Abstract
Change of osmolality surrounding spawned sperm from isotonic to hypotonic causes the initiation of sperm motility in the common carp. Here we show that membrane-permeable cAMP does not initiate motility of carp sperm that is quiescent in isotonic solution, and that motility of the demembranated sperm can be reactivated without cAMP. Furthermore, the cAMP level does not change during the initiation of sperm motility, and inhibitors of protein kinase do not affect sperm motility, suggesting that no cAMP-dependent system is necessary for the regulation of sperm motility. Sperm motility could not be initiated in Ca2+-free hypoosmotic solutions, and significant increase in the intracellular Ca2+ level was observed by a Ca-sensitive fluorescence dye during hypoosmolality-induced active motion period. The demembranated sperm cells were fully reactivated in the solutions containing 10−7 to 10−5 M Ca2+. Ca2+ channel blockers such as verapamil and ω-conotoxin reversibly inhibited the initiation of sperm motility, suggesting that Ca2+ influx is the prerequisite for the initiation of carp sperm motility. Motility of intact sperm was completely blocked; however, that of the demembranated sperm was not inhibited by the calmodulin inhibitor W7, suggesting that the calmodulin bound close to the plasma membrane participated in the initiation of sperm motility. Flow cytometric membrane potential measurements and spectrophotometric measurements by using fluorescence dyes showed transient membrane hyperpolarization on hypoosmolality-induced motility. This article discusses the role of membrane hyperpolarization on removal of inactivation of Ca2+ channels, leading to Ca2+ influx at the initiation of carp sperm motility.
It is well known that changes in the osmotic pressure around cells trigger the signal transduction systems regulating cell volumes to maintain the homeostasis of cells (1). Moreover, Morisawa and Suzuki have found another unique osmolality-dependent regulation of cell function in sperm cells (2), although the harmful effect of hyper- and hypoosmolality has long been known (1, 3). Spermatozoa that are quiescent in electrolyte or nonelectrolyte solutions isotonic to the seminal plasma become motile when the sperm are diluted with hypotonic solution in freshwater teleosts (2, 4), including the common carp (4–5). These findings suggest that environmental osmotic changes around sperm at spawning are the factors triggering the initiation of sperm motility.
Stimulation by environmental osmolality has to cross the plasma membrane to confer motility to sperm. Márián et al. have demonstrated that the environmental osmotic change modifies the membrane structure of the common carp (6). Other studies on flagellar movement also show rapid morphological changes at the initiation of sperm motility in the carp (7). Krasznai et al. have shown that hypoosmotic shock changes the membrane potential through the opening of the voltage-gated potassium channels (8) and, as a consequence, the intracellular ion concentration will also change in the carp as shown in another freshwater fish, zebrafish (9). It is also suggested that the activation of sperm motility is followed by the alkalization of the intracellular milieu in the carp (10) and zebrafish (9). However, it has been reported that the second messengers, cAMP and Ca2+ (11), are not necessary for the hypoosmolality-induced initiation of sperm motility in the common carp. Phosphodiesterase inhibitors—of which the treatment causes increase in intracellular cAMP—do not initiate motility of carp sperm that is quiescent in isotonic solution (12), and motility of the demembranated sperm can be reactivated without cAMP (5, 11, 12). No inhibitory effect of Ca2+ fluxes at the plasma membrane has been observed with native sperm, and demembranated sperm have had no effect on Ca2+ concentration in the reactivating medium (11). Although intensive research studies are going on studying the role of cAMP and Ca2+ in the mechanism of the initiation, activation, and chemotaxis of sperm motility in many animal species, such as sea urchins (13), tunicates (14), salmonid fish (15, 16), and mammals (17–19), the unique transmembrane signaling underlying the osmotic pressure-induced initiation of sperm motility in fresh water is still ignored. In the present study, we show that Ca2+ influx plays an important role in the initiation of carp sperm motility.
Materials and Methods
Solutions and Animals.
Calcium green1-AM, bis-(1,3-dibutylbarbituric acid)trimethine oxonol (oxonol) was purchased from Molecular Probes. 3,3′-dipropylthiadicarbocyanine iodide (DiSC3) (5), carbonyl cyanide m-chlorophenylhydrazone (CCCP), and valinomycin, A 23187 were from Sigma; DTT and Cremophor EL were from Nacalai Tesque (Kyoto); verapamil, methoxyverapamil, flunarizine, N2,2–0-dibutyrilguanosine 3:5 cyclic monophosphate sodium salt, N1,2–0-D dibutyriladenosine 3:5 cyclic monophosphate sodium salt, and ionomycin were purchased from Sigma or Research Biochemicals (Natick, MA). Other calcium channel blockers and intracellular Ca2+ mobilizers—cyclopiazonic acid and thapsigargin—were obtained from Alomone Labs (Jerusalem). W-7, W-5, H-7, H-8, and H-89 were purchased from Seikagaku Kogyo (Tokyo). All other reagents, including 4-aminopyridine (4-AP), were from Wako Biochemicals (Osaka). W-7, W-5, and calcium green were dissolved in DMSO.
