Abstract
The aim of the present study was to determine whether inhibition of cyclic nucleotide phosphodiesterase (PDE) modulates the stimulated generation of the cytokines, interleukin-4 (IL-4) and IL-13, from human basophils. This was addressed by evaluating the effects of both nonselective and selective inhibitors of PDEs on the generation of cytokines from basophils.
The nonselective PDE inhibitors, isobutyl-methylxanthine (IBMX) and theophylline, attenuated the IgE-mediated generation of IL-4 and IL-13 and, also, the release of histamine from basophils.
The effects of the isoform-selective inhibitors, 8-methoxymethyl-IBMX (PDE 1 inhibitor), siguazodan (PDE3 inhibitor), rolipram (PDE4 inhibitor), denbufylline (PDE4 inhibitor), Org 30029 (mixed PDE3 and 4 inhibitor) and zaprinast (PDE5 inhibitor), were studied. Of these selective compounds, only rolipram, denbufylline and Org 30029 inhibited the IgE-dependent generation of IL-4, IL-13 and histamine from basophils to a statistically significant (P<0.05) degree.
The effects of isoform-selective inhibitors on basophils activated by IL-3 were evaluated. The IL-3-induced generation of IL-4, IL-13 and histamine was inhibited to a statistically significant (P<0.05) extent, only by compounds that act as inhibitors of PDE4.
These data suggest that inhibition of PDE4 can regulate the generation of cytokines from human basophils.
Keywords: Basophils, phosphodiesterases, interleukin-4, interleukin-13, theophylline, rolipram
Introduction
A role for the human basophil in the mediation of allergic disorders, such as asthma, has been suggested (Warner & Kroegel, 1994; Schroeder et al., 1995). Basophils, along with mast cells, express high-affinity IgE receptors and the IgE-mediated activation of basophils by allergen can lead to the generation of a variety of proinflammatory mediators such as histamine and cysteinyl-leukotrienes (Warner & Kroegel, 1994; Schroeder et al., 1995). More recently, it has also been established that basophils are a major source of interleukin-4 (IL-4) and IL-13 and that the generation of these cytokines can be induced by IgE-dependent and non-IgE-mediated mechanisms (Brunner et al., 1993; MacGlashan et al., 1994; Gibbs et al., 1996; Redrup et al., 1998; Shimizu et al., 1998; Ochensberger et al., 1999). Both IL-4 and IL-13 are known to have important roles in the mediation of allergic disorders not least by promoting and sustaining IgE production (Wills-Karp et al., 1998; Corry, 1999).
Among the most effective inhibitors of basophil function are agents that elevate cAMP (Bourne et al., 1972; 1974). Both receptor-mediated activators of adenylate cyclase and inhibitors of phosphodiesterase (PDE), the enzyme that degrades cyclic nucleotides, are known to be effective inhibitors of mediator release from basophils (Lichtenstein & Margolis, 1968).
More recent studies suggest that PDE exists as multiple molecular forms (Beavo et al., 1994; Soderling & Beavo, 2000; Conti et al., 2003). At least 11 PDE families have been identified differing in substrate specificity, requirements for activation and susceptibility to inhibition. Isoform-selective inhibitors are available and have been used widely to determine whether different PDEs are involved in regulating discrete processes (Beavo & Reifsnyder, 1990; Nicholson & Shahid, 1994). This approach has led to an appreciation that the major isoform of PDE regulating the activity of inflammatory cells is the cAMP-specific PDE, PDE4 (Teixera et al., 1997; Souness et al., 2000). Our own data in human basophils also suggest that PDE4 is important as PDE4-selective inhibitors, such as rolipram, prevent the IgE-mediated release of histamine and the generation of cysteinyl-leukotrienes from basophils (Peachell et al., 1992; Weston et al., 1997). Moreover, we have also reported that basophils contain a cAMP hydrolytic activity that is inhibited by rolipram but not by alternative PDE inhibitors that act at isoforms other than PDE4 (Weston et al., 1997). The aim of the present study was to determine whether PDE4 also regulates the generation of the cytokines, IL-4 and IL-13, from activated basophils.
Methods
Buffers
Phosphate-buffered saline (PBS) contained (mM): NaCl 137; Na2HPO4.12H2O 8; KCl 2.7; KH2PO4 1.5. PIPES buffer contained (mM): PIPES 22; NaCl 110; KCl, 5. The pH of both buffers was titrated to 7.3.
Preparation of inhibitors and stimuli
Theophylline and isobutyl-methylxanthine (IBMX) were prepared daily as stock solutions (2 mM) in buffer. Rolipram, denbufylline, 8-methoxymethyl-IBMX, zaprinast and Org 30029 (N-hydroxy-5,6-dimethoxy-benzo[b]thiophene-2-carboximidamide HCl) were all prepared as stock solutions (100 mM) in dimethyl sulphoxide (DMSO) and stored at 4°C. Siguazodan was prepared weekly as a stock solution (1 mM) in buffer and stored at 4°C. Dibutyryl-cAMP, dibutyryl-cGMP, 8-bromo-cAMP and 8-bromo-cGMP were prepared daily as stock solutions (10 mM) in buffer. The Sp isomer of 8-(4-chlorophenylthio)adenosine-3′, 5′-cyclic monophosphorothioate (Sp-8-CPT-cAMPS) and Sp isomer of 8-(4-chlorophenylthio)guanosine-3′, 5′-cyclic monophosphorothioate (Sp-8-CPT-cGMPS) were prepared as stock solutions (10 mM) in buffer and stored in appropriate aliquots at −20°C. IL-3 was prepared as a stock solution (100 μg ml−1) in distilled water and stored at −20°C. Neat polyclonal goat anti-human IgE antibody was made up in distilled water and stored at 4°C. All drugs and stimuli were diluted to the desired concentration in buffer just prior to use.
