Abstract
It has been established that polycations cause airway hyperresponsiveness (AHR) to methacholine by inducing a deficiency of constitutive nitric oxide synthase (cNOS)-derived bronchodilating nitric oxide (NO). Since a deficiency of cNOS-derived NO also contributes to allergen-induced AHR after the early asthmatic reaction (EAR) and since this AHR is associated with the release of polycationic proteins from infiltrated eosinophils in the airways, we hypothesized that endogenous polycations underlie or at least contribute to the allergen-induced NO deficiency and AHR.
Using a guinea-pig model of allergic asthma, we addressed this hypothesis by examining the effect of the polyanion heparin, acting as a polycation antagonist, on the responsiveness to methacholine of isolated perfused tracheae from unchallenged control animals and from animals 6 h after ovalbumin challenge, that is, after the EAR.
A 2.0-fold AHR (P<0.001) to intraluminal administration of methacholine was observed in airways from allergen-challenged animals compared to control. Incubation of these airways with 250 U ml−1 heparin completely normalized the observed hyperresponsiveness (P<0.001), whereas the responsiveness to methacholine of airways from unchallenged control animals was not affected.
The effect of heparin on airways from allergen-challenged guinea-pigs was dose-dependently (0.1 and 1.0 mM) reversed by the NOS inhibitor L-NAME (P<0.01).
These results indicate that endogenous (presumably eosinophil-derived) polycations are involved in allergen-induced NO deficiency and AHR after the EAR, probably by inhibition of L-arginine transport.
Keywords: L-Arginine, heparin, polycations, cationic amino-acid transporter, nitric oxide synthase, eosinophils, early asthmatic reaction, airway hyperresponsiveness, allergic asthma, guinea-pig
Introduction
It has been well established that a deficiency of epithelial constitutive nitric oxide synthase (cNOS)-derived nitric oxide (NO) contributes to allergen-induced airway hyperresponsiveness (AHR) in both guinea-pigs (De Boer et al., 1996; 1999) and asthmatic patients (Ricciardolo et al., 2001). In airways from allergen-challenged guinea-pigs, it was demonstrated that a reduced availability of L-arginine is involved in the observed NO deficiency and subsequent AHR after the early asthmatic reaction (EAR) (De Boer et al., 1999). Since the intracellular L-arginine concentration is under control of specific cationic amino-acid transporter systems (Closs, 1996), one of the mechanisms leading to a reduced availability of L-arginine after the EAR could be reduced cellular uptake of this amino acid.
Interestingly, it has been demonstrated that polycations like major basic protein (MBP), as well as its analogue poly-L-arginine, inhibit L-arginine uptake through cationic amino acid (y+) transporters, and decrease NO synthesis in rat alveolar macrophages and tracheal epithelial cells (Hammermann et al., 1999). Polycation-induced inhibition of L-arginine uptake in these cells was prevented by the polyanion heparin, acting as a polycation antagonist (Hammermann et al., 1999). The functional relevance of this finding was indicated by our previous study, demonstrating that poly-L-arginine causes AHR to methacholine in a perfused guinea-pig tracheal tube preparation by inducing a deficiency of cNOS-derived NO in response to this agonist (Meurs et al., 1999). Accordingly, the poly-L-arginine-induced NO deficiency and AHR were reversed by heparin (Meurs et al., 1999).
The development of allergen-induced AHR after the EAR in guinea-pigs is associated with the release of polycationic proteins by infiltrated eosinophils in the airways (Santing et al., 1994a). In asthmatic patients, influx and activation of eosinophils are observed as early as 3 h after allergen challenge (Aalbers et al., 1993). Furthermore, elevated levels of MBP have been demonstrated in the bronchoalveolar lavage (BAL) fluid of asthmatic patients, which were correlated with the severity of AHR (Wardlaw et al., 1988). MBP-induced dysfunction of the airway epithelium has been implicated in the development of AHR in allergic asthma (Gleich et al., 1993). In vitro, it has been demonstrated that MBP is detrimental to airway epithelial cells and induces pathological alterations comparable to those observed in asthmatic patients (Motojima et al., 1989). Furthermore, intratracheal instillation or inhalation of MBP or synthetic polycations such as poly-L-arginine and poly-L-lysine caused increased airway responsiveness to methacholine in rats (Coyle et al., 1993; Uchida et al., 1993) and guinea-pigs (Yahata et al., 2002), without overt epithelial damage (Coyle et al., 1993; Uchida et al., 1993). In addition, it was demonstrated that heparin suppressed polycation-induced AHR, indicating that cation–anion interactions may modulate airway responsiveness (Coyle et al., 1993; Yahata et al., 2002).
In the present study, we hypothesized that endogenous polycations like eosinophil-derived MBP contribute to the allergen-induced NO deficiency and AHR. This hypothesis was investigated by measuring the effect of heparin on methacholine-induced airway constriction and NO deficiency in perfused tracheal preparations from unchallenged control guinea-pigs and from animals 6 h after ovalbumin-challenge, that is, after the EAR, in the absence and presence of the NOS-inhibitor Nω-nitro-L-arginine methyl ester (L-NAME).