Fish Physiological Solution (FPS) consisted of 140 mM NaCl, 10 mM KCl, 1 mM CaCl2, and 20 mM Hepes, pH 8.5. Calcium-free FPS (NoCaFPS) contained 140 mM NaCl, 10 mM KCl, and 5 mM EGTA, 20 mM Hepes, pH 8.5, and activating solution (AS) 25 mM KCl, 25 mM NaCl, 1 mM CaCl2, 10 mM Hepes, pH 8.5. The calcium-free activating solution (NoCaAS) consisted of 25 mM KCl, 25 mM NaCl, and 5 mM EGTA. Male common carp (Cyprinus carpio) were purchased from a commercial source and kept in an indoor aquarium at 25°C. The photoperiod was adjusted to intervals of 14 hr light and 10 hr dark. Spermiation was induced by intraperitoneal injection of 1 mg/kg body weight of acetone-dried pituitary gland dissolved in FPS. From 10 to 20 hr after the injection, the milt was collected by gently pressing the abdomen. Care was taken to avoid contamination of the milt with water or urine.
cAMP Measurements.
The cAMP enzyme-immunoassay system (dual range BIOTRAK) and the cAMP enzyme-immunoassay kit of Amersham (Buckinghamshire, England) were used. Semen was diluted 2,000-fold (5 × 106 cell/ml) in FPS or AS. After the necessary incubation time, each 100 μl of the suspension was mixed with 10 μl of 1 M HCl to stop cAMP synthesis and incubated at 90°C for 1 min to lyse the cells. After centrifuging at 13,000 × g for 1 min, each 100 μl of supernatant was put into the wells contained in the kit to quantify cAMP as described in the manual. The cAMP level of each sample was calculated by measuring 450 nm absorbance with a microplate reader (Model 550, Bio-Rad).
Sperm Motility Measurements.
The semen was suspended in 2,000× of the experimental media with appropriate compounds, and images of track of the sperm were taken through a high-sensitivity video camera [Hamamatsu (Ichinocho, Japan) 2400–07] mounted on a phase contrast microscope (Nicon-Optiphot) with an inverted contrast objective lens (Olympus Splan NH). The percentage of motile sperm and their velocity were analyzed by using an automated semen analyzer, cellsoft (CRYO Resources) from the image of sperm taken within 30 sec after suspending the sperm in the experimental media. In this period, sperm showed almost straightforward motility (11), their velocity being around 210 μm/sec. Velocity was calculated by summing up the distance between every successive frame of the sperm trajectory and dividing the sum by the time interval during which the sperm was tracked. Four frames were used for measuring velocity. To remove the plasma membrane of the spermatozoa, 10 μl of semen was added to 0.2 ml of the extracting solution containing 150 mM KCl, 2 mM MgCl2, 0.5 mM EDTA, 5 mM Tris⋅HCl, 0.5 mM DTT, and 0.04% (wt/vol) Triton X-100, pH 8.0. Gentle stirring was carried out for 30 sec on ice, then 20 μl of the demembranated sperm suspension was mixed with 0.5 ml of the reactivating solution containing 0.15 M KCl, 20 mM Tris⋅HCl, 0.5 mM DTT, 2% (wt/vol) polyethylenglycol (molecular weight 6.000), 1 mM MgATP, and various concentrations of EGTA and CaCl2 at room temperature (20–25°C). The percentage of motile sperm and velocity were measured as described above, by using cellsoft. To test the effect of Ca2+ on the motility of intact sperm, two methods were used.
(i) The semen was diluted 50-fold in NoCaFPS and centrifuged at 1,000 rpm for 5 min, the precipitate was suspended in the same volume of NoCaFPS, the suspension was diluted in AS or NoCaAS containing 5 mM EGTA, and then CaCl2 was added to the sperm suspension in NoCaAS at the free Ca2+ concentration of 10−4 M. Sperm motility was estimated in each preparation.
(ii) The semen was diluted 50-fold in NoCaFPS and incubated for 30 min, then one volume of the suspension was diluted in 3 volumes of AS containing 1 mM Ca2+ or 3 volumes of distilled water and 5 mM EGTA, and then the final concentration of 10−4 M Ca2+ was added to the sperm in distilled water.
By using the intact and demembranated sperm, Ca2+ concentrations in the experimental media or reactivating solutions, respectively, were set up in a range of 10−10 to 10−2 M in combination with Ca2+ and EGTA by the chelator software of Fabiato and Fabiato (20). Every experiment was repeated three times, and the mean and SD were calculated.
Intracellular Calcium Measurements.
The semen was diluted to 5 × 107 cell/ml density in FPS containing 20 μM of calcium green1-AM and 0.025% Cremophor (stock solution; 25% in DMSO) and incubated for 2 hr at room temperature. Then the cells were centrifuged at 2,000 rpm for 5 min, and the precipitate was resuspended in FPS. The ester bond of calcium green1-AM was hydrolyzed for 1 hr at room temperature; 0.1 ml of calcium green-loaded sperm suspension was added in 1 ml of FPS with or without ion channel blockers, and the fluorescence intensity was monitored at 488/535-nm excitation/emission wavelength by using a Perkin–Elmer fluorescence spectrophotometer. Then 2 ml of distilled water was added without changing the pH of the suspension. Because fluorescence intensity decreased by the dilution, the level of the recorder was adjusted to the initial levels by increasing the amplification of the recorder setup three times. Calcium green did not affect sperm motility. Flow cytometric measurement of intracellular Ca2+ was carried out by using calcium green fluorescent dye (21). Cell loading was done as described above. The semen was diluted in 2 ml (106 cell/ml) of experimental medium in a test tube. The extracellular Ca2+ concentration was set up in a range of 10−10 to 10−3 M in combination Ca2+ and EGTA by the chelator software of Fabiato and Fabiato (20). For the calibration of the intracellular Ca2+ concentration, A-23187 Ca2+ ionophore in 1 μM concentration was added to the solutions. Fluorescence was excited with the 488-nm line at 200–400 mW power with a Becton Dickinson FACS III flowcytometer by using an argon ion laser. The output optics contained a combination of a 529-nm longpass filter and 540-nm band filter. Data of dead sperm cells were excluded from the analysis.