Basophil isolation
Basophil-enriched preparations were obtained by Percoll density gradient centrifugation. Briefly, whole venous blood was layered over a two-step discontinuous Percoll gradient consisting of 15 ml of 62% Percoll overlaid with 15 ml of 53% Percoll and centrifuged (250 × g, 15 min). A basophil-rich layer (5–15% purity), located 1 cm above the 53/62% interface, was harvested. The contaminant cells were lymphocytes and monocytes. These cells were washed three times in PIPES buffer and then used in experiments investigating the release of histamine, IL-4 and IL-13. On occasion, basophils were further purified (⩾90% purity) by immunomagnetic bead separations according to methods that have been described in detail elsewhere (MacGlashan et al., 1994; Schroeder et al., 2001). These basophils of high purity were used in some of the functional studies. Basophil purities were determined using an alcian blue stain (Gilbert & Ornstein, 1975).
Mediator release
The release of histamine, IL-4 and IL-13 was assessed from basophil-enriched preparations activated with either anti-human IgE or IL-3. The effects of PDE inhibitors on the generation of these mediators were also determined. Mediator release experiments were performed in RPMI 1640 buffer supplemented with BSA (1 mg ml−1), gentamicin (10 μg ml−1) and calcium chloride (made up to 1 mM). Basophils (80,000–300,000 basophils per sample) were incubated (15 or 30 min) with an inhibitor or buffer before challenge with a stimulus, typically, in a total reaction volume of 220 μl. Cells incubated in buffer alone served as measures of spontaneous mediator release, and all values cited for stimulus-induced mediator generation were corrected by subtracting this spontaneous mediator release.
In experiments monitoring IL-4 generation, basophils were activated for 4 h with an optimal releasing concentration of anti-IgE (1 : 100,000 or 1 : 10,000). In experiments monitoring IL-13 generation, basophils were activated for longer (20 or 24 h). These conditions for optimal generation of IL-4 and IL-13 generation have been reported by others (MacGlashan et al., 1994; Gibbs et al., 1996; Redrup et al., 1998) and were confirmed by us in a series of preliminary experiments. In some experiments, cells were activated with IL-3 (100 ng ml−1) for 24 h for the generation of mediators in the presence and absence of PDE inhibitors.
After activation, the cells were centrifuged (450 × g, 4 min) and the supernatants saved and analysed for mediator release. For the analysis of histamine content, an aliquot (50 μl) of the supernatant, diluted in PBS buffer (950 μl), was analysed using a modification (Ennis, 1991) of the automated fluorometric technique of Siraganian (1974). The remainder of the supernatant was assayed for IL-4 and IL-13 content by enzyme-linked immunosorbent assay (ELISA). The limit of sensitivity was 0.2 and 0.5 pg ml−1 for the IL-4 and IL-13 assays, respectively. The OD of samples was measured at 450 nM using a Dynatech plate reader.
Materials
The following were purchased from the sources indicated; 8-bromo-cAMP, 8-bromo-cGMP, DMSO, goat anti-human IgE, PIPES (free acid), Percoll, BSA, IBMX, theophylline, rolipram, zaprinast and siguazodan (Sigma, Poole, U.K.); gentamicin and RPMI 1640 (Gibco BRL, Dundee, U.K.); 8-methoxymethyl-IBMX (LC Laboratories, Woburn, MA, U.S.A.); IL-3 (Peprotech, Rocky Hill, NJ, U.S.A.); Sp-8-CPT-cAMPS and Sp-8-CPT-cGMPS (Biolog Life Science Institute, Bremen, Germany); ELISA kits for human IL-4 and IL-13 (Mast Diagnostics, Amsterdam, Netherlands).
The following compounds were gifts: denbufylline (SKB, U.K.); Org 30029 (Organon, U.K.).
Data analysis
Data are expressed as means±s.e.m. EC50 values were determined using GraphPad Prism software (version 2). In order to establish whether drug treatments caused statistically significant effects, either paired t-tests or ANOVA, followed by Dunnett's test, was performed. In all instances, the raw data were subjected to statistical analyses.