Methods
Animals
Outbred specified pathogen-free guinea-pigs (Harlan, Heathfield, U.K.), weighing 700–900 g, were used in this study. Animals were actively IgE-sensitized to ovalbumin at the age of 4 weeks as previously described (Van Amsterdam et al., 1989). In short, 0.5 ml of an allergen solution containing 100 μg ml−1 ovalbumin and 100 mg ml−1 Al(OH)3 in saline was injected intraperitoneally, while another 0.5 ml was divided over seven intracutaneous injection sites in the proximity of lymph nodes in the paws, lumbar regions and the neck. The animals were used experimentally 4–8 weeks after sensitization. The animals were group-housed in individual cages in climate-controlled animal quarters and given water and food ad libitum, while a 12-h on/12-h off light cycle was maintained. All protocols described in this study were approved by the University of Groningen Committee for Animal Experimentation.
Allergen provocation
Ovalbumin provocations were performed by inhalation of aerosolized solutions. The provocations were performed in a specially designed animal cage, in which the guinea-pigs could move freely (Santing et al., 1992). The volume of the cage was 9 l, which ensured fast replacement of the air inside the cage with aerosol and vice versa. A DeVilbiss nebulizer (type 646, DeVilbiss, Somerset, PA, U.S.A.) driven by an airflow of 8 l min−1 provided the aerosol required, with an output of 0.33 ml min−1. Allergen provocations were performed by inhalation of an aerosol concentration of 0.5 mg ml−1 ovalbumin in saline. Allergen inhalations were discontinued when the first signs of respiratory distress were observed. Anti-histamines were not needed to prevent the development of anaphylactic shock. Previous studies measuring pleural pressure changes in ovalbumin-sensitized, permanently instrumented, unrestrained guinea-pigs have indicated that the allergen-induced EAR induced by this procedure is maximal within 20 min and lasts for up to 5 h (Santing et al., 1992; 1994b). At 6 h after ovalbumin challenge, the guinea-pigs were killed for tracheal perfusion experiments or BAL. Unchallenged or saline-challenged animals, respectively, served as controls.
Tracheal perfusion
The animals were killed by a sharp blow on the head and exsanguinated. The tracheae were rapidly removed and placed in Krebs–Henseleit (KH) solution (37°C) of the following composition (mM): NaCl 117.50, KCl 5.60, MgSO4 1.18, CaCl2 2.50, NaH2PO4 1.28, NaHCO3 25.00, D-glucose 5.50; gassed with 5% CO2 and 95% O2; pH 7.4. The tracheae were prepared free of serosal connective tissue and cut into two halves of approximately 17 mm before mounting in a perfusion setup, as described previously (De Boer et al., 1996). To this aim, the tracheal preparations were attached at each end to stainless steel perfusion tubes fixed in a Delrin perfusion holder. The holder with the trachea was then placed in a water-jacketed organ bath (37°C) containing 20 ml of gassed KH (the serosal or extraluminal (EL) compartment). The lumen was perfused with recirculating KH from a separate 20 ml bath (mucosal or intraluminal (IL) compartment) at a constant flow rate of 12 ml min−1. Two axially centred side-hole catheters connected with pressure transducers (TC-XX, Viggo-Spectramed B.V., Bilthoven, The Netherlands) were situated at the distal and proximal ends of the trachealis to measure hydrostatic pressures (Poutlet and Pinlet, respectively). The signals were fed into a differential amplifier to obtain the difference between the two pressures (ΔP=Pinlet−Poutlet), which was plotted on a flatbed chart recorder (BD 41, Kipp en Zonen, Delft, The Netherlands). ΔP reflects the resistance of the tracheal segment to perfusion and is a function of the mean diameter of the trachea between the pressure taps. The transmural pressure in the trachea was set at 0 cm H2O. At the perfusion flow rate used, a baseline ΔP of 0.1–1.0 cm H2O was measured, depending on the diameter of the preparation. After a 45 min equilibration period with three washes with fresh KH (both IL and EL), 1 μM isoproterenol was added to the EL compartment for maximal smooth muscle relaxation to assess basal tone. After three washes during at least 30 min, the trachea was exposed to EL 40 mM KCl in KH to obtain a receptor-independent reference response. Subsequently, the preparation was washed four times with KH during 45 min until basal tone was reached, and a cumulative concentration–response curve was made with IL methacholine. When used, heparin (from porcine intestinal mucosa; 250 U ml−1) was applied to both the IL and EL reservoirs, while L-NAME (0.1 or 1.0 mM) was applied to the IL reservoir, both 40 min prior to agonist addition.
