Abstract
Neutrophils are thought to play a major role in the mediation of reperfusion injury. CXC chemokines are known inducers of neutrophil recruitment. Here, we assessed the effects of Repertaxin, a novel low molecular weight inhibitor of human CXCL8 receptor activation, on the local, remote and systemic injuries following intestinal ischaemia and reperfusion (I/R) in the rat.
Pre-incubation of rat neutrophils with Repertaxin (10−11–10−6 M) inhibited the chemotaxis of neutrophils induced by human CXCL8 or rat CINC-1, but not that induced by fMLP, PAF or LTB4, in a concentration-dependent manner. Repertaxin also prevented CXCL8-induced calcium influx but not CXCL8 binding to purified rat neutrophils.
In a model of mild I/R injury (30 min of ischaemia and 30 min of reperfusion), Repertaxin dose-dependently (3–30 mg kg−1) inhibited the increase in vascular permeability and neutrophil influx. Maximal inhibition occurred at 30 mg kg−1.
Following severe I/R injury (120 min of ischaemia and 120 min of reperfusion), Repertaxin (30 mg kg−1) markedly prevented neutrophil influx, the increase in vascular permeability both in the intestine and the lungs. Moreover, there was prevention of haemorrhage in the intestine of reperfused animals.
Repertaxin effectively suppressed the increase in tissue (intestine and lungs) and serum concentrations of TNF-α and the reperfusion-associated lethality.
For comparison, we also evaluated the effects of an anti-CINC-1 antibody in the model of severe I/R injury. Overall, the antibody effectively prevented tissue injury, systemic inflammation and lethality. However, the effects of the antibody were in general of lower magnitude than those of Repertaxin.
In conclusion, CINC-1 and possibly other CXC chemokines, acting on CXCR2, have an important role during I/R injury. Thus, drugs, such as Repertaxin, developed to block the function of the CXCR2 receptor may be effective at preventing reperfusion injury in relevant clinical situations.
Keywords: Inflammation, neutrophils, TNF-α, chemokine receptor antagonists, systemic inflammatory response syndrome
Introduction
Reperfusion is the treatment of choice to save viable tissue following acute ischaemia of a vascular territory. Nevertheless, reperfusion of ischaemic tissues may be associated with a severe inflammatory response (Willerson, 1997). Among the cell types involved in the injury following reperfusion of an ischaemic tissue, neutrophils are of major importance (Cornejo et al., 1997; Willerson, 1997). Thus, neutrophils have been shown to mediate both local and remote organ injury after ischaemia and reperfusion (I/R) of hindlimbs (Kyriakides et al., 1999; Merchant et al., 2003), liver (Jaeschke et al., 1990), intestine (Xiao et al., 1997; Souza et al., 2000a, 2000b), kidneys (Weight et al., 1996) and myocardium (Baxter, 2002; Kohtani et al., 2002). Several mediators of the inflammatory process have been shown to participate in the cascade of events leading to I/R injury, including lipids mediators, vasoactive peptides, neuropeptides and cytokines, especially TNF-α (Gilmont et al., 1996; Souza et al., 2000a, 2000b, 2002b, 2002c, 2003). Among inflammatory mediators shown to activate neutrophils and induce their recruitment in vivo, much interest has been placed on the role of CXC-chemokines. Members of the CXC branch of the chemokine family have four invariant cysteines, the first two of which are separated by one other amino acid (X). Chemokines containing glutamic acid-leucine (ELR) immediately preceding the CXC motif are potent neutrophil chemoattractants (Baggiolini et al., 1995). In humans, IL-8 is the most studied member of this chemokine family, which activates both CXCR1 and CXCR2, seven transmembrane receptors that transduce the signals of CXC-ELR+ chemokines (Baggiolini et al., 1995). It appears that rats (and other rodents) lack a homologue of CXCL8 but possess other CXC-ELR+ chemokines, namely cytokine-induced neutrophil chemoatractant-1 (CINC-1) and macrophage inflammatory protein-2. The latter chemokines act preferentially on CXCR2.
Previous studies have demonstrated that CXC chemokines are released following I/R injury of several tissues (Kalfin et al., 1993; Chandrasekar et al., 2001; Kataoka et al., 2002; Ren et al., 2003). Furthermore, studies using anti-CINC antibodies or anti-CXCR2 receptor antibodies have shown that such strategies ameliorated reperfusion injury (Boyle et al., 1998; Tsuruma et al., 1998; Yagihashi et al., 1998; Miura et al., 2001). Thus, blockade of the action of CXC-ELR+ chemokines or their receptors appears to be a valid strategy for the treatment of injuries associated with the reperfusion of a vascular territory. Repertaxin is a novel low molecular weight inhibitor of human CXCL8 receptor activation (Figure 1, Bertini et al., 2004). Here, we assessed the effects of Repertaxin on the local, remote and systemic injuries following intestinal I/R in the rat. Initial experiments were conducted with rat neutrophils to confirm the specificity and potency of Repertaxin at inhibiting rat CXCR2 on neutrophils. Dose–response experiments were conducted in a model of mild neutrophil-dependent I/R injury previously described by our group (Souza et al., 2000a). We then tested the effectiveness of our strategy in a model of more severe I/R injury in which local and systemic changes are observed (Souza et al., 2000b). For comparison, we compared the effects of Repertaxin to that of anti-CINC-1 antibodies.
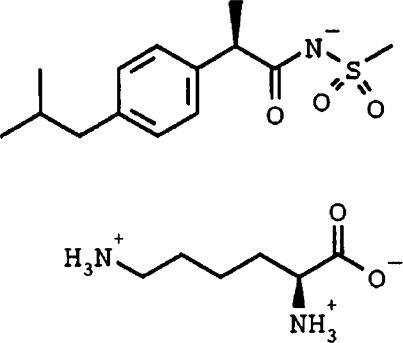
Figure 1.
Chemical structure of Repertaxin (R(-)-2-(4-isobutylphenyl)propionyl methansulphonamide). As shown in the figure, Repertaxin is salified with L-lysine.
Methods
Animals
Male Wistar rats (200–220 g) obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All procedures described here had prior approval from the local animal ethics committee.
