Abstract
CB1 receptor cellular signal transduction is dependent on the expression of G proteins to which the receptor couples, the potential for precoupling of particular G proteins to the receptors either by scaffolding mechanisms or colocalization in lipid raft domains, and the effector mechanisms that these transducer molecules regulate. This discourse will evaluate studies of efficacy for CB1 receptor-Gi/o activation at the molecular level. Evidence for brain regional differences in CB1 receptor signal transduction efficacy and agonist selectivity for G proteins will be summarized. The possibility that CB1 receptors interact with Gs or Gq will be evaluated, and questions with regard to the constitutive activity and G protein sequestration will be posed.
Keywords: Aminoalkylindoles, anandamide, cyclic AMP, Ca2+ channels, CP55940, cerebellar membranes, CB1 receptor(−/−) transgenic mice, G protein-coupled receptors, GTPγS binding, levonantradol, radioligand binding assays, receptor immunoprecipitation, Δ9-tetrahydrocannabinol, WIN55212-2
Introduction
Cannabinoid receptors comprise two cloned types of G protein-coupled receptors (GPCR), the CB1 receptor found predominantly in the brain and other nervous tissue and the CB2 receptor found predominantly in immune cells. Studies of these receptor types have been recently defined and discussed by the IUPHAR Committee on Nomenclature (Howlett et al., 2002). As described in that review (Howlett et al., 2002), these receptor types respond to cannabinoid compounds such as Δ9-tetrahydrocannabinol (Δ9-THC), its metabolite 11-OH-Δ9-THC, and synthetic analogs that have been developed by numerous laboratories (e.g. HU210, levonantradol). When the classical A–B(pyran)–C tricyclic ring structure was broken, a series of A–C bicyclic analogs (e.g. CP55940) and more structurally rigid A–C–D tricyclic analogs were created, and these series of compounds have become known as nonclassical cannabinoid compounds. In addition, a series of aminoalkylindole and related agonists for CB1 and CB2 receptors have been developed based on a series of drugs designed by Sterling Research Group. WIN55212-2 is the prototype of this series. Finally, a series of arachidonic acid derivatives (arachidonyl ethanolamide (anandamide), 2-arachidonoyl glycerol, noladin ether) have been found in nature to behave as agonists for the cannabinoid receptors, and these endogenously synthesized eicosanoid agonists are referred to as ‘endocannabinoids'. Synthetic analogs have been developed for research investigation (e.g. (R)-methanandamide).
Considerable evidence exists to support the idea that CB1 receptors couple through Gi/o proteins to inhibit adenylyl cyclase, regulate ion channels, activate mitogen-activated protein kinase (MAPK), and modulate several other pathways, and these studies have been reviewed recently (Pertwee, 1999; Howlett et al., 2002; Mukhopadhyay et al., 2002). GPCR-Gi/o activation is generally considered to be due to agonist-stimulated G protein dissociation to allow the release of a G protein subunit to regulate effector proteins. The nature of the effector molecule is important in determining the way a cell will respond to the G protein stimulus. The isoform of adenylyl cyclase determines whether the response will be an inhibition due to interaction with the released αi (isoforms 1,3,8,5,6) or stimulation due to the release of the βγ dimers (isoforms 2,4,7) (Rhee et al., 1998). Only certain types of Ca2+ channels respond to CB1 receptor stimulation (Mackie & Hille, 1992; Mackie et al., 1995; Twitchell et al., 1997).
Some CB1 receptor signal transduction pathways utilize Gi/o proteins to serve as scaffolding proteins to localize regulatory enzymes such as phosphatidylinositol-3-kinase (PI3K). This could lead to the regulation of MAPK pathways and subsequent regulatory events (Wartmann et al., 1995; Bouaboula et al., 1997; Sanchez et al., 1998; Gomez et al., 2000). Other signaling pathways do not utilize G proteins as transducers. Sanchez et al. (2001) showed that sphingomyelinase activation by the CB1 receptor was mediated by the adaptor protein Fan but not by G proteins.
This level of complexity notwithstanding, CB1 receptor-mediated signal transduction by the endogenous eicosanoid ligands and a variety of plant products and synthetic ligands is generally governed by the pharmacodynamic principles of agonist affinity (ability to bind to the receptor) and efficacy (ability to evoke a functional response). For the purposes of the present review, we will consider the immediate response evoked as the ligand interacts with the receptor, or ‘intrinsic efficacy', and the discussion will predominantly be limited to the initiation of the cellular signal transduction pathways mediated by G proteins.
Studies of a repertoire of classical and nonclassical cannabinoid agonists provided a relatively good correlation between affinity for the CB1 receptor in brain membranes ([3H]CP55940 displacement) and activity in in vitro assays (adenylyl cyclase) (Devane et al., 1988; Howlett et al., 1990) and in vivo responses (Compton et al., 1993; Melvin et al., 1993; 1995). Similarly, within a series of aminoalkylindole compounds, good correlations between ligand binding affinity and ability to produce a biological response were obtained (Compton et al., 1992; Shim et al., 1998). At the level of adenylyl cyclase regulation, it appeared that the lower potency cannabinoid agonists also exhibited a lower efficacy for the response (see Figure 2c in review by Mukhopadhyay et al., 2002). However, a confounding factor in the interpretation of efficacy results that could not be addressed until the advent of an antagonist for the CB1 receptor was the high lipid solubility and poor aqueous solubility of these compounds that does not correlate with biological activity (Thomas et al., 1990) (see also review by Makriyannis & Rapaka, 1990). The resulting high membrane-media partition coefficient drives high concentrations of poorly potent compounds into biological membranes where perturbation of the lipid structure could be expected to alter biological functions (Hillard et al., 1985; 1990). When membrane perturbation was examined specifically as a potential negative influence on the signal transduction activity of poorly active cannabinoid agonists, the results were difficult to interpret (Hillard et al., 1990; Howlett et al., 1989). The eicosanoid endocannabinoid anandamide was shown to behave as a partial agonist in the inhibition of adenylyl cyclase (Childers et al., 1994), and this provided the first clear indication that differences in efficacy could be observed for CB1 receptor agonists to regulate this signal transduction pathway.
Evidence for efficacy differences among CB1 receptor agonists in the regulation of Ca2+ channels was reported for anandamide in the inhibition of N-type Ca2+ currents using electrophysiological measures in morphologically differentiated N18 mouse neuroblastoma cells (Mackie et al., 1993). In those studies (Mackie et al., 1993) and experiments that determined Ca2+ influx fluorometrically (Sugiura et al., 1997b), maximally active concentrations of anandamide produced less than the maximal response that could be achieved with WIN55212-2. Anandamide was able to antagonize the response to WIN55212-2, indicating that it behaved as a partial agonist (Mackie et al., 1993). In contrast, in cultured rat hippocampal neurons, the maximal inhibition of Ca2+ currents carried by N- and P/Q-type channels in response to anandamide was similar to that of WIN55212-2 and CP55940 (Twitchell et al., 1997). Shen et al. (1996) noted that CB1 receptor-mediated glutamatergic neurotransmission in cultured rat hippocampal neurons was maximally reduced in response to anandamide and WIN55212-2, but that CP55940 behaved as a partial agonist. Anandamide was also as effective as WIN55212-2 in inhibiting the Ca2+ current carried by Q-type channels (as well as stimulating the inwardly rectifying K+ current) in cultured AtT20 mouse pituitary cells expressing exogenous CB1 receptors (Mackie et al., 1995). The differences in anandamide's efficacy between these experimental cell types was postulated to be related to the density of the CB1 receptors or the G proteins that transduce the response (Mackie et al., 1995).
