Abstract
Serotonin-2 receptor antagonists, like ritanserin, greatly enhance deep slow wave sleep (SWS-2) and low-frequency EEG power in humans and rodents. 5-HT2A and 5-HT2C receptors may be involved in these effects, but the role of the 5-HT2B receptor is still unclear.
To investigate the role of the 5-HT2B receptor in regulation of the sleep–wake cycle, the subtype-selective antagonist SB-215505 (0.1, 0.3 and 1.0 mg kg−1 i.p.) was administered to Sprague–Dawley rats at light onset (beginning of passive phase). EEG, EMG and motor activity were recorded during the subsequent 8 h.
SB-215505 dose-dependently increased wakefulness (W) at the expense of the intermediate stage of sleep, paradoxical sleep (PS) and SWS-2 in the first hour. Parallel to increased W, significantly increased motor activity was found. Spectral analysis of the EEG in W showed a dose-dependent decrease in power density in the 3–8 Hz frequency range (maximum effect at 6 Hz). In light slow wave sleep and SWS-2, the drug reduced low-frequency (<8 Hz) EEG power, suggesting decreased sleep intensity after SB-215505 treatment. In PS, the drug dose-dependently decreased EEG power solely in the theta (6–9 Hz) band, primarily affecting the peak power value (7 Hz).
The well-known SWS-2 enhancing effect of 5-HT2 receptor antagonists is mediated by 5-HT2A and/or 5-HT2C receptors. In contrast, blockade of 5-HT2B receptors increases motor activity and W along with decreased theta activity during W and PS. Activation of 5-HT2B receptors may contribute to initiation of sleep and to theta generation during W and PS under physiological conditions.
Keywords: Serotonin, 5-HT2B receptor, SB-215505, spectral analysis, motor activity, theta activity, sleep, vigilance, fast Fourier transformation, EEG analysis
Introduction
Serotonin (5-HT) was one of the first neurotransmitters associated with physiological regulation of the sleep–wake cycle (Jouvet, 1999). There is overwhelming evidence that 5-HT2 receptor antagonists like ritanserin greatly enhance slow wave sleep (stages 3 and 4) in humans (Idzikowski et al., 1986; 1987; 1991; Clarenbach et al., 1988; Dijk et al., 1989; Sharpley et al., 1990; 1994) and increase deep slow wave sleep (SWS-2) in rats (Dugovic & Wauquier, 1987; Dugovic et al., 1989a; 1989b; Silhol et al., 1992; Stutzmann et al., 1992; Detari et al., 1999; Kantor et al., 2000; 2003). The 5-HT2 agonists, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and 1-(3-chlorophenyl)piperazine (m-CPP), have opposite sleep–waking effects from 5-HT2 receptor antagonists: they increase wakefulness (W), decrease slow wave sleep and REM sleep (Monti et al., 1990; Kantor et al., 2000). In addition to the sleep–waking effects, ritanserin and seganserin increase low-frequency EEG activity administered at the beginning of the passive phase in rats (Borbely et al., 1988; Kantor et al., 2002) and humans (Dijk et al., 1989). Ritanserin, which binds selectively to 5-HT2 receptors (Leysen et al., 1985), cannot differentiate among subtypes of the 5-HT2 receptor family, namely 5-HT2A, 5-HT2B and 5-HT2C receptors (Bonhaus et al., 1997). In a recent study (Sebban et al., 2002), M100907, a subtype-selective 5-HT2A receptor antagonist, caused similar EEG effects to ritanserin. In another study (Smith et al., 2002), the 5-HT2C antagonist 6-chloro-5-methyl-1-(5-quinolylcarbamoyl) indoline (SB-243213) was found to produce similar sleep–waking effects to ritanserin, namely increased SWS-2 and decreased paradoxical sleep (PS) in rats. However, the role of 5-HT2B receptors in sleep and EEG processes has not been described. To investigate the possible role of the 5-HT2B receptor in regulation of the sleep–wake cycle, we analysed vigilance states and motor activity in rats and examined EEG spectra in different vigilance states after a selective blockade of this receptor subtype by the 5-HT2B antagonist SB-215505.
Methods
Animals and surgery
All animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Permission was given by the local ethical committee.
Male Sprague–Dawley rats (Crl:CDRBR, Charles River, Hungary), weighing 230–260 g at implantation, were used in the experiments. Rats were kept in single cages with food and water available ad libitum, and maintained in a 12 h light/dark cycle (lights on from 0900 to 21 00 hours, daylight type fluorescent tubes, 18 W, approximately 300 lx), at an ambient temperature of 21°C. Animals were equipped with EEG and EMG electrodes as described earlier (Kantor et al., 2000; 2002; Filakovszky et al., 2001). Briefly, stainless steel screw electrodes were implanted epidurally over the left frontal cortex (L: 2.0 mm and A: 2 mm to bregma) and over the left parietal cortex (L: 2.0 mm and A: 2.0 mm to lambda) for fronto-parietal EEG recordings. The ground electrode was placed over the cerebellum. In addition, EMG electrodes (stainless steel spring electrodes embedded in silicon rubber, Plastics One Inc., Roanoke, U.S.A.) were placed in the muscles of the neck. Surgery was performed under halothane (2%) anaesthesia (Fluotec 3) using a Kopf stereotaxic instrument. After a 10-day recovery period, the rats were attached to the polygraph by a flexible recording cable and an electric swivel, fixed above the cages, permitting free movement of the animals. In order to habituate the animals to the recording conditions, the rats were attached to the polygraph and received intraperitoneal (i.p.) injections of physiological saline for 5 days before the experiments. The animals remained connected to the recording cables throughout the study.