In vitro neutrophil chemotaxis
The chemotactic activity for neutrophils obtained from the blood of rats was assayed using the 48-well microchemotaxis chamber technique, as previously described (Bignold & Ferrante, 1987; DeForge et al., 1992). Briefly, the lower compartment of the chamber was loaded with aliquots of medium, CXCL8, CINC-1, fMLP, PAF or LTB4, while the upper compartment of the chamber was loaded with the purified neutrophils (resuspended in RPMI medium). Neutrophils (>90% purity, >95 viability) were purified over a Percoll gradient, as previously described (Ramos et al., 2003), and were incubated for 10 min with vehicle (saline) or Repertaxin (10−11–10−6 M) prior to addition of the chemoattractants. The two compartments were separated by a 5.0 μm polycarbonate filter (Poretics Products, Osmonics, CA, U.S.A.). Following 1 h of incubation at 37°C, the filter was removed, fixed and stained. The migrated cells in 10-high-power fields were counted by light microscopy.
Intracellular calcium measurements
PMN (107 ml−1) were resuspended in RPMI 1640 and incubated with 1 mM FURA-2AM at 37°C for 15 min. Next, PMN were washed and resuspended in HBSS containing 1.2 mM CaCl2 and incubated in the presence of vehicle or 1 mM Repertaxin for 15 min at room temperature. PMN were stimulated with vehicle, CXCL8 (100 ng ml−1), as previously described (Brandolini et al., 1996). FURA-2 fluorescence was measured in a Jasco FP-750 spectrophotometer (Jasco Corporation, Tokyo, Japan). Samples were excited at 340 and 380 nm, and emission at 505 nm was continuously recorded. Intracellular calcium concentration was determined as previously described (Bizzarri et al., 1995).
hCXCL8 binding to rat neutrophils
[125I]CXCL8 (specific activity 2200 Ci mmol−1; Amersham Pharmacia Biotech, Buckinghamshire, U.K.) binding on rat PMN was performed as previously described (Bizzarri et al., 2001). Rat PMN (4 × 107 cells ml−1) were incubated at 37°C for 15 min in the presence of vehicle or 1 mM Repertaxin. Next, 0.8 nM [125I]CXCL8 and serial dilutions of unlabeled CXCL8 were added to 2 × 106 rat PMN in 100 ml of binding medium and incubated at 4°C for 2 h under gentle agitation, as previously described (Bizzarri et al., 2001). Scatchard analysis and calculations were performed with the LIGAND program (Munson & Rodbard, 1980).
I/R injury
Rats were anaesthetized with urethane (140 mg kg−1, i.p. ) and laparotomy was performed. This procedure was sufficient to keep the animals under anaesthesia until the end of the experiment. The superior mesenteric artery (SMA) was isolated and ischaemia was induced by totally occluding the SMA for 30 or 120 min. After ischaemia, reperfusion was initiated by removal of the occlusion. Animals made ischaemic for 30 or 120 min were allowed to reperfuse for 30 (mild injury) or 120 (severe injury) min, respectively. The durations of I/R were based upon previous experiments (Souza et al., 2000a, 2000b) and were optimal for mild and severe reperfusion injuries. Sham-operated animals were used as controls for the reperfusion-induced injury. Lethality was accompanied and, at the end of the experiment, animals were killed by cervical dislocation. Inflammatory parameters were assessed only in animals that were alive at 120 min after reperfusion.
Initial experiments were carried out in the mild reperfusion injury model to examine the dose-dependent effects of CXCR2 inhibitor (Repertaxin, 3–30 mg kg−1). In these experiments, Repertaxin was administered i.v. just prior to the reperfusion of the SMA. We then tested the effects of the administration of Repertaxin (30 mg kg−1, i.v., just prior to reperfusion), Repertaxin vehicle (saline, 1 ml kg−1), anti-CINC (0.5 ml of hyperimmune serum/animal, s.c., 60 min before reperfusion) or non-immune serum (0.5 ml) in the model of severe I/R injury. The drug or antibodies used in the present study had no significant effects on basal parameters (data not shown), and to simplify the graphs presented basal data obtained in vehicle- or drug-treated animals have been pooled for presentation. Similarly, results obtained in reperfused animals treated with Repertaxin vehicle or nonimmune serum were not different and were pooled to simply presentation (data not shown).
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability (Souza et al., 2000a). Evans blue (20 mg kg−1) was administered i.v. (1 ml kg−1) via a femoral vein 2 min prior to reperfusion of the ischaemic artery. At 30 (in the mild injury model) or 120 min (in the severe injury model) after reperfusion, fragments of the duodenum (10 cm) were cut open and allowed to dry in a petri dish for 24 h at 37°C. The dry weight of the tissue was determined and Evans blue extracted using 3 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the optical density (OD) of the extract with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue in μg per 100 mg of tissue. The right ventricle was flushed with 20 ml of phosphate-buffered saline to wash the intravascular Evans blue in the lungs. The left lung was then excised and used for Evans blue extraction. The right lung was used for the determination of myeloperoxidase (MPO) as described below.
MPO levels
The extent of neutrophil accumulation in the intestine and right lung tissue was measured by assaying MPO activity as previously described (Matos et al., 1999). Briefly, a fragment of duodenum and the flushed right lungs of animals that had undergone I/R injury were removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (1 g of tissue per 19 ml of buffer) was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M NaEDTA), centrifuged at 260 × g for 10 min and the pellet underwent hypotonic lysis (15 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After a further centrifugation, the pellet was then resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide and re-homogenized. Aliquots (1 ml) of the suspension were transferred into 1.5-ml Eppendorf tubes, followed by three freeze–thaw cycles using liquid nitrogen. These were then centrifuged for 15 min at 10,000 × g, the pellet was resuspended to 1 ml and samples of intestine and lung were diluted prior to the assay. MPO activity in the resuspended pellet was assayed by measuring the change in OD at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as the total number of neutrophils by comparing the OD of tissue supernatant with that of rat peritoneal neutrophils processed in the same way. To this end, neutrophils were collected from the peritoneum of rats 8–12 h after injection of 3 ml of 5% casein. A standard curve of neutrophil numbers versus OD was obtained by processing casein-elicited neutrophils (>95% purity by using this methodology), as above and assaying for MPO activity.
Determination of the concentration of circulating leukocytes
The total number of circulating leukocytes and neutrophils was evaluated in blood samples obtained via a cannula in the femoral artery. Samples were collected prior to ischaemia (time 0), 120 min after ischaemia and 30 and 120 min after reperfusion. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution and differential counts by evaluating the percentage of each leukocyte on blood films stained with May–Grunwald–Giemsa.
Measurement of haemoglobin levels
The levels of haemoglobin in tissue were used as an index of tissue haemorrhage. Tissues were carefully washed with excess saline to remove blood attached to the intestinal epithelia or serosa. No attempt was made to perfuse the vessels with saline as no obvious hyperaemia was present. After washing, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's colour reagent according to the instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 min at 3000 × g and filtered using 0.2 μm filters. The resulting solution was read using an ELISA plate reader at 520 nm and compared against a standard curve of haemoglobin.
