Abstract
Ranolazine is a novel anti-ischemic drug that prolongs the QT interval. To evaluate the potential mechanisms and consequences, we studied: (i) Ranolazine's effects on HERG and IsK currents in Xenopus oocytes with two-electrode voltage clamp; (ii) effects of ranolazine, compared to D-sotalol, on effective refractory period (ERP), QT interval and ventricular rhythm in a dog model of acquired long QT syndrome; and (iii) effects on selected native currents in canine atrial myocytes with whole-cell patch-clamp technique.
Ranolazine inhibited HERG and IsK currents with different potencies. HERG was inhibited with an IC50 of 106 μmol l−1, whereas the IC50 for IsK was 1.7 mmol l−1.
D-Sotalol caused reverse use-dependent ERP and QT interval prolongation, whereas ranolazine produced modest, nonsignificant increases that plateaued at submaximal doses. Neither drug affected QRS duration. D-Sotalol had clear proarrhythmic effects, with all D-sotalol-treated dogs developing torsades de pointes (TdP) ventricular tachyarrhythmias, of which they ultimately died. In contrast, ranolazine did not generate TdP.
Effects on IKr and IKs were similar to those on HERG and IsK. Ranolazine blocked ICa with an IC50 of ∼300 μmol l−1. INa was unaffected.
We conclude that ranolazine inhibits IKr by blocking HERG currents, inhibits ICa at slightly larger concentrations, and has modest and self-limited effects on the QT interval. Unlike D-sotalol, ranolazine does not cause TdP in a dog model. The greater safety of ranolazine may be due to its ability to inhibit ICa at concentrations only slightly larger than those that inhibit IKr, thus producing offsetting effects on repolarization.
Keywords: Ranolazine, dog model, acquired LQTS, patch clamp, two-electrode voltage clamp, arrhythmogenesis, ionic basis
Introduction
Ranolazine is an interesting anti-anginal and anti-ischemic agent in clinical development, (Chaitman et al., 2004a, 2004b). Although the mechanism(s) underlying the anti-ischemic effect of ranolazine has not yet been elucidated, it has been reported that this piperazine derivative increases myocardial glucose oxidation and decreases fatty acid oxidation (Clarke et al., 1993, 1996; McCormack et al., 1996). Reduced lactate production and a greater amount of ATP formed per O2 consumed (McCormack et al., 1996) could account for preservation of cellular viability under ranolazine therapy (Clarke et al., 1993; Gralinski et al., 1994).
Clinical studies in patients with chronic stable angina have shown that ranolazine increases the total time that patients can exercise during symptom-limited treadmill exercise tests, as well as the time to onset of angina (Jain et al., 1990), in a concentration-dependent fashion (Wolff, 2000). One study failed to demonstrate the beneficial effects of ranolazine (Thadani et al., 1994), a result that has been attributed to insufficient plasma concentrations (Pepine & Wolff, 1999). Ranolazine has been shown to be effective in monotherapy or in combination with β-blockers or calcium antagonists, without affecting the heart rate and arterial blood pressure (Cocco et al., 1992; Pepine & Wolff, 1999; Louis et al., 2002; Chaitman et al., 2004a, 2004b). In a primate model of ischemia–reperfusion, ranolazine prevented the release of myocardial enzymes, suggesting a cardioprotective drug effect (Allely & Alps, 1990). Although Black et al. (1994) were unable to detect a reduction in infarct size after regional myocardial ischemia and subsequent reperfusion in a dog model, the same group found substantial cardioprotective effects in the isolated perfused rabbit heart (Gralinski et al., 1994). A recent study found that ranolazine significantly reduced infarct size and cardiac troponin T release in rats subjected to left anterior descending artery occlusion–reperfusion (Zacharowski et al., 2001). In dogs with heart failure, ranolazine improved left ventricular function without increasing myocardial oxygen consumption (Chandler et al., 2002).
Very little is known about the effects of ranolazine on cardiac electrophysiology. Although Allely & Alps (1988) did not find any effect of ranolazine on myocardial conduction in anesthetized dogs, clinical trials have shown a slight but clear prolongation of the QT interval in the ECG (Chaitman et al., 2004a, 2004b). Drug-induced long QT syndrome.
(LQTS) might lead to potentially fatal ventricular arrhythmias (Roden et al., 1996). It is therefore of importance to understand the effects of ranolazine on cardiac electrophysiology, and to appreciate the potential mechanism of any effects based on changes in ionic currents.
The present study was designed to evaluate the electrophysiological actions of ranolazine and the drug's effect on cardiac rhythm in a dog model of TdP. Inhibition of outward potassium (K+) currents is known to cause prolongation of the QT interval (Keating & Sanguinetti, 2001). To assess ranolazine's effect on outward K+ currents in a system free of contamination by overlapping currents, we performed voltage-clamp studies of heterologously expressed HERG and IsK subunits encoding the main repolarizing currents IKr and IKs, respectively, in Xenopus oocytes. To determine the drug effects on inward and outward currents in a native system, we performed patch-clamp studies on isolated canine atrial myocytes. To assess in vivo actions and possible arrhythmogenic potential, the effects of ranolazine were studied in a dog model of LQTS (Derakhchan et al., 1998) and compared to those of the IKr blocking class III antiarrhythmic drug D-sotalol.
Methods
Heterologous expression of HERG and IsK in Xenopus oocytes
HERG and IsK cRNAs were synthesized with the mMESSAGE mMACHINE kit (Ambion Inc., Austin, TX, U.S.A.) using SP6 and T7 promoters, respectively. cRNAs were injected into stage IV–V Xenopus oocytes (ng cRNA/oocyte: HERG 6, IsK 1), followed by two-electrode voltage-clamp recordings 24–48 h after cRNA injection. Currents were elicited at room temperature by 4-s voltage steps at 0.1 Hz from a holding potential of −80 mV to membrane potentials ranging from −50 to +40 mV in 10-mV increments, using a GeneClamp 500 amplifier and pClamp® 6.0 software (Axon Instruments, Inc., Union City, California, U.S.A.). The external (bath) solution contained (mmol l−1): 2 KCl, 96 NaCl, 1 MgCl2, 5 HEPES, 1.8 CaCl2 (pH adjusted to 7.4 with NaOH). Stock solutions were added to bath solutions as needed to obtain the final test concentrations. Currents from Xenopus oocytes expressing HERG were recorded before (control) and after application of 10, 30, 100 μmol l−1 and 1 mmol l−1 ranolazine. Currents from Xenopus oocytes expressing IsK were recorded before and after 100, 300 μmol l−1, 1 and 3 mmol l−1 ranolazine. Drug-containing solutions were superfused until steady-state block occurred (generally ∼15 min) before repeating full voltage-clamp protocols. Glass microelectrodes (3-mol l −1 KCl-filled) had 1.3–2.0 MΩ resistances. IHERG amplitude was determined by back-extrapolating a two-exponential fit of tail currents to the end of the depolarizing pulse. IsK current amplitude was measured at the end of the test pulse.