Bronchoalveolar lavage
Animals were anaesthetized with 20 mg ml−1 Brietal-sodium, 35 mg kg−1 ketamine hydrochloride and 6 mg kg−1 Rompun i.p., which ensured a fast, deep anaesthesia. The lungs were gently lavaged via a tracheal canula with 5 ml of sterile saline at 37°C, followed by three subsequent aliquots of 8 ml saline. The recovered samples were placed on ice, and centrifuged at 200 × g for 10 min at 4°C. The combined pellets were resuspended to a final volume of 1.0 ml in RPMI-1640 medium and total cell numbers were counted in a Bürker-Türk chamber. For cytological examination, cytospin preparations were stained with May-Grünwald and Giemsa. A cell differentiation was performed by counting at least 400 cells in duplicate.
Eosinophil peroxidase assay
BAL cells were centrifuged and resuspended in Hanks balanced salt solution (HBSS) to a final density of 2.5 × 106 cells ml−1 and incubated with medium for 30 min at 37°C. Cell incubation was stopped by placing the samples on ice, followed by immediate centrifugation and subsequent decantation of the supernatant for measurement of eosinophil peroxidase (EPO) activity. After decantation the cells were lysed, centrifuged and the supernatant was collected to measure the remaining intracellular EPO content.
The EPO activity in cell supernatants and cell lysates was analysed according to the kinetic assay described by White et al. (1991), which is based on the oxidation of O-phenylenediamine (OPD) by EPO in the presence of hydrogen peroxide (H2O2). The substrate was made by dissolving 0.018% H2O2 and 16 mM OPD in 100 mM Tris (hydroxymethyl)-aminomethane-HCl buffer, pH 8.0, containing 0.1% Triton X-100, immediately prior to use. Horseradish peroxidase (HRP) in increasing concentrations was used as a reference. For the assay, 50 μl of supernatants obtained from intact or lysed cells or 50 μl of HRP solution was combined with 75 μl of substrate in a polystyrene 96-well microplate and placed into a thermoregulating microplate absorbance spectrophotometer (Thermomax, Molecular Devices, Menlo Park, CA, U.S.A.) at 37°C. Absorbance at 490 nm was measured every 15 s for 30 min; the velocity of the reaction (as a measure of EPO activity) was calculated by interpolation between 20 successive points (5 min) utilizing customized software (Softmax v2.01, Molecular Devices). All samples were assayed in quadruplicate.
Data analysis
To compensate for differences in baseline ΔP and in ΔP changes in response to contractile stimuli due to variation in resting internal diameter of the preparations used, IL responses of the tracheal tube preparations to methacholine were expressed as a percentage of the response induced by EL administration of 40 mM KCl. The contractile effect of 10 mM methacholine (highest concentration) was defined as Emax (De Boer et al., 1996; 1999). Using this Emax, the sensitivity to methacholine was evaluated as pEC50 (−log EC50) value. EPO activity was expressed as a per cent of the total amount of EPO (amount of EPO in the supernatant of intact cells+the amount present in the supernatant of the lysed cells). Results are expressed as means±s.e.m. Statistical analysis was performed using the Student's t-test for unpaired observations. Differences were considered statistically significant at P<0.05.
Materials
Ovalbumin (grade III), aluminium hydroxide, (−)-isoprenaline hydrochloride, heparin sodium salt from porcine intestinal mucosa (grade IA), L-NAME, OPD dihydrochloride, HRP and May-Grünwald and Giemsa stain were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.) and methacholine chloride from Aldrich (Milwaukee, WI, U.S.A.). Brietal-sodium (methohexital) was purchased from Eli Lilly (Amsterdam, The Netherlands), Ketamine hydrochloride from Parke-Davis (Barcelona, Spain), Rompun (2-(2.6-xylidino)-5.6-dihydro-4H-1.3-thiazine-hydrochloride, methylparaben) from Bayer (Leverkusen, Germany), and RPMI-1640 medium and HBSS from Gibco Life Technologies (Praisley, Scotland).
Results
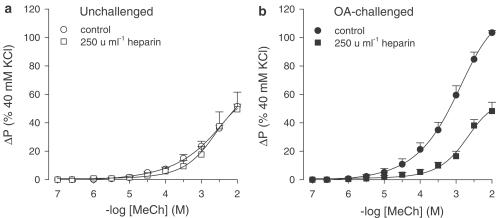
In line with previous studies (De Boer et al., 1996; 1999; Meurs et al., 2002), a 2.0-fold increase in airway responsiveness to methacholine (Emax) was observed in perfused tracheal preparations from ovalbumin-challenged guinea-pigs obtained after the EAR, as compared with unchallenged controls (P<0.001), without an effect on the sensitivity (pEC50) to the agonist (Figure 1a, b; Table 1). Preincubation with heparin (250 U ml−1; IL and EL) completely normalized the observed AHR to methacholine in preparations from ovalbumin-challenged guinea-pigs (P<0.001; Figure 1b, Table 1), while heparin did not affect the response to methacholine in unchallenged control airways (Figure 1a, Table 1). In both conditions, the pEC50 was not affected by heparin (Table 1).