Results
Effects of nonselective PDE inhibitors on cytokine generation
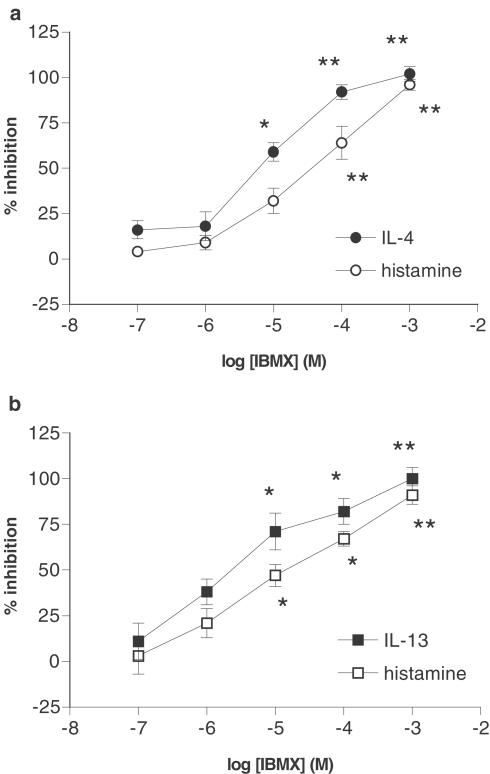
The effects of the nonselective PDE inhibitor, IBMX, on the generation of histamine and IL-4 from basophils activated with anti-IgE for 4 h were determined (Figure 1a). IBMX inhibited the generation of histamine and IL-4 concentration-dependently although IBMX was more potent as an inhibitor of IL-4 (log EC50 for IL-4 inhibition; −5.1±0.1) generation than histamine release (log EC50 for inhibition of histamine release; −4.4±0.2). The effects of IBMX on the generation of histamine and IL-13, following activation of basophils with anti-IgE for 24 h, were determined (Figure 1b). IBMX was also an effective inhibitor of IgE-mediated IL-13 generation (log EC50; −5.6±0.3) and histamine release (log EC50; −5.0±0.3).
Figure 1.
Effect of IBMX on mediator generation. The effects of IBMX on the generation of (a) IL-4 and histamine and (b) IL-13 and histamine from human basophils were determined. Cells were incubated for 15 min with IBMX before challenge with anti-IgE (1 : 100,000 or 1 : 10,000) for (a) 4 h or (b) 24 h. Values are expressed as the % inhibition of the control releases, which were for (a), 73±31 pg of IL-4 per 106 basophils and 42±6% histamine release and for (b), 8±2 pg of IL-13 per 106 basophils and 21±6% histamine release. Values are means±s.e.m., n=5 for both (a) and (b). Asterisks denote; *P<0.05, **P<0.01.
Effects of selective PDE inhibitors on cytokine generation
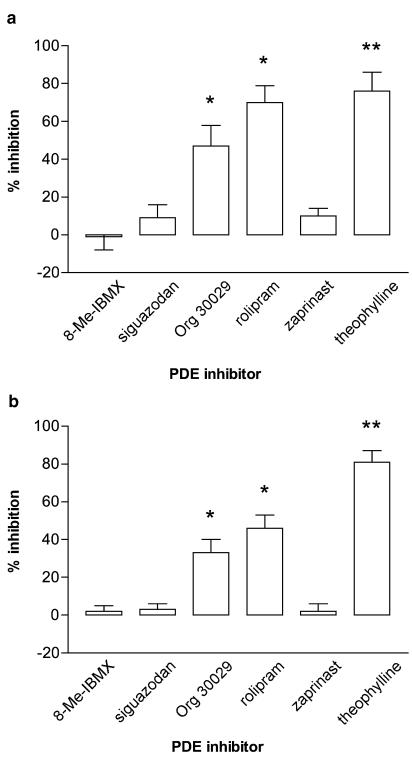
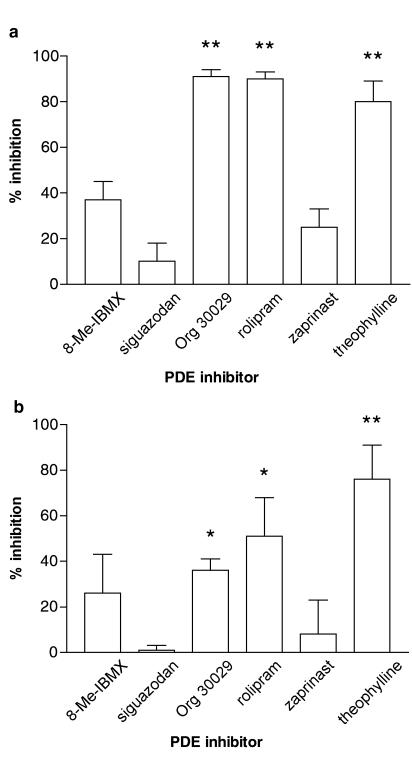
The effects of selective PDE inhibitors on mediator generation were also determined. Basophils were incubated (15 min) with either 8-methoxymethyl-IBMX (PDE1-selective), siguazodan (PDE3-selective), Org 30029 (mixed PDE3/4 inhibitor), rolipram (PDE4-selective), zaprinast (PDE5-selective) or theophylline (nonselective) before challenge with anti-IgE for 4 h, for histamine and IL-4 generation (Figure 2), or 20 h, for histamine and IL-13 generation (Figure 3). The data show that only those PDE inhibitors with activity at PDE4 (rolipram, Org 30029) inhibited histamine release and the generation of both cytokines to a statistically significant extent (P<0.05 at least).
Figure 2.
Effect of selective PDE inhibitors on (a) IL-4 generation and (b) histamine release from basophils. Cells were incubated for 15 min with the inhibitors, 8-methoxymethyl-IBMX (8-Me-IBMX; PDE1 inhibitor), siguazodan (PDE3 inhibitor), Org 30029 (mixed PDE3/4 inhibitor), rolipram (PDE4 inhibitor) zaprinast (PDE5 inhibitor) or theophylline (nonselective) before challenge with anti-IgE (1 : 100,000 or 1 : 10,000) for 4 h. All inhibitors were used at 10 μM except theophylline which was used at 1 mM. Values are expressed as the % inhibition of the control releases, which were for (a), 53±16 pg of IL-4 per 106 basophils and for (b), 22±5% histamine release. Values are means±s.e.m., n=7. Asterisks denote; *P<0.05, **P<0.01.