In vivo studies
Adult mongrel dogs were pre-treated with Atravet® 0.07 mg kg−1 sc (acepromazine maleate USP sterile, Ayerst, DIN 00053023). After 15 min, animals were anesthetized with Ketalean® 5.3 mg kg−1 i.v.
(ketamine hydrochloride USP, Bimeda MTC, DIN 00612316) and diazepam 0.25 mg kg−1 i.v. (Sabex Inc., DIN 00399728), followed by isoflurane 1–2% (Isoflurane USP, Abbott, DIN 02032384), intubated and mechanically ventilated. AV block was produced with radiofrequency ablation. D-Sotalol was administered intravenously at a loading dose of 8 mg kg−1 and a maintenance dose of 4 mg kg−1 h−1 (n=5). Five dogs received ranolazine as a 0.5 mg kg−1 intravenous load, followed by a first, a second and a third continuous intravenous infusion of 1, 3 and 15 mg kg−1 h−1, respectively. At 20 min after the beginning of the maintenance infusion (for D-sotalol) or 30 min after the start of each intravenous infusion rate (for ranolazine), electrophysiological measurements (right ventricular effective refractory period (ERP), QT and QRS intervals) were obtained at basic cycle lengths (BCLs) of 300, 400, 600 and 1000 ms. Phenylephrine challenges were given as boluses (10, 20, 30, 40 and 50 μg kg−1) at each D-sotalol or ranolazine infusion rate to induce ventricular tachyarrhythmias as previously described (Derakhchan et al., 1998).
Voltage-clamp experiments in native myocytes
Adult mongrel dogs (20–30 kg) were anesthetized with pentobarbital sodium (30 mg kg−1 intravenously). Their hearts were quickly removed and immersed in Tyrode's solution equilibrated with 100% O2 at room temperature. Left atrial myocytes were isolated as previously described (Li et al., 1996; 1999; 2000; Lu et al., 1998) and maintained in a high-K+ storage solution for use the same day.
General voltage-clamp techniques were performed as previously described in detail (Li et al., 1996; 1999; 2000; Lu et al., 1998). The standard Tyrode solution for cell isolation and patch-clamp studies of IKr and IKs contained (mmol l−1) 136 NaCl, 5.4 KCl, 1 MgCl2, 1 CaCl2, 0.33 NaH2PO4, 5 HEPES, and 10 dextrose (pH adjusted to 7.4 with NaOH). The high-K+ storage solution contained (mmol l−1) 20 KCl, 10 KH2PO4, 10 dextrose, 70 glutamic acid, 10 β-hydroxybutyric acid, 10 taurine, 10 EGTA, and 0.1% albumin (pH adjusted to 7.4 with KOH). The extracellular solution used to record ICa contained (mmol l−1) 136 tetraethylammonium chloride (TEA), 5.4 CsCl, 1 MgCl2, 0.33 NaH2PO4, 2 CaCl2, 10 dextrose, and 10 HEPES. The extracellular solution used to record INa contained (mmol l−1) 132.5 CsCl, 5 NaCl, 1 MgCl2, 1 CaCl2, 11 dextrose, and 20 HEPES. The pipette solution used to study K+ currents contained (mmol l−1) 110 potassium aspartate, 20 KCl, 1 MgCl2, 5 Mg2-ATP, 10 HEPES, 5 phosphocreatine, 0.1 GTP, and 10 EGTA (pH adjusted to 7.2 with KOH). The pipette solution used to record ICa contained (mmol l−1) 20 CsCl, 110 cesium aspartate, 10 HEPES, 10 EGTA, 1 MgCl2, 5 Mg2-ATP, 5 phosphocreatine, and 0.1 GTP (lithium salt). The pipette solution used to record INa contained (mmol l−1) 135 CsF, 5 NaCl, 5 HEPES, 10 EGTA, and 5 Mg2-ATP. The pHs of internal and external solutions for studies of ICa and INa were adjusted to 7.2 and 7.4, respectively, with the use of CsOH. IK was studied in the presence of 0.2 mmol l−1 CdCl2 to block ICa and 2 mmol l−1 4-AP to inhibit Ito and IKur.d. IKs was measured in the presence of 5 μmol l−1 E-4031 to block IKr. Chromanol 293B (50 μmol l−1) was added to the superfusate for IKr recording to block IKs. INa was studied in the presence of 100 μmol l−1 CdCl2 to inhibit ICa. Dofetilide and atropine (1 μmol l−1) and CdCl2 (200 μmol l−1) were added to block IKr, acetylcholine-dependent K+ current and ICa, respectively. All chemicals were obtained from Sigma-Aldrich, St Louis, MO, U.S.A. Ranolazine dihydrochloride was obtained from CV Therapeutics, Inc., Palo Alto, CA, U.S.A., lot number E3-ML-003. The stock solutions were prepared in methanol and water, and kept in the refrigerator (4°C).