Figure 1.
Methacholine (MeCh; IL)-induced constriction of intact perfused tracheal preparations obtained from (a) unchallenged and (b) ovalbumin (OA)-challenged guinea-pigs, in the absence and presence of 250 U ml−1 heparin. Results are the means±s.e.m. of 5–8 experiments.
Table 1.
Effects of heparin in the absence and presence of L-NAME on the responsiveness to methacholine of intact perfused tracheae from unchallenged and ovalbumin-challenged guinea-pigs
| Unchallenged | Ovalbumin-challenged | |||||
| Emax (% KCl) | pEC50 (−log M) | n | Emax (% KCl) | pEC50 (−log M) | n | |
| Control | 51.6±1.5 | 2.94±0.14 | 5 | 103.4±1.5* | 3.16±0.13 | 8 |
| 250 U ml−1 heparin | 49.5±12.0 | 2.80±0.05 | 5 | 48.2±6.2† | 2.98±0.14 | 5 |
| +0.1 mM L-NAME | N.D. | N.D. | 60.7±10.2† | 2.95±0.06 | 4 | |
| +1.0 mM L-NAME | N.D. | N.D. | 101.2±13.1‡ | 3.20±0.27 | 5 | |
Results are the means±s.e.m. of n experiments. N.D., not determined.
P<0.001 compared with unchallenged
P<0.001 compared with control
P<0.01 compared with 250 U ml−1 heparin.
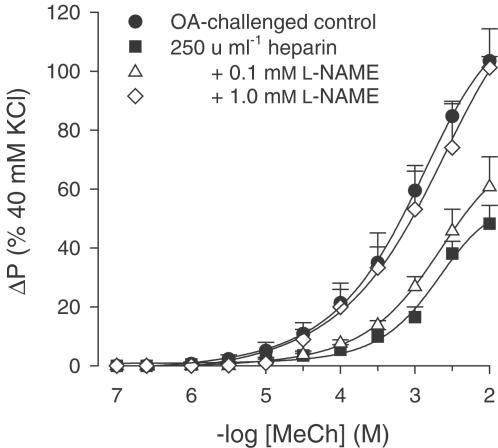
In the presence of heparin, the normalized responsiveness of ovalbumin-challenged tracheae was reversed in a concentration-dependent fashion by coincubation with the NOS inhibitor L-NAME to the level of hyperresponsiveness of ovalbumin-challenged control airways after the EAR (Figure 2; Table 1).
Figure 2.
Methacholine (MeCh; IL)-induced constriction of intact perfused tracheae from ovalbumin (OA)-challenged guinea-pigs, in the absence and presence of 250 U ml−1 heparin, alone or in combination with 0.1 or 1.0 mM L-NAME. Results are the means±s.e.m. of 4–8 experiments.
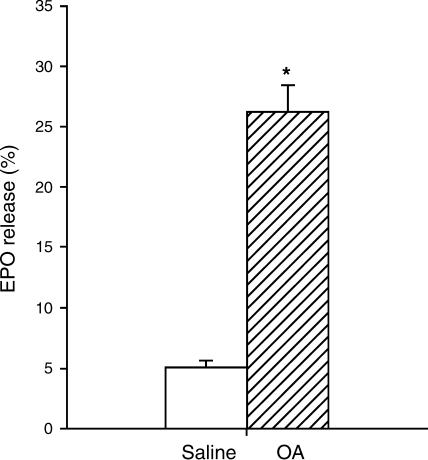
To establish eosinophil activation at 6 h after allergen challenge, release of EPO activity by BAL cells was measured. Figure 3 demonstrates that the spontaneous release of EPO by eosinophils obtained from ovalbumin-challenged animals was five-fold enhanced compared to those obtained after saline challenge (P<0.001), indicating endogenous eosinophil activation at this time point. Also, the number of eosinophils in the BAL was significantly enhanced after allergen challenge (6.1±1.0 × 106 vs 2.6±0.5 × 106 in controls, P<0.05).
Figure 3.
Spontaneous EPO release from BAL eosinophils obtained from sensitized guinea-pigs at 6 h after saline or ovalbumin (OA) challenge, expressed as the % of total cellular EPO content. 100% represents 21.9±5.2 and 22.5±3.6 ng ml−1 EPO 106 cells−1 for saline- and OA-challenged animals, respectively. Results are the means±s.e.m. of 5–8 experiments. *P<0.001 compared to saline.
Discussion
It has been demonstrated that MBP as well as its synthetic analogue poly-L-arginine can inhibit L-arginine transport through specific cationic amino-acid transporters in rat alveolar macrophages and tracheal epithelial cells, thereby limiting NO synthesis in these cells (Hammermann et al., 1999). In line with these observations, we have previously found that poly-L-arginine causes hyperresponsiveness to methacholine in perfused guinea-pig tracheal preparations by causing a deficiency of agonist-induced NO (Meurs et al., 1999). Polycation-induced inhibition of L-arginine transport as well as NO deficiency and AHR were all inhibited by the polyanion heparin, indicating that the positive charge of the polycations may be important for these effects (Hammermann et al., 1999; Meurs et al., 1999).