Data acquisition
EEG, EMG and motor activity were recorded for 8 h, starting at light onset. The signals were amplified (amplification factors approximately 5000 for EEG and motor activity, 20,000 for EMG), conditioned by analogue filters (filtering below 0.53 Hz and above 30 Hz at 6 dB octave−1) and subjected to analogue to digital conversion with a sampling rate of 64 Hz. The digitized signals were displayed on a monitor and stored on the computer for further analysis.
Scoring of vigilance states
The vigilance states were scored visually for 4 s periods over 1–5 h as follows: W, the EEG is characterized by low-amplitude activity at beta (14–30 Hz) and alpha (8–13 Hz) frequencies accompanied by high EMG and motor activity; light slow wave sleep (SWS-1), high-voltage slow cortical waves (0.5–4 Hz) interrupted by low-voltage fast EEG activity (spindles, 6–15 Hz) accompanied by reduced EMG and motor activity; SWS-2, continuous high-amplitude slow cortical waves (0.5–4 Hz) with reduced EMG and motor activity; intermediate stage of sleep (IS), a brief stage (mean 3 s) just prior to PS and sometimes just after it characterized by unusual association of high-amplitude spindles (mean 12.5 Hz) and low-frequency (5.4 Hz) theta rhythm; PS, low-amplitude and high-frequency EEG activity with regular theta waves (6–9 Hz) accompanied by silent EMG and motor activity with occasional twitching (Gottesmann, 1992; Kantor et al., 2000; 2002; Filakovszky et al., 2001).
In addition to the visual scoring, 4 s periods of the polygraphic recordings were classified using sleep analysis software (SleepSign for Animal, Kissei Comtec America Inc., U.S.A.) for 8 h. The results obtained from this software were compared to those obtained by visual analysis: six animals, 4 × 30 min each (from 0000 to 0030, 0100 to 0130, 0230 to 0300 and 0430 to 0500 hours). The values obtained by the software yielded strong correlations with those obtained by visual inspection (W, r=0.972; SWS-1, r=0.806; SWS-2, r=0.933; IS, r=0.716; PS, r=0.841; P<0.01 for all parameters, respectively).
EEG power spectral analysis
EEG power spectra were computed for consecutive 4 s epochs in the frequency range 0.25–30 Hz (fast Fourier transformation routine, Hanning window; frequency resolution 0.25 Hz). Epochs with artefacts were discarded on the basis of the polygraph records. Adjacent 0.25 Hz bins were summed into 1 Hz bins, and those above 30 Hz were omitted. Bins are marked by their upper limits; thus, 2 Hz refers to 1.25–2.00 Hz. The values of consecutive 4 s EEG epochs in W, SWS-1, SWS-2 and PS, respectively, were averaged in the first hour after treatment to obtain the power density values for these vigilance states.
Analysis of motor activity
To assess motor activity, the potentials generated in electromagnetic transducers activated by the movements of the recording cable were used and averaged for consecutive 4 s epochs (Svensson et al., 1987). The absolute value or full-wave rectified signal was derived from the original motor activity signal. The unwanted baseline noise was removed by shifting the rectified signal below zero reference (submerging the band of noise under zero), re-establishing zero at the baseline, and then taking the positive half-wave rectification of the re-zeroed signal. After this, the periods above the zero reference line were quantified, obtaining the time of motor activity. The values obtained by this method yielded very strong correlations with those obtained by visual inspection and scoring (12 × 2 h intervals, r=0.93, P<0.01).
Drugs
All animals (10 rats) received vehicle and two doses of SB-215505 (0.1, 0.3 and 1.0 mg kg−1, i.p.) treatment in a random order (4 days between treatments). The solutions were administered i.p. in the first 5 min at the beginning of the light period. SB-215505 (kindly donated by Dr Guy A. Kennett) was dissolved in 40% 2-hydroxypropyl-β-cyclodextrin (Research Biochemicals International, Natick, MA, U.S.A.) solution and administered in a volume of 1 ml kg−1.
Statistical analysis
To analyse statistically the time course of changes in each vigilance state, data from each hour were submitted to multivariate analysis of variance (MANOVA) for repeated measures with two main factors: treatment (vehicle and SB-215505 1.0 mg kg−1) and time (1–5 h). When the effects of different treatments (vehicle, SB-215505 0.1, 0.3 and 1.0 mg kg−1) on sleep/W were compared, MANOVA for repeated measures was used. Values of motor activity were also evaluated by MANOVA for repeated measures. The EEG power spectral values were submitted to MANOVA for repeated measures with two main factors: treatment (vehicle, SB-215505 0.1, 0.3 and 1.0 mg kg−1) and frequency bins (1–30 Hz). Tukey's honest significant difference test was used for post hoc comparisons.