Before series resistance (Rs) compensation, time constants of the decay of the capacitive surge averaged 580±21 μs (Cm, 77.4±2.6 pF; n=18) for cells used to study IKr, 515±98 μs (Cm, 76.7±3.3 pF; n=15) for cells used to study IKs, 696±45 μs (Cm, 88.8±6.8 pF; n=12) for cells used to study ICa, and 454±53 μs (Cm, 73.7±5.0 pF; n=6) for cells used to study INa. Rs values were 7.5±0.2, 6.7±0.2, 7.9±0.2, and 6.4±1.0 MΩ for electrodes used to study IKr, IKs, ICa, and INa, respectively. After compensation, the time constants were reduced to 216±10, 195±10, 241±14, and 235±9 μs, and Rs values were reduced to 2.9±0.1, 2.7±0.1, 2.8±0.1, and 3.3±0.4 MΩ for electrodes used to study IKr, IKs, ICa, and INa, respectively. IKr amplitude was determined by back-extrapolating a two-exponential fit of tail currents to the end of the depolarizing pulse. Currents were recorded at physiological temperatures (35–37°C) unless otherwise stated. Recordings were performed before (control) and after 10 min of superfusion with ranolazine. Reversal of effects was confirmed upon drug washout.
Data analysis
Data were analyzed with Axon Clampfit 6, Graphpad Prism 3 (Graphpad Software, San Diego, CA, U.S.A.) and Microsoft Excel 2000 (Microsoft Corporation, Redmond, WA, U.S.A). Group data are presented as the mean±s.e.m. Statistical comparisons between groups were made with Student's t-test and a two-tailed probability <0.05 was taken to indicate statistical significance. Nonlinear curve fitting was performed with the use of the algorithm provided in Clampfit 6 or Prism 3.
Results
Effects of ranolazine on HERG current in Xenopus oocytes
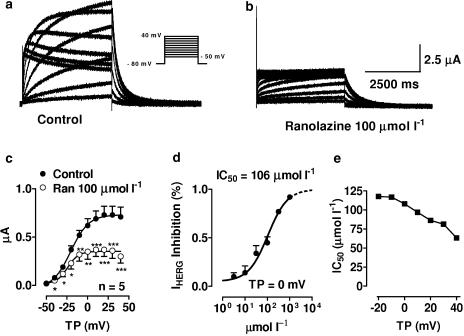
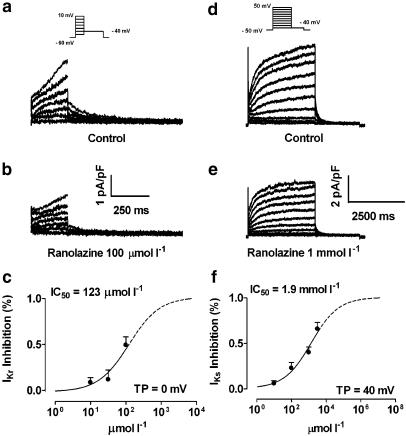
Figures 1a and b show original HERG current (IHERG) recordings before (a) and after (b) application of 100 μmol l−1 ranolazine. Mean tail current–voltage relations of IHERG obtained from five oocytes under control conditions (filled circles) and in the presence (open circles) of 100 μmol l−1 ranolazine are shown in (c). Ranolazine significantly decreased IHERG over the voltage range from −40 to 40 mV. Panel d shows ranolazine concentration–response relationships at a membrane potential of 0 mV. Block was concentration dependent with an IC50 of 106 μmol l−1 at a test potential of 0 mV. Panel e depicts the voltage dependence of IHERG block by ranolazine. At test potentials between –20 and +40 mV, IC50's ranged from 60 to 120 μmol l−1. Overall, 100 μM ranolazine inhibited IHERG by ∼50%.
Figure 1.
Inhibition of IHERG current by ranolazine. (a, b) Currents from a representative cell before (a) and after application of 100 μmol l−1 ranolazine (b). Currents were elicited by the protocol shown in the inset. (c) Mean current–voltage relationships of IHERG tail currents under control conditions (filled circles) and in the presence of 100 μmol l−1 ranolazine (open circles). (d) Mean concentration–response curve at a test potential of 0 mV. Results are mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 vs control, n=5. (e) 50% inhibition of IHERG by ranolazine (IC50; y-axis) as a function of test potential (x-axis).
Effects of ranolazine on IsK current in Xenopus oocytes
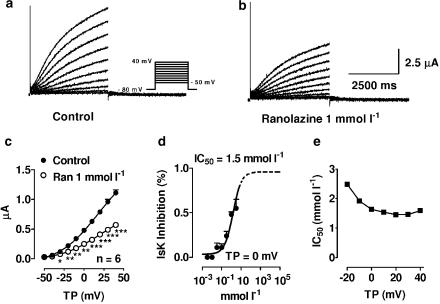
Original IsK current recordings before and after 1 mmol l−1 ranolazine are shown in Figure 2, panels a and b, respectively. Mean current–voltage relationships of IsK current obtained from six Xenopus oocytes before (filled circles) and after 1 mmol l−1 ranolazine (open circles) are illustrated in panel c. Panel d shows the ranolazine concentration–response curve at a membrane potential of 0 mV. Measurements could not be obtained with concentrations greater than 3 mmol l−1 because of limited solubility. To calculate the IC50 of IsK inhibition by ranolazine, we assumed a maximum inhibition of 100% at a concentration of 10 mol l−1 ranolazine. The extrapolated part of the concentration–response curve in panel d is represented by a dotted line. Ranolazine inhibited IsK currents in a concentration-dependent fashion, with an IC50 of 1.7 mmol l−1 at a test potential of 0 mV. At test potentials between −20 and +40 mV, IC50's were between 1.5 and 2.5 mmol l−1, as shown in panel e. No clear voltage dependence of block was observed.
Figure 2.
Inhibition of IsK by ranolazine. (a, b) Currents from a representative cell under control conditions (a) and in the presence of 1 mmol l−1 ranolazine (b). Currents were elicited by the protocol shown in the inset. (c) Mean current–voltage relationships of IsK under control conditions (filled circles) and in the presence of 1 mmol l−1 ranolazine (open circles). (d) Mean concentration–response curve at a test potential of 0 mV. Results are mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 vs control, n=6. (e) 50% inhibition of IsK by ranolazine (IC50; y-axis) as a function of test potential (x-axis).