Membrane Potential Measurements.
The semen was diluted 2,000-fold in 1 ml (5 × 106 cell/ml) of experimental medium in a cuvette, and 0.5 μM final concentration of DiSC3 (5) was added to the suspension. The mitochondrial potential was dissipated by the addition of 1 μM CCCP, and the fluorescence was then monitored with a fluorescence spectrophotometer (Hitachi 650–10S, Tokyo) at 620/670 nm excitation/emission wavelength pair (22, 23). The depolarization of the plasma membrane increased the fluorescence intensity under this condition. DiSC3 (5) did not affect sperm motility. Flow cytometric membrane potential measurements were carried out as described in detail by Krasznai et al. (24). The semen was diluted 2,000-fold in 2 ml (5 × 106 cell/ml) of experimental medium in a test tube, and 1 μM final concentration of oxonol was added to the suspension. Oxonol fluorescence was excited with the 488-nm line at 200–400 mW power with a Becton Dickinson FACS III flowcytometer by using an argon ion laser. The output optics contained a combination of a 529-nm longpass filter and 540-nm band filter. Data of dead sperm cells were excluded from the analysis. Oxonol did not affect sperm motility.
Results
Membrane-permeable cyclic nucleotids, dibutyril cGMP (100 μM), and dibutyril cAMP (100 μM) did not induce the initiation of sperm motility in isotonic FPS. Motility of the demembranated sperm could be initiated by Mg-ATP in the absence of cAMP. These results confirmed those previously reported (11). Furthermore, the cAMP level of the sperm was very low (152.8 ± 12.6 fM/mg protein) in the isotonic FPS as compared with other cells such as trout sperm (11–16 pM/mg protein) (25). It did not change significantly during the active motion period in the hypotonic medium even if it contained low (10−7 M) or high (10−5 M) Ca2+. Inhibitors of protein kinase A (10 μM H-89) and protein kinase A and G (100 μM H-8) as well as protein kinase C (100 μM H-7) did not affect sperm motility. These results suggest that cyclic nucleotide-dependent protein phosphorylation is not involved in the hypoosmolality-induced initiation of sperm motility in the carp.
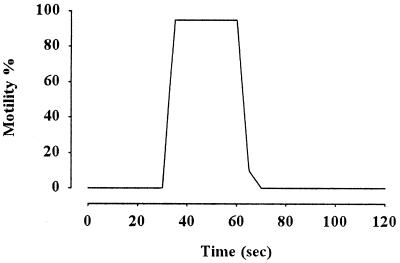
When carp sperm were washed in NoCaFPS, and then 1 volume of the suspension was transferred to 3 volumes of hypotonic AS, the sperm exhibited excellent motility with 91% motile sperm, velocity being 210 μm/sec. However, when sperm were suspended in NoCaFPS, no motility occurred. Subsequent addition of Ca2+ (final external Ca2+ concentration estimated by chelator software was 10−4 M) caused immediate initiation of sperm motility (Fig. 1). The sperm suspended in Ca-free medium without washing were also immotile, and the addition of Ca2+ caused the initiation of sperm motility. These results suggest that extracellular Ca2+ is a prerequisite for the initiation of sperm motility in the carp.
Figure 1.
Requirement of extracellular Ca2+ for the initiation of carp sperm motility. Sperm washed in NoCaFPS as described in Materials and Methods was diluted in NoCaAS containing 5 mM EGTA at t = 0 time, and sperm motility was observed under microscope. At t = 30 sec, 10−4 M CaCl2 was added to the suspension. Sperm initiated motility and maintained maximum motility for 30 sec, and then motility decreased. Washed sperm suspended in AS without EGTA started motility simultaneously with the suspension (data not shown). Typical pattern of five experiments is shown.
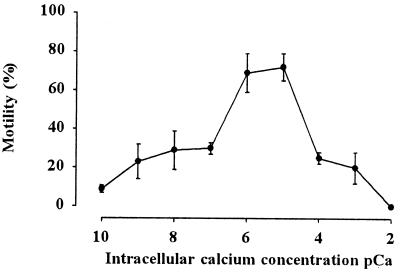
The demembranated sperm showed almost straightforward motility in the range of Ca2+ concentration between 19−9 − 10−3 M (Fig. 2), as described before (11). However, the motility in the reactivating medium containing 10−9 to 10−7 M or 10−4 to 10−3 M Ca2+ was almost 30% in the present study. The spermatozoa exhibited motility similar to the intact sperm in the range of 10−7 to 10−5 M Ca2+. Over 80% sperm were motile with velocity around 100 μm/sec in this Ca2+ range.