Results
Vigilance states and motor activity
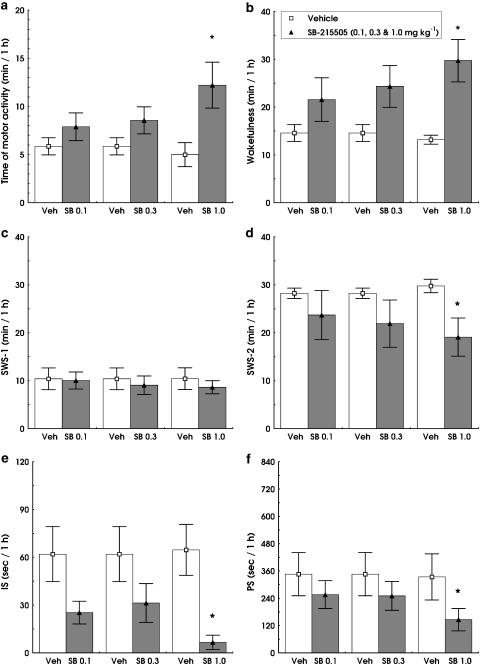
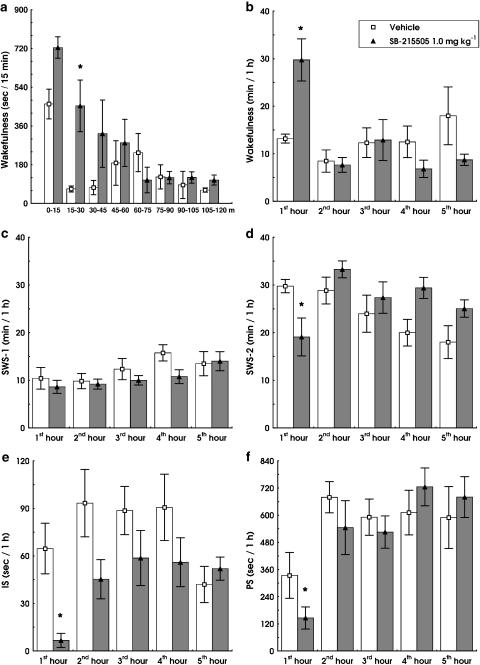
SB-215505 (0.1, 0.3 and 1.0 mg kg−1 i.p.) dose-dependently increased W (F3,12=4.288; P<0.05) decreased IS (F3,12=6.798; P<0.01) and PS (F(3,12)=3.689; P<0.05) compared to vehicle (Figure 2b, e and f). The drug significantly decreased SWS-2 relative to controls (F1,5=6.857, P<0.05; Figure 2d) at the highest dose (1.0 mg kg−1 i.p.). Duration of SWS-1 was not significantly affected by SB-215505 (Figures 1c and 2c). The effect of the drug was restricted to the first hour, with maximal effects between 15 and 45 min (Figure 1a). The time × treatment interaction was significant for W (F4,20=3.128, P<0.001; Figure 1b) and a strong trend was found for SWS-2 (F4,20=2.714, P=0.059; Figure 1d). No significant changes were found in vigilance states at later time points up to 8 h analysed by the SleepSign software.
Figure 2.
Dose–response effects of SB-215505 (0.1, 0.3 and 1.0 mg kg−1 i.p.) on motor activity (a) and different vigilance states in conscious, freely moving rats in the first hour after treatment. W (b), SWS-1 (c), SWS-2 (d), IS (e) and PS (f). All columns represent mean values (±s.e.m., n=6). *Significant effect of SB-215505 compared to vehicle, P<0.05.
Figure 1.
Effect of SB-215505 (1.0 mg kg−1 i.p.) on different vigilance states in conscious, freely moving rats. Time of W in each 15 min period within the first 2 h after treatment (a). Time of W (b), SWS-1 (c), SWS-2 (d), IS (e) and PS (f) per hour within the first 5 h after treatment. All columns represent mean values (±s.e.m., n=6). *Significant effect of SB-215505 compared to vehicle, P<0.05.
Parallel to the increased W, we found a dose-dependent increase in motor activity (F3,12=3.613, P<0.05; Figure 2a).
EEG power in different vigilance states
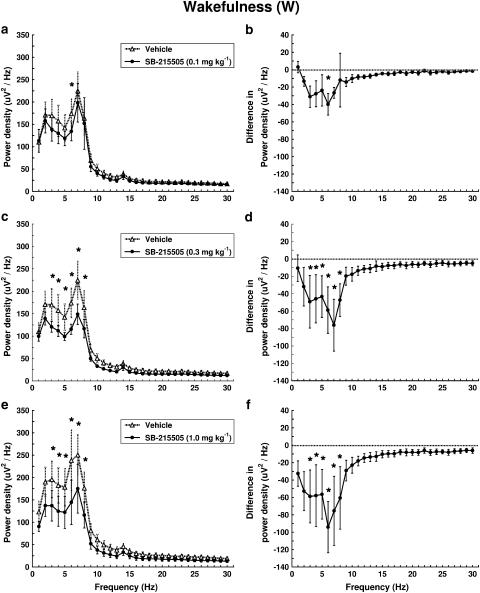
Effects of SB-215505 on EEG power were analysed separately in W, SWS-1, SWS-2 and PS. In W, the drug dose-dependently decreased EEG power mainly in the frequency range of 3–8 Hz (Figure 3). A significant treatment × frequency interaction was found (F29,116=1.716, P<0.05; F29,116=3.827, P<0.01; F29,116=3.545, P<0.01) for 0.1, 0.3 and 1 mg kg−1 i.p., respectively. The most prominent decrease was observed at 6 Hz; this was already present at the lowest dose (0.1 mg kg−1 i.p.) of the drug (Figure 3a and b).
Figure 3.
Dose–response effect of SB-215505 on EEG power spectra in W in conscious, freely moving rats in the first hour after treatment. Each point represents mean values (±s.e.m.) of 1 Hz bins in a 1 h recording period. The curves connect absolute power density values after vehicle and drug treatment (a, c, e) or the differences in drug treatment compared to vehicle treatment (dashed line) (b, d, f). *Significant effect of SB-215505 compared to vehicle, P<0.05. (a, b) 0.1 mg kg−1 i.p. (n=5); (c, d) 0.3 mg kg–1 i.p. (n=5); (e, f) 1.0 mg kg−1 i.p. (n=5).
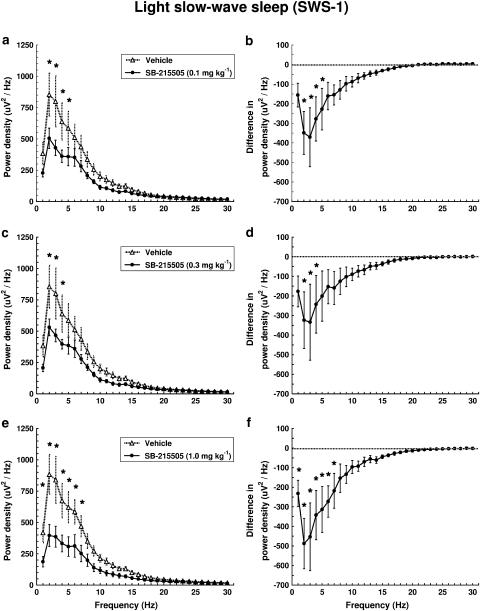
In SWS-1, the drug reduced low-frequency (<8 Hz) EEG power with a maximal effect at 2–4 Hz (Figure 4e and f). Significant treatment × frequency interactions were found (F29,145=6.371, P<0.01; F29,145=3.236, P<0.01; F29,145=8.331, P<0.01) for 0.1, 0.3 and 1 mg kg−1 i.p., respectively.