Effects of ranolazine on QT intervals and arrhythmia induction in anesthetized dogs: comparison with D-sotalol
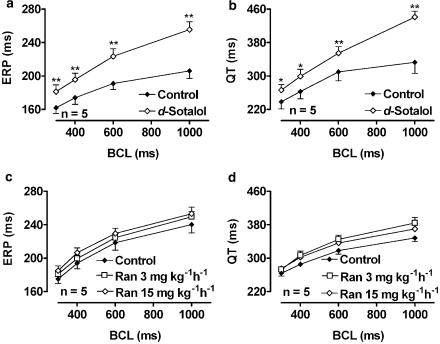
Figure 3 shows the effects of D-sotalol and ranolazine on ERP and QT interval as a function of BCL. D-Sotalol and ranolazine had quite different effects on repolarization. D-Sotalol increased right ventricular ERP and QT interval in a reverse use-dependent fashion (panels a and b, respectively). Ranolazine, on the other hand, had only a modest, statistically nonsignificant, tendency to increase ERP and QT interval (c and d). For example, at a BCL of 1000 ms, D-sotalol increased the QT interval from 333±27 to 441±14 ms (a 32% increase), whereas at the same cycle length the maximum increase by ranolazine (at the submaximal infusion rate of 3 mg kg−1h−1) was from 348±9 to 384±14 ms (a 10% increase). Neither drug significantly affected QRS duration (data not shown).
Figure 3.
Effects of D-sotalol and ranolazine on right ventricular ERP and QT interval as a function of BCL. (a, b) Effect of D-sotalol on ERP (a) and QT duration (b). Filled diamonds: Control, n=5. Open diamonds: D-Sotalol bolus of 8 mg kg−1, followed by a maintenance dose of 4 mg kg−1 h−1, n=5. (c, d) Effects of ranolazine on ERP (c) and QT duration (d). Filled diamonds: Control, open squares: ranolazine 3 mg kg−1 h−1, open diamonds: ranolazine 15 mg kg−1 h−1. *P<0.05, **P<0.01, n=5.
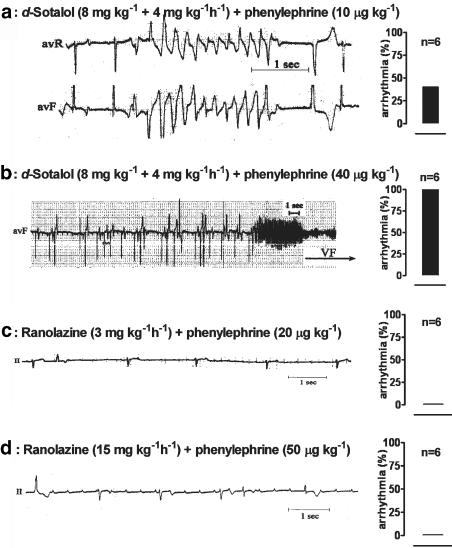
Figure 4 illustrates the arrhythmogenicity of D-sotalol upon challenge with phenylephrine 10 μg kg−1 (a) and phenylephrine 40 μg kg−1 (b) and ranolazine at an infusion rate of 3 mg kg−1 h−1 with phenylephrine at 20 μg kg−1 (c) and at 15 mg kg−1 h−1 with phenylephrine 50 μg kg−1 (d). The occurrence of tachyarrhythmia (percentage of animals) upon challenge with the dose of phenylephrine shown is provided in the bar graph in the right panel of each original recording. D-Sotalol had clear proarrhythmic effects such as bigeminy, trigeminy, TdP (a), and TdP degenerating to ventricular fibrillation (b). One of five dogs had TdP without phenylephrine challenge, and all five had TdP upon phenylephrine challenge (mean lowest dose causing TdP was 28±8 μg kg–1). All dogs receiving D-sotalol eventually died from TdP degenerating to ventricular fibrillation. In contrast, no TdP or ventricular fibrillation was observed during ranolazine infusion with or without i.v. bolus injections of phenylephrine (panels c and d).
Figure 4.
Arrhythmogenic effects of D-sotalol and ranolazine. TdP was induced by challenge with phenylephrine, which was administered as an intravenous bolus of 10–50 μg kg−1. (a) D-Sotalol 8 mg kg−1 bolus followed by continuous infusion of 4 mg kg−1 h−1 and phenylephrine 10 μg kg−1 bolus. (b) D-Sotalol 8 mg kg−1 bolus followed by continuous infusion of 4 mg kg−1 h−1 and phenylephrine 40 μg kg−1 bolus. (c) Ranolazine 3 mg kg−1 and phenylephrine 20 μg kg−1 bolus. (d) Ranolazine 15 mg kg−1 and phenylephrine 50 μg kg−1 bolus. The bar graphs on the right-hand panel of each ECG illustrate the number of animals (in %; n=6 in each group) developing TdP in each series of experiments.
Effects of ranolazine on IKr and IKs in native myocytes
Experiments to study IKr were performed in the presence of 50 μmol l−1 chromanol 293B to suppress contamination by IKs. Recordings were obtained at 0.1 Hz with 200-ms pulses from –60 to +10 mV, followed by 2-s repolarizations to −40 mV to observe tail currents, after verifying that tail currents were fully suppressed by 5 μmol l−1 E-4031. Figure 5 shows representative recordings before (a) and after (b) 100 μmol l−1 ranolazine. Ranolazine decreased IKr tail current density by 8.2, 15.2 and 49.3% at 10, 30, and 100 μmol l−1, respectively, at a test potential of 0 mV. As for IHERG, block of IKr was concentration-dependent (c) with about 50% block at 100 μmol l−1 at a test potential of 0 mV.
Figure 5.
IKr and IKs inhibition by ranolazine. (a, b) IKr from a representative cell before (a) and after application of 100 μmol l−1 ranolazine (b). Currents were elicited by the protocol shown in the inset. (c) Concentration–response curve of mean data at a test potential of 0 mV. *P<0.05, **P<0.01 vs control, n=10. (d, e) Representative IKs recordings before (d) and after 1 mmol l−1 ranolazine (e). (f) Concentration–response curve of mean data at a test potential of +40 mV. *P<0.05, **P<0.01 vs control, n=6.
Experiments to study IKs were performed in the presence of 5 μmol l−1 E-4031 to prevent contamination by IKr. IKs was elicited by 3-s depolarizing pulses from −50 to +50 mV, followed by 2-s repolarizations to −40 mV to observe tail currents. Original IKs recordings before and after 1 mmol l−1 ranolazine are shown in Figures 5d and e, respectively. Mean step current density–voltage relations were not significantly affected by 10 and 30 μmol l−1 ranolazine. Block of IKs step current was concentration-dependent (Figure 5f), with an IC50 of 1.9 mmol l−1 at 40 mV, similar to that of IsK current.