Measurement of cytokine levels in serum, intestine and lungs
TNF-α, IL-1β, IL-6, IL-10 and CINC levels were measured in serum and intestine of animals using ELISA techniques previously described (Hagan et al., 1993; Rees et al., 1999a, 1999b; Francischi et al., 2000). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analysed at a 1 : 3 dilution in PBS. In all, 100 mg of duodenum or lung of sham-operated and reperfused animals were homogenized in 1 ml of PBS (0.4 M NaCl and 10 mM NaPO4) containing anti-proteinases (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000 × g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in PBS. ELISA plates (Nunc MaxiSorb) were coated with a sheep anti-rat TNF-α/IL-1β/IL-6 or IL-10 polyclonal antibodies (1–2 μg ml−1) overnight. The plates were washed thrice and then blocked with 1% bovine serum albumin. After a further wash, plates were incubated with samples or recombinant rat cytokine and incubated overnight. The biotinylated polyclonal antibodies were used at a 1 : 1000 to 1 : 2000 dilution and the assays had a sensitivity of 16 pg ml−1.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, hexadecyltrimethylammonium bromide, 3,3,5,5, tetramethyl-benzidine, Percoll. Repertaxin (R( )-2-(4-isobutylphenyl)propionyl methansulphonamide) was from Dompé, L'Aquila, Italy (Figure 1). Anti-CINC-1 antibodies were raised in rabbits and shown to be optimally inhibitory at the dose used, as previously described (Lorenzetti et al., 2002).
Statistical analysis
Results are shown as the mean±s.e.m. Percent inhibition of a given parameter was calculated by subtracting the background levels obtained in sham-operated animals. Differences were evaluated by using analysis of variance (ANOVA) followed by Student–Newman–Keuls post-hoc analysis. Results with P<0.05 were considered significant. For survival curves, differences between groups at different time points were compared using Fisher's exact test and considered significant when P<0.05.
Results
Effects of Repertaxin on chemoattractant-induced neutrophil chemotaxis in vitro
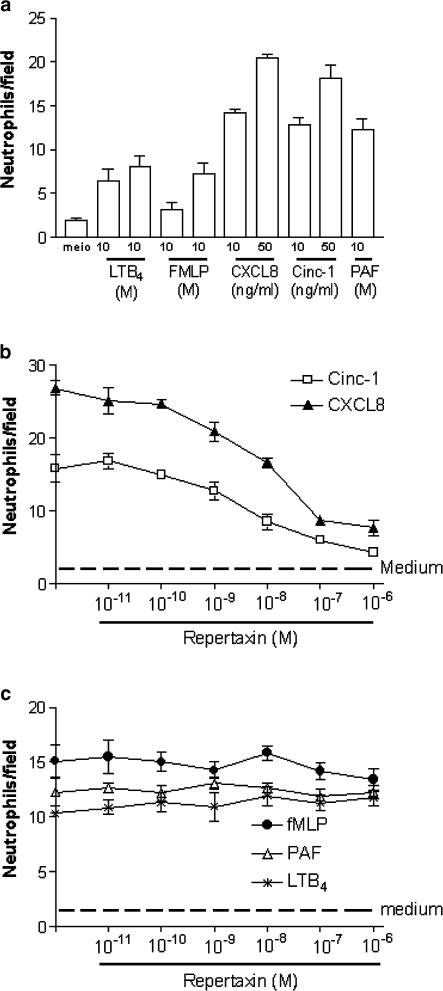
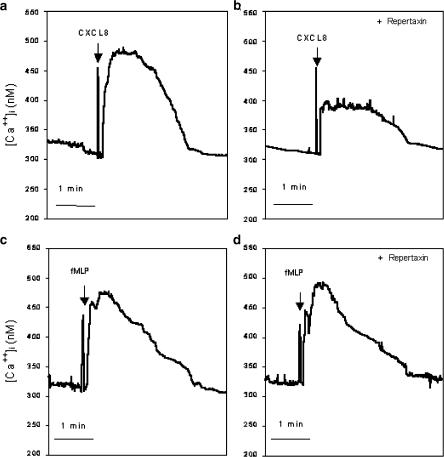
Initial experiments were carried out in vitro with rat neutrophils to assess whether Repertaxin was able to inhibit CXC-ELR+ chemokine-induced neutrophil recruitment. Neutrophils purified from rat blood migrated in response to various concentrations of human IL-8 (CXCL8), rat CINC-1 (CXCL1-3), fMLP, PAF and LTB4 (Figure 2a). Pre-incubation of neutrophils with Repertaxin inhibited the recruitment of neutrophils induced by CXCL8 or CINC-1 in a concentration-dependent manner (Figure 2b). The IC50 of Repertaxin for the inhibition of CINC-1- and CXCL8-induced migration was 6 and 30 nM, respectively. In contrast, Repertaxin had no significant effect on the recruitment induced by fMLP, PAF or LTB4 (Figure 2c). In experiments evaluating intracellular Ca2+ concentration in rat neutrophils, repertaxin (10−6 M) effectively inhibited the response elicited by CXCL8 (100 ng ml−1) (Figure 3a, b). In contrast, the drug failed to affect the elevation in intracellular Ca2+ concentration induced by fMLP (Figure 3c, d). In binding experiments of [125I]-CXCL8 to rat neutrophils, the Kd values in the presence or in the absence of Repertaxin (1 mM) were 8.98±1.07 × 10−9 and 8.99±1.61 × 10−9 M, respectively (mean±s.d., n=3). These results show that Repertaxin is a noncompetitive specific inhibitor of rat neutrophil migration induced by CXC-ELR+-chemokines.
Figure 2.
Effects of Repertaxin on the chemotaxis of neutrophils induced by LTB4, fMLP, CXCL8, CINC-1 or PAF. These experiments were assayed in a 48-well microchemotaxis chamber, as described in the Methods section. Neutrophils were incubated for 10 min with vehicle (saline) or increasing concentration of Repertaxin (10−11–10−6 M) prior to addition of chemoattractants. In (b) and (c), the concentrations of agonists were as follows: CINC-1 (50 ng ml−1), CXCL8 (50 ng ml−1), fMLP (10−6 M), PAF (10−6 M), LTB4 (10−7 M). Results are the number of neutrophils per field and are expressed the mean±s.e.m. of at least 10 fields in each group.
Figure 3.