Figure 2.
Effect of Ca2+ concentration on the motility of the demembranated sperm. Sperm were demembranated with Triton X-100 and reactivated in the reactivation medium containing 10−10 to 10−2 M of Ca2+, as described in Materials and Methods. Note that the sperm exhibited high motility at 10−6 and 10−5 M Ca2+ concentration. Velocity of the motile sperm at each Ca2+ concentration was almost the same (around 100 μm/sec). Bar represents mean ± SD (n = 5).
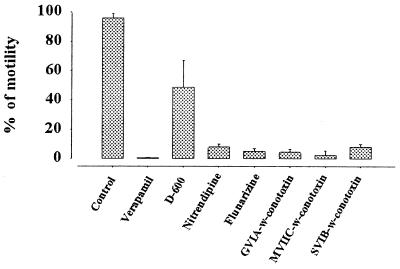
Ca2+ channel blockers whose half-blocking concentrations are usually in the range 20–50 μM (26) inhibited the initiation of sperm motility. When sperm were incubated in FPS containing verapamil and then transferred into the AS containing inhibitors in the same concentration, motility was completely inhibited. A similar block occurred after the same treatment with nitrendipine and flunarizine (Fig. 3). D-600 resulted in a 50% decrease of the motile fraction. ω-Conotoxin GVIA, ω-conotoxin MVIIC, and ω-conotoxin SVIB reduced the motile fraction to 2.4%–8%. After each blocker was washed out from the sperm incubated in the presence of blockers for 3 min by centrifugation and the precipitated sperm were resuspended in AS, sperm motility recovered up to 80–100%. Nimodipine, PLTX-II, ω-agatoxin, calciludine, nifedipine, ω-conotoxin MVIIA, and waglorine had no inhibitory effect on sperm motility, even if administered in 5 to 10 times higher dose than the suggested effective reference concentration.
Figure 3.
Effect of Ca2+ channel blockers on the initiation of sperm motility. Semen was diluted in FPS (see Materials and Methods) containing Ca2+ channel blocker, 100 μM verapamil, nitrendipine, and flunarizine, 200 μM D-600; 2 μM ω-conotoxin GVIA, ω-conotoxin MVIIC, and ω-conotoxin SVIB, and incubated for 3 min, and then 5 μl of the sperm suspension was diluted in 50 μl of AS containing each blocker and sperm motility was measured. Five to ten times higher dose than the effective blocking dose (up to 50 μM) of nimodipine, PLTX-II, ω-agatoxin, calciludine, nifedipine, ω-conotoxin MVIIA, and waglorine had no inhibitory effect on sperm motility (data not shown). Control was taken in the absence of Ca2+ channel blocker. Bar represents mean ± SD (n = 5).
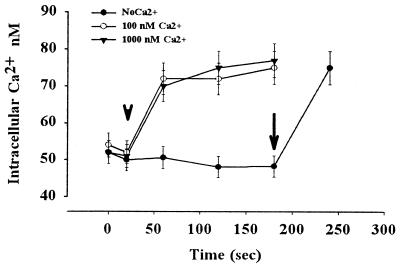
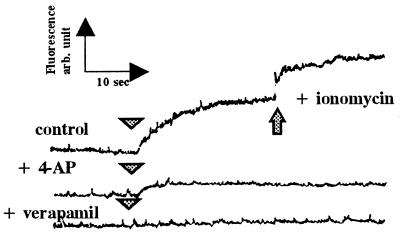
The intracellular Ca2+ concentration of the carp sperm immotile in FPS was 48 ± 3 nM, and it increased to approximately 78 ± 3 nM when sperm motility was initiated by hypotonic treatment in the presence of 0.1–1 μM external Ca2+ (Fig. 4). Intracellular Ca2+ did not change in the absence of external Ca2+, but the subsequent addition of Ca2+ caused an increase in intracellular Ca2+ and sperm motility. When the sperm were loaded with calcium green and the fluorescence intensity was monitored, the intensity increased concomitantly with the initiation of sperm motility by the decrease of osmolality surrounding sperm (Fig. 5). Within a few seconds of hypoosmolality treatment, a drastic increase in Ca2+ concentration occurred. The calcium green treatment had no harmful effect on sperm motility. The Ca2+ channel blocker verapamil and a voltage-gated potassium channel blocker 4-aminopyriridine completely blocked the increase of intracellular Ca2+ and sperm motility (Fig. 5).
Figure 4.
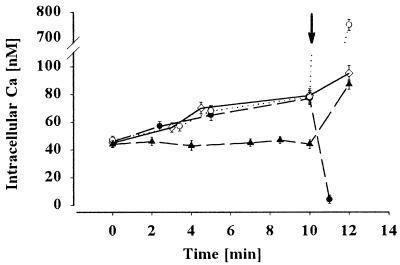
Flow cytometric measurement shows the increase in intracellular Ca2+ concentration during the hypoosmolality-induced initiation of sperm motility in the carp sperm. The semen was diluted in fish physiological solution (see Materials and Methods) containing no Ca2+ (closed circle), 100 nM Ca2+ (open circle), and 1,000 nM Ca2+ (closed triangle). Then distilled water with each concentration of Ca2+ calculated by the Fabiato software (20) was added (arrowhead). Sperm motility was initiated if the external media contained Ca2+ but not in the absence of Ca2+. When calcium was added (100 nM of free Ca2+ final) to the sperm suspended in NoCaFPS (arrow), the intracellular Ca2+ increased, and sperm initiated their motility. Note the very fast increase in Ca2+ on hypoosmolality-induced initiation of sperm motility. Bar represents mean ± SD of 10,000 sperm cells of three experiments.