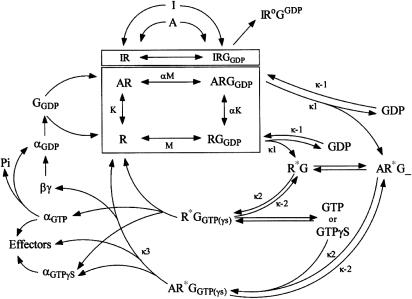
This review describes the research addressing the molecular mechanism(s) by which CB1 receptor ligands evoke varying efficacy in their regulation of G proteins. For these purposes, the term efficacy will refer to the maximal functional signal transduction response produced by a particular ligand. Efficacy will be examined based on our current understanding of the G protein activation cycle as developed for other GPCRs (see theoretical discussion by Waelbroeck, 1999). Figure 1 summarizes the ‘ternary complex model' of agonist (A)–receptor (R)–G protein (G) distribution in a steady-state model (Leff, 1995), combined with the catalytic pathway by which agonists activate the receptor-G protein (ARGGDP) complex to promote GDP dissociation (AR*G_), receptor–heterotrimer dissociation (to AR, GαGTP (or GαGTPγS) and Gβγ), and activation of effectors. The model also depicts possible mechanisms for the constitutive activity (RGGDP to R*G_ conversion in the absence of A) and inverse agonist (I) effects to promote an inactive ternary complex (IRoGGDP).
Figure 1.
Ternary complex equilibrium model for agonist (A)–receptor (R)–G protein (G) interactions, coupled with the G protein activation cycle. Details with regard to the mechanisms depicted here are provided in the text. According to the ternary complex model, receptors (R), G proteins (G) and ligands can form an equilibrium (depicted within the box). The free CB1 receptor (R) and the CB1 receptor-Gα complex (RGGDP) exist in equilibrium in the absence of exogenously added agonist (A) or inverse agonist (I) ligands (central box). This facile association is believed to be responsible for the constitutive activity reported for CB1 receptors. The influence of agonist ligands (A) or inverse agonist ligands (I) on this equilibrium expands the equilibrium to include the AR and ARGGDP complexes (main box) or the IR and IRGGDP complexes (upper box). Outside the ternary complex equilibrium box, GTP and its analogs exchange for GDP in the absence of agonists (R*G_ and R*GGTPγS) or in the presence of agonists (AR*G_ and AR*GGTPγS. The presence of GTP or its analog facilitates dissociation of the Gαi and Gβγ proteins from the receptors. The activation cycle is reinitiated by the hydrolysis of GTP, and recombination of Gαi and Gβγ proteins to form the heterotrimer (GGDP). GTPγS alone can promote dissociation of the G proteins from the CB1 receptor, indicating that some RGGDP complexes can spontaneously become activated in the absence of agonists, allowing GDP release (transiently empty G protein R*G_) and GTPγS binding. Once GTPγS binds, the GαGTPγS dissociates irreversibly and the GTPγS cannot be hydrolyzed, such that the GαGTPγS can no longer re-enter the equilibrium reaction. In the presence of an inverse agonist (I), the IR and IRGGDP complexes exist. The IRGGDP is believed to form an inactive complex, which in the figure is depicted as exiting the ternary complex equilibrium box, and sequestering G proteins in an inactive (IRoGGDP) state. This state was originally proposed by Bouaboula et al. (1997) to describe a mechanism for the CB1 receptor to ‘sequester' Gi proteins, thereby explaining their data that basal signal transduction through the MAPK or adenylyl cyclase pathways were blocked in the presence of SR141716 (see text).
Efficacy for CB1 receptor-Gi/o activation
By examination of the first step in signal transduction after the ligand interacts with the CB1 receptor, efficacy differences have been characterized for GDP/GTP exchange measured as 35S-labeled guanosine 5′-O-(3-thio)-triphosphate ([35S]GTPγS) binding to activated G proteins. This assay determines the ability of the agonist to activate the GPCR-G protein complex to release GDP from the Gα subunit and allow the binding of the nonhydrolyzable GTP analog [35S]GTPγS. Conditions in these assays favored a reduced basal GDP/GTP exchange in order to maximize the response to agonists (150 mM NaCl, 2–9 mM Mg2+, and 10–100 μM GDP) (Selley et al., 1996; Breivogel et al., 1998). [35S]GTPγS binding to G proteins in rat cerebellar membranes (Selley et al., 1996; Breivogel et al., 1998; Griffin et al., 1998; Kearn et al., 1999) and mouse whole brain membranes (Burkey et al., 1997) was stimulated to a maximal extent by CP55940, WIN55212-2, HU210, and levonantradol; to a fraction of the maximum by methanandamide and analogs (and CP55940 in some experiments); poorly by anandamide, Δ9-THC and 11-OH-Δ9-THC; and not at all by cannabinol or SR141716. A study of rat cerebellar membranes that was performed with lower concentrations of Na+ and limiting Mg2+ yielded similar results (Petitet et al., 1997). Disparities between the concentration for half-maximal response (EC50) compared with the inhibition constants in heterologous radioligand competition assays (Ki) as a measure of affinity suggest that efficacy differences could be more complex than can be assessed simply by examination of the maximal response (Griffin et al., 1998). Burkey et al. (1997) analyzed the data using a formula combining the factors of: (1) maximal effect of the agonist (maximum response in a biological assay, Emax) compared with that of CP55940; and (2) the EC50 for G protein activation relative to the Ki determined from heterologous displacement of [3H]SR141716. They proposed a relative efficacy for cannabinoid agonists of: CP55940 : HU210 : Δ9-THC=1 : 0.5 : 0.27; and anandamide=0.39.