Figure 4.
Dose–response effect of SB-215505 on EEG power spectra in SWS-1 in conscious, freely moving rats in the first hour after treatment. Each point represents mean values (±s.e.m.) of 1 Hz bins in a 1 h recording period. The curves connect absolute power density values after vehicle and drug treatment (a, c, e) or the differences in drug treatment compared to vehicle treatment (dashed line) (b, d, f). *Significant effect of SB-215505 compared to vehicle, P<0.05. (a, b) 0.1 mg kg−1 i.p. (n=6); (c, d) 0.3 mg kg−1 i.p. (n=6); (e, f) 1.0 mg kg−1 i.p. (n=6).
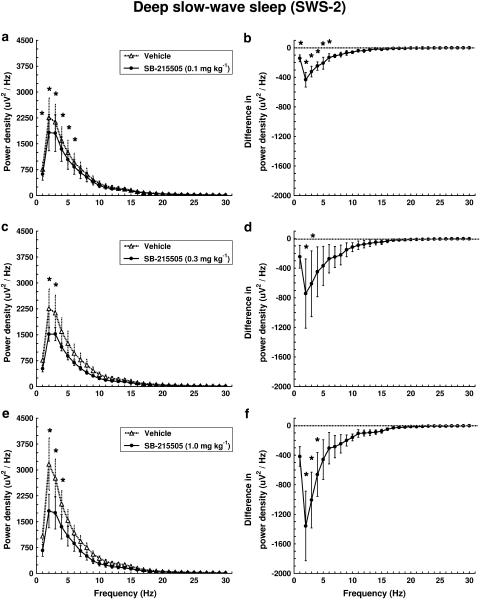
Spectral analysis of the EEG in SWS-2 after SB-215505 treatment showed a dose-dependent decrease in power density mainly at 2–3 Hz compared to control treatment (Figure 5). The treatment × frequency interaction was highly significant for all three doses (F29,116=13.499, P<0.01; F29,116=2.126, P<0.01; F29,145=6.200, P<0.01).
Figure 5.
Dose–response effect of SB-215505 on EEG power spectra in SWS-2 in conscious, freely moving rats in the first hour after treatment. Each point represents mean values (±s.e.m.) of 1 Hz bins in a 1 h recording period. The curves connect absolute power density values after vehicle and drug treatment (a, c, e) or the differences in drug treatment compared to vehicle treatment (dashed line) (b, d, f). *Significant effect of SB-215505 compared to vehicle, P<0.05. (a, b) 0.1 mg kg−1 i.p. (n=5); (c, d) 0.3 mg kg−1 i.p. (n=5); (e, f) 1.0 mg kg−1 i.p. (n=6).
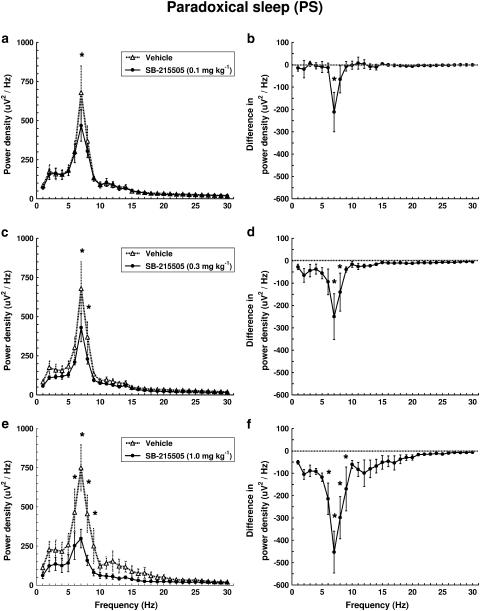
In PS, the drug decreased EEG power only in the theta band (6–9 Hz) in a dose-dependent manner (Figure 6). A highly significant treatment × frequency interaction was found at all three doses (F29,116=3.340, P<0.01; F29,116=3.917, P<0.01; F29,116=8.241, P<0.01). The drug decreased most prominently the frequency with the highest power value (7 Hz) during this stage, and this effect was already present at the lowest dose (0.1 mg kg−1 i.p.) of the drug (Figure 6a and b).
Figure 6.
Dose–response effect of SB-2715505 on EEG power spectra in PS in conscious, freely moving rats in the first hour after treatment. Each point represents mean values (±s.e.m.) of 1 Hz bins in a 1 h recording period. The curves connect absolute power density values after vehicle and drug treatment (a, c, e) or the differences in drug treatment compared to vehicle treatment (dashed line) (b, d, f). *Significant effect of SB-215505 compared to vehicle, P<0.05. (a, b) 0.1 mg kg−1 i.p. (n=5); (c, d) 0.3 mg kg−1 i.p. (n=5); (e, f) 1.0 mg kg−1 i.p. (n=5).