Previously, we have also observed that NO deficiency, caused by L-arginine limitation, is involved in the development of allergen-induced AHR after the EAR (De Boer et al., 1996; 1999). By using heparin, we did now find evidence that endogenous polycations, including eosinophil-derived MBP, could be involved in these processes. Thus, in a concentration which did not affect basal airway responsiveness to methacholine, heparin completely normalized the increased responsiveness in ovalbumin-challenged tracheae. This normalized airway responsiveness towards methacholine was dose-dependently reversed to hyperresponsiveness by the NOS inhibitor L-NAME, indicating that heparin had decreased the AHR by restoring the agonist-induced, cNOS-derived NO production. The maximal effect of L-NAME in the heparin-treated, ovalbumin-challenged preparations was similar to the maximal increase in responsiveness seen with L-NAME in unchallenged preparations (Meurs et al., 2000). A possible explanation for the observation that a higher concentration of L-NAME (1.0 mM) was needed to fully inhibit NO production in the heparin-treated, ovalbumin-challenged airways than in control preparations (0.1 mM) (De Boer et al., 1996; 1999; Meurs et al., 2000; 2002) is that heparin might scavenge L-NAME as well. Indeed, it has been demonstrated that heparin may bind monovalent cations (Lerner & Torchia, 1986).
The effect of heparin in reverting NO deficiency is presumably caused by preventing inhibition of L-arginine transport by endogenous polycations, including eosinophil-derived MBP. To ascertain that activation of eosinophil degranulation does indeed occur at 6 h after allergen challenge (after the EAR), we measured the activity of EPO – one of the major polycationic and cytotoxic granule constituents of the eosinophil in addition to MBP – in the supernatant of isolated BAL cells. Indeed, the spontaneous release of EPO by eosinophils from allergen-challenged animals was considerably increased compared to controls, which corresponds with our previous finding of increased EPO activity in the (cell-free) BAL fluid at 6 h after ovalbumin challenge (Santing et al., 1994a). Moreover, the increased spontaneous EPO release of the isolated eosinophils ex vivo indicates sustained activation of these cells, and suggests that eosinophil-derived polycations and subsequent inhibition of L-arginine uptake will not disappear during preparation and equilibration of the perfused airways before heparin treatment.
Inhaled unfractionated or low-molecular-weight heparins have previously been demonstrated to inhibit allergen-induced early and late asthmatic reactions in allergic sheep (Ahmed et al., 1994; 2000) and guinea-pigs (Yahata et al., 2002), as well as exercise- and allergen-induced asthmatic reactions in asthmatic patients (Ahmed et al., 1993; Bowler et al., 1993; Diamant et al., 1996). Moreover, heparin has been shown to inhibit AHR to methacholine, histamine and leukotriene D4 in asthmatics (Ceyhan & Celikel, 1995; 2000; Stelmach et al., 2003) and to various cholinergic agonists in allergen-challenged sheep (Ahmed et al., 1994; Molinari et al., 1998) and guinea-pigs (Yahata et al., 2002) in vivo. Restoration of bronchodilating NO production as indicated by our study is presumably involved. However, other non-anticoagulant, anti-inflammatory actions have also been implicated in the effects of heparin. Thus, in sensitized guinea-pigs, unfractionated and various modified heparins inhibited allergen-induced infiltration of eosinophils into the lung (Seeds & Page, 2001).
Low-molecular-weight heparin is known to act as a competitive inhibitor of inositol 1,4,5-trisphosphate (InsP3) receptors in mast cells and may thereby exert an inhibitory role on histamine release (Ghosh et al., 1988; Lucio et al., 1992; Ahmed et al., 1997). Indeed, the prevention of exercise-induced bronchoconstriction in patients with asthma was considered to be related to inhibition of the InsP3-dependent stimulus–secretion coupling in mast cells (Ahmed et al., 1993; Garrigo et al., 1996). Inhibition of InsP3-induced Ca2+ release by heparin has also been found in (tracheal) smooth muscle cells (Ghosh et al., 1988; Chilvers et al., 1990). However, since heparin did not at all affect methacholine responsiveness in our control preparations, a direct effect of the polyanion on airway smooth muscle Ca2+ signaling and contraction can be excluded.
It has also been demonstrated that the polyanions heparin and poly-L-glutamate could recover neuronal autoinhibitory M2 muscarinic receptor function and vagally-induced AHR in antigen-challenged guinea-pigs, presumably by neutralizing eosinophil-derived MBP, which is an endogenous allosteric antagonist of the M2 receptor (Fryer & Jacoby, 1992; 1998). However, restoration of prejunctional M2 receptor function cannot explain the action of heparin in our preparation, since only postjunctional M3-muscarinic receptors were involved in the response of methacholine. In addition to reducing AHR, heparin is known to possess antiproliferative activity for airway smooth muscle cells (Halayko et al., 1998). Since airway remodelling, including increased airway smooth muscle mass, is involved in the pathology of asthma, the antiproliferative activity of heparin together with its ability to reduce AHR makes heparin of interest in the therapy of asthma.