Figure 3.
Effect of selective PDE inhibitors on (a) IL-13 generation and (b) histamine release from basophils. Cells were incubated for 15 min with the inhibitors, 8-methoxymethyl-IBMX (8-Me-IBMX; PDE1 inhibitor), siguazodan (PDE3 inhibitor), Org 30029 (mixed PDE3/4 inhibitor), rolipram (PDE4 inhibitor) zaprinast (PDE5 inhibitor) or theophylline (nonselective) before challenge with anti-IgE (1 : 100,000 or 1 : 10,000) for 20 h. All inhibitors were used at 10 μM except theophylline which was used at 1 mM. Values are expressed as the % inhibition of the control releases, which were for (a), 5±1 pg of IL-13 per 106 basophils and for (b), 19±6% histamine release. Values are means±s.e.m., n=4. Asterisks denote; *P<0.05, **P<0.01.
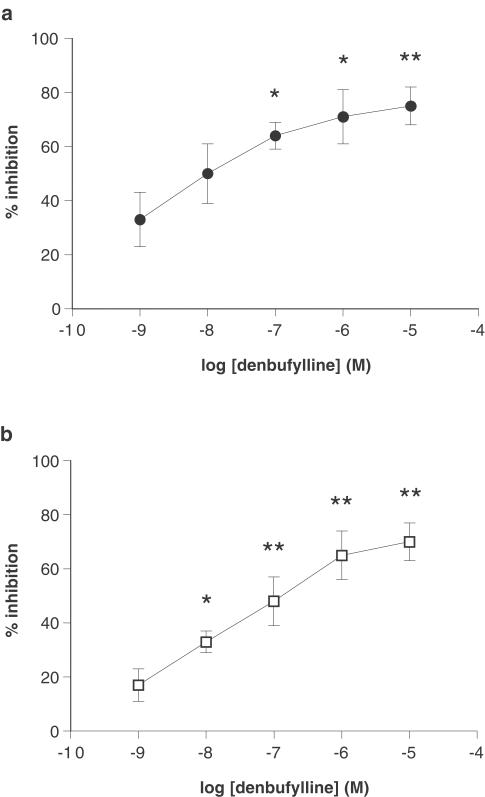
In order to confirm whether inhibition of PDE4 affects the generation of cytokines, the effect of an alternative PDE4-selective inhibitor, denbufylline, was assessed (Figure 4). Denbufylline attenuated the generation of IL-4 (log EC50; −8.4±0.2) and IL-13 (log EC50; −7.0±0.5) in a concentration-dependent manner.
Figure 4.
Effect of denbufylline on cytokine generation from basophils. Cells were incubated for 15 min with denbufylline before challenge with anti-IgE (1 : 100,000 or 1 : 10,000) for 4 h for IL-4 generation (a) or 24 h for IL-13 generation (b). Values are expressed as the % inhibition of the control releases, which were for (a), 73±31 pg of IL-4 per 106 basophils and for (b), 8±2 pg of IL-13 per 106 basophils. Data for both IL-4 and IL-13 were generated from the same set of donors but on different days. Values are means±s.e.m., n=5. Asterisks denote; *P<0.05, **P<0.01.
Effects of IL-3 on the generation of mediators from basophils
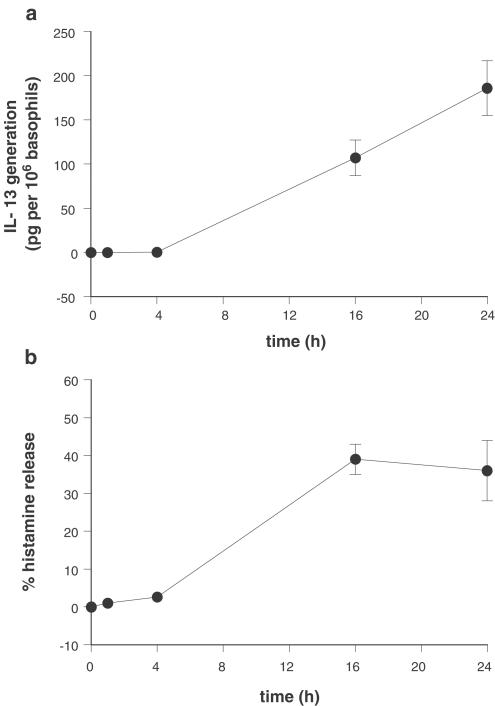
Previous studies have shown that stimulation of basophils with IL-3 induces the generation of IL-13 and modest levels of IL-4 (Redrup et al., 1998; Ochensberger et al., 1999). Reports suggest that IL-3 weakly induces histamine release from basophils after 4 h of activation (Redrup et al., 1998). However, preliminary experiments of our own in nine donors (three atopics) indicated that activation of basophils for 24 h with IL-3 (100 ng ml−1) caused not only IL-13 generation in eight out of nine donors (range, 49–462 pg of IL-13 per 106 basophils; mean±s.e.m., 194±43 pg of IL-13 per 106 basophils; n=8) but also induced histamine release in eight out of nine donors (range, 16–56%; mean±s.e.m., 31±5%; n=8) and some modest levels of IL-4 generation in six out of nine donors (5–27 pg of IL-4 per 106 basophils; mean±s.e.m., 14±3 pg of IL-4 per 106 basophils, n=6). Further studies indicated that both the release of histamine and the generation of IL-13 were time dependent (Figure 5). Significant (P<0.05) levels of mediator generation were observed after ⩾16 h of activation with IL-3.