Discussion and conclusions
The central role of the 5-HT2B receptor has been a controversial topic for a long time due to lack of evidence for the presence of 5-HT2B receptors in the CNS (Kursar et al., 1992; Pompeiano et al., 1994). Low levels of the 5-HT2B receptor mRNA have been detected recently in mouse (Loric et al., 1992), rat (Pompeiano et al., 1994) and human brains (Kursar et al., 1994; Schmuck et al., 1994; Bonhaus et al., 1995). By performing immunohistochemistry with antiserum raised against the 5-HT2B receptor protein, the expression of the receptor has been identified in both mouse (Choi & Maroteaux, 1996) and rat (Duxon et al., 1997) brains. In the mouse, a lower level of immunoreactivity was also found in the cerebrum (Choi & Maroteaux, 1996), while in the rat the presence of the 5-HT2B receptor has been demonstrated in discrete nuclei in the cerebellum, lateral septum (LS), dorsal hypothalamic nucleus (DHN) and medial amygdala (Duxon et al., 1997).
The function of the 5-HT2B receptor is unclear due to the lack of selective drugs available. In rodent studies, stimulation of 5-HT2B receptors has been reported to have a modest anxiolytic effect (Kennett et al., 1996). In addition, the 5-HT2B receptor agonist BW 723C86 caused hyperphagia and reduced grooming (Kennett et al., 1997).
The 5-HT2B receptor antagonist SB-215505 exhibits at least 95-fold selectivity for 5-HT2B over 5-HT2C in the rat (Kennett et al., 1998b). Pretreatment with SB-215505 (1 and 3 mg kg−1 p.o.) effectively opposed the antipunishment effect of the 5-HT2B receptor agonist BW 723C86 in the rat Vogel conflict test (Kennett et al., 1998a). Furthermore, SB-215505 (1 mg kg−1 i.p.) failed to affect m-CPP-induced behavioural effects mediated by 5-HT2C receptors (Graf et al., 2003). In contrast, pretreatment with SB-242084 (0.1 and 0.3 mg kg−1 i.p.), a subtype-selective 5-HT2C receptor antagonist, dose-dependently inhibited these effects of m-CPP (Graf et al., 2003).
In our study, SB-215505 dose-dependently increased W at the expense of IS, PS and SWS-2. Thus, the blockade of 5-HT2B receptors resulted in the opposite sleep/waking effect than the previously reported 5-HT2 antagonists like ritanserin (Dugovic et al., 1989b; Detari et al., 1999; Kantor et al., 2000; 2002) or the 5-HT2C receptor antagonist SB-243213 (Smith et al., 2002). This may be the consequence of different CNS distribution and/or cellular localization of the 5-HT2 receptor subtypes.
A large body of experimental data has confirmed the key role played by the posterior hypothalamic area (PHA) in neocortical activation (or desynchronization) and behavioural arousal (Moruzzi, 1972; Vanni-Mercier et al., 2003). The presence of 5-HT2B receptors has been described on multipolar and bipolar soma in the DHN (Duxon et al., 1997), which forms the extreme rostral border of the PHA (Abrahamson & Moore, 2001). In addition, a large population of GABA- and GAD-immunoreactive neurons is present at the border between the PHA and dorsomedial hypothalamic nucleus (Abrahamson & Moore, 2001). Therefore, it is interesting to speculate that 5-HT2B receptors are localized on GABA-ergic neurons in the DHN and these neurons send their inhibitory projections to the waking centre of the PHA. Thus, under physiological conditions, 5-HT may increase GABA-ergic inhibition of the waking centre throughout 5-HT2B receptors. Consequently, blockade of these receptors may stop inhibition of the waking centre followed by an increase in W.
Although SB-215505 did not change the amount of SWS-1, we found a depressed low-frequency EEG activity (mainly between 2 and 4 Hz) during this stage. Similar to SWS-1, the drug dose-dependently decreased EEG power in SWS-2 mainly at 2–3 Hz compared to control treatment. The decrease in low-frequency EEG activity (primarily in the delta range) both in SWS-1 and SWS-2 reflects a reduced sleep intensity that may be a consequence of a fragmentation of sleep. These EEG changes are in contrast to those produced by 5-HT2 receptor antagonists (Borbely et al., 1988; Dijk et al., 1989; Kantor et al., 2002) or the subtype-selective 5-HT2A receptor antagonist M100907 (Sebban et al., 2002). Therefore, the decreased delta power after selective blockade of the 5-HT2B receptor implies that ritanserin-induced increase in delta activity (Borbely et al., 1988; Kantor et al., 2002) may be a result of 5-HT2A receptor blockade. However, the role of 5-HT2C receptors cannot be excluded (Smith et al., 2002). The selective 5-HT2A receptor antagonist M100907 at low doses (0.01 and 0.05 mg kg−1 s.c.) increased EEG power density in the low-frequency range (Sebban et al., 2002) and the rate of this increase was two-fold higher than that caused by ritanserin (Borbely et al., 1988; Kantor et al., 2002; Sebban et al., 2002). Consequently, the smaller increase in slow wave activity after ritanserin treatment may be a consequence of a parallel 5-HT2B receptor blockade.