Recently, we have demonstrated that another mechanism, involved in L-arginine limitation and subsequent NO deficiency and AHR, is increased activity of arginase, which hydrolyses L-arginine to L-ornithine and urea and thus limits the L-arginine availability to NOS (Meurs et al., 2002; 2003). Interestingly, like MBP and poly-L-arginine, the arginase product L-ornithine has also been demonstrated to inhibit L-arginine transport (Schapira et al., 1998). Thus, at least two mechanisms – increased arginase activity and inhibition of L-arginine uptake caused by endogenous polycations and possibly L-ornithine – are involved in the observed L-arginine limitation after the EAR. Since increasing the L-arginine availability by restoring L-arginine uptake after heparin incubation normalizes the AHR after the EAR, it follows that arginase activity is becoming increasingly important in regulating the substrate availability for cNOS under conditions of substrate limitation. Therefore, restoration of the production of bronchodilating, cNOS-derived NO can be obtained by eliminating substrate competition by using an arginase inhibitor (Meurs et al., 2002) or increasing the L-arginine availability by restoration of the L-arginine uptake. It should be mentioned that a direct effect of heparin on NOS or arginase cannot explain the restoration of the AHR, since incubation of unchallenged control tracheae with heparin did not affect the responsiveness to methacholine.
In conclusion, using a guinea-pig model of allergic asthma, we have demonstrated that endogenous (eosinophil-derived) polycations may be importantly involved in allergen-induced NO deficiency and AHR after the EAR, presumably by inhibition of cellular L-arginine uptake through cationic amino-acid transporters. Restoration of bronchodilating cNOS-derived NO production may be one of the mechanisms involved in the previously observed beneficial effects of heparin on AHR in allergic asthma.
Acknowledgments
This work was supported by the Netherlands Asthma Foundation (NAF grant 00.24).
Abbreviations
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- cNOS
constitutive nitric oxide synthase
- EAR
early asthmatic reaction
- EL
extraluminal
- Emax
maximal effect
- EPO
eosinophil peroxidase
- HBSS
Hanks balanced salt solution
- HRP
horseradish peroxidase
- IL
intraluminal
- InsP3
inositol 1,4,5-trisphosphate
- KH
Krebs–Henseleit
- L-NAME
Nω-nitro-L-arginine methyl ester
- MBP
major basic protein
- OPD
O-phenylenediamine
- ΔP
differential (hydrostatic) pressure
- Pinlet
(hydrostatic) pressure at the inlet
- Poutlet
(hydrostatic) pressure at the outlet
- pEC50
−log of the concentration causing 50% of the effect
References
- AALBERS R., KAUFFMAN H.F., VRUGT B., KOETER G.H., DE MONCHY J.G. Allergen-induced recruitment of inflammatory cells in lavage 3 and 24 h after challenge in allergic asthmatic lungs. Chest. 1993;103:1178–1184. doi: 10.1378/chest.103.4.1178. [DOI] [PubMed] [Google Scholar]
- AHMED T., CAMPO C., ABRAHAM M.K., MOLINARI J.F., ABRAHAM W.M., ASHKIN D., SYRISTE T., ANDERSSON L.O., SVAHN C.M. Inhibition of antigen-induced acute bronchoconstriction, airway hyperresponsiveness, and mast cell degranulation by a nonanticoagulant heparin: comparison with a low molecular weight heparin. Am. J. Respir. Crit. Care Med. 1997;155:1848–1855. doi: 10.1164/ajrccm.155.6.9196085. [DOI] [PubMed] [Google Scholar]
- AHMED T., GARRIGO J., DANTA I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N. Engl. J. Med. 1993;329:90–95. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- AHMED T., SYRISTE T., MENDELSSOHN R., SORACE D., MANSOUR E., LANSING M., ABRAHAM W.M., ROBINSON M.J. Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation. J. Appl. Physiol. 1994;76:893–901. doi: 10.1152/jappl.1994.76.2.893. [DOI] [PubMed] [Google Scholar]
- AHMED T., UNGO J., ZHOU M., CAMPO C. Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J. Appl. Physiol. 2000;88:1721–1729. doi: 10.1152/jappl.2000.88.5.1721. [DOI] [PubMed] [Google Scholar]
- BOWLER S.D., SMITH S.M., LAVERCOMBE P.S. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am. Rev. Respir. Dis. 1993;147:160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- CEYHAN B., CELIKEL T. Effect of inhaled heparin on methacholine-induced bronchial hyperreactivity. Chest. 1995;107:1009–1012. doi: 10.1378/chest.107.4.1009. [DOI] [PubMed] [Google Scholar]