Childer's laboratory (Sim et al., 1996) analyzed their data by comparing the maximal effect (Emax) for [35S]GTPγS binding to G proteins with the Bmax (maximum binding calculated in a radioligand equilibrium binding assay) for high-affinity agonist ([3H]WIN55212-2) binding to CB1 receptors, as a determinant of the receptor/transducer catalytic amplification ratio. The receptor affinity assays were performed in the absence of guanine nucleotides in order to capture the AR*G_ complex which has a high affinity for agonists (see Figure 1). The G protein activation assays were performed in a reaction mixture containing 100 mM NaCl, 3 mM Mg2+, and 20 μM GDP to maximize the response to the agonist to activate the ARGGDP complex. Examination of rat striatal membranes indicated that the receptor/transducer catalytic amplification ratio was only 3 for CB1 agonist-mediated G protein activation compared with a ratio of about 20 for μ-opioid and δ-opioid receptors in the same membranes (Sim et al., 1996). When similar analyses were performed in membranes from other distinct brain regions, the amplification ratios ranged from 2.5 to 6, with the greatest amplification being in brain regions having relatively sparse CB1 receptors (Breivogel et al., 1997). These studies showed that each agonist-occupied CB1 receptor was able to activate fewer G proteins compared with an agonist-occupied opioid receptor, particularly if there were excess measurable CB1 receptors. Thus, the question arises as to the role of the receptors (R and RGGDP) that were not measured in the high-affinity state for agonists.
In order to address the question of the role of the receptors that were not occupied by agonists in a high-affinity state, further analyses required an examination of receptor/transducer amplification ratios based on the total receptors (R, RGGDP, and R*G_). Several studies have reported that modifiers of the G protein allosteric regulation of the receptor's affinity for the agonist (nonhydrolyzable GTP analogs and NaCl) had no effect on the affinity for the radiolabeled antagonist in rat brain membranes (Rinaldi-Carmona et al., 1996; Houston & Howlett, 1998; Kearn et al., 1999). The assumption from these studies was that [3H]SR141716 would bind to uncoupled receptors devoid of G proteins (R) as well as to coupled RGGDP or R*G_ states (Breivogel et al., 1997). The amplification ratios obtained by comparing Emax for G protein activation with the Bmax for antagonist binding ranged from 2 to 8 for different brain regions. Those regions that exhibited low amplification ratios based on the Bmax of high-affinity agonist states (AR*G_) exhibited similarly low amplification ratios when the Bmax of ‘total' receptors (AR, ARGGDP, and AR*G_) was used for the calculations. For many brain regions, the high-affinity Bmax determined by agonist binding was similar to the total receptor Bmax determined by antagonist binding, suggesting that under the equilibrium binding conditions chosen, the CB1 receptors were well coupled to G proteins (RGGDP and R*G_). Exceptions occurred in those regions of very sparse density of receptors, in which the fraction of receptors in the high-affinity state was only about half the total estimated receptors.
Childer's laboratory noted that the amplification ratios for G protein exchange failed to correlate with the fraction of receptors in high-affinity (AR*G_) states (Breivogel et al., 1997). To understand this, these researchers performed a more detailed kinetic analysis of the mechanism of CB1 receptor activation of G proteins through an analysis of rates of [35S]GTPγS binding to G proteins (Breivogel et al., 1998). In the absence of agonist, the rate of association of [35S]GTPγS decreased with the concentration of GDP (from 0 to 30 μM), indicating that GDP and GTPγS were competing for the guanine nucleotide binding site. The CB1 agonist WIN55212-2 functioned to overcome the low rate of association at higher GDP concentrations, consistent with a role for the agonist to destabilize the GDP interaction with the ARGαGDP complex. This would be consistent with the WIN55212-2-occupied CB1 receptor serving as the guanine nucleotide exchange factor to destabilize the GαGDP complex. The important conclusion from these experiments was that the Emax values for various CB1 agonists obtained from equilibrium [35S]GTPγS binding in the presence of 30 μM GDP correlated well with low-affinity Ki values for GDP competition with [35S]GTPγS. Thus, the differences in efficacy for various agonists could be attributed to the ability of the agonist to destabilize GDP binding.
Hillard's laboratory (Kearn et al., 1999) performed an analysis of CB1 receptor agonist affinity states and efficacy, interpreting their data according to a two-state equilibrium ternary complex model of agonist binding and receptor activation (Leff, 1995). In their analysis, the CB1 receptor was considered to exist in a state having either a low affinity for agonist (R) or a high affinity for agonist (R*). The R* state would be preferentially stabilized when occupied by the agonist (Leff, 1995). According to this scenario, the AR* complex would interact with GGDP leading to activation. Ligand binding of a radiolabeled agonist, for example, [3H]CP55940 at its Kd concentration, would detect the R* state exclusively, and thus provide a measure of the high-affinity Kd(high) for agonists. The assumption was made that the radiolabeled antagonist [3H]SR141716 would have the same affinity for both R and R* states, and thus heterologous competition could be used to detect the two affinity states for agonists. When the GTP analog GppNHp was added to the equilibrium mixture, the binding of agonist A to R*GGDP would promote dissociation of GDP and association of GppNHp, with dissociation of GαGppNHp from AR* as a consequence. Uncoupled R* or AR* would isomerize to the ground state R and AR, and would accumulate as such because this analog of GTP is not subject to the hydrolysis necessary to form the G protein heterotrimer and reinitiate the cycle. Thus, heterologous competition with [3H]SR141716 in the presence of GppNHp could be used to determine the high-affinity Kd(high), low-affinity Kd(low), and the percent of receptors in the high-affinity state for different agonists. It should be noted here that these assumptions might not be strictly correct if the radiolabeled [3H]SR141716 is able to bind with high affinity to a novel receptor state, Ro, envisioned as an ‘inverse agonist' state. Using these equilibrium assumptions, the fraction of receptors in the high agonist affinity R* state at the maximally effective concentration for each of six agonist ligands was found to correlate well with the maximal activity for [35S]GTPγS binding to the G protein (Kearn et al., 1999). How these receptor affinity states exist in association with G proteins was further analyzed by Breivogel & Childers (2000)).
Breivogel & Childers (2000) analyzed the relationship between receptor occupancy and activation of G proteins using identical assay components for both assays: 100 mM NaCl, 3 mM MgCl2, 0.5 nM SR141716, 0.05 nM GTPγS, and 50 μM GDP. They examined membranes from rat cerebellum, hippocampus, and hypothalamus for the activities of five agonists (WIN55212-2, levonanatradol, CP55940, methanandamide, and Δ9-THC) to bind to the CB1 receptor assessed by competition with [3H]SR141716 and to stimulate [35S]GTPγS binding. The cerebellum and hippocampus, having similar receptor densities, exhibited similar efficacy profiles determined by Emax for stimulation of [35S]GTPγS binding. When the agonist radioligand competition curves were analyzed, three affinity states (high, intermediate, and low) were recognized for agonists (except methanandamide which exhibited two: intermediate and low). Each agonist was characterized by a different fraction of receptors in each state. When activation of G proteins was examined, two apparent Kact values could be discerned that generally corresponded to the fractions of receptors exhibiting intermediate and low affinity for agonist, respectively. In this analysis, the fraction of receptors in the high-affinity state for agonists corresponded to the basal [35S]GTPγS binding activity. Within the scheme depicted in Figure 1, it can be speculated that a considerable fraction of the receptor exists coupled with G proteins (RGGDP), and that a certain fraction of these could spontaneously achieve a conformation that allows dissociation of GDP (R*G_). If agonists were introduced into this equilibrium mixture, this state would be characterized by high affinity for those agonists that exhibit high efficacy (AR*G_). The dissociation of GDP from the guanine nucleotide binding site allows facile association of [35S]GTPγS without the necessity of agonists triggering GDP release. The majority of the precoupled receptors remain as the RGGDP form, which exhibits an intermediate affinity for agonists, and requires the stimulus of the agonist interaction to promote isomerization to the AR*GGDP state that triggers dissociation of GDP and allows subsequent association of [35S]GTPγS. Those receptors that remain uncoupled from G proteins (R) bind the agonist with low affinity. However, when agonist is bound (AR), these receptors can subsequently couple to GGDP, leading to the dissociation of GDP and subsequent association of [35S]GTPγS. For a low efficacy agonist such as (R)-methanandamide, the agonist interaction with the receptor exhibited little difference in affinity for either the uncoupled R or precoupled RGGDP receptor states, and so both pathways could be taken to build the ARGGDP complex, and these would appear to exhibit the same agonist affinity and ability to stimulate [35S]GTPγS binding.