Figure 5.
Kinetics of IL-3-induced mediator generation from basophils. Basophils were challenged with IL-3 (100 ng ml−1) for time periods up to 24 h and the generation of (a) IL-13 and (b) histamine were monitored. Values are means±s.e.m., n=4. IL-4 generation was also monitored in these experiments but only one donor generated IL-4 to an appreciable degree.
Since basophil-enriched (5–15% basophils) preparations have been used in these studies, the possibility that IL-3 may stimulate leucocytes, other than or in addition to basophils, to secrete cytokines cannot be excluded. This contrasts with the situation for the stimulus, anti-IgE, which is likely to activate basophils relatively exclusively owing to the selective expression of IgE by basophils but not other leucocytes (MacGlashan et al., 1994; Gibbs et al., 1996). In order to determine the specificity of IL-3, basophils of high purity (⩾90%) were challenged with IL-3 and cytokine generation was evaluated (Table 1). The data indicate that basophils are likely to be the main source of IL-4 and IL-13 when a mixed leucocyte preparation is challenged with IL-3.
Table 1.
IL-3-induced generation of IL-13 and IL-4 from purified basophils
| pg per 106 basophils | ||
|---|---|---|
| Basophil purity (%) | IL-13 | IL-4 |
| 97±3 | 40±16 | 28±14 |
| 9±3* | 57±18 | 23±6 |
Basophils were purified by immunomagnetic bead separations and then challenged with IL-3 (100 ng ml−1) for 24 h, after which, supernatants were harvested and evaluated for cytokine generation. In parallel, the residual mononuclear cell preparations, containing basophils*that had not been captured by the purification step, were also challenged with IL-3. Values are means±s.e.m., n=3.
Effects of PDE inhibitors on mediator generation induced by IL-3
The effects of nonselective and selective PDE inhibitors on IL-3-induced mediator generation were also determined (Table 2). Theophylline was an effective inhibitor of the IL-3-mediated generation of histamine, IL-4 and IL-13. Of the isoform-selective PDE inhibitors, only compounds with activity at PDE4 (rolipram, Org 30029) inhibited the IL-3-dependent generation of mediators to a statistically significant (P<0.05) degree.
Table 2.
Effect of PDE inhibitors on the generation of histamine, IL-13 and IL-4 from basophils activated with IL-3
| % inhibition | |||
|---|---|---|---|
| PDE inhibitor | Histamine | IL-13 | IL-4 |
| 8-Me-IBMX | 31±7 | 16±8 | 9±15 |
| siguazodan | 27±7 | −2±7 | −1±2 |
| Org 30029 | 52±11* | 32±6** | 64±7* |
| rolipram | 66±12** | 56±5** | 81±3** |
| zaprinast | 14±7 | 10±4 | 34±11 |
| theophylline | 94±4** | 65±6** | 92±4** |
Basophils were incubated for 15 min with the inhibitors, 8-methoxymethyl-IBMX (8-Me-IBMX; PDE1 inhibitor), siguazodan (PDE3 inhibitor), Org 30029 (mixed PDE3/4 inhibitor), rolipram (PDE4 inhibitor), zaprinast (PDE5 inhibitor) or theophylline (nonselective) before challenge with IL-3 (100 ng ml−1) for 24 h. All inhibitors were used at 10 μM except theophylline, which was used at 1 mM. Values are expressed as the % inhibition of the control releases, which were 28±6% histamine release, 237±48 pg of IL-13 per 106 basophils, 24±8 pg of IL-4 per 106 basophils. Although IL-3 induced histamine release in all seven experiments, in one experiment no IL-13 generation was detected and in three experiments no IL-4 generation was obtained. Thus, values are means±s.e.m. from seven (histamine), six (IL-13) and four (IL-4) experiments. Asterisks denote
P<0.05
P<0.01.
In order to confirm that inhibition of PDE4 affects the IL-3-dependent generation of cytokines, the effect of an alternative PDE4-selective inhibitor, denbufylline, was assessed. Basophils were incubated (15 min) with or without denbufylline (10 μM) before challenge with IL-3 (100 ng ml−1). After 24 h, supernatants were harvested and the effects of denbufylline, on the generation of cytokines, were determined. Denbufylline inhibited, to a statistically significant (P<0.05) degree, the elaboration of IL-13 and IL-4 by 81±3 and 87±2%, respectively (n=4).