Figure 5.
Effects of ion channel blockers on intracellular Ca2+ and sperm motility. Semen was diluted in FPS containing calcium green1-AM to load the dye as described in Materials and Methods. The dye-loaded sperm was suspended in FPS with or without 5 mM 4-AP or 100 μM verapamil for 5 min, and then distilled water with or without the blockers was added (arrowhead). The changes of fluorescence were monitored by fluorescence spectrophotometer as described in Materials and Methods. Note that intensive increase in intracellular Ca2+ occurs within 10 sec after exposure of sperm to hypotonic environment in which sperm motility is initiated. Verapamil and 4-AP block both sperm motility and the increase in intracellular Ca2+.
The effect of Ca2+ mobilizers on the intracellular free Ca2+ concentration was also tested. The cells were loaded with calcium green fluorescence dye, and the intracellular free Ca2+ concentration was measured in a flow cytometer as described in Materials and Methods. After application of the Ca2+ mobilizer thapsigargin to the quiescent sperm cells diluted in FPS, the intracellular free Ca2+ concentration increased from approximately 50 nM to about 80 nM (Fig. 6) regardless of the Ca2+ concentration of the environment. By adding Ca2+ ionophore A-23187 in 1 μM concentration to the cells, the intracellular Ca2+ concentration changed to the extracellular value. Another intracellular Ca2+ mobilizer, cyclopiazonic acid, showed the same effect as that of the thapsigargin. The calmodulin inhibitor W-7 (1 mM) completely blocked, whereas 1 mM W-5 did not affect, carp sperm motility. When the sperm were demembranated and the motility of the sperm were initiated by ATP in the presence of W-7 and W-5, motility similar to that of the control cells was observed (data not shown), suggesting that calmodulin bound close to the plasma membrane.
Figure 6.
Effect of the Ca2+ mobilizer thapsigargin on the intracellular Ca2+concentration of the quiescent cells. Sperm were loaded with calcium green1-AM as described in Materials and Methods and suspended in NoCaFPS (closed circle) or FPS containing 100 nM Ca2+ (open diamond) or 1,000 nM Ca2+ (open circle), and 50 μM thapsigargin and fluorescence from the cells was monitored for 10 min. Control cells were suspended in FPS containing 100 nM Ca2+ (closed circle), and at t = 10 min, 5 μM A 23187 calcium ionophore was added to the suspension and the fluorescence from the sperm cells was measured. When 5 μM A 23187 was added to the sperm cells containing different calcium concentrations and thapsigargin (arrow), the intracellular calcium concentration reached that of the extracellular value.
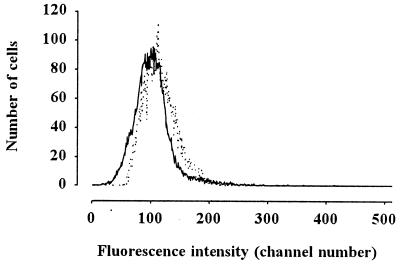
The hyperpolarization of the sperm on hypoosmolality-induced motility was proven by flow cytometric membrane potential measurement by using a dye, oxonol. When initiating sperm motility by hypoosmosis, the oxonol fluorescence from the sperm cells decreased, indicating the hyperpolarization of the cells. (Fig. 7). This hyperpolarization could be eliminated by the potassium channel blocker 4-aminopyridine, but the Ca2+ channel blocker verapamil did not block membrane hyperpolarization. These findings suggest that an increase in potassium permeability is responsible for the hyperpolarization, which is followed by a Ca2+ influx.
Figure 7.
Flow cytometric membrane potential measurement showed hyperpolarization of carp sperm during hypoosmolality-induced initiation of motility. Fluorescence from oxonol-loaded sperm was monitored as described in Materials and Methods. Dotted line shows the oxonol fluorescence from sperm immotile in FPS. Continuous line was recorded 1 min after dilution of sperm into AS. Decrease in fluorescence of oxonol exhibits the hyperpolarization of the plasma membrane.
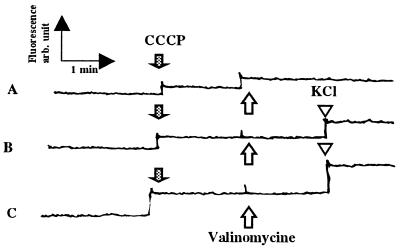
Valinomycin, a potassium ionophore (2 μM), depolarized sperm plasma membrane in the presence of 150 mM extracellular KCl (Fig. 8). Valinomycin did not affect the membrane potential in the solution containing 75 mM KCl and 75 mM NaCl or 10 mM KCl and 140 mM NaCl. Valinomycin, in a range of 0.2 to 20 μM, did not initiate carp sperm motility. When sperm motility was initiated by the activating solution, valinomycin did not affect sperm motility. A high concentration of the ionophore (200 μM) decreased the hypoosmolality-induced sperm motility to 4% of the control.