Brain regional differences in CB1 receptor signal transduction efficacy
CB1 agonist WIN55212-2 stimulation of [35S]GTPγS binding exhibited a range of amplification ratios across various regions of the brain even when adjusted for the density of receptors in those individual brain regions (Sim et al., 1996; Breivogel et al., 1997). One might look to the different types of G proteins within the Gi/o family that are available to interact with CB1 receptors within brain membranes for further explanation. Studies of Gi-mediated stimulation of GTPase activity and inhibition of adenylyl cyclase demonstrated brain regional differences in modulation by Na+, suggesting that the receptor-G protein coupling could be under different regulatory control in different neuronal types (Pacheco et al., 1994). Little or no inhibition of adenylyl cyclase could be detected in certain brain regions in which the CB1 receptor could nevertheless activate G proteins, suggesting that Gi proteins coupled to CB1 receptors are coupled to selective effectors or that their coupling efficiency is reduced (Breivogel & Childers, 2000). These findings might be attributed to differential profiles of G proteins (availability of subtypes, or receptor-G protein sequestration) to which the CB1 receptor couples in different brain regions.
The possibility that brain regional efficacy differences can be related to CB1 receptor density has been examined in a study of transgenic heterozygote CB1 (+/−) compared with wild-type CB1 (+/+) mice (Selley et al., 2001). In those studies, the receptor Bmax was reduced by half in each of the brain regions tested, but the Emax of WIN55212-2 to stimulate [35S]GTPγS binding was reduced by only 25% in most regions except for the striatum. The EC50 for WIN55212-2 stimulation was significantly increased only in the cerebellum and striatum. This meant that the reduction of CB1 receptor density in the heterozygote led to an increase in amplification ratio by two-fold in most brain regions, indicative of an increase in the receptor-G protein coupling efficiency. The relative efficacies of the partial agonists (R)-methanandamide and Δ9-THC did not change with the decrement in receptor density, suggesting that these ligands were not able to influence the receptor-G protein equilibrium to overcome the receptor loss (Selley et al., 2001).
A credible alternative explanation for regional differences in the response to ligands is that an alternative receptor in addition to the CB1 receptor recognizes these agonists. Studies of the transgenic CB1 (−/−) knockout mouse have indicated that anandamide and WIN55212-2, but not classical or nonclassical cannabinoid ligands, stimulate [35S]GTPγS binding in the absence of CB1 receptors, and this response was not specifically antagonized by SR141716 (Breivogel et al., 2001). Although the CB1 (−/−) knockout response to WIN55212-2 was small compared with that of the CB1 receptor in wild-type CB1 (+/+) mice, it was nevertheless significant in certain brain regions (Breivogel et al., 2001). The possibility for an alternative receptor is consistent with the biological responses to anandamide that can be observed in the presence of SR141716 (Adams et al., 1998) or these CB1 (−/−) animals (DiMarzo et al., 2000; Baskfield et al., 2004). Additional evidence for an alternative receptor for WIN55212-2 is that [3H]WIN55212-2 binding sites exist in certain brain regions in the CB1 (−/−) knockout mouse (Breivogel et al., 2001) and might also exist in cultured NG108-15 cells (Stark et al., 1997). The pharmacology of this novel non-CB1, non-CB2 receptor for anandamide and WIN55212-2 has not been characterized, and signal transduction beyond that of G protein activation has not been reported. Until further studies elucidate the genotypic and phenotypic properties that distinguish this alternative receptor from the CB1 receptor, interpretations that attribute differences in pharmacological responses to G proteins profiles must be made with caution.
Agonist selectivity for G proteins
Evidence for differential regulation of different Gi subtypes by different agonists was provided by Houston & Howlett (1998), who examined rat brain membrane CB1 receptors for multiple agonist affinity states detected by competition for [3H]SR141716 binding. Under standard assay conditions (4 mM Mg2+, no guanine nucleotides), the cannabinoid desacetyllevonantradol and the aminoalkylindole WIN55212-2 both bound in two discrete affinity states, representing approximately 30% of the receptors in a high-affinity state for the agonist, and the remainder in a low-affinity state. A difference in the affinity states for the two agonists was obvious in the ratios of the two Ki values and in the modulation by Na+ and GTP analogs. Under conditions of Na+ regulation (100 mM NaCl), both affinity components for desacetyllevonantradol were reduced in affinity, particularly the component representing receptors in the low-affinity state. However, the addition of NaCl had little influence on WIN55212-2 binding affinities. The addition of GTPγS reduced affinity in the high-affinity state, indicative of an accumulation of GαGTPγS and increase in the population of receptors in the uncoupled R state. This shift of equilibrium was complete for WIN55212-2, but not for desacetyllevonantradol. This finding might suggest that two or more different populations of G proteins that couple to the CB1 receptor are modulated differently, with WIN55212-2 modulating all G protein subtypes and desacetyllevonantradol affecting only a subset of G proteins.
Glass & Northup (1999) found differences in the way that CB1 receptor agonists could regulate purified Gi versus Go proteins. They studied CB1 receptors that were expressed in Sf9 insect cells by isolating the membranes and treating them with the chaotrope urea in order to uncouple and remove G proteins. By this means, they were able to reconstitute receptor-G protein coupling by adding purified G protein subunits to the membranes. The activation of specific G proteins by agonist-occupied CB1 receptors was monitored by determining [35S]GTPγS binding to the Gα proteins in the presence of saturating concentrations of βγ proteins. These assays were performed in the presence of an excess of βγ proteins, 100 mM NaCl and 4 μM GDP and <1 nM concentrations of [35S]GTPγS, conditions that limited the amount of GDP-[35S]GTPγS exchange that would occur in the absence of agonists. In these studies, both Gi and Go proteins were activated most efficaciously by HU210 and least efficaciously (60%) by Δ9-THC. Significant G-protein-specific differences were observed for WIN55212-2 and anandamide, which exhibited maximal or near-maximal efficacy for Gi, but only about 70% maximal efficacy for Go. The potency order remained unchanged, suggesting that the binding to the receptor remained the same irrespective of the G protein being activated.