Effects of ranolazine on ICa in native myocytes
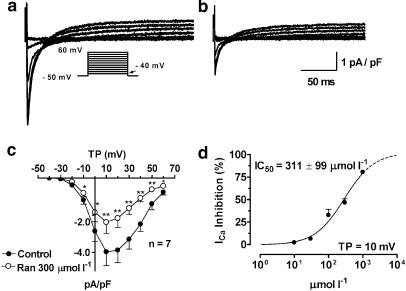
ICa was recorded upon 240-ms depolarizing pulses from −50 mV to voltages ranging from –40 to +60 mV. Figures 6a and b show original recordings of ICa before and after application of 300 μmol l−1 ranolazine, respectively. Mean current density–voltage relationships at 1 Hz under control conditions (filled circles) and in the presence of 300 μmol l−1 ranolazine (a concentration close to the IC50, open circles) are shown in (c). At low concentrations (10 and 30 μmol l−1), no significant change was found, but at higher concentrations (100, 300 μmol l−1 and 1 mmol l−1), ICa density was significantly reduced, with block increasing as drug concentration increased. Panel d illustrates the concentration-dependent inhibition of ICa by ranolazine. At the voltage associated with maximum current (+10 mV), EC50 values averaged 311±99 μmol l−1.
Figure 6.
ICa inhibition by ranolazine. (a, b) ICa current recordings before (a) and after application of 300 μmol l−1 ranolazine (b). Currents were elicited from a holding potential of −50 mV to test potentials between −40 mV (arrow) and +60 mV as shown by the protocol in the inset. (c) Mean current–voltage relationships under control conditions (filled circles) and in the presence of 300 μmol l−1 ranolazine (open circles). (d) Mean concentration–response curve at a test potential of 10 mV. Results are mean±s.e.m. *P<0.05 and **P<0.01 vs control, n=8.
Effects of ranolazine on INa in native myocytes
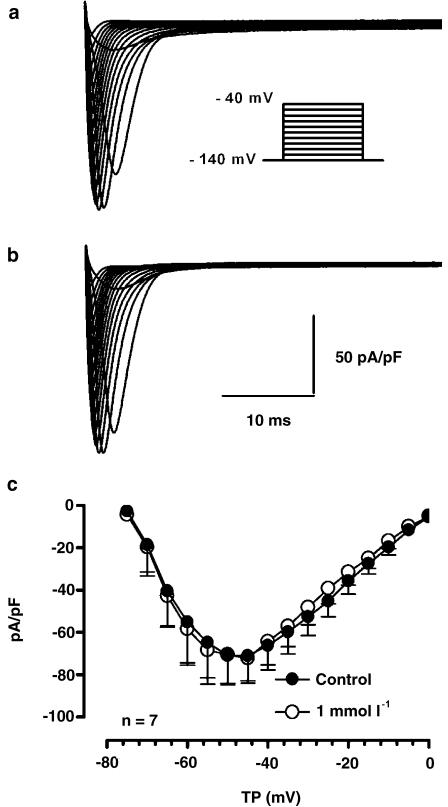
INa was recorded at 17°C during 40-ms depolarizations applied from a holding potential of –140 mV to a test potential of up to −40 mV in 10 mV steps at 1 and 2 Hz. Figure 7 shows original recordings of INa before (a) and after (b) application of 1 mmol l−1 ranolazine. In the cell shown, ranolazine did not alter INa at either 1 or 2 Hz. A similar lack of effect was observed in a total of six cells. Panel c shows the density–voltage relation of INa before (filled circles) and after (open circles) exposure to 1 mmol l−1 ranolazine at 1 Hz.
Figure 7.
Effect of ranolazine on INa. (a, b) Currents from a representative cell before (a) and after 1 mmol l−1 ranolazine (b). Currents were elicited by the protocol shown in the inset. (c) Mean current–voltage relationship under control conditions (filled circles) and in the presence of 1 mmol l−1 ranolazine (open circles), n=6. Results are mean±s.e.m.
Discussion
Ranolazine is a promising new agent for the treatment of myocardial ischemia (Conti, 2003), with particular advantages for the management of patients with congestive heart failure (Ferrari et al., 2003). The drug causes some degree of QT interval prolongation, which has been a concern in development. In the present study, we evaluated ranolazine's effects on a variety of ionic currents. Results in Xenopus oocytes pointed to inhibition of delayed-rectifier currents, with selectivity for HERG current over IsK. This was confirmed in native cardiomyocytes, and in addition significant effects on ICa were identified. Experiments in a dog model confirmed ranolazine's ability to increase the QT interval, but showed that, in contrast to the more selective IKr blocker D-sotalol, ranolazine's QT–prolonging action reached a maximum at a modest level and failed to increase despite increasing dose, and that unlike D-sotalol ranolazine failed to produce significant ventricular proarrhythmia. To our knowledge, the present study is the first evaluation in the literature of ranolazine's ion-channel-blocking actions and the first comparison of ranolazine's in vivo electrophysiological actions with those of a class III compound known to cause TdP.
Ion current-blocking effects of ranolazine
The delayed rectifier current IK is a key repolarizing current of the cardiac action potential. It consists of the rapidly activating component IKr and the slowly activating component IKs (Sanguinetti & Jurkiewicz, 1990; 1991). The pore-forming subunit HERG is believed to coassemble with the regulatory subunit MiRP1 to form IKr (Sanguinetti et al., 1995; Trudeau et al., 1995; Abbott et al., 1999), although there is some contradictory evidence regarding the role of MiRP1 (Weerapura et al., 2002). KvLQT1 coassembles with minK (or IsK) to form IKs (Barhanin et al., 1996; Sanguinetti et al., 1996). Dysfunction of delayed rectifier potassium channels commonly underlies prolongation of the QT interval in the ECG and is associated with inherited cardiac arrhythmias (Sanguinetti, 1999). Drug-induced block of HERG has been identified as a common cause of TdP and occasionally sudden cardiac death (Mitcheson et al., 2000). ICa is a significant contributor to the early afterdepolarizations implicated in drug-induced TdP (Nattel & Quantz, 1988). Drugs that block ICa as well as IKr, such as verapamil and amiodarone, are less likely to produce TdP than pure IKr blockers, possibly because ICa inhibition prevents early afterdepolarization generation despite substantial repolarization delay (Nattel & Quantz, 1988; Nattel & Talajic, 1988; Zhang et al., 1999). The concomitant block of ICa and IKr may explain ranolazine's lack of TdP induction in our in vivo dog model. Indeed, preliminary data have been presented that suggest similarities in the ionic actions of ranolazine and amiodarone (Zygmunt et al., 2002).