Effects of cyclic nucleotide analogues on the generation of mediators
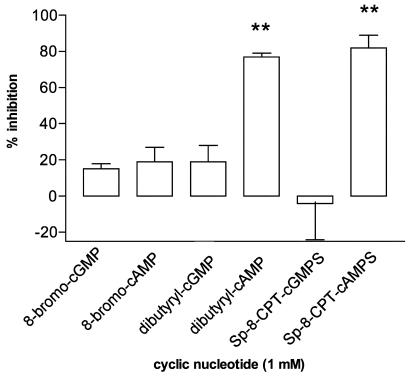
Our experiments indicate that inhibitors targeting the cAMP-specific PDE, PDE4, are effective at attenuating the generation of mediators from basophils. This suggests an inhibitory mechanism mediated by cAMP. To assess this, the effects of several analogues (1 mM) of cAMP were investigated in basophils along with analogues of cGMP for comparative purposes. In initial experiments, it was established that both dibutyryl-cAMP and Sp-8-CPT-cAMPS were effective inhibitors of IgE-mediated histamine release from basophils, whereas 8-bromo-cAMP was relatively ineffective (Figure 6). None of the corresponding cGMP analogues, dibutyryl-cGMP, 8-bromo-cGMP or Sp-8-CPT-cGMPS was an effective inhibitor of IgE-mediated histamine release. In further studies, the effects of Sp-8-CPT-cAMPS (1 mM) on the generation of IL-4, IL-13 and histamine from basophils, induced by both anti-IgE and IL-3, were determined. Sp-8-CPT-cAMPS was a very effective inhibitor of cytokine generation and histamine release from basophils induced by either stimulus (Table 3).
Figure 6.
Effects of cyclic nucleotides on histamine release from basophils. Cells were incubated without or with analogues of cAMP or cGMP for 30 min before challenge with anti-IgE (1 : 100,000 or 1 : 10,000) for a further 45 min. Values are expressed as the % inhibition of the control histamine release, which was 34±7%. Values are means±s.e.m., n=4. Asterisks denote; **P<0.01.
Table 3.
Effects of Sp-8-CPT-cAMPS on cytokine generation and histamine release from basophils
| % inhibition | |||
|---|---|---|---|
| Stimulus | Histamine | IL-13 | IL-4 |
| Anti-IgE | 64±5 | 80±7 | 81±7 |
| IL-3 | 98±2 | 87±7 | 76±7 |
Cells were incubated for 30 min without or with Sp-8-CPT-cAMPS (1 mM) and then challenged with either anti-IgE (1 : 100,000) or IL-3 (100 ng ml−1) for 24 h. After this time, supernatants were collected and assayed for histamine, IL-4 and IL-13 content. Values are expressed as % inhibition of the control releases, which were for anti-IgE, 20±3% histamine release, 34±7 pg IL-13 per 106 basophils, 17±2 pg IL-4 per 106 basophils and for IL-3, 15±4% histamine release, 856±307 pg IL-13 per 106 basophils, 36±12 pg IL-4 per 106 basophils. Sp-8-CPT-cAMPS inhibited the generation of all mediators, induced by both stimuli, to a statistically significant (P<0.05 at least) extent. Values are means±s.e.m., n=4–6.
Discussion
Recent studies show that PDE exists as multiple molecular forms (Beavo et al., 1994; Soderling & Beavo, 2000; Conti et al., 2003). Moreover, the tissue distribution of these isoforms varies quite widely. In the context of inflammation, it has been established that most inflammatory cells are regulated by the cAMP-specific PDE, PDE4 (Souness et al., 2000). As a consequence, it has been proposed that inhibitors of PDE4 may be useful anti-inflammatory agents (Teixera et al., 1997; Souness et al., 2000).
The basophil is thought to be involved in allergic-type diseases (Warner & Kroegel, 1994; Schroeder et al., 1995). It follows that stabilising the activity of this cell, by preventing the generation of mediators that promote inflammation, could be therapeutically useful. It has long been recognised that nonselective PDE inhibitors, such as the methylxanthines, are effective inhibitors of histamine release from basophils (Lichtenstein & Margolis, 1968). Later studies also demonstrated that compounds, such as theophylline, are effective at preventing the de novo generation of leukotrienes following basophil activation (Peachell et al., 1988). More recent studies have shown that the basophil is an important source of IL-4 and IL-13 (Brunner et al., 1993; MacGlashan et al., 1994; Gibbs et al., 1996; Redrup et al., 1998; Shimizu et al., 1998; Ochensberger et al., 1999). The present study has shown that theophylline and IBMX are effective inhibitors of the stimulated generation of IL-4 and IL-13 from basophils, confirming observations made by others (Shichijo et al., 1997; Gibbs et al., 1998). These data suggest that inhibition of PDE prevents not only the generation of histamine and leukotrienes from basophils but also cytokine generation as well.
Further studies were performed to determine the isoform of PDE that regulates cytokine generation from human basophils by employing isoform-selective inhibitors. Of these compounds, only those drugs acting at PDE4, namely, rolipram, denbufylline and Org 30029, inhibited the IgE-mediated generation of IL-4 and IL-13. These data indicate that the major isoform of PDE that regulates the IgE-triggered generation of cytokines from basophils is PDE4 and that this isoform also regulates the generation of histamine and leukotrienes (Weston et al., 1997) from basophils. Interestingly, cytokine generation induced by IL-3, a mechanistically discrete activator of basophils (Redrup et al., 1998), was also attenuated by PDE4-selective inhibitors and not by inhibitors selective for other isoforms. These data suggest that PDE4 can regulate the IgE- and non-IgE-dependent generation of a wide spectrum of proinflammatory mediators from the basophil.