Studies of Prather et al. (2000) demonstrated differences in Gi/o-subtype activation in response to a single cannabinoid receptor agonist, WIN55212-2. These studies utilized GDP-[32P]azidoanilidoGTP exchange. The covalent binding of this GTP analog to the G protein would preclude any reverse reaction, so that the labeled proteins could be isolated by immunoprecipitation or separation and identification after sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS–PAGE). In those studies, Gαi1/ i3, Gαi2, Gαo1, Gαo2, and Gαo3 were distinguishable bands on immunoblots and autoradiographs, and could be quantitated densitometrically. Using this measure of G protein activation, a maximally active concentration of WIN55212-2 stimulated greater incorporation of [32P]azidoanilidoGTP into Gαo1 compared with Gαo2 or Gαo3 for all areas of the rat brain examined. However, the EC50 for WIN55212-2 to activate the various G protein subtypes differed by 36-fold, from 100 nM for Gαi1 and Gαo3 to 3.7 μM for Gαo2. Thus, it could be concluded that low concentrations of the CB1 agonist WIN55212-2 can activate certain Gα subtypes without activating others.
Mukhopadhyay & Howlett (2000) studied selective receptor-Gi-subtype interactions by quantitating the Gαi and Gβγ proteins that co-immunoprecipitate with the CB1 receptor from a detergent extract of N18TG2 membranes in the presence of ligands. An equilibrium dissociation between ARGGDP and AR plus GGDP was observed in response to the aminoalkylindole WIN55212-2 for all three RGαi complexes, the cannabinoid desacetyllevonantradol for Gαi1 and Gαi2, and the eicosanoid (R)-methanandamide for Gαi3. However, desacetyllevonantradol maintained RGαi3 complexes and (R)-methanandamide maintained RGαi1 and RGαi2 complexes even in the presence of GTPγS. The biaryl pyrazole SR141716 maintained all three RGαi complexes, but supported some equilibrium mixtures in the presence of GTPγS. Gβ proteins exhibited the same association/dissociation pattern as the Gα proteins. These results can be explained by invoking the existence of an inverse agonist (I)-supported inactive state (IRoGGDP), in the ternary complex equilibrium model (see Figure 1). In this model, WIN55212-2 behaves as an agonist for all three Gi subtypes, and SR141716 behaves as an inverse agonist for all three Gi subtypes. However, desacetyllevonantradol behaves as an agonist for Gi1 and Gi2 and an inverse agonist for Gi3, and (R)-methanandamide behaves as an inverse agonist for Gi1 and Gi2 and an agonist for Gi3. These ligand-selective G protein responses imply that multiple conformations of the receptor could be evoked by ligands in order to regulate individual G proteins.
The observation that a unique pattern of functional interaction is ligand specific (i.e. the behavior as an agonist or inverse agonist at a given Gi subtype is not the same for each ligand) has great biological significance because it implies that ligands can direct cellular signal transduction pathways via one Gi subtype at the expense of inactivation of another. The functional consequences of CB1 receptor stimulation within cells possessing multiple Gi/o subtypes would be both ligand- and Gi/o-subtype specific.
Evidence that CB1 receptors interact with Gq or Gs
Several studies have suggested that phospholipase C can be stimulated by CB1 receptors (Sugiura et al., 1996; 1997a; Netzeband et al., 1999), which might suggested the possibility for a coupling to Gq, were it not for the observation that these responses were blocked by pertussis toxin. Furthermore, recombinant CB1 receptors failed to alter the production of inositol phospholipids when expressed in host cells that would have allowed for stimulation of phospholipase C (Felder et al., 1992; 1995). In a hippocampal preparation, cannabinoid compounds inhibited neurotransmitter-stimulated inositol phospholipid production (Nah et al., 1993). Thus, CB1 receptor coupling to the Gq family is not well supported by the available research findings.
The question of whether CB1 receptors can interact with Gs has been suggested from findings that, under conditions of pertussis toxin treatment that prevents the receptor's interaction with Gi/o proteins, a stimulation of cyclic AMP accumulation was observed in cultured neurons and CHO cells expressing recombinant CB1 receptors (Glass & Felder, 1997; Felder et al., 1998). In striatal cell cultures, combinations of dopaminergic and cannabinergic stimulation resulted in an increase in cyclic AMP (Glass & Felder, 1997) and WIN55212-2 produced an increase in basal cyclic AMP production in globus pallidus slice preparations (Maneuf & Brotchie, 1997).
A study using a recombinant model system of HEK293 cells stably transfected with the D2 dopamine receptor and transiently transfected with the human CB1 indicated that the expression of D2 dopamine receptors was sufficient to convert the inhibition of forskolin-stimulated cyclic AMP production by CP55940 to a stimulation of cyclic AMP production (Jarrahian et al., 2004). This would be consistent with the uncovering of a cryptic ability of the CB1 receptor to couple to Gs in addition to Gi. Evidence supporting this notion included the observation within this experimental model that pretreatment with pertussis toxin eliminated the component of inhibition of cyclic AMP accumulation but did not affect the stimulation of cyclic AMP. Interestingly, the converse did not occur: the D2 dopaminergic inhibition of forskolin-stimulated cyclic AMP accumulation was not affected by the expression of CB1 receptors (Jarrahian et al., 2004). However, stimulation of the CB1 receptors by CP55940 did increase the net cyclic AMP accumulation, consistent with a stimulation of Gs by the agonist-stimulated CB1 receptor. The mechanism for this response could be explained by invoking the ability D2 dopamine receptors to sequester Gi proteins such that these transducers would no longer be available to couple to the CB1 receptors, leaving the CB1 receptors to couple to Gs proteins, which would be readily available endogenously in this model cell system. Evidence in support of this explanation is that overexpression of Gαi1 but not Gαo allowed the inhibition of cyclic AMP accumulation by CP55940-stimulated CB1 receptors to prevail (Jarrahian et al., 2004). Additional evidence was that when the D2 receptor coupling to Gi was compromised by persistent agonist stimulation (18 h treatment of the cells with the D2 agonist quinpirole), the CB1 receptor-Gi inhibition was the prevalent response (Jarrahian et al., 2004). The CB1 receptor-Gs stimulation was not robust, being only 30% of forskolin's maximal response (Jarrahian et al., 2004). The Gs response required greater occupancy of the CB1 receptors than did the Gi response, inasmuch as inhibition of cyclic AMP accumulation occurred at 10–1000 nM CP55940, but stimulation of cyclic AMP accumulation required 0.1–10 μM (Jarrahian et al., 2004).