Effects of Repertaxin on the increase in intracellular Ca2+ in neutrophils induced by CXCL8 or fMLP. Neutrophils were incubated for 10 min with vehicle (saline) or Repertaxin (10−6 M) prior to addition of CXCL8 (100 ng ml−1) or fMLP (10−6 M). Results are representative of at least three determinations using each chemoattractant in the presence or absence of Repertaxin.
Dose-dependent effects of Repertaxin in a model of mild I/R injury
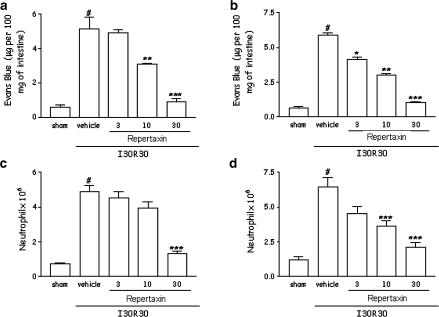
The next experiments in a model of mild I/R injury were designed to investigate the dose-dependent effects of Repertaxin in a model of reperfusion injury and, hence, the putative role of CXCR2 in the system. As clearly observed in Figure 4, postischaemic treatment of animals with Repertaxin inhibited in a dose-dependent manner both the increase in vascular permeability and the recruitment of neutrophils in the intestine (Figure 4a, b) and lungs (Figure 4c, d) following reperfusion of the ischaemic SMA. Repertaxin appeared to be more potent against reperfusion-induced vascular permeability than neutrophil influx in the intestine, but not in the lung (Figure 4). Moreover, 50% inhibition only occurred when doses greater than 10 mg kg−1 were used and the drug was equieffective and markedly prevented tissue injury when used at 30 mg kg−1.
Figure 4.
Dose-dependent effects of the treatment with Repertaxin on the increase in vascular permeability and recruitment of neutrophils in the intestine and lungs following mild ischaemia (30 min) and reperfusion (30 min) injury of the SMA. Changes in vascular permeability in the (a) intestine and (b) lungs were assessed by evaluating the extravasation of Evans blue dye. Neutrophil recruitment in the (c) intestine and (d) lungs was assessed by evaluating tissue levels of MPO. Repertaxin (3–30 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (I/R) received drug vehicle (saline). Results are shown as μg Evans blue or as the number of neutrophils per 100 mg of tissue, and are the mean±s.e.m. of at least 5–6 animals in each group. *P<0.01 when compared to sham-operated animals; #P<0.05 when compared to mild I/R animals.
Effects of Repertaxin on the local, remote and systemic injuries in a model of severe I/R injury
The next series of experiments was carried out in a model of severe I/R injury, where, in addition to the changes in vascular permeability and neutrophil accumulation, we could observe tissue haemorrhage, leucopoenia, increase in the levels of cytokine in tissue and blood and significant lethality (Souza et al., 2000b).
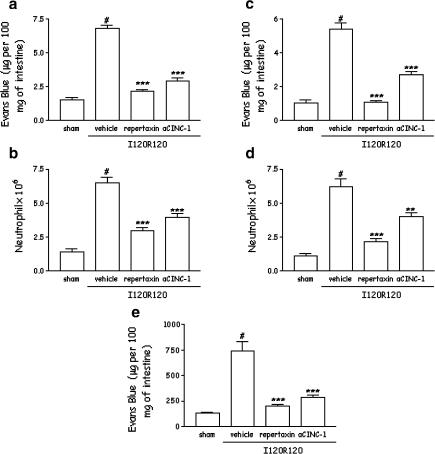
For the experiments evaluating the role of Repertaxin during severe I/R injury, the drug was used at a dose shown to be maximally inhibitory in the mild I/R injury model (30 mg kg−1). Postischaemic treatment with Repertaxin virtually abolished the increase in vascular permeability and neutrophil recruitment in the intestine and in the lung following severe I/R injury (Figure 5). Treatment with Repertaxin also abolished the intestinal increase of haemoglobin, a marker of tissue haemorrhage (Figure 5).
Figure 5.
Effects of the treatment with Repertaxin or anti-CINC-1 on the increase in vascular permeability, recruitment of neutrophils and haemorrhage in the intestine and lung following severe ischaemia (120 min) and reperfusion (120 min) injury of the SMA. Changes in vascular permeability in the (a) intestine and (b) lungs were assessed by evaluating the extravasation of Evans blue dye. Neutrophil recruitment in the (c) intestine and (d) lungs was assessed by evaluating tissue levels of MPO. Haemorrhage was evaluating by haemoglobin content in the intestine (e). Repertaxin (30 mg kg−1) was given i.v. 5 min prior to reperfusion, and the anti-CINC-1 antibody (aCINC-1) was given s.c. 60 min prior reperfusion. Control animals received saline (vehicle) or nonimune serum. Results are shown as μg Evans blue, as the number of neutrophils or μg haemoglobin per 100 mg of tissue and are the mean±s.e.m. of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals; # P< 0.05 when compared to vehicle I/R animals.
We have previously shown an increase in the concentration of blood neutrophils during the ischaemic period and a rapid drop in neutrophil levels once reperfusion occurs (Souza et al., 2000b). The concentration of circulating neutrophils at 120 min of ischaemia was similar and markedly greater in both Repertaxin and vehicle-treated than sham-operated animals (sham, 2.1±0.4 neutrophils × 106 ml−1 of blood; 120 min after ischaemia, 16.0±1.1 neutrophils; 120 min after in Repertaxin-treated animals, 15.0±1.2; n=5–6). This is consistent with the administration of Repertaxin at the end of the ischaemic period. In vehicle-treated animals, reperfusion of the ischaemic SMA induced a rapid fall of circulating neutrophils to levels observed in sham-operated animals. Pretreatment with Repertaxin reversed by approximately 40% the rapid neutropaenia that occurred 120 min after reperfusion (sham, 2.3±0.2 neutrophils × 106 ml−1 of blood; 120 min after reperfusion, 0.3±0.02 neutrophils; 120 min after reperfusion in Repertaxin-treated animals, 4.8±0.5; n=5–6, P<0.05).
