234-3. [DOI] [PubMed] [Google Scholar]
- WEILLER C., MAY A., LIMMROTH V., JUPTNER M., KAUBE H., SCHAYCK R.V., COENEN H.H., DIENER H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural blood vessel diameter in the anaesthetized rat. Cephalalgia. 1997;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WOLFF H.G. Headache and Other Head Pain. New York: Oxford University Press; 1948. [Google Scholar]
- YU L.-C., HANSSON P., LUNDEBERG T. The calcitonin gene-related peptide antagonist CGRP8–37 increases the latency to withdrawal responses bilaterally in rats with unilateral experimental mononeuropathy, an effect reversed by naloxone. Neuroscience. 1996;71:523–531. doi: 10.1016/0306-4522(95)00428-9. [DOI] [PubMed] [Google Scholar]