The CB1 receptor interaction with Gs has also been demonstrated in CHO cells stably expressing recombinant human CB1 receptors (Bonhaus et al., 1998). In order to observe receptor coupling to Gs, the cells were pretreated with pertussis toxin such that the Gi/o proteins were unable to interact with the receptor. The agonists exhibited a different order of efficacies when tested for Gi versus Gs regulation of forskolin-stimulated cyclic AMP production (Bonhaus et al., 1998). The inhibition of the forskolin-stimulated cyclic AMP accumulation by Gi was maximal in response to full agonists, HU210, CP55940, and WIN55212-2 (in order of potency), and only 50 and 75% of maximal by partial agonists, Δ9-THC, and anandamide, respectively. Following pertussis toxin treatment, stimulation of cyclic AMP accumulation, presumably by Gs, was increased by 100% above the forskolin-stimulated value by WIN55212-2. Cyclic AMP accumulation was increased by only about 50% by HU210 and CP55940, and about 35% by Δ9-THC and anandamide, suggesting that these compounds behave as partial agonists for this response. The potency order was the same whether a decrease or an increase in cyclic AMP was being measured, even though HU210 and CP55940 exhibited relatively lower maximal activities when coupled to Gs. The CB1 receptor antagonist SR141716 behaved as a competitive inhibitor with equal ability to antagonize both responses.
In contrast to the regulation of cyclic AMP production, no evidence has been found to support a direct CB1 receptor-Gs or Gq interaction in vitro in equilibrium association or G protein activation studies. In studies of solubilized CB1 receptor from brain or N18TG2 cell membranes, no indications have been found that Gs or Gq could be stably associated with the receptor, as has been shown to be the case for the Gi/o family (Mukhopadhyay et al., 2000). In studies of membranes from Sf9 cells expressing CB1 receptors, no activation of reconstituted Gαq was found under conditions in which the activation of reconstituted Gi and Go was quite robust (Glass & Northup, 1999).
A mechanism for the activation of Gs by the CB1 receptor has been explored. An Ala-Leu sequence in IL3 is believed to interact with Gs, but does not form the appropriate helical structure to interact with Gi (Ulfers et al., 2002). To demonstrate this, stimulation of cyclic AMP production was observed when host cells bearing the mutant CB1 receptor Ala-Leu sequence were treated with pertussis toxin to block interaction of Gi proteins with the receptor. In the absence of functional Gi proteins after pertussis toxin pretreatment, the mutant CB1 receptor was able to couple to Gs (Abadji et al., 1999), suggesting that the mutant CB1 receptor could associate with Gs as might be pred icted from sequence homologies with Gs-coupled receptors.
Cannabinoid receptor constitutive activity and inverse agonism of SR141716
The constitutive activity for GPCRs in the absence of an agonist has been noted in recombinant expression systems. This is believed to be due to the high concentrations of exogenously expressed receptor that drive the R plus GGDP equilibrium reaction in the forward direction, thereby increasing the concentration RGGDP and any R*G_ (see Figure 1) that would be generated due to spontaneous isomerization in the absence of an agonist (Kenakin, 1997). Constitutive activity for exogenously expressed CB1 receptors has been demonstrated (Bouaboula et al., 1997; Landsman et al., 1997; MacLennan et al., 1998; Pan et al., 1998), indicating that activity in the absence of stimulation by an exogenously applied agonist is possible. Evidence that native CB1 receptors exhibit constitutive activity is relatively sparse, but alteration of experimental conditions can allow such observations (Meschler et al., 2000; Sim-Selley et al., 2001). Studies from Sim-Selley's laboratory have further explored this ‘inverse agonist' component of the action of SR141716 (Sim-Selley et al., 2001). Relatively high concentrations of SR141716 were required to observe the inverse agonist behavior in [35S]GTPγS binding assays, even under assay conditions made more appropriate to observe constitutively activated receptors (Sim-Selley et al., 2001). Further evidence for constitutive activity in endogenously expressed CB1 receptor systems will be necessary to support the notion that this is a phenomenon that occurs in neurons in the body.
Bouaboula et al. (1997) proposed that the mechanism of the inverse agonist ability of SR141716 was a stabilization of the receptor-G protein complex that prevents G protein activation (IRoGGDP as depicted in Figure 1). They further proposed that SR141716-occupied CB1 receptors could sequester Gi/o proteins away from other signal transduction pathways that are presumed to share G proteins. Studies from Lewis' laboratory on the regulation of Ca2+ channels by CB1 receptor and Gi/o proteins have provided evidence favoring the sequestration of G proteins (Pan et al., 1998; Vasquez & Lewis, 1999). In superior cervical ganglia neurons expressing recombinant CB1 receptors, the responses to norepinephrine or somatostatin via their endogenous receptors were tempered by the presence of CB1 receptors, suggesting a shared pool of Gi/o proteins (Vasquez & Lewis, 1999). Additional support for the sequestration of G proteins needs to be demonstrated for natively expressed receptors for ion channel regulation and for other effector responses. These studies should be forthcoming now that demonstrations in recombinant systems have opened the possibility that constitutive activity and G protein sequestration exists.
Goals for studies of efficacy
This review has concentrated on describing potential explanations for differences in intrinsic efficacy for ligands that interact with the CB1 cannabinoid receptor type. Much less information is available for a comparable analysis of the CB2 cannabinoid receptor. Intrinsic efficacy is a property of the ligand itself, and reflects the ability of the ligand to interact with the receptor and produce a response. The molecular mechanism for this response would have to involve conformational changes in the receptor protein that lead to conformational activation of the G protein heterotrimer. The nature of the conformational changes induced by ligands for GPCRs is a subject of great research activity. Efficacy to produce a biological response in in vivo systems involves determinants other than the intrinsic efficacy of the ligand. Efficacy in in vivo systems also includes parameters that define the ability of the particular in vivo system to produce a maximum stimulus for the response. Factors that influence the stimulus efficacy are related to the involvement of multiple cell types in a pathway toward the end point of the response, as well as environmental factors associated with the response mechanism and pharmacokinetic parameters of drug distribution and metabolism within the in vivo system. The goal of research activities in the area of pharmacodynamics is to discover drugs that can produce a maximal therapeutic response while at the same time evoking a minimum of untoward side effects. Unfortunately, we are currently suffering from an inadequate understanding of all the factors involved in the stimulus efficacy in in vivo systems, and are only beginning to translate our understanding of intrinsic efficacy to the development of novel ligands having therapeutic value in CNS pharmacology.
Acknowledgments
The effort put forth for writing this review, as well as the majority of the studies reported in this review, were supported by grants from the National Institute on Drug Abuse. The assistance of Tu Luan in producing the figure is gratefully appreciated.