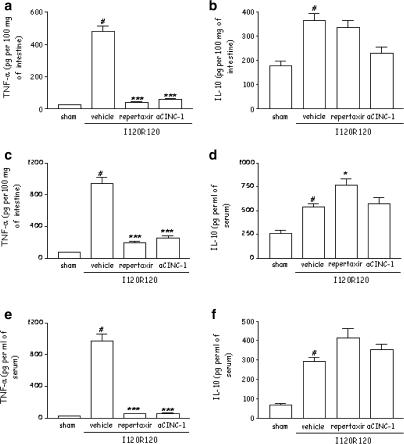
The levels of pro-inflammatory cytokines – IL-1β, IL-6 and TNF-α – and of the anti-inflammatory cytokine IL-10 are markedly elevated in serum and tissues after severe I/R injury (Figure 6, Table 1) (Souza et al., 2000b). Postischaemic treatment with Repertaxin significantly inhibited the elevations of TNF-α in tissue and serum after severe I/R injury (Figure 6a, c, e). Interestingly, pretreatment with Repertaxin was accompanied by an increase in the concentrations of IL-10 in the lung but not in intestine and serum above that observed after severe I/R injury (Figure 6b, d, f). Overall, pre-treatment with Repertaxin prevented the increase in concentrations of IL-6 in tissues and serum and augmented the increase in concentrations of IL-1β in tissues (Table 1). Repertaxin did not alter the concentrations of IL-1β in serum (Table 1).
Figure 6.
Effects of the treatment with Repertaxin or anti-CINC-1 on the concentrations of TNF-α and IL-10 in the intestine, lung and serum following severe ischaemia (120 min) and reperfusion (120 min) of the SMA. The concentrations of TNF-α (a, c, e) and IL-10 (b, d, f) were assessed in the intestine (a, b), lung (c, d) and serum (e, f) by using specific ELISA. Repertaxin (30 mg kg−1) was given i.v. 5 min prior to reperfusion and the anti-CINC-1 antibody (aCINC-1) was given s.c. 60 min prior to reperfusion. Control animals received saline (vehicle) or nonimune serum. Results are shown as pg TNF-α or IL-10 per ml of plasma or as pg TNF-α or IL-10 per 100 mg of tissue, and are the mean ±s.e.m. of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals; # P< 0.05 when compared to severe I/R animals.
Table 1.
Effects of the treatment with Repertaxin or anti-CINC-1 polyclonal antibody on the concentration of IL-1β and IL-6 in a model of severe ischaemia and reperfusion injury in rats
| IL-1β | IL-6 | |||||
|---|---|---|---|---|---|---|
| Intestine | Lung | Serum | Intestine | Lung | Serum | |
| Sham | 49±3 | 553±47 | 360±34 | 18±2 | 17±3 | 240±21 |
| Vehicle | 930±121* | 1331±11* | 1155±136* | 936±123* | 853±76* | 1716±205* |
| Repert | 1643±211*# | 1821±94*# | 955±81* | 530±40*# | 462±51*# | 291±23*# |
| aCINC | 1619±114*# | 993±108* | 935±87* | 816±72* | 447±63*# | 265±21*# |
Results in tissue and serum are expressed as pg per 100 mg of tissue and pg ml−1, respectively. Repert=Repertaxin and aCINC=anti-CINC-1 polyclonal antibody. Results are shown as pg IL-1β or IL-6 per ml of plasma or as pg IL-1β or IL-6 per 100 mg of tissue, and are the mean±s.e.m. of 5–6 animals in each group.
P<0.01 when compared to sham-operated animals;
P< 0.01 when compared to severe I/R animals.
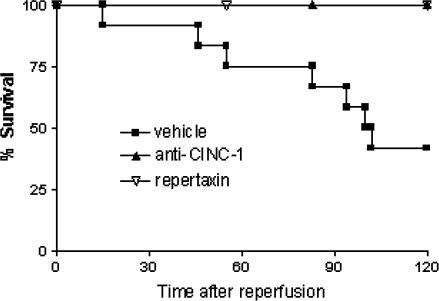
Our previous studies have shown that severe reperfusion injury is accompanied by significant TNF-α-dependent lethality, reaching 60% in most experiments (Souza et al., 2001). In the present series of experiments, 55% of animals were dead after 120 min of reperfusion (Figure 7). Treatment with Repertaxin prevented lethality and 100% of animals were alive at 120 min (Figure 7).
Figure 7.
Effects of the treatment with Repertaxin or anti-CINC-1 on the lethality following severe I/R of the SMA. Repertaxin (30 mg kg−1) was given i.v. 5 min prior to reperfusion, and the anti-CINC-1 antibody was given s.c. 60 min prior reperfusion. Control animals received saline (vehicle) or non-imune serum. Survival was monitored as indicated and animals were killed after 120 min.
Effects of the treatment with antibodies anti-CINC on the local, remote and systemic injuries in a model of severe I/R injury
As tissue and systemic inflammation was suppressed and lethality abolished in Repertaxin-treated rat and CINC-1 is one of the ligands at this receptor, it was of interest to examine whether similar effects could be observed after treatment with anti-CINC-1 antibodies. The treatment with anti-CINC-1 60 min prior to the reperfusion virtually abolished the increases in vascular permeability and influx of neutrophils in the intestine and lungs following intestinal I/R (Figure 5). The reperfusion-induced intestinal haemorrhage, as assessed by extravasation of haemoglobin, was abrogated in anti-CINC-1-treated animals (Figure 5). As in mice treated with Repertaxin, pretreatment with anti-CINC-1 also reversed by approximately 40% the rapid neutropaenia that occurred 120 min after reperfusion (sham, 2.1±0.4 neutrophils × 106 ml−1 of blood; 120 min after reperfusion, 0.3±0.02 neutrophils; 120 min after reperfusion in anti-CINC-treated animals, 4.9±0.5; n=5–6, P<0.05).
Anti-CINC-1 also prevented the reperfusion-induced increase in TNF-α concentrations in tissue and serum (Figure 6). Our previous studies have shown a strong correlation between serum concentrations of TNF-α and lethality (Souza et al., 2001, 2002a). Consistent with these results, treatment of mice with anti-CINC prevented the lethality that followed reperfusion of the ischaemic mesenteric artery (Figure 7). Anti-CINC failed to enhance significantly the increases in IL-10 production in the lungs, intestine and serum following reperfusion of the ischaemic SMA (Figure 6). Furthermore, pretreatment with anti-CINC prevented the increase in concentrations of IL-6 in tissues and serum, whereas this treatment had little effects on the concentrations of IL-1β (Table 1).