Figure 8.
Effect of valinomycin on the membrane potential of sperm in solutions containing different extracellular K+. Sperm loaded with DiSC3 (5) as described in Materials and Methods were suspended in FPS containing 150 mM KCl (A), 75 mM KCl + 75 mM NaCl (B), or 10 mM KCl + 140 mM NaCl (C), which is isotonic to the seminal plasma (300 milliosmolal). Sperm were immotile in these media. After cancelled potential from mitochondria with CCCP (closed arrow), valinomycin was added (open arrow), and fluorescence was monitored. KCl at the concentration of 10 mM was added (open arrowhead).
Discussion
The spermatozoa of freshwater fishes, including carp, are immotile in the seminal plasma, with osmolality around 300 milliosmolal (4), or in the experimental solutions isotonic to it (2). The sperm become motile when suspended into hypotonic experimental solutions containing electrolytes such as NaCl, KCl (2, 4–6), or a nonelectrolyte, mannitol (2, 4). At the initiation of sperm motility, ATP is hydrolyzed by dynein ATPases, and its contents decrease coupled to the movement, as described in various animals, for example trout (27) and carp (5). In addition to the requirement of an energy source, cAMP has long been implicated as an activator of sperm motility through its stimulating effect on the motile apparatus. Morisawa and Okuno (15) first demonstrated that cAMP was required before ATP to trigger the initiation of sperm motility in rainbow trout. However, present results suggest that cAMP does not play a major role in the regulation of sperm motility in carp, because demembranated sperm could be reactivated by the addition of ATP in the absence of cAMP (11), and membrane-permeable cyclic nucleotids as well as IBMX (12) were not effective in initiating carp sperm motility. Furthermore, the cAMP level of the sperm was very low and did not change significantly during hyperosmolality-induced initiation of sperm motility. Protein kinase A, A and G, as well as protein kinase C inhibitors did not block the motility of the sperm, suggesting that cAMP-dependent protein phosphorylation is not critical for the initiation of sperm motility in carp.
An increase in intracellular calcium is a prerequisite for the initiation of sperm motility in rainbow trout (28–31). It was also shown by Okuno and Morisawa (32) that demembranated trout sperm showed vigorous motility in the reactivating solution containing Ca2+ in a concentration below 10−8 M, suggesting that the initiation of fish sperm motility requires a transient influx of Ca2+, although its origin, from intracellular storage or of extracellular origin, is controversial (29). The present results suggest that the presence of extracellular Ca2+ is necessary for the initiation of sperm motility in carp. The sperm washed and preincubated with a calcium-free solution could not be activated in calcium-free AS, but the addition of 10−4 M Ca2+ immediately initiated sperm motility (Fig. 1). Furthermore, demembranated and reactivated carp sperm showed similar motility to that of the intact sperm if the reactivating solution contained 10−5 to 10−7 M Ca2+ (Fig. 2). The intracellular Ca2+ level remarkably increased on hypoosmolality-induced initiation of carp sperm motility. The intracellular Ca2+ concentration in the quiescent sperm was approximately 50 nM, and it increased to 80 nM during the hypoosmolality-induced initiation of sperm motility (Fig. 4). Fig. 5 also shows that vigorous Ca2+ rise occurred in the earliest period after hypoosmolality treatment of the sperm. The increase in intracellular Ca2+ by one-third within a few seconds would constitute the signaling for the initiation of sperm motility (2). General Ca2+ channel blockers, verapamil, nifedipine, nitrendipine, D-600, and N-type or Q-type Ca2+ channel blockers, ω-conotoxin GVIA, ω-conotoxin MVIIC, and ω-conotoxin SVIB, inhibited sperm motility at a reasonable concentration to inhibit Ca2+ channels (Fig. 3), and verapamil completely eliminated the Ca2+ increase and sperm motility (Fig. 5). Sperm motility recovered if the blockers were removed by washing. All these results strongly suggest that Ca2+ influx through Ca2+ channel and the resulting increase in intracellular Ca2+ are the events triggering the initiation of carp sperm motility.
Ca2+ mobilizers cause an increase in intracellular Ca2+ (Fig. 6), but this Ca2+ increase itself does not cause the initiation of sperm motility, suggesting that the entrance of extracellular Ca2+ has a critical role in the initiation of sperm motility.