Spectral analysis of the EEG in W showed a dose-dependent decrease in EEG power in the frequency range of 3–8 Hz with a maximal effect at 6 Hz after SB-215505 treatment. In addition, the drug selectively decreased neocortical theta activity (6–9 Hz) during PS, affecting most prominently the frequency with the highest power value (7 Hz) during this stage. It has been shown that the neocortical theta rhythm of rats is passively spread from the underlying hippocampus (Gerbrandt et al., 1978). Thus, the theta reducing effect of the 5-HT2B receptor blockade described here can be explained by the distribution of this receptor in the CNS. The 5-HT2B receptor was identified on the neurons of the LS (Duxon et al., 1997) and it is generally accepted that the rhythm generator (i.e. pacemaker) of hippocampal-enthorinal theta oscillation is the septal complex (Stewart & Fox, 1990; Lee et al., 1994; Leranth & Kiss, 1996). It is well established that the rhythmically discharging cells of the medial septum/vertical limb of the diagonal band nucleus (MS/DBv) that fire synchronously with the theta rhythm are responsible for its generation in hippocampal formation (Vertes & Kocsis, 1997). Furthermore, it has been proposed that the LS, which receives the most important afferents from the hippocampus, contributes to theta rhythm generation by giving a feedback input from the hippocampus to the MS/DBv (Raisman, 1966; Risold & Swanson, 1997a; 1997b; Pedemonte et al., 1998). Moreover, infusion of carbachol, a cholinergic agonist, into the LS induced theta rhythm in the hippocampus (Monmaur et al., 1993) while ibotenic lesion of the LS significantly attenuated the hippocampal theta rhythm about 50% bilaterally, at both surface and deep electrodes (Leung et al., 1994). LS receives dense serotonergic input from several parts of the raphe nuclei including dorsal raphe (Dinopoulos et al., 1993; Risold & Swanson, 1997a; 1997b; Leger et al., 2001) and it has been demonstrated that approximately 55% of dorsal raphe neurons discharge synchronously with the theta rhythm of the hippocampus during awake-moving or rapid eye movement sleep (Kocsis & Vertes, 1992). Accordingly, these findings and our present data suggest that serotonin, acting through 5-HT2B receptors of the LS, plays an important role in theta rhythm generation under physiological conditions.
Parallel to sleep and EEG changes, we found increased motor activity after the highest dose of SB-215505. In addition, a slight but not significant increase in time of self-grooming and number of grooming bouts was observed at the 1 mg kg−1 dose (Graf et al., 2003). SB-215505 did not induce penile erections but there were some signs of restlessness noted by the observer; this was not scored (Graf et al., 2003). These data are consistent with the effect of the 5-HT2B receptor agonist BW 723C86 described previously, namely the drug tended to suppress locomotor activity and rearing at higher doses (Kennett et al., 1997). Thus, it is possible that the 5-HT2B receptor plays an important role in the regulation of motor activity. Expression of 5-HT2B receptors on cerebellar Purkinje cells provides further evidence to support this hypothesis (Duxon et al., 1997).
In conclusion, our studies show that the selective 5-HT2B receptor antagonist SB-215505 administered at light onset dose-dependently increases W and motor activity, and decreases IS, PS and SWS-2 in the first hour after treatment. Spectral analysis of the EEG in SWS-1 and SWS-2 shows a dose-dependent decrease in low-frequency EEG power (<8 Hz), reflecting reduced sleep intensity after the treatment. In addition, the drug significantly reduced theta activity in W and PS in a dose-dependent manner, suggesting an important role of the 5-HT2B receptor in theta rhythm generation.
These data indicate that the well-known SWS-2 enhancing effect of 5-HT2 receptor antagonists is mediated by 5-HT2A and/or 5-HT2C receptors. In contrast, blockade of 5-HT2B receptors may increase W along with a decreased theta activity during W and PS. The results suggest that, under physiological conditions, activation of 5-HT2B receptors may contribute to initiation of sleep as well as to theta generation during W and PS.
Acknowledgments
These studies were supported by the Fifth Framework Programme of the European Community, QLG-CT-2002-00809, the Hungarian Research Fund Grants T020500 and M 27976, Ministry of Welfare Research Grant T058/2003, the Fund Management of Ministry of Education OMFB 01926/2002 and the Postdoctoral Ph.D. Fellowship Program of Semmelweis University, Ministry of Culture and Education, Hungary. SB-215505 was kindly provided by Dr G. Kennett, Winnersh, Wokingham, U.K.
Abbreviations
- DHN
dorsal hypothalamic nucleus
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- IS
intermediate stage of sleep
- LS
lateral septum
- m-CPP
1-(3-chlorophenyl)piperazine
- MS/DBv
medial septum/vertical limb of the diagonal band nucleus
- PHA
posterior hypothalamic area
- PS
paradoxical sleep
- SB-215505
6-chloro-5-methyl-1-(5-quinolylcarbamoyl) indoline
- SWS-1
light slow wave sleep
- SWS-2
deep slow wave sleep
- W
wakefulness
References
- ABRAHAMSON E.E., MOORE R.Y. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., BACH C., DESOUZA A., SALAZAR F.H., MATSUOKA B.D., ZUPPAN P., CHAN H.W., EGLEN R.M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHAUS D.W., WEINHARDT K.K., TAYLOR M., DESOUZA A., MCNEELEY P.M., SZCZEPANSKI K., FONTANA D.J., TRINH J., ROCHA C.L., DAWSON M.W., FLIPPIN L.A., EGLEN R.M. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- BORBELY A.A., TRACHSEL L., TOBLER I. Effect of ritanserin on sleep stages and sleep EEG in the rat. Eur. J. Pharmacol. 1988;156:275–278. doi: 10.1016/0014-2999(88)90332-9. [DOI] [PubMed] [Google Scholar]