Drug-induced LQTS
Acquired LQTS is a potentially lethal side effect of common medications and is most often caused by block of cardiac HERG channels (Roden et al., 1996). In our study, ranolazine inhibited IHERG expressed in Xenopus oocytes in a concentration- and voltage-dependent fashion. Drug-induced inhibition of IKs along with IKr can be particularly potent in delaying repolarization, because of loss of ‘repolarization reserve' (Biliczki et al., 2002). Ranolazine effects on IsK occurred only at concentrations expected to be higher than those achieved in man, and about an order of magnitude greater than those on HERG, indicating that ranolazine is a very weak IsK blocker. Inhibition of native IKr and IKs was consistent with results of heterologous expression of HERG and IsK in Xenopus oocytes. Like IHERG, IKr was inhibited with an IC50 in the range of 100 μM. IKs was less potently inhibited by ranolazine than were HERG/IKr currents. The effects of ranolazine on IHERG and IKr provide a potential explanation for the drug's QT-prolonging effects in man (Chaitman et al., 2004a, 2004b).
To assess ranolazine's potential to cause ventricular proarrhythmia, we evaluated its effects in a dog model of LQTS and compared the results to the IKr blocking class III antiarrhythmic drug D-sotalol. D-Sotalol had clear proarrhythmic effects, with all D-sotalol-treated dogs developing TdP and ultimately dying due to arrhythmia. In contrast, ranolazine did not produce TdP. The only ventricular arrhythmias occurring in the presence of ranolazine were isolated, brief runs of accelerated ventricular rhythms, not more than would be expected by phenylephrine infusion alone. D-Sotalol produced marked reverse use-dependent prolongation of ERP and QT interval, whereas ranolazine produced very modest increases. Ranolazine's QT-prolonging action became maximal at 3 mg kg−1 h−1 and decreased thereafter at 15 mg kg−1 h−1. In a primate model of local ischaemia with reperfusion, ranolazine infusion at this dose resulted in the prevention of cardiac enzyme release, suggesting reduced ischemic damage (Allely & Alps, 1990). In a rat model, infusion of 9.6 mg kg−1 h−1 (a dosage similar to the high ranolazine infusion in our in vivo dog experiments) resulted in 33% reduction in myocardial infarct size compared to control rats. Troponin T release was also significantly attenuated by this ranolazine dosage (Zacharowski et al., 2001), indicating cardioprotective effects of ranolazine at the dosages tested. Therefore, our data show that, despite its effect on outward K+ currents, ranolazine is not arrhythmogenic in our dog model at therapeutically effective dosages.
A potential explanation for the lack of arrhythmogenicity despite ranolazine's HERG-blocking effect is concurrent inhibition of inward currents. Ranolazine had no effect on INa, but inhibited the inward calcium current ICa with an IC50 in the range of 300 μmol l−1. Consistent with our findings, Allen & Chapman (1996) observed ∼11.3% inhibition of peak ICa by 100 μmol l−1 in guinea-pig ventricular myocytes. Thus, ICa is inhibited at concentrations just higher than those that block IKr/HERG. This result may explain the limited maximum APD/QT prolongation produced by the drug. At low and therapeutic clinical concentrations, IKr block is minimal and very little, if any, APD/QT change is seen. At maximum clinical concentrations, IKr block might become measurable and modest APD/QT prolongation occurs. At higher concentrations, IKr block would be expected to increase, but ICa block would be expected to become manifest, counteracting the tendency of IKr inhibition to cause APD/QT prolongation and limiting arrhythmogenic effects of the drug. An additional potential mechanism for ranolazine-limited repolarization-delaying action is its ability to suppress late (slowly-inactivating) INa (Zygmunt et al., 2002), which likely contributes to the drug's ability to suppress TdP induced by an INa inactivation inhibitor, anemone toxin (ATX-II), in the guinea-pig heart (Wu et al., 2004).
Potential limitations
We have not examined the effects of ranolazine on the transient outward current (Ito) at either the level of cloned Ito subunits or native current. Ito contributes primarily to early repolarization and Ito inhibition tends to reduce the overall action potential because of secondary changes, primarily IKr activation, due to a raised plateau voltage (Courtemanche et al., 1998). It is therefore unlikely that Ito inhibition contributes importantly to ranolazine's ability to delay repolarization in dogs or man. Zygmunt et al. (2002) found in preliminary studies that 100 μmol l−1 ranolazine causes about 17% inhibition of Ito in ventricular myocytes.
We did not find any inhibition of INa by ranolazine. However, we cannot fully exclude the possibility that the drug may have INa-inhibiting actions that are not manifest under the conditions needed for INa study in isolated myocytes: low temperature (17°C) to achieve voltage control and negative holding potentials to remove inactivation.
We have not measured the effects of D-sotalol on ionic currents. D-Sotalol is known to block IK (Carmeliet, 1985), specifically the IKr component (Sanguinetti & Jurkiewicz, 1990). Feng et al. (1997) showed that D-sotalol does not have any effect on Ito or the ultrarapid delayed rectifier IKur.
We studied ranolazine's effects on HERG and IsK current in Xenopus oocytes, as well as on corresponding native currents in canine cardiomyocytes. The oocyte studies provide information on drug block in an isolated system in which the problem of overlapping currents, which require the use of blocking drugs and selected voltage protocols to suppress in native systems, is minimized. On the other hand, studies in native cardiac cell systems provide information about the currents of interest in their cellular environment, but are subject to the limitations of complex interventions to minimize contaminating currents and potential distortions of effects from nonspecific drug actions and incomplete current separation. Thus, we consider oocyte and native myocyte studies to provide complementary information. Their concordance in the present study is reassuring in terms of the relative IKr- and IKs- blocking properties of ranolazine.