The plasma membrane of carp sperm is remarkably depolarized in the semen, because K+ concentration of the seminal plasma (82.4 mM) (4) is higher than that of intracellular K+ concentration of the sperm (60.5 mM) (33, 34). At the hypoosmolality-induced initiation of sperm motility, a decrease in extracellular osmotic pressure and K+ concentration occur, resulting in a transient membrane hyperpolarization (Figs. 7 and 8). In addition, when the cells are incubated in high K+ containing FPS, the addition of valinomycin depolarizes the cells (Fig. 9). This depolarization is because high extracellular K+ results in depolarization, but prolonged depolarization results in the inactivation of the K+ channels where they do not conduct; therefore, the K+ concentration difference between the two sides of the plasma membrane does not contribute to the membrane potential. An increase in K+ permeability by the K+ ionophore therefore results in the shift of the membrane potential to the direction calculated from the Nernst equation. A similar case can be observed when the extracellular K+ concentration is 75 mM. In this solution, the K+ channels of the sperm cells are also in an inactive state, because the intracellular K+ concentration of the sperm cells is very similar to, or almost identical with, that of the extracellular solution. Therefore, an increase in the K+ permeability by valinomycin does not change the membrane potential, because it is already 0 mV. The most interesting case is when the extracellular medium contains only 10 mM K+. Under this condition, the K+ channels regularly open and close, maintaining the membrane potential at the level of the K+ equilibrium potential. A further increase in K+ permeability by applying valinomycin therefore does not change the membrane potential (Fig. 8). At hypoosmosis-induced initiation of sperm motility, the recovery from inactivation may result in an opening and closing of K+ channels in the plasma membrane of the sperm. Because of the possibility that these cells are of a very small size and have a limited number of K+ channels, the opening or closing of a single channel results in a remarkable local hyperpolarization or depolarization of the plasma membrane. These transient depolarizations may open Ca2+ channels, resulting in an influx of Ca2+ that leads to the activation of the flagellar motility of carp sperm (Figs. 4, 5, and 6). The block of sperm motility in a calcium-free activating solution strongly supports this hypothesis. The observation that similar sperm motility and increase in intracellular Ca2+ level were obtained in different extracellular concentrations of Ca2+ suggests that Ca2+-induced Ca2+ release from intracellular stores is involved in the initiation of sperm motility in freshwater teleosts.
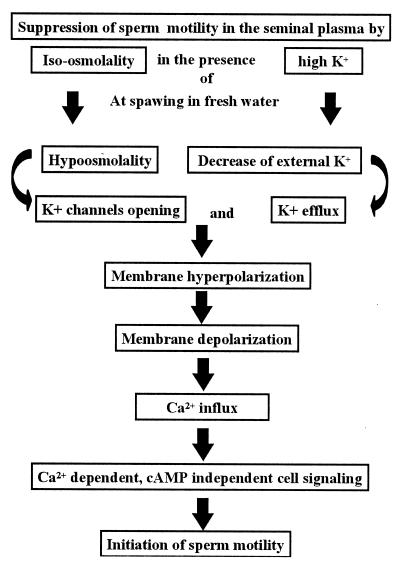
Figure 9.
Suggested cell-signaling mechanism for the initiation of carp sperm motility. Sperm in the male reproductive organ are surrounded by isoosmotic and high K+ environment of the seminal plasma. On spawning into the environment with hypoosmolality and K+-deficient fresh water, hypoosmolality-induced initiation of sperm motility occurs through a Ca2+-dependent cAMP-independent cell signaling, as shown.
Membrane hyperpolarization (29) through the direct effect of transmembrane K+ efflux (30, 31) is the first trigger for the initiation of sperm motility in salmonid fishes. Membrane hyperpolarization also triggers the activation of ascidian sperm motility through hyperpolarization-induced direct activation of adenylyl cyclase to synthesize cAMP in Ciona (35). Concerning the carp, it seems likely that the change of external osmolality is the first trigger before K+ efflux in the initiation of sperm motility (Fig. 9), because an isotonic nonelectrolyte solution containing mannitol as well as electrolyte Na+ can suppress sperm motility in the absence of K+ (2). After K+ channel opening by osmotic stimulus, membrane hyperpolarization—which is inhibited by 4-AP, an inhibitor for both K+ channel and sperm motility—may subsequently occur by the efflux of K+ during the decrease in external K+ at spawning of the sperm in fresh water. Because 4-AP also inhibited the increase in intracellular Ca2+ concentration, and verapamil did not inhibit membrane hyperpolarization, hyperpolarization may be upstream of the Ca2+ channel opening, which is inhibited by verapamil, an inhibitor of Ca2+ channel and sperm motility. At spawning, the quick hyperpolarization of the plasma membrane removes inactivation of Ca2+ channels as described above, resulting in a Ca2+ influx (the former is inhibited by 4-AP, the latter by verapamil). Thereafter, Ca2+-dependent and cAMP-independent cell signalings will trigger the initiation of sperm motility in the freshwater teleosts, common carp.
Acknowledgments
We thank Dr. Y. Oka Misaki (Marine Biological Station, the University of Tokyo) and Dr. G. Panyi (University Medical School of Debrecen, Hungary) for helpful advice. This work was supported by OTKA grant T 22435 to Z.K., by OMFB-Japan 5/98 grant to Z.K. and M.M., and by grant no. 09440276 from the Ministry of Education, Science, Culture, and Sports of Japan to M.M.