- CHOI D.S., MAROTEAUX L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett. 1996;391:45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- CLARENBACH P., BIRMANNS B., JAURSCH-HANCKE C.The effect of ritanserin on sleep and hormones in man Sleep ‘86 1988Stuttgart: Gustav Fischer Verlag; 355–358.ed. Koella, W.P., Obal, F., Schultz, H. & Visser, P. pp [Google Scholar]
- DETARI L., SZENTGYORGYI V., HAJNIK T., SZENASI G., GACSALYI I., KUKORELLI T. Differential EEG effects of the anxiolytic drugs, deramciclane (EGIS-3886), ritanserin and chlordiazepoxide in rats. Psychopharmacology (Berl.) 1999;142:318–326. doi: 10.1007/s002130050895. [DOI] [PubMed] [Google Scholar]
- DIJK D.J., BEERSMA D.G., DAAN S., VAN DEN HOOFDAKKER R.H. Effects of seganserin, a 5-HT2 antagonist, and temazepam on human sleep stages and EEG power spectra. Eur. J. Pharmacol. 1989;171:207–218. doi: 10.1016/0014-2999(89)90109-x. [DOI] [PubMed] [Google Scholar]
- DINOPOULOS A., DORI I., PARNAVELAS J.G. Serotonergic innervation of the mature and developing lateral septum of the rat: a light and electron microscopic immunocytochemical analysis. Neuroscience. 1993;55:209–222. doi: 10.1016/0306-4522(93)90467-t. [DOI] [PubMed] [Google Scholar]
- DUGOVIC C., LEYSEN J.E., JANSSEN P.F., WAUQUIER A. The light–dark cycle modulates the effects of ritanserin on sleep–wakefulness patterns in the rat. Pharmacol. Biochem. Behav. 1989a;34:533–537. doi: 10.1016/0091-3057(89)90554-6. [DOI] [PubMed] [Google Scholar]
- DUGOVIC C., WAUQUIER A. 5-HT2 receptors could be primarily involved in the regulation of slow-wave sleep in the rat. Eur. J. Pharmacol. 1987;137:145–146. doi: 10.1016/0014-2999(87)90196-8. [DOI] [PubMed] [Google Scholar]
- DUGOVIC C., WAUQUIER A., LEYSEN J.E., MARRANNES R., JANSSEN P.A. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl.) 1989b;97:436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- DUXON M.S., FLANIGAN T.P., REAVLEY A.C., BAXTER G.S., BLACKBURN T.P., FONE K.C. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- FILAKOVSZKY J., KANTOR S., HALASZ P., BAGDY G. 8-OH-DPAT and MK-801 affect epileptic activity independently of vigilance. Neurochem. Int. 2001;38:551–556. doi: 10.1016/s0197-0186(00)00120-0. [DOI] [PubMed] [Google Scholar]
- GERBRANDT L.K., LAWRENCE J.C., ECKARDT M.J., LLOYD R.L. Origin of the neocortically monitored theta rhythm in the curarized rat. Electroencephalogr. Clin. Neurophysiol. 1978;45:454–467. doi: 10.1016/0013-4694(78)90290-0. [DOI] [PubMed] [Google Scholar]
- GOTTESMANN C. Detection of seven sleep–waking stages in the rat. Neurosci. Biobehav. Rev. 1992;16:31–38. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- GRAF M., KANTOR S., ANHEUER Z.E., MODOS E.A., BAGDY G. m-CPP-induced self-grooming is mediated by 5-HT2C receptors. Behav. Brain Res. 2003;142:175–179. doi: 10.1016/s0166-4328(02)00404-7. [DOI] [PubMed] [Google Scholar]
- IDZIKOWSKI C., COWEN P.J., NUTT D., MILLS F.J. The effects of chronic ritanserin treatment on sleep and the neuroendocrine response to L-tryptophan. Psychopharmacology (Berl.) 1987;93:416–420. doi: 10.1007/BF00207228. [DOI] [PubMed] [Google Scholar]
- IDZIKOWSKI C., MILLS F.J., GLENNARD R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;378:164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- IDZIKOWSKI C., MILLS F.J., JAMES R.J. A dose–response study examining the effects of ritanserin on human slow wave sleep. Br. J. Clin. Pharmacol. 1991;31:193–196. doi: 10.1111/j.1365-2125.1991.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOUVET M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- KANTOR S., GERBER K., HALASZ P., BAGDY G. The role of 5-HT2A, 5-HT2C and 5HT3 serotonin receptors in the regulation of sleep. J. Physiol. 2000;526:66P–67P. [Google Scholar]
- KANTOR S., JAKUS R., BODIZS R., HALASZ P., BAGDY G. Acute and long-term effects of the 5-HT2 receptor antagonist ritanserin on EEG power spectra, motor activity, and sleep: changes at the light–dark phase shift. Brain Res. 2002;943:105–111. doi: 10.1016/s0006-8993(02)02698-7. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., AINSWORTH K., TRAIL B., BLACKBURN T.P. BW 723C86, a 5-HT2B receptor agonist, causes hyperphagia and reduced grooming in rats. Neuropharmacology. 1997;36:233–239. doi: 10.1016/s0028-3908(96)00171-2. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., BRIGHT F., TRAIL B., BAXTER G.S., BLACKBURN T.P. Effects of the 5-HT2B receptor agonist, BW 723C86, on three rat models of anxiety. Br. J. Pharmacol. 1996;117:1443–1448. doi: 10.1111/j.1476-5381.1996.tb15304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNETT G.A., TRAIL B., BRIGHT F. Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology. 1998a;37:1603–1610. doi: 10.1016/s0028-3908(98)00115-4. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., TRAIL B., RILEY G., BICKERDIKE M.J., RANSON J., FORBES I.T., PRICE G.W., BAXTER G.S., HUNTER A.J.SB 215505, a selective 5-HT2B receptor antagonist in rats Soc. Neurosci. Abstr. 1998b24541.12 [Google Scholar]
- KOCSIS B., VERTES R.P. Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J. Neurophysiol. 1992;68:1463–1467. doi: 10.1152/jn.1992.68.4.1463. [DOI] [PubMed] [Google Scholar]