We studied ranolazine effects in a specific dog model of TdP. The complete lack of proarrhythmia with ranolazine, in contrast to the clear proarrhythmic effects of D-sotalol under the same conditions, is reassuring. However, our results were obtained from healthy animals. Owing to decreased repolarization reserve in certain pathological conditions such as heart failure or congenital LQTS, otherwise weak K+ channel block and repolarization lengthening might cause proarrhythmia. Therefore, our data cannot exclude the potential occurrence of Tdp in such circumstances. It must also be kept in mind that these results were obtained in a specific animal model of proarrhythmia and extrapolation to man should be appropriately cautious.
Conclusions
Our study shows that ranolazine inhibits IKr, IKs, and ICa, with a relative potency IKr>ICa>IKs. The IKr- inhibiting effects account for the drug's ability to cause QT prolongation, and the ICa- blocking actions occurring at slightly higher concentrations may explain the plateauing and subsequent decrease in QT prolongation at larger drug doses. Ranolazine did not cause TdP in a dog model of acquired LQTS, in contrast to the clear proarrhythmia resulting from the comparison drug, D-sotalol.
Acknowledgments
HERG and IsK cDNA were kind gifts from Dr Michael Sanguinetti, Utah. We thank Chantal St-Cyr, Evelyn Landry, and Xiao Fan Yang for excellent technical assistance and France Thériault for secretarial help with the manuscript. Gernot Schram was supported by the Canadian Institutes of Health Research (CIHR)/Aventis Pharmaceuticals Fellowship. Joachim R. Ehrlich was supported by the Heart and Stroke Foundation of Canada studentship.
Abbreviations
- APD
action potential duration
- ATP
adenosine triphosphate
- AV block
atrioventricular block
- BCL
basic cycle length
- Cm
membrane capacitance
- ECG
electrocardiogram
- EGTA
ethylene glycol-bis[β-aminoethyl ether]-N,N,N′N′-tetraacetic acid
- ERP
effective refractory period
- HEPES
4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid
- HERG
human ether-a-gogo-related gene
- Hz
Hertz
- IC50
half-maximal inhibitory concentration
- ICa
inward calcium current
- IKr
rapid component of the delayed rectifier potassium current
- IKs
slow component of the delayed rectifier potassium current
- IKur,d
ultrarapid component of the delayed rectifier potassium current in dogs
- INa
inward sodium current
- Ito
transient outward current
- i.v.
intravenous
- LQTS
long QT syndrome
- MΩ
mega ohm
- MiRP1
min K-related peptide 1
- pF
pico Farad
- Rs
series resistance
- s.e.m.
standard error of the mean
- TEA
tetraethylammonium chloride
- TdP
torsade de pointes
References
- ABBOTT G.W., SESTI F., SPLAWSKI I., BUCK M.E., LEHMANN M.H., TIMOTHY K.W., KEATING M.T., GOLDSTEIN S.A. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- ALLELY M.C., ALPS B.J. The effects of the novel anti-anginal compound RS 43285 on myocardial conduction in the anaesthetized dog. Br. J. Pharmacol. 1988;93:375–382. doi: 10.1111/j.1476-5381.1988.tb11444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLELY M.C., ALPS B.J. Prevention of myocardial enzyme release by ranolazine in a primate model of ischaemia with reperfusion. Br. J. Pharmacol. 1990;99:5–6. doi: 10.1111/j.1476-5381.1990.tb14641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN T.J., CHAPMAN R.A. Effects of ranolazine on L-type calcium channel currents in guinea-pig single ventricular myocytes. Br. J. Pharmacol. 1996;118:249–254. doi: 10.1111/j.1476-5381.1996.tb15395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARHANIN J., LESAGE F., GUILLEMARE E., FINK M., LAZDUNSKI M., ROMEY G. KvLQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- BILICZKI P., VIRAG L., IOST N., PAPP J.G., VARRO A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S.C., GRALINSKI M.R., MCCORMACK J.G., DRISCOLL E.M., LUCCHESI B.R. Effect of ranolazine on infarct size in a canine model of regional myocardial ischemia/reperfusion. J. Cardiovasc. Pharmacol. 1994;24:921–928. doi: 10.1097/00005344-199424060-00009. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Electrophysiologic and voltage clamp analysis of the effects of sotalol on isolated cardiac muscle and Purkinje fibers. J. Pharmacol. Exp. Ther. 1985;232:817–825. [PubMed] [Google Scholar]
- CHAITMAN B.R., PEPINE C.J., PARKER J.O., SKOPAL M.J., CHUMAKOVA G., KUCH J., WANG W., SKETTINO S.L., WOLFF A.A. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina. JAMA. 2004b;21:309–315. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- CHAITMAN B.R., SKETTINO S.L., PARKER J.O., HANLEY P., MELUZIN J., KUCH J., PEPINE C.J., WANG W., NELSON J.J., HEBERT D.A., WOLFF A.A., for the MARISA Investigators Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J. Am. Coll. Cardiol. 2004a;43:1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- CHANDLER M.P., STANLEY W.C., MORITA H., SUZUKI G., ROTH B.A., BLACKBURN B., WOLFF A., SABBAH H.N. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ. Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- CLARKE B., SPEDDING M., PATMORE L., MCCORMACK J.G. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br. J. Pharmacol. 1993;109:748–750. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE B., WYATT K.M., MCCORMACK J.G. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J. Mol. Cell. Cardiol. 1996;28:341–350. doi: 10.1006/jmcc.1996.0032. [DOI] [PubMed] [Google Scholar]
- COCCO G., ROUSSEAU M.F., BOUVY T., CHERON P., WILLIAMS G., DETRY J.M., POULEUR H. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J. Cardiovasc. Pharmacol. 1992;20:131–138. [PubMed] [Google Scholar]