Discussion
Several studies, including that of our own group, have demonstrated that intestinal I/R injury in rats is dependent on neutrophil recruitment (Ma et al., 1993; Lefer et al., 1996; Omata et al., 1997; Ritter et al., 1998; Souza et al., 2000a, 2000b; Onai et al., 2003). For example, the inhibition of selectins or integrins expressed on neutrophils is capable of inhibiting neutrophil influx and, consequently, decreases reperfusion injury to the tissues (Souza et al., 2000a, 2000b). It is suggested that strategies that limit neutrophil accumulation and/or activation may be a useful adjuvant in the treatment of ischaemic disorders. One possible strategy to prevent neutrophil influx/activation is the inhibition and/or antagonism of mediators that activate neutrophils. Among the mediators known to activate neutrophils very potently and effectively are CXC-ELR+ chemokines (Baggiolini et al., 1995). These chemokines act by activating CXCR1 (absent in rodents) and CXCR2 receptors on the surface of neutrophils. Indeed, several studies have now shown that anti-CXC-ELR+ or anti-CXCR2 antibodies prevent I/R injury in several vascular beds (Boyle et al., 1998; Tsuruma et al., 1998; Yagihashi et al., 1998; Miura et al., 2001). Here, we tested a novel inhibitor of human CXCL8 receptors, Repertaxin, for its ability to prevent neutrophil chemotaxis in vitro and intestinal I/R injury in rats.
The chemoattractant mediators PAF, LTB4, fMLP and CXC chemokines were effective inducers of neutrophil recruitment in vitro. Treatment with Repertaxin prevented the chemotaxis of neutrophils induced by CINC-1 or CXCL8, but failed to alter the effects of PAF, LTB4 or fMLP. Repertaxin has been shown to be a noncompetitive allosteric inhibitor of human CXCR1 and CXCR2. The drug did not affect binding of radiolabelled CXCL8 to human PMN, whereas it inhibited CXCL8 (but not fMLP)-induced Ca+2 mobilization and tyrosine kinase activation, suggesting that Repertaxin affects CXCL8 receptor-induced signal transduction in human PMN (Bertini et al., 2004). Similarly, we show that Repertaxin prevented CXCL8-induced Ca+2 mobilization in rat neutrophils, but failed to alter CXCL-8 binding to these cells. Altogether these studies confirm our previous findings in human neutrophils (Bertini et al., 2004) and suggest that repertaxin is also a noncompetitive allosteric inhibitor of rat CXCR2.
Initial experiments in a model of mild I/R injury showed that Repertaxin dose-dependently inhibited both the local (intestine) and remote (lung) increase in vascular permeability and neutrophil accumulation. As the local influx of neutrophils is a determinant in the development of reperfusion injury following ischaemia, the capacity of Repertaxin to modulate the recruitment of neutrophils may underlie the beneficial effects of the drug in this model of mild reperfusion-induced injury. Importantly, Repertaxin was administered at the end of the ischaemic period and just prior to reperfusion, thus mimicking closely the clinical situation.
In the model of more severe ischaemia–reperfusion injury, in addition to the vascular permeability and neutrophil influx, there are marked systemic alterations that include hypotension, elevated levels of pro-inflammatory cytokines, neutropaenia and death (Souza et al., 2000b). It was, thus, of interest to examine whether CXCR2 inhibitor would also function in this model of more severe injury. Pretreatment with Repertaxin markedly inhibited both the neutrophil accumulation and increase in vascular permeability. Not only was the site of injury (i.e. the intestine) protected, but there was also marked protection of the reperfusion injury to the lungs. The inhibition of neutrophil recruitment into tissue was reflected by the partial capacity of Repertaxin treatment to reverse the neutropaenia observed during reperfusion. Moreover, Repertaxin greatly attenuated intestinal pathology, as attested by the decrease in haemorrhage.
After prolonged reperfusion injury, there is a marked local and systemic release of pro-inflammatory cytokines, including TNF-α, IL-6 and IL-1β (Souza et al., 2001, 2003). Of these cytokines, TNF-α appears to play a major pathophysiological role, as its inhibition prevents tissue injury and lethality (Souza et al., 2001, 2002c). Interestingly, we have previously shown that the local influx of neutrophils is an important player in the cascade of events leading to tissue, but not systemic, TNF-α production. On the other hand, the initial tissue release of TNF-α, possibly mast cell-derived, is essential for neutrophil influx to occur. An amplification circuit is thus installed in which neutrophil influx facilitates TNF-α production and TNF-α production facilitates neutrophil influx (Souza et al., 2001, 2002c). Inhibition of CXCR2 is accompanied by virtual abolishment of the increase in concentration of TNF-α in tissues of reperfused animals. Thus, the ability of Repertaxin shown to modulate both neutrophil influx and TNF-α production could be contributing to the beneficial effects of these drugs in the system. In addition to abolishing the increase in tissue concentrations of TNF-α, Repertaxin prevented the increase in concentration of TNF-α in serum. As systemic concentrations of TNF-α appear to be the best correlate of lethality in our system (Souza et al., 2001, 2002c), the latter results are consistent with the ability of Repertaxin to prevent lethality. Interestingly, we have previously shown that the inhibition of selectins was capable of inhibiting reperfusion-induced neutrophil influx and tissue lesions, without however decreasing systemic TNF-α and lethality (Souza et al., 2000a, 2000b). Thus, the inhibition of neutrophil influx by Repertaxin was more efficacious than inhibition with the selectin inhibitor fucoidin. One unproven possibility to explain the latter findings is that Repertaxin, but not fucoidin, prevented the activation of circulating neutrophils and, consequent, systemic production of TNF-α and TNF-α-dependent lethality.
In agreement with the literature (Yao et al., 1997; Yamamoto et al., 2001), the concentrations of IL-1β and IL-6 in tissue and serum were elevated following intestinal ischaemia–reperfusion injury. Repertaxin prevented the reperfusion-induced increase in IL-6 production. In our system, the levels of IL-6 do not correlate with disease severity (Souza et al., 2002b), but the effects of Repertaxin on IL-6 may be an additional beneficial effect of this CXCR2 inhibitor. In contrast to its inhibitory effect on IL-6 production, Repertaxin did not have an effect on the increases in the concentration of IL-1β in serum and actually enhanced the tissue concentrations of the cytokine. One important additional finding was the ability of the pretreatment with Repertaxin to enhance the concentrations of IL-10 in lung following severe reperfusion-associated injury. We have previously shown that IL-10 was a major protective endogenous cytokine during I/R injury in rats, and that IL-1β was a major force driving IL-10 production (Souza et al., 2003). It is unclear why inhibition of CXCR2 function would facilitate the production of IL-1β and consequent production of IL-10. Nevertheless, the increase of IL-10 concentration may play a role in the protective effects afforded by Repertaxin in our model of intestinal I/R injury.