- CEYHAN B.B., CELIKEL T. Effect of inhaled low molecular weight heparin on methacholine-induced bronchoconstriction. Int. J. Clin. Pharmacol. Ther. 2000;38:446–451. doi: 10.5414/cpp38446. [DOI] [PubMed] [Google Scholar]
- CHILVERS E.R., CHALLISS R.A., WILLCOCKS A.L., POTTER B.V., BARNES P.J., NAHORSKI S.R. Characterisation of stereospecific binding sites for inositol 1,4,5-trisphosphate in airway smooth muscle. Br. J. Pharmacol. 1990;99:297–302. doi: 10.1111/j.1476-5381.1990.tb14698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSS E.I. CATs, a family of three distinct mammalian cationic amino acid transporters. Amino Acids. 1996;11:193–208. doi: 10.1007/BF00813860. [DOI] [PubMed] [Google Scholar]
- COYLE A.J., ACKERMAN S.J., IRVIN C.G. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am. Rev. Respir. Dis. 1993;147:896–900. doi: 10.1164/ajrccm/147.4.896. [DOI] [PubMed] [Google Scholar]
- DE BOER J., DUYVENDAK M., SCHUURMAN F.E., POUW F.M., ZAAGSMA J., MEURS H. Role of L-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea-pigs. Br. J. Pharmacol. 1999;128:1114–1120. doi: 10.1038/sj.bjp.0702882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER J., MEURS H., COERS W., KOOPAL M., BOTTONE A.E., VISSER A.C., TIMENS W., ZAAGSMA J. Deficiency of nitric oxide in allergen-induced airway hyperreactivity to contractile agonists after the early asthmatic reaction: an ex vivo study. Br. J. Pharmacol. 1996;119:1109–1116. doi: 10.1111/j.1476-5381.1996.tb16011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMANT Z., TIMMERS M.C., VAN D.V., PAGE C.P., VAN DER MEER F.J., STERK P.J. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. Am. J. Respir. Crit. Care Med. 1996;153:1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- FRYER A.D., JACOBY D.B. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J. Clin. Invest. 1992;90:2292–2298. doi: 10.1172/JCI116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER A.D., JACOBY D.B. Muscarinic receptors and control of airway smooth muscle. Am. J. Respir. Crit. Care Med. 1998;158:S154–S160. doi: 10.1164/ajrccm.158.supplement_2.13tac120. [DOI] [PubMed] [Google Scholar]
- GARRIGO J., DANTA I., AHMED T. Time course of the protective effect of inhaled heparin on exercise-induced asthma. Am. J. Respir. Crit. Care Med. 1996;153:1702–1707. doi: 10.1164/ajrccm.153.5.8630624. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., EIS P.S., MULLANEY J.M., EBERT C.L., GILL D.L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J. Biol. Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- GLEICH G.J., ADOLPHSON C.R., LEIFERMAN K.M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- HALAYKO A.J., RECTOR E., STEPHENS N.L. Airway smooth muscle cell proliferation: characterization of subpopulations by sensitivity to heparin inhibition. Am. J. Physiol. 1998;274:L17–L25. doi: 10.1152/ajplung.1998.274.1.L17. [DOI] [PubMed] [Google Scholar]
- HAMMERMANN R., HIRSCHMANN J., HEY C., MOSSNER J., FOLKERTS G., NIJKAMP F.P., WESSLER I., RACKE K. Cationic proteins inhibit L-arginine uptake in rat alveolar macrophages and tracheal epithelial cells. Implications for nitric oxide synthesis. Am. J. Respir. Cell Mol. Biol. 1999;21:155–162. doi: 10.1165/ajrcmb.21.2.3574. [DOI] [PubMed] [Google Scholar]
- LERNER L., TORCHIA D.A. A multinuclear NMR study of the interactions of cations with proteoglycans, heparin, and ficoll. J. Biol. Chem. 1986;261:12706–12714. [PubMed] [Google Scholar]
- LUCIO J., D'BROT J., GUO C.B., ABRAHAM W.M., LICHTENSTEIN L.M., KAGEY-SOBOTKA A., AHMED T. Immunologic mast cell-mediated responses and histamine release are attenuated by heparin. J. Appl. Physiol. 1992;73:1093–1101. doi: 10.1152/jappl.1992.73.3.1093. [DOI] [PubMed] [Google Scholar]
- MEURS H., HAMER M.A., PETHE S., VADON-LE GOFF S., BOUCHER J.L., ZAAGSMA J. Modulation of cholinergic airway reactivity and nitric oxide production by endogenous arginase activity. Br. J. Pharmacol. 2000;130:1793–1798. doi: 10.1038/sj.bjp.0703488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEURS H., MAARSINGH H., ZAAGSMA J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol. Sci. 2003;24:450–455. doi: 10.1016/S0165-6147(03)00227-X. [DOI] [PubMed] [Google Scholar]