Abbreviations
- DiSC3
3,3′-dipropylthiadicarbocyanine iodide
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- FPS
fish physiological solution
- NoCaFPS
calcium-free FPS
- AS
activating solution
- 4-AP
4-aminopyridine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040558097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040558097
References
- 1.Hoffmann E K, Dunham P B. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 2.Morisawa M, Suzuki K. Science. 1980;210:1145–1147. doi: 10.1126/science.7444445. [DOI] [PubMed] [Google Scholar]
- 3.Drevius L O, Erickson H. Exp Cell Res. 1966;42:136–156. doi: 10.1016/0014-4827(66)90327-2. [DOI] [PubMed] [Google Scholar]
- 4.Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K. J Exp Biol. 1983;107:95–103. doi: 10.1242/jeb.107.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Perchec G, Jeulin C, Cosson J, Andre F, Billard R. J Cell Sci. 1995;108:747–753. doi: 10.1242/jcs.108.2.747. [DOI] [PubMed] [Google Scholar]
- 6.Márián T, Krasznai Z, Balkay L, Balázs M, Emri M, Bene L, Trón L. J Histochem Cytochem. 1993;41:291–297. doi: 10.1177/41.2.8419464. [DOI] [PubMed] [Google Scholar]
- 7.Perchec G, Cosson M P, Cosson J, Jeulin C, Billard R. Cell Motil Cytoskeleton. 1996;35(2):113–120. doi: 10.1002/(SICI)1097-0169(1996)35:2<113::AID-CM4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Krasznai Z, Márián T, Balkay L, Gáspár R, Trón L. Aquaculture. 1995;129:123–128. [Google Scholar]
- 9.Takai H, Morisawa M. J Cell Sci. 1995;108:1175–1181. doi: 10.1242/jcs.108.3.1175. [DOI] [PubMed] [Google Scholar]
- 10.Márián T, Krasznai Z, Balkay L, Emri M, Trón L. Cytometry. 1997;27:374–382. doi: 10.1002/(sici)1097-0320(19970401)27:4<374::aid-cyto9>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Perches-Poupard G P, Gatti J L, Cosson J, Jeulin C, Fierville F, Billard R. J Reprod Fertil. 1997;110(2):315–327. doi: 10.1530/jrf.0.1100315. [DOI] [PubMed] [Google Scholar]
- 12.Cosson M P, Gagnon C. Cell Motil Cytoskeleton. 1988;10:518–527. [Google Scholar]
- 13.Cook S P, Brokaw C J, Muller C H, Babcock D F. Dev Biol. 1994;165:10–19. doi: 10.1006/dbio.1994.1229. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Inaba K, Ishida K, Morisawa M. Dev Growth Differ. 1994;36:589–595. doi: 10.1111/j.1440-169X.1994.00589.x. [DOI] [PubMed] [Google Scholar]
- 15.Morisawa M, Okuno M. Nature (London) 1982;295:703–704. doi: 10.1038/295703a0. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H, Yamamoto K, Yonekawa H, Morisawa M. J Biol Chem. 1987;262:16692–16698. [PubMed] [Google Scholar]
- 17.Lindenmann C B. Cell. 1978;13:9–18. doi: 10.1016/0092-8674(78)90133-2. [DOI] [PubMed] [Google Scholar]
- 18.Tash J H, Means A R. Biol Reprod. 1983;28:75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- 20.Fabiato A, Fabiato F. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 21.Eberhard M, Erne P. Biochem Biophys Res Commun. 1991;15:209–215. doi: 10.1016/s0006-291x(05)81278-1. [DOI] [PubMed] [Google Scholar]
- 22.Babcock D F, Bosma M M, Battaglia D E, Darszon A. Proc Natl Acad Sci USA. 1992;89:6001–6005. doi: 10.1073/pnas.89.13.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Y, Clark E N, Florman H. Dev Biol. 1995;171:554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- 24.Krasznai Z, Márián T, Balkay L, Emri M, Trón J. J Photochem Photobiol. 1995;28:93–99. doi: 10.1016/1011-1344(94)07099-a. [DOI] [PubMed] [Google Scholar]
- 25.Morisawa M, Ishida K. J Exp Zool. 1987;242:199–204. doi: 10.1002/jez.1402420211. [DOI] [PubMed] [Google Scholar]
- 26.Hille B. Ionic Channels of Excitable Membranes. 2nd Ed. Sunderland, MA: Sinauer; 1991. pp. 108–114. [Google Scholar]
- 27.Christen R, Gatti J L, Billard R. Eur J Biochem. 1987;166:667–671. doi: 10.1111/j.1432-1033.1987.tb13565.x. [DOI] [PubMed] [Google Scholar]
- 28.Cosson M P, Billard R, Letellier L. Cell Motil Cytoskel. 1989;14:424–434. [Google Scholar]
- 29.Boitano S, Omoto C K. J Cell Sci. 1991;98:343–349. doi: 10.1242/jcs.98.3.343. [DOI] [PubMed] [Google Scholar]
- 30.Tanimoto S, Kudo Y, Nakazawa T, Morisawa M. Mol Rep Dev. 1994;39:409–414. doi: 10.1002/mrd.1080390409. [DOI] [PubMed] [Google Scholar]
- 31.Tanimoto S, Morisawa M. Dev Growth Differ. 1988;30(2):117–124. doi: 10.1111/j.1440-169X.1988.00117.x. [DOI] [PubMed] [Google Scholar]
- 32.Okuno M, Morisawa M. Cell Motil Cytoskeleton. 1989;14:194–200. [Google Scholar]
- 33.Balkay L, Márián T, Emri M, Krasznai Z, Trón L. Cytometry. 1997;27(4):374–382. doi: 10.1002/(sici)1097-0320(19970401)27:4<374::aid-cyto9>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Emri M, Márián T, Trón L, Balkay L, Krasznai Z. Aquaculture. 1998;167:85–94. [Google Scholar]
- 35.Izumi H, Márián T, Inaba K, Oka Y, Morisawa M. Dev Biol. 1999;213:246–256. doi: 10.1006/dbio.1999.9367. [DOI] [PubMed] [Google Scholar]