As rat CINC-1 is one the CXC-ELR+ chemokines capable of binding to rat CXCR2, we evaluated comparatively the effects of an anti-CINC-1 antibody in our system. The anti-CINC-1 antibody was very effective at inhibiting oedema formation, intestinal haemorrhage and TNF-α concentration, likely by inhibiting the recruitment and/or activation of neutrophils. However, the effect of anti-CINC-1 on IL-6, IL-1β and IL-10 levels was less intense when compared with treatment with Repertaxin. Moreover, the drug appeared to be slightly more effective than the antibody, especially in the lung, suggesting that other CXC-ELR+ chemokines acting on the CXCR2 receptors were also relevant for neutrophil influx and tissue injury in our model.
Altogether, our results suggest that CINC-1 and, possibly, other CXC-ELR+ chemokines, acting on CXCR2, have an important role during I/R injury. Thus, drugs, such as Repertaxin, developed to block the function of the CXCR2 receptor may be effective at preventing reperfusion injury in relevant clinical situations.
Acknowledgments
We are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo as Pesquisas do Estado de Minas Gerais (FAPEMIG) and Fundação de Amparo as Pesquisas do Estado de São Paulo (FAPESP) for financial support.
Abbreviations
- CINC-1
cytokine-induced neutrophil chemoattractant-1
- IL
Interleukin
- I/R
ischaemia and reperfusion
- LTB4
leukotriene B4
- PAF
platelet activating factor
- PMN
polymorphonuclear cells
- SMA
superior mesenteric artery
- MPO
myeloperoxidase
References
- BAGGIOLINI M., LOETSCHER P., MOSER B. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F. The neutrophil as a mediator of myocardial ischemia–reperfusion injury: time to move on. Basic Res. Cardiol. 2002;97:268–275. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- BERTINI R., ALLEGRETTI M., BIZZARRI C., MORICONI A., LOCATI M., ZAMPELLA G., CERVELLERA M.N., DI CIOCCIO V., CESTA M.C., GALLIERA E., MARTINEZ F.O., DI BITONDO R., TROIANI G., SABBATINI V., D'ANNIBALLE G., ANACARDIO R., CUTRIN J.C., CAVALIERI B., MAINIERO F., STRIPPOLI R., VILLA P., DI GIROLAMO M., MARTIN F., GENTILE M., SANTONI A., CORDA D., GHEZZI P., POLI G., MANTOVANI A., COLOTTA F.A new class of non-competitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury Proc. Nat. Acad. Sci. U.S.A. 2004(in press) [DOI] [PMC free article] [PubMed]
- BIGNOLD L.P., FERRANTE A. Mechanism of separation of polymorphonuclear leukocytes from whole blood by the one-step Hypaque–Ficoll method. J. Immunol. Methods. 1987;96:29–33. doi: 10.1016/0022-1759(87)90363-2. [DOI] [PubMed] [Google Scholar]
- BIZZARRI C., BERTINI R., BOSSÙ P., SOZZANI S., MANTOVANI A., VAN DAMME J., TAGLIABUE A., BORASCHI D. Single-cell analysis of macrophage chemotactic protein-1-regulated cytosolic Ca2+ increase in human adherent monocytes. Blood. 1995;86:2388–2394. [PubMed] [Google Scholar]
- BIZZARRI C., PAGLIEI S., BRANDOLINI L., MASCAGNI P., CASELLI G., TRANSIDICO P., SOZZANI S., BERTINI R. Selective inhibition of interleukin-8-induced neutrophil chemotaxis by ketoprofen isomers. Biochem. Pharmacol. 2001;61:1429–1437. doi: 10.1016/s0006-2952(01)00610-4. [DOI] [PubMed] [Google Scholar]
- BOYLE E.M., JR, KOVACICH J.C., HEBERT C.A., CANTY T.G., JR, CHI E., MORGAN E.N., POHLMAN T.H., VERRIER E.D. Inhibition of interleukin-8 blocks myocardial ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 1998;116:114–121. doi: 10.1016/S0022-5223(98)70249-1. [DOI] [PubMed] [Google Scholar]
- BRANDOLINI L., BERTINI R., BIZZARRI C., SERGI R., CASELLI G., ZHOU D., LOCATI M., SOZZANI S. IL-1 beta primes IL-8-activated human neutrophils for elastase release, phospholipase D activity, and calcium flux. J. Leukoc. Biol. 1996;59:427–434. doi: 10.1002/jlb.59.3.427. [DOI] [PubMed] [Google Scholar]
- CHANDRASEKAR B., SMITH J.B., FREEMAN G.L. Ischemia–reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- CORNEJO C.J., WINN R.K., HARLAN J.M. Anti-adhesion therapy. Adv. Pharmacol. 1997;39:99–142. doi: 10.1016/s1054-3589(08)60070-8. [DOI] [PubMed] [Google Scholar]
- DEFORGE L.E., KENNEY J.S., JONES M.L., WARREN J.S., REMICK D.G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- FRANCISCHI J.N., YOKORO C.M., CUNHA F.Q., TAFURI W.L., TEIXEIRA M.M. Effects of the PDE4 inhibitor rolipram in a rat model of arthritis. Eur. J. Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- GILMONT R.R., DARDANO A., ENGLE J.S., ADAMSON B.S., WELSH M.J., LI T., REMICK D.G., SMITH D.J., JR, REES R.S. TNF-alpha potentiates oxidant and reperfusion-induced endothelial cell injury. J. Surg. Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- HAGAN P., POOLE S., BRISTOW A.F. Endotoxin-stimulated production of rat hypothalamic interleukin-1 beta in vivo and in vitro, measured by specific immunoradiometric assay. J. Mol. Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- JAESCHKE H., FARHOOD A., SMITH C.W. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- KALFIN R.E., ENGELMAN R.M., ROUSOU J.A., FLACK J.E., III, DEATON D.W., KREUTZER D.L., DAS D.K. Induction of interleukin-8 expression during cardiopulmonary bypass. Circulation. 1993;88:II401–II406. [PubMed] [Google Scholar]
- KATAOKA M., SHIMIZU H., MITSUHASHI N., OHTSUKA M., WAKABAYASHI Y., ITO H., KIMURA F., NAKAGAWA K., YOSHIDOME H., SHIMIZU Y., MIYAZAKI M. Effect of cold-ischemia time on C–X–C chemokine expression and neutrophil accumulation in the graft liver after orthotopic liver transplantation in rats. Transplantation. 2002;73:1730–1735. doi: 10.1097/00007890-200206150-00007. [DOI] [PubMed] [Google Scholar]
- KOHTANI T., ABE Y., SATO M., MIYAUCHI K., KAWACHI K. Protective effects of anti-neutrophil antibody against myocardial ischemia/reperfusion injury in rats. Eur. Surg. Res. 2002;34:313–320. doi: 10.1159/000063073. [DOI] [PubMed] [Google Scholar]