Abbreviations
- A
agonist
- Bmax
maximum binding calculated in a radioligand equilibrium binding assay
- EC50
concentration of ligand that produces half of the maximum response
- Emax
maximum response in a biological assay
- GGDP or G_
G protein heterotrimer with a GDP bound or an empty nucleotide binding site, respectively
- GαGXP
Gα monomer possessing a guanine nucleotide (GDP, GTP, GTPγS) bound to the nucleotide binding site
- Gβγ
Gβγ dimer
- GPCR
G protein-coupled receptor
- GppNHp
guanylyl-imidodiphosphate
- GTPγS
guanosine 5′-O-(3-thio)-triphosphate
- I
inverse agonist
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol-3-kinase
- R or R*
receptor in ground state or in a conformation which is able to release GDP from G proteins, respectively
- SDS–PAGE
sodium dodecylsulfate–polyacrylamide gel electrophoresis
- THC
tetrahydrocannabinol
References
- ABADJI V., LUCAS-LENARD J.M., CHIN C., KENDALL D.A. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of Gs. J. Neurochem. 1999;72:2032–2038. doi: 10.1046/j.1471-4159.1999.0722032.x. [DOI] [PubMed] [Google Scholar]
- ADAMS I.B., COMPTON D.R., MARTIN B.R. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J. Pharmacol. Exp. Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- BASKFIELD C.Y., MARTIN B.R., WILEY J.L. Differential effects of delta-9-tetrahydrocannabinol and methanandamide on operant behavior in CB1 knockout and wild type mice. J. Pharmacol. Exp. Ther. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., CHANG L.K., KWAN J., MARTIN G.R. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J. Pharmacol. Exp. Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.P., LE FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., CHILDERS S.R. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J. Pharmacol. Exp. Ther. 2000;295:328–336. [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI M., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- BREIVOGEL C.S., SELLEY D.E., CHILDERS S.R. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J. Biol. Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., SIM L.J., CHILDERS S.R. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- BURKEY T.H., QUOCK R.M., CONSROE P., EHLERT F.J., HOSOHATA Y., ROESKE W.R., YAMAMURA H.I. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur. J. Pharmacol. 1997;336:295–298. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- CHILDERS S.R., SEXTON T., ROY M.B. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem. Pharmacol. 1994;47:711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- COMPTON D.R., GOLD L.H., WARD S.J., BALSTER R.L., MARTIN B.R. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- COMPTON D.R., RICE K.C., DE COSTA B.R., RAZDAN R.K., MELVIN L.S., JOHNSON M.R., MARTIN B.R. Cannabinoid structure–activity relationships: correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- DEVANE W.A., DYSARZ F.A., III, JOHNSON M.R., MELVIN L.S., HOWLETT A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- DIMARZO V., BREIVOGEL C.S., TAO Q., BRIDGEN D.T., RAZDAN R.K., ZIMMER A.M., ZIMMER A., MARTIN B.R. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., GLASS M., MACKIE K.P., FAHEY K.J., CULLINAN G.J., HUNDEN D.C., JOHNSON D.W., CHANEY M.O., KOPPEL G.A., BROWNSTEIN M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J. Pharmacol. Exp. Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- FELDER C.C., VELUZ J.S., WILLIAMS H.L., BRILEY E.M., MATSUDA L.A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol. Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- GLASS M., FELDER C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASS M., NORTHUP J.K. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol. Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- GOMEZ D.P., VELASCO G., GUZMAN M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN G., ATKINSON P.J., SHOWALTER V.M., MARTIN B.R., ABOOD M.E. Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J. Pharmacol. Exp. Ther. 1998;285:553–560. [PubMed] [Google Scholar]
- HILLARD C.J., HARRIS R.A., BLOOM A.S. Effects of the cannabinoids on physical properties of brain membranes and phospholipid vesicles: fluorescence studies. J. Pharmacol. Exp. Ther. 1985;232:579–588. [PubMed] [Google Scholar]
- HILLARD C.J., POUNDS J.J., BOYER D.R., BLOOM A.S. Studies of the role of membrane lipid order in the effects of delta 9-tetrahydrocannabinol on adenylate cyclase activation in heart. J. Pharmacol. Exp. Ther. 1990;252:1075–1082. [PubMed] [Google Scholar]
- HOUSTON D.B., HOWLETT A.C. Differential receptor-G-protein coupling evoked by dissimilar cannabinoid receptor agonists. Cell Signal. 1998;10:667–674. doi: 10.1016/s0898-6568(98)00013-8. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., CHAMPION T.M., WILKEN G.H., MECHOULAM R. Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., SCOTT D.K., WILKEN G.H. Regulation of adenylate cyclase by cannabinoid drugs. Insights based on thermodynamic studies. Biochem. Pharmacol. 1989;38:3297–3304. doi: 10.1016/0006-2952(89)90628-x. [DOI] [PubMed] [Google Scholar]
- JARRAHIAN A., WATTS V.J., BARKER E.L. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2004;308:880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- KEARN C.S., GREENBERG M.J., DICAMELLI R., KURZAWA K., HILLARD C.J. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J. Neurochem. 1999;72:2379–2387. doi: 10.1046/j.1471-4159.1999.0722379.x. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G protein stoichiometry. Trends Pharmacol. Sci. 1997;18:456–464. doi: 10.1016/s0165-6147(97)01136-x. [DOI] [PubMed] [Google Scholar]
- LANDSMAN R.S., BURKEY T.H., CONSROE P., ROESKE W.R., YAMAMURA H.I. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur. J. Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- LEFF P. The two-state model of receptor activation. Trends Pharmacol. Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- MACKIE K., HILLE B. Cannabinoids inhibit N-type calcium channels in neuroblastoma–glioma cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKIE K., DEVANE W.A., HILLE B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- MACKIE K., LAI Y., WESTENBROEK R., MITCHELL R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKRIYANNIS A., RAPAKA R.S. The molecular basis of cannabinoid activity. Life Sci. 1990;47:2173–2184. doi: 10.1016/0024-3205(90)90147-j. [DOI] [PubMed] [Google Scholar]
- MANEUF Y.P., BROTCHIE J.M. Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br. J. Pharmacol. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELVIN L.S., MILNE G.M., JOHNSON M.R., SUBRAMANIAM B., WILKEN G.H., HOWLETT A.C. Structure-activity relationships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs. Mol. Pharmacol. 1993;44:1008–1015. [PubMed] [Google Scholar]
- MELVIN L.S., MILNE G.M., JOHNSON M.R., WILKEN G.H., HOWLETT A.C. Structure–activity relationships defining the ACD-tricyclic cannabinoids: cannabinoid receptor binding and analgesic activity. Drug Des. Discov. 1995;13:155–166. [PubMed] [Google Scholar]
- MESCHLER J.P., KRAICHELY D.M., WILKEN G.H., HOWLETT A.C. Inverse agonist properties of N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A) and 1-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxylic acid phenylamide (CP-272871) for the CB(1) cannabinoid receptor. Biochem. Pharmacol. 2000;60:1315–1323. doi: 10.1016/s0006-2952(00)00447-0. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY S., HOWLETT A.C. Chemically distinct agonists promotes differential cannabinoid receptor-G protein dissociation. Pharmacologist. 2000.