- KURSAR J.D., NELSON D.L., WAINSCOTT D.B., BAEZ M. Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol. Pharmacol. 1994;46:227–234. [PubMed] [Google Scholar]
- KURSAR J.D., NELSON D.L., WAINSCOTT D.B., COHEN M.L., BAEZ M. Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Mol. Pharmacol. 1992;42:549–557. [PubMed] [Google Scholar]
- LEE M.G., CHROBAK J.J., SIK A., WILEY R.G., BUZSAKI G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- LEGER L., CHARNAY Y., HOF P.R., BOURAS C., CESPUGLIO R. Anatomical distribution of serotonin-containing neurons and axons in the central nervous system of the cat. J. Comp. Neurol. 2001;433:157–182. [PubMed] [Google Scholar]
- LERANTH C., KISS J. A population of supramammillary area calretinin neurons terminating on medial septal area cholinergic and lateral septal area calbindin-containing cells are aspartate/glutamatergic. J. Neurosci. 1996;16:7699–7710. doi: 10.1523/JNEUROSCI.16-23-07699.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG L.S., MARTIN L.A., STEWART D.J. Hippocampal theta rhythm in behaving rats following ibotenic acid lesion of the septum. Hippocampus. 1994;4:136–147. doi: 10.1002/hipo.450040204. [DOI] [PubMed] [Google Scholar]
- LEYSEN J.E., GOMMEREN W., VAN GOMPEL P., WYNANTS J., JANSSEN P.F., LADURON P.M. Receptor-binding properties in vitro and in vivo of ritanserin: a very potent and long acting serotonin-S2 antagonist. Mol. Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- LORIC S., LAUNAY J.M., COLAS J.F., MAROTEAUX L. New mouse 5-HT2-like receptor. Expression in brain, heart and intestine. FEBS Lett. 1992;312:203–207. doi: 10.1016/0014-5793(92)80936-b. [DOI] [PubMed] [Google Scholar]
- MONMAUR P., AYADI K., BRETON P. Hippocampal EEG responses induced by carbachol and atropine infusions into the septum and the hippocampus in the urethane-anaesthetized rat. Brain Res. 1993;631:317–324. doi: 10.1016/0006-8993(93)91551-3. [DOI] [PubMed] [Google Scholar]
- MONTI J.M., PINEYRO G., ORELLANA C., BOUSSARD M., JANTOS H., LABRAGA P., OLIVERA S., ALVARINO F. 5-HT receptor agonists 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and 8-OH-DPAT increase wakefulness in the rat. Biog. Amines. 1990;7:145–151. [Google Scholar]
- MORUZZI G. The sleep–waking cycle. Ergeb. Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- PEDEMONTE M., BARRENECHEA C., NUNEZ A., GAMBINI J.P., GARCIA-AUSTT E. Membrane and circuit properties of lateral septum neurons: relationships with hippocampal rhythms. Brain Res. 1998;800:145–153. doi: 10.1016/s0006-8993(98)00517-4. [DOI] [PubMed] [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- RAISMAN G. The connexions of the septum. Brain. 1966;89:317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- RISOLD P.Y., SWANSON L.W. Chemoarchitecture of the rat lateral septal nucleus. Brain Res. Brain Res. Rev. 1997a;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- RISOLD P.Y., SWANSON L.W. Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev. 1997b;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- SCHMUCK K., ULLMER C., ENGELS P., LUBBERT H. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 1994;342:85–90. doi: 10.1016/0014-5793(94)80590-3. [DOI] [PubMed] [Google Scholar]
- SEBBAN C., TESOLIN-DECROS B., CIPRIAN-OLLIVIER J., PERRET L., SPEDDING M. Effects of phencyclidine (PCP) and MK 801 on the EEGq in the prefrontal cortex of conscious rats; antagonism by clozapine, and antagonists of AMPA-, α1- and 5-HT2A-receptors. Br. J. Pharmacol. 2002;135:65–78. doi: 10.1038/sj.bjp.0704451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARPLEY A.L., ELLIOTT J.M., ATTENBURROW M.J., COWEN P.J. Slow wave sleep in humans: role of 5-HT2A and 5-HT2C receptors. Neuropharmacology. 1994;33:467–471. doi: 10.1016/0028-3908(94)90077-9. [DOI] [PubMed] [Google Scholar]
- SHARPLEY A.L., SOLOMON R.A., FERNANDO A.I., DA ROZA DAVIS J.M., COWEN P.J. Dose-related effects of selective 5-HT2 receptor antagonists on slow wave sleep in humans. Psychopharmacology (Berl.) 1990;101:568–569. doi: 10.1007/BF02244239. [DOI] [PubMed] [Google Scholar]
- SILHOL S., GLIN L., GOTTESMANN C. Study of the 5-HT2 antagonist ritanserin on sleep–walking cycle in the rat. Pharmacol. Biochem. Behav. 1992;41:241–243. doi: 10.1016/0091-3057(92)90091-s. [DOI] [PubMed] [Google Scholar]
- SMITH M.I., PIPER D.C., DUXON M.S., UPTON N. Effect of SB-243213, a selective 5-HT(2C) receptor antagonist, on the rat sleep profile: a comparison to paroxetine. Pharmacol. Biochem. Behav. 2002;71:599–605. doi: 10.1016/s0091-3057(01)00702-x. [DOI] [PubMed] [Google Scholar]
- STEWART M., FOX S.E. Firing relations of lateral septal neurons to the hippocampal theta rhythm in urethane anesthetized rats. Exp. Brain Res. 1990;79:92–96. doi: 10.1007/BF00228876. [DOI] [PubMed] [Google Scholar]
- STUTZMANN J.M., EON B., LUCAS M., BLANCHARD J.C., LADURON P.M. RP 62203, a 5-hydroxytryptamine2 antagonist, enhances deep NREM sleep in rats. Sleep. 1992;15:119–124. doi: 10.1093/sleep/15.2.119. [DOI] [PubMed] [Google Scholar]
- SVENSSON K., ALFOLDI P., HAJOS M., RUBICSEK G., JOHANSSON A.M., CARLSSON A., OBAL F., JR Dopamine autoreceptor antagonists: effects on sleep–wake activity in the rat. Pharmacol. Biochem. Behav. 1987;26:123–129. doi: 10.1016/0091-3057(87)90544-2. [DOI] [PubMed] [Google Scholar]
- VANNI-MERCIER G., GIGOUT S., DEBILLY G., LIN J.S. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav. Brain Res. 2003;144:227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- VERTES R.P., KOCSIS B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]