- KYRIAKIDES C., AUSTEN W., JR, WANG Y., FAVUZZA J., KOBZIK L., MOORE F.D., JR, HECHTMAN H.B. Membrane attack complex of complement and neutrophils mediate the injury of acid aspiration. J. Appl. Physiol. 1999;87:2357–2361. doi: 10.1152/jappl.1999.87.6.2357. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., FLYNN D.M., BUDA A.J. Effects of a monoclonal antibody directed against P-selectin after myocardial ischemia and reperfusion. Am. J. Physiol. 1996;270:H88–H98. doi: 10.1152/ajpheart.1996.270.1.H88. [DOI] [PubMed] [Google Scholar]
- LORENZETTI B.B., VEIGA F.H., CANETTI C.A., POOLE S., CUNHA F.Q., FERREIRA S.H. Cytokine-induced neutrophil chemoattractant 1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivity in rats. Eur. Cytokine Netw. 2002;13:456–461. [PubMed] [Google Scholar]
- MA X.L., WEYRICH A.S., LEFER D.J., BUERKE M., ALBERTINE K.H., KISHIMOTO T.K., LEFER A.M. Monoclonal antibody to L-selectin attenuates neutrophil accumulation and protects ischemic reperfused cat myocardium. Circulation. 1993;88:649–658. doi: 10.1161/01.cir.88.2.649. [DOI] [PubMed] [Google Scholar]
- MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1 or PAF receptor blockade on the lung injury induced by scorpion venom in rats. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- MERCHANT S.H., GURULE D.M., LARSON R.S. Amelioration of ischemia–reperfusion injury with cyclic peptide blockade of ICAM-1. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1260–H1268. doi: 10.1152/ajpheart.00840.2002. [DOI] [PubMed] [Google Scholar]
- MIURA M., FU X., ZHANG Q.W., REMICK D.G., FAIRCHILD R.L. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am. J. Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- OMATA M., MATSUI N., INOMATA N., OHNO T. Protective effects of polysaccharide fucoidin on myocardial ischemia–reperfusion injury in rats. J. Cardiovasc. Pharmacol. 1997;30:717–724. doi: 10.1097/00005344-199712000-00003. [DOI] [PubMed] [Google Scholar]
- ONAI Y., SUZUKI J., NISHIWAKI Y., GOTOH R., BERENS K., DIXON R., YOSHIDA M., ITO H., ISOBE M. Blockade of cell adhesion by a small molecule selectin antagonist attenuates myocardial ischemia/reperfusion injury. Eur. J. Pharmacol. 2003;481:217–225. doi: 10.1016/j.ejphar.2003.09.040. [DOI] [PubMed] [Google Scholar]
- RAMOS C.D., HELUY-NETO N.E., RIBEIRO R.A., FERREIRA S.H., CUNHA F.Q. Neutrophil migration induced by IL-8-activated mast cells is mediated by CINC-1. Cytokine. 2003;21:214–223. doi: 10.1016/s1043-4666(03)00050-4. [DOI] [PubMed] [Google Scholar]
- REES G.S., BALL C., WARD H.L., GEE C.K., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat IL-6:expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999a;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- REES G.S., GEE C.K., WARD H.L., BALL C., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999b;10:383–392. [PubMed] [Google Scholar]
- REN G., DEWALD O., FRANGOGIANNIS N.G. Inflammatory mechanisms in myocardial infarction. Curr. Drug Targets Inflamm. Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- RITTER L.S., COPELAND J.G., MCDONAGH P.F. Fucoidin reduces coronary microvascular leukocyte accumulation early in reperfusion. Ann. Thorac. Surg. 1998;66:2063–2072. doi: 10.1016/s0003-4975(98)00823-6. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000a;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000b;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., GUABIRABA R., PINHO V., BRISTOW A., POOLE S., TEIXEIRA M.M. IL-1-driven endogenous IL-10 production protects against the systemic and local acute inflammatory response following intestinal reperfusion injury. J. Immunol. 2003;170:4759–4766. doi: 10.4049/jimmunol.170.9.4759. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., MENDONÇA V.A., DE A., CASTRO M.S., POOLE S., TEIXEIRA M.M. Role of tachykinin NK receptors on the local and remote injuries following ischaemia and reperfusion of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2002a;135:303–312. doi: 10.1038/sj.bjp.0704464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., PINHO V., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effect of a BLT receptor antagonist in a model of severe ischemia and reperfusion injury in the rat. Eur. J. Pharmacol. 2002b;440:61–69. doi: 10.1016/s0014-2999(02)01313-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., SOARES A.C., PINHO V., TORLONI H., REIS L.F., TEIXEIRA M.M., DIAS A.A. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am. J. Pathol. 2002c;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUMA T., YAGIHASHI A., TARUMI K., HIRATA K. Anti-rat IL-8 (CINC) monoclonal antibody administration reduces ischemia–reperfusion injury in small intestine. Transplant. Proc. 1998;30:2644–2645. doi: 10.1016/s0041-1345(98)00765-9. [DOI] [PubMed] [Google Scholar]
- WEIGHT S.C., BELL P.R., NICHOLSON M.L. Renal ischaemia–reperfusion injury. Br. J. Surg. 1996;83:162–170. [PubMed] [Google Scholar]
- WILLERSON J.T. Pharmacologic approaches to reperfusion injury. Adv. Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]
- XIAO F., EPPIHIMER M.J., WILLIS B.H., CARDEN D.L. Complement-mediated lung injury and neutrophil retention after intestinal ischemia–reperfusion. J. Appl. Physiol. 1997;82:1459–1465. doi: 10.1152/jappl.1997.82.5.1459. [DOI] [PubMed] [Google Scholar]
- YAGIHASHI A., TSURUMA T., TARUMI K., KAMESHIMA T., YAJIMA T., YANAI Y., WATANABE N., HIRATA K. Prevention of small intestinal ischemia–reperfusion injury in rat by anti-cytokine-induced neutrophil chemoattractant monoclonal antibody. J. Surg. Res. 1998;78:92–96. doi: 10.1006/jsre.1998.5367. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., TANABE M., WAKABAYASHI G., SHIMAZU M., MATSUMOTO K., KITAJIMA M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia–reperfusion injury of the rat small intestine. J. Surg. Res. 2001;99:134–141. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- YAO Y.M., BAHRAMI S., REDL H., FUERST S., SCHLAG G. IL-6 release after intestinal ischemia/reperfusion in rats is under partial control of TNF. J. Surg. Res. 1997;70:21–26. doi: 10.1006/jsre.1997.5074. [DOI] [PubMed] [Google Scholar]