Although the present study strongly suggests that PDE4 is important in regulating basophil activity, the possibility that other isoforms might be involved cannot be excluded as selective inhibitors to PDEs 1, 3, 4 and 5 alone have been used in this study due to the unavailability of inhibitors that act selectively at alternative isoforms. However, it is interesting to note that maximal inhibition of the generation of IL-4 and IL-13 observed with rolipram is very similar to that seen with theophylline irrespective of the stimulus employed (anti-IgE or IL-3) to stimulate cytokine generation. This contrasts with the situation when comparing the effects of theophylline and rolipram as inhibitors of histamine release induced by either stimulus where theophylline is a more effective inhibitor than rolipram. These data could suggest that the main, if not only, isoform of PDE-regulating cytokine generation is PDE4 but that for the regulation of histamine release, theophylline may act not only at PDE4 but some other isoform of PDE or may have actions unrelated to PDE inhibition.
Since PDE4 is a cAMP-specific PDE, inhibition of PDE4 would be expected to elevate cAMP intracellularly and, by activating cAMP-dependent protein kinase (PKA), cellular activity would be modulated. To investigate the role of cAMP further, we studied the effects of a number of analogues of cAMP and, in a comparative context, analogues of cGMP also on the IgE-mediated release of histamine from basophils. None of the cGMP analogues studied had any effect on the release of histamine arguing against a role for cGMP, and cGMP-specific PDEs, in the regulation of basophil activity. By contrast, dibutyryl-cAMP was an effective inhibitor of histamine release from basophils. However, concerns have been raised that the effects of this analogue may not be due to activation of PKA but rather because of the intracellular conversion of the molecule to butyrate (Schwede et al., 2000). In these same experiments, 8-bromo-cAMP was also studied but this analogue was an ineffective inhibitor of histamine release from basophils questioning the specificity of the activity of dibutyryl-cAMP. However, 8-bromo-cAMP, although recognised as a superior probe to dibutyryl-cAMP in terms of target specificity and resistance to hydrolysis, is less cell-permeant than dibutyryl-cAMP such that lack of activity with 8-bromo-cAMP could be due to cell exclusion (Schwede et al., 2000). This reasoning is supported by studies with an alternative highly lipophilic and nonhyrolysable analogue, Sp-8-CPT-cAMPS (Schwede et al., 2000; Spicuzza et al., 2001), which was an effective inhibitor of both histamine release and the generation of cytokines. Overall, these findings support a role for the cAMP-PKA pathway in the modulation of basophil activity.
An interesting element of the present study is the recognition that IL-3 can induce substantial levels of histamine release, and that the kinetics of this release are very slow requiring in excess of 4 h. This contrasts with IgE-mediated histamine release from basophils that is complete after about 30–40 min following challenge. This suggests that the mechanisms by which anti-IgE and IL-3 induce histamine release from basophils differ. These findings indicate that IL-3, alone, may be very capable of inducing the release of mediators from basophils, not just cytokines but histamine too, as well as priming the cells for activation by both IgE- and non-IgE-dependent mechanisms (Redrup et al., 1998; Ochensberger et al., 1999). These properties of IL-3 may be quite important at allergic loci where basophils may be present and the concentration of IL-3 may be elevated (Brunner et al., 1993).
To conclude, the present study has shown that inhibition of PDE4 attenuates the stimulated release of cytokines from human basophils. This suggests that anti-inflammatory drugs, targeting PDE4, are likely to be effective stabilisers of the activity of basophils.
Acknowledgments
We thank Dr Ian Sabroe for helpful comments and suggestions. This work was supported, in part, by Asthma UK.
Abbreviations
- DMSO
dimethyl sulphoxide
- IBMX
isobutyl-methylxanthine
- IL
interleukin
- PBS
phosphate-buffered saline
- PDE
phosphodiesterase
- Sp-8-CPT-cAMPS
Sp isomer of 8-(4-chlorophenylthio)adenosine-3′, 5′-cyclic monophosphorothioate
- Sp-8-CPT-cGMPS
Sp isomer of 8-(4-chlorophenylthio)guanosine-3′, 5′-cyclic monophosphorothioate
References
- BEAVO J.A., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- BEAVO J.A., REIFSNYDER D.H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- BOURNE H.R., LICHTENSTEIN L.M., MELMON K.L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3′5′-adenosine monophosphate in human leukocytes. J. Immunol. 1972;108:695–705. [PubMed] [Google Scholar]
- BOURNE H.R., LICHTENSTEIN L.M., MELMON K.L., HENNEY C.S., WEINSTEIN Y., SHEARER G.M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184:9–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- BRUNNER T., HEUSSER C.H., DAHINDEN C.A. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J. Exp. Med. 1993;177:605–611. doi: 10.1084/jem.177.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI M., RICHTER W., MEHATS C., LIVERA G., PARK J.-Y., JIN C. Cyclic AMP-specific phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- CORRY D.B. IL-13 in allergy: home at last. Curr. Opin. Immunol. 1999;11:610–614. doi: 10.1016/s0952-7915(99)00025-4. [DOI] [PubMed] [Google Scholar]