- MUKHOPADHYAY S., MCINTOSH H.H., HOUSTON D.B., HOWLETT A.C. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharmacol. 2000;57:162–170. [PubMed] [Google Scholar]
- MUKHOPADHYAY S., SHIM J.Y., ASSI A.A., NORFORD D., HOWLETT A.C. CB(1) cannabinoid receptor-G protein association: a possible mechanism for differential signaling. Chem. Phys. Lipids. 2002;121:91–109. doi: 10.1016/s0009-3084(02)00153-6. [DOI] [PubMed] [Google Scholar]
- NAH S.Y., SAYA D., VOGEL Z. Cannabinoids inhibit agonist-stimulated formation of inositol phosphates in rat hippocampal cultures. Eur. J. Pharmacol. 1993;246:19–24. doi: 10.1016/0922-4106(93)90004-s. [DOI] [PubMed] [Google Scholar]
- NETZEBAND J.G., CONROY S.M., PARSONS K.L., GRUOL D.L. Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J. Neurosci. 1999;19:8765–8777. doi: 10.1523/JNEUROSCI.19-20-08765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHECO M.A., WARD S.J., CHILDERS S.R. Differential requirements of sodium for coupling of cannabinoid receptors to adenylyl cyclase in rat brain membranes. J. Neurochem. 1994;62:1773–1782. doi: 10.1046/j.1471-4159.1994.62051773.x. [DOI] [PubMed] [Google Scholar]
- PAN X., IKEDA S.R., LEWIS D.L. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- PETITET F., JEANTAUD B., CAPET M., DOBLE A. Interaction of brain cannabinoid receptors with guanine nucleotide binding protein: a radioligand binding study. Biochem. Pharmacol. 1997;54:1267–1270. doi: 10.1016/s0006-2952(97)00384-5. [DOI] [PubMed] [Google Scholar]
- PRATHER P.L., MARTIN N.A., BREIVOGEL C.S., CHILDERS S.R. Activation of cannabinoid receptors in rat brain by WIN 55212-2 produces coupling to multiple G protein alpha-subunits with different potencies. Mol. Pharmacol. 2000;57:1000–1010. [PubMed] [Google Scholar]
- RHEE M.H., BAYEWITCH M., AVIDOR-REISS T., LEVY R., VOGEL Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J. Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., PIALOT F., CONGY C., REDON E., BARTH F., BACHY A., BRELIERE J.C., SOUBRIE P., LE FUR G. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996;58:1239–1247. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- SANCHEZ C., GALVE-ROPERH I., RUEDA D., GUZMAN M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol. Pharmacol. 1998;54:834–843. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- SANCHEZ C., RUEDA D., SEGUI B., GALVE-ROPERH I., LEVADE T., GUZMAN M. The CB(1) cannabinoid receptor of astrocytes is coupled to sphingomyelin hydrolysis through the adaptor protein fan. Mol. Pharmacol. 2001;59:955–959. doi: 10.1124/mol.59.5.955. [DOI] [PubMed] [Google Scholar]
- SELLEY D.E., RORRER W.K., BREIVOGEL C.S., ZIMMER A.M., ZIMMER A., MARTIN B.R., SIM-SELLEY L.J. Agonist efficacy and receptor efficiency in heterozygous CB1 knockout mice: relationship of reduced CB1 receptor density to G-protein activation. J. Neurochem. 2001;77:1048–1057. doi: 10.1046/j.1471-4159.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- SELLEY D.E., STARK S., SIM L.J., CHILDERS S.R. Cannabinoid receptor stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding in rat brain membranes. Life Sci. 1996;59:659–668. doi: 10.1016/0024-3205(96)00347-5. [DOI] [PubMed] [Google Scholar]
- SHEN M., PISER T.M., SEYBOLD V.S., THAYER S.A. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIM J.Y., COLLANTES E.R., WELSH W.J., SUBRAMANIAM B., HOWLETT A.C., EISSENSTAT M.A., WARD S.J. Three-dimensional quantitative structure-activity relationship study of the cannabimimetic (aminoalkyl)indoles using comparative molecular field analysis. J. Med. Chem. 1998;41:4521–4532. doi: 10.1021/jm980305c. [DOI] [PubMed] [Google Scholar]
- SIM L.J., SELLEY D.E., XIAO R., CHILDERS S.R. Differences in G-protein activation by mu- and delta-opioid, and cannabinoid, receptors in rat striatum. Eur. J. Pharmacol. 1996;307:97–105. doi: 10.1016/0014-2999(96)00211-7. [DOI] [PubMed] [Google Scholar]
- SIM-SELLEY L.J., BRUNK L.K., SELLEY D.E. Inhibitory effects of SR141716A on G-protein activation in rat brain. Eur. J. Pharmacol. 2001;414:135–143. doi: 10.1016/s0014-2999(01)00784-1. [DOI] [PubMed] [Google Scholar]
- STARK S., PACHECO M.A., CHILDERS S.R. Binding of aminoalkylindoles to noncannabinoid binding sites in NG108-15 cells. Cell Mol. Neurobiol. 1997;17:483–493. doi: 10.1023/A:1026306804802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., KONDO S., NAKANE S., KONDO H., WAKU K., ISHIMA Y., WATANABE K., YAMAMOTO I. Is the cannabinoid CB1 receptor a 2-arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J. Biochem.(Tokyo) 1997a;122:890–895. doi: 10.1093/oxfordjournals.jbchem.a021838. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., KONDO S., TONEGAWA T., NAKANE S., KISHIMOTO S., YAMASHITA A., WAKU K. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma × glioma hybrid NG108-15 cells. Biochem. Biophys. Res. Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., KONDO S., TONEGAWA T., NAKANE S., KISHIMOTO S., YAMASHITA A., WAKU K. Inhibition by 2-arachidonoylglycerol, a novel type of possible neuromodulator, of the depolarization-induced increase in intracellular free calcium in neuroblastoma × glioma hybrid NG108-15 cells. Biochem. Biophys. Res. Commun. 1997b;233:207–210. doi: 10.1006/bbrc.1997.6425. [DOI] [PubMed] [Google Scholar]
- THOMAS B.F., COMPTON D.R., MARTIN B.R. Characterization of the lipophilicity of natural and synthetic analogs of delta 9-tetrahydrocannabinol and its relationship to pharmacological potency. J. Pharmacol. Exp. Ther. 1990;255:624–630. [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- ULFERS A.L., MCMURRY J.L., MILLER A., WANG L., KENDALL D.A., MIERKE D.F. Cannabinoid receptor-G protein interactions: G(alphai1)-bound structures of IC3 and a mutant with altered G protein specificity. Protein Sci. 2002;11:2526–2531. doi: 10.1110/ps.0218402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASQUEZ C., LEWIS D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAELBROECK I. Kinetics versus equilibrium: the importance of GTP in GPCR activation. Trends Pharmacol. Sci. 1999;20:477–481. doi: 10.1016/s0165-6147(99)01394-2. [DOI] [PubMed] [Google Scholar]
- WARTMANN M., CAMPBELL D., SUBRAMANIAN A., BURSTEIN S.H., DAVIS R.J. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett. 1995;359:133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]