- MEURS H., MCKAY S., MAARSINGH H., HAMER M.A., MACIC L., MOLENDIJK N., ZAAGSMA J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br. J. Pharmacol. 2002;136:391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEURS H., SCHUURMAN F.E., DUYVENDAK M., ZAAGSMA J. Deficiency of nitric oxide in polycation-induced airway hyperreactivity. Br. J. Pharmacol. 1999;126:559–562. doi: 10.1038/sj.bjp.0702372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINARI J.F., CAMPO C., SHAKIR S., AHMED T. Inhibition of antigen-induced airway hyperresponsiveness by ultralow molecular-weight heparin. Am. J. Respir. Crit. Care Med. 1998;157:887–893. doi: 10.1164/ajrccm.157.3.9708027. [DOI] [PubMed] [Google Scholar]
- MOTOJIMA S., FRIGAS E., LOEGERING D.A., GLEICH G.J. Toxicity of eosinophil cationic proteins for guinea-pig tracheal epithelium in vitro. Am. Rev. Respir. Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L., TIMMERS M.C., GEPPETTI P., VAN SCHADEWIJK A., BRAHIM J.J., SONT J.K., DE GOUW H.W., HIEMSTRA P.S., VAN KRIEKEN J.H., STERK P.J. Allergen-induced impairment of bronchoprotective nitric oxide synthesis in asthma. J. Allergy Clin. Immunol. 2001;108:198–204. doi: 10.1067/mai.2001.116572. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., HOEKSTRA Y., PASMAN Y., ZAAGSMA J., MEURS H. The importance of eosinophil activation for the development of allergen-induced bronchial hyperreactivity in conscious, unrestrained guinea-pigs. Clin. Exp. Allergy. 1994a;24:1157–1163. doi: 10.1111/j.1365-2222.1994.tb03322.x. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., MEURS H., VAN DER MARK T.W., REMIE R., OOSTEROM W.C., BROUWER F., ZAAGSMA J. A novel method to assess airway function parameters in chronically instrumented, unrestrained guinea-pigs. Pulmon. Pharmacol. 1992;5:265–272. doi: 10.1016/0952-0600(92)90069-s. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., OLYMULDER C.G., ZAAGSMA J., MEURS H. Relationships among allergen-induced early and late phase airway obstructions, bronchial hyperreactivity, and inflammation in conscious, unrestrained guinea pigs. J. Allergy Clin. Immunol. 1994b;93:1021–1030. doi: 10.1016/s0091-6749(94)70051-6. [DOI] [PubMed] [Google Scholar]
- SCHAPIRA R.M., WIESSNER J.H., MORRISEY J.F., ALMAGRO U.A., NELIN L.D. L-arginine uptake and metabolism by lung macrophages and neutrophils following intratracheal instillation of silica in vivo. Am. J. Respir. Cell Mol. Biol. 1998;19:308–315. doi: 10.1165/ajrcmb.19.2.2814. [DOI] [PubMed] [Google Scholar]
- SEEDS E.A., PAGE C.P. Heparin inhibits allergen-induced eosinophil infiltration into guinea-pig lung via a mechanism unrelated to its anticoagulant activity. Pulmon. Pharmacol. Ther. 2001;14:111–119. doi: 10.1006/pupt.2000.0277. [DOI] [PubMed] [Google Scholar]
- STELMACH I., JERZYNSKA J., STELMACH W., MAJAK P., BRZOZOWSKA A., GORSKI P., KUNA P. The effect of inhaled heparin on airway responsiveness to histamine and leukotriene D4. Allergy Asthma Proc. 2003;24:59–65. [PubMed] [Google Scholar]
- UCHIDA D.A., ACKERMAN S.J., COYLE A.J., LARSEN G.L., WELLER P.F., FREED J., IRVIN C.G. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am. Rev. Respir. Dis. 1993;147:982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- VAN AMSTERDAM R.G., BROUWER F., ZAAGSMA J. Analysis of the beta-adrenoceptor mediated inhibition of IgG1 and IgE dependent guinea-pig anaphylactic tracheal smooth muscle contraction. Agents Actions. 1989;26:48–51. doi: 10.1007/BF02126559. [DOI] [PubMed] [Google Scholar]
- WARDLAW A.J., DUNNETTE S., GLEICH G.J., COLLINS J.C., KAY A. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am. Rev. Respir. Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- WHITE S.R., KULP G.V.P., SPAETHE S.M., VAN ALSTYNE E., LEFF A.R. A kinetic assay for eosinophil peroxidase activity in eosinophils and eosinophil conditioned media. J. Immunol. Methods. 1991;44:257–263. doi: 10.1016/0022-1759(91)90094-v. [DOI] [PubMed] [Google Scholar]
- YAHATA T., NISHIMURA Y., MAEDA H., YOKOYAMA M. Modulation of airway responsiveness by anionic and cationic polyelectrolyte substances. Eur. J. Pharmacol. 2002;434:71–79. doi: 10.1016/s0014-2999(01)01528-x. [DOI] [PubMed] [Google Scholar]