- ENNIS M. Current techniques of histamine determination. Automated fluorometric assays. Handbook Exp. Pharmacol. 1991;97:31–38. [Google Scholar]
- GIBBS B.F., HAAS H., FALCONE F.H., ALBRECHT C., VOLLRATH I.B., NOLL T., WOLFF H.H., AMON U. Purified human blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur. J. Immunol. 1996;26:2493–2498. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- GIBBS B.F., VOLLRATH I.B., ALBRECHT C., AMON U., WOLFF H.H. Inhibition of interleukin-4 and interleukin-13 release from immunologically activated human basophils due to the actions of anti-allergic drugs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:573–578. doi: 10.1007/pl00005210. [DOI] [PubMed] [Google Scholar]
- GILBERT H.S., ORNSTEIN L. Basophil counting with a new staining method using Alcian Blue. Blood. 1975;46:279–282. [PubMed] [Google Scholar]
- LICHTENSTEIN L.M., MARGOLIS S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968;161:902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- MACGLASHAN D.W., WHITE J.M., HUANG, S-K, ONO S.J., SCHROEDER J.T., LICHTENSTEIN L.M. Secretion of IL-4 from human basophils: the relationship between IL-4 mRNA and protein in resting and stimulated basophils. J. Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- NICHOLSON C.D., SHAHID M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes – their potential utility in the therapy of asthma. Pulmon. Pharmacol. 1994;7:1–18. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- OCHENSBERGER B., TASSERA L., BIFRARE D., RIHS S., DAHINDEN C.A. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur. J. Immunol. 1999;29:11–22. doi: 10.1002/(SICI)1521-4141(199901)29:01<11::AID-IMMU11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- PEACHELL P.T., MACGLASHAN D.W., LICHTENSTEIN L.M., SCHLEIMER R.P. Regulation of human basophil and lung mast cell function by cAMP. J. Immunol. 1988;140:571–579. [PubMed] [Google Scholar]
- PEACHELL P.T., UNDEM B.J., SCHLEIMER R.P., MACGLASHAN D.W., LICHTENSTEIN L.M., CIESLINSKI L.B., TORPHY T.J. Preliminary identification and role of phosphodiesterase isozymes in human basophils. J. Immunol. 1992;148:2503–2510. [PubMed] [Google Scholar]
- REDRUP A.C., HOWARD B.P., MACGLASHAN D.W., KAGEY-SOBOTKA A., LICTHTENSTEIN L.M., SCHROEDER J.T. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J. Immunol. 1998;160:1957–1964. [PubMed] [Google Scholar]
- SCHROEDER J.T., KAGEY-SOBOTKA A., LICHTENSTEIN L.M. The role of the basophil in allergic inflammation. Allergy. 1995;50:463–472. doi: 10.1111/j.1398-9995.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- SCHROEDER J.T., SCHLEIMER R.P., LICHTENSTEIN L.M., KREUTNER W. Inhibition of cytokine generation and mediator release by human basophils treated with desloratadine. Clin. Exp. Allergy. 2001;31:1369–1377. doi: 10.1046/j.1365-2222.2001.01130.x. [DOI] [PubMed] [Google Scholar]
- SCHWEDE F., MARONDE E., GENIESER H.-G., JASTORFF B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol. Ther. 2000;87:199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- SHICHIJO M., SHIMIZU Y., HIRAMATSU K., INAGAKI N., TAGAKI K., NAGAI H. Cyclic AMP-elevating agents inhibit mite-antigen-induced IL-4 and IL-13 release from basophil-enriched leukocyte preparation. Int. Arch. Allergy Immunol. 1997;114:348–353. doi: 10.1159/000237693. [DOI] [PubMed] [Google Scholar]
- SHIMIZU Y., SHICHIJO M., HIRAMATSU K., TAKEUCHI M., NAGAI H., TAGAKI K. Mite-antigen-induced IL-4 and IL-13 production by basophils derived from atopic asthma patients. Clin. Exp. Allergy. 1998;28:497–503. doi: 10.1046/j.1365-2222.1998.00267.x. [DOI] [PubMed] [Google Scholar]
- SIRAGANIAN R.P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal. Biochem. 1974;57:283–287. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., ALDOUS D., SARGENT C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. doi: 10.1016/s0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- SPICUZZA L., BELVISI M.G., BIRRELL M.A., BARNES P.J., HELE D.J., GIEMBYCZ M.A. Evidence that the anti-spasmogenic effect of the β-adrenoceptor agonist, isoprenaline, on guinea pig trachealis is not mediated by cyclic AMP-dependent protein kinase. Br. J. Pharmacol. 2001;133:1201–1212. doi: 10.1038/sj.bjp.0704213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXERA M.M., GRISTWOOD R.W., COOPER N., HELLEWELL P.G. Phosphodiesterase (PDE) 4 inhibitors: anti-inflammatory drugs of the future. Trends Pharmacol. Sci. 1997;18:164–171. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- WARNER J.A., KROEGEL C. Pulmonary immune cells in health and disease: mast cells and basophils. Eur. Respir. J. 1994;7:1326–1341. doi: 10.1183/09031936.94.07071326. [DOI] [PubMed] [Google Scholar]
- WESTON M.C., ANDERSON N., PEACHELL P.T. Effects of phosphodiesterase inhibitors on human lung mast cell and basophil function. Br. J. Pharmacol. 1997;121:287–295. doi: 10.1038/sj.bjp.0701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS-KARP M., LUYIMBAZI J., XU X., SCHOFIELD B., NEBEN T.Y., KARP C.L., DONALDSON D.D. Interleukin 13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]