- CONTI C.R. Partial fatty acid oxidation (pFOX) inhibition: a new therapy for chronic stable angina. Clin. Cardiol. 2003;26:161–162. doi: 10.1002/clc.4960260402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COURTEMANCHE M., RAMIREZ R.J., NATTEL S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am. J. Physiol. 1998;275:H301–H321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- DERAKHCHAN K., CARDINAL R., BRUNET S., KLUG D., PHARAND C., KUS T., SASYNIUK B.I. Polymorphic ventricular tachycardias induced by D-sotalol and phenylephrine in canine preparations of atrioventricular block: initiation in the conduction system followed by spatially unstable re-entry. Cardiovasc. Res. 1998;38:617–630. doi: 10.1016/s0008-6363(98)00036-4. [DOI] [PubMed] [Google Scholar]
- FENG J., WANG Z., LI G.R., NATTEL S. Effects of class III antiarrhythmic drugs on transient outward and ultra-rapid delayed rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 1997;281:384–392. [PubMed] [Google Scholar]
- FERRARI R., CICCHITELLI G., MERLI E., ANDREADOU I., GUARDIGLI G. Metabolic modulation and optimization of energy consumption in heart failure. Med. Clin. North. Am. 2003;87:493–507. doi: 10.1016/s0025-7125(02)00184-0. [DOI] [PubMed] [Google Scholar]
- GRALINSKI M.R., BLACK S.C., KILGORE K.S., CHOU A.Y., MCCORMACK J.G., LUCCHESI B.R. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc. Res. 1994;28:1231–1237. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- JAIN D., DASGUPTA P., HUGHES L.O., LAHIRI A., RAFTERY E.B. Ranolazine (RS-43285) – a preliminary-study of a new antianginal agent with selective effect on ischemic myocardium. Eur. J. Clin. Pharmacol. 1990;38:111–114. doi: 10.1007/BF00265967. [DOI] [PubMed] [Google Scholar]
- KEATING M.T., SANGUINETTI M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- LI D., MELNYK P., FENG J., WANG Z., PETRECCA K., SHRIER A., NATTEL S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- LI G.R., FENG J., YUE L., CARRIER M., NATTEL S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ. Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- LI G.R., YANG B., FENG J., BOSCH R.F., CARRIER M., NATTEL S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am. J. Physiol. 1999;276:H98–H106. doi: 10.1152/ajpheart.1999.276.1.H98. [DOI] [PubMed] [Google Scholar]
- LOUIS A.A., MANOUSOS I.R., COLETTA A.P., CLARK A.L., CLELAND J.G. Clinical trials update: The Heart Protection Study, IONA, CARISA, ENRICHD, ACUTE, ALIVE, MADIT II and REMATCH. Impact of nicorandil on angina. Combination assessment of ranolazine in stable angina. Enhancing recovery in coronary heart disease patients. Assessment of cardioversion using transoesophageal echocardiography. Azimilide post-infarct survival evaluation. Randomised evaluation of mechanical assistance for treatment of chronic heart failure. Eur. J. Heart Fail. 2002;4:111–116. doi: 10.1016/s1388-9842(01)00240-9. [DOI] [PubMed] [Google Scholar]
- LU Y., YUE L., WANG Z., NATTEL S. Effects of the diuretic agent indapamide on Na+, transient outward, and delayed rectifier currents in canine atrial myocytes. Circ. Res. 1998;83:158–166. doi: 10.1161/01.res.83.2.158. [DOI] [PubMed] [Google Scholar]
- MCCORMACK J.G., BARR R.L., WOLFF A.A., LOPASCHUK G.D. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., LIN M., CULBERSON C., SANGUINETTI M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATTEL S., QUANTZ M.A. Pharmacological response of quinidine induced early afterdepolarisations in canine cardiac Purkinje fibres: insights into underlying ionic mechanisms. Cardiovasc. Res. 1988;22:808–817. doi: 10.1093/cvr/22.11.808. [DOI] [PubMed] [Google Scholar]
- NATTEL S., TALAJIC M. Recent advances in understanding the pharmacology of amiodarone. Drugs. 1988;36:121–131. doi: 10.2165/00003495-198836020-00001. [DOI] [PubMed] [Google Scholar]
- PEPINE C.J., WOLFF A.A. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am. J. Cardiol. 1999;84:46–50. doi: 10.1016/s0002-9149(99)00190-3. [DOI] [PubMed] [Google Scholar]
- RODEN D.M., LAZZARA R., ROSEN M., SCHWARTZ P.J., TOWBIN J., VINCENT G.M. Multiple mechanisms in the long-QT syndrome: current knowledge, gaps, and future directions. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C. Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia. Ann. N.Y. Acad. Sci. 1999;868:406–413. doi: 10.1111/j.1749-6632.1999.tb11302.x. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., CURRAN M.E., ZOU A., SHEN J., SPECTOR P.S., ATKINSON D.L., KEATING M.T. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JIANG C., CURRAN M.E., KEATING M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am. J. Physiol. 1991;260:H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- THADANI U., EZEKOWITZ M., FENNEY L., CHIANG Y.K. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris Ranolazine Study Group. Circulation. 1994;90:726–734. doi: 10.1161/01.cir.90.2.726. [DOI] [PubMed] [Google Scholar]
- TRUDEAU M.C., WARMKE J.W., GANETZKY B., ROBERTSON G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- WEERAPURA M., NATTEL S., CHARTIER D., CABALLERO R., HEBERT T.E. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current Is MiRP1 the missing link. J. Physiol. 2002;540:15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF A.A.MARISA: Monotherapy assessment of ranolazine in stable angina J. Am. Coll. Cardiol. 200035408A(abstract) [Google Scholar]
- WU L., SHRYOCK J.C., SONG Y., LI Y., ANTZELEVITCH C., BELARDINELLI L.Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome J. Pharmacol. Exp. Ther. 2004(Epub ahead of print) [DOI] [PubMed]
- ZACHAROWSKI K., BLACKBURN B., THIEMERMANN C. Ranolazine, a partial fatty acid oxidation inhibitor, reduces myocardial infarct size and cardiac troponin T release in the rat. Eur. J. Pharmacol. 2001;418:105–110. doi: 10.1016/s0014-2999(01)00920-7. [DOI] [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT A.C., THOMAS G.P., BELARDINELLI L., BLACKBURN B., ANTZELEVITCH C.Ranolazine produces ion channel effects similar to those observed with chronic amiodarone in canine ventricular myocytes Pacing Clin. Electrophysiol. 200224626(abstract) [Google Scholar]