Abstract
Vasopeptidase inhibition (i.e., the simultaneous inhibition of both angiotensin-converting enzyme (ACE) and neutral endopeptidase) can ameliorate diabetic nephropathy. We investigated whether this nephroprotection is mediated by the bradykinin B2 receptor.
In all, 43 obese Zucker diabetic fatty (ZDF/Gmi-fa/fa) rats aged 21 weeks were separated into four groups and treated for 26 weeks with either placebo, the bradykinin B2 receptor antagonist icatibant (500 μg kg−1 day−1 s.c. infusion), the vasopeptidase inhibitor AVE7688 (45 mg kg−1 day−1 in chow), or AVE7688 plus icatibant. Nephropathy was assessed as albuminuria at age 31 and 39 weeks, and by histopathologic scoring at the end of the treatment period.
All animals had established diabetes mellitus (blood glucose >20 mmol l−1) and marked albuminuria at baseline. Blood glucose was not influenced by any treatment. Icatibant alone did not influence albuminuria (8.6±1.6 vs placebo 9.5±1.3 mg kg−1 h−1). AVE7688 reduced albuminuria at week 31 markedly to 1.1±0.1 mg kg−1 h−1 and reduced glomerular and tubulo-interstitial kidney damage at week 47. In the AVE7688 plus icatibant group, proteinuria was significantly higher than in the AVE7688 only group (2.0±0.6 mg kg−1 h−1), but still reduced compared to placebo. In addition, icatibant partly antagonized the tubulo-interstitial protection mediated by AVE7688.
We conclude that vasopeptidase inhibition provides nephroprotection in rats with type II diabetic nephropathy, which is partly mediated by bradykinin B2 receptor activation.
Keywords: Zucker diabetic fatty rat, diabetic nephropathy, vasopeptidase inhibition, AVE7688
Introduction
Diabetic nephropathy is a devastating disease characterized by progressive impairment of glomerular function and, ultimately, end-stage renal disease. As a result of the diabetes epidemic in Westernized countries, the incidence of diabetic nephropathy is predicted to increase substantially over the next decade. In fact, type II diabetes has already become the most common single cause for renal replacement therapy in most industrialized countries (Ismail et al., 1999). Several landmark trials have demonstrated convincingly that blockade of the renin–angiotensin system, including inhibition of ACE, is effective to retard the progression of diabetic nephropathy and to prevent end-stage renal failure (Lewis et al., 1993; 2001; HOPE Study Investigators, 2000; Brenner et al., 2001). Part of the pharmacological action of ACE inhibitors is based on inhibition of bradykinin degradation, leading to increased stimulation of the bradykinin B2 receptor (Wirth et al., 1997). Blockade of the bradykinin B2 receptor partially prevented the cardiovascular benefits of ACE inhibition in different disease models, demonstrating the pharmacological relevance of bradykinin signaling in cardiovascular disease (for a review, see Linz et al., 1995). As bradykinin is degraded by both ACE and neutral endopeptidase, simultaneous inhibition of both enzymes by the novel vasopeptidase inhibitors is supposed to increase renal bradykinin concentrations and protect the diabetic kidney even more effectively than selective ACE inhibition alone (Molinaro et al., 2002). However, the relative role of bradykinin in the therapeutic action of vasopeptidase inhibitors in diabetic nephropathy has not been investigated so far. Recent data indicate that indeed, vasopeptidase inhibitors may be superior over pure ACE inhibitors, possibly related to their greater potency in increasing tissue bradykinin concentrations. The vasopeptidase inhibitor omapatrilat has shown superior nephroprotection over selective ACE inhibition in nondiabetic nephropathy (Taal et al., 2001) and in a hypoinsulinaemic, hypertensive model (Tikkanen et al., 1998; Davis et al., 2003). In the Zucker diabetic fatty (ZDF) rat, a type II diabetes animal model, the vasopeptidase inhibitor AVE7688 prevents nephropathy when treatment is started early (Schäfer et al., 2003) and reduces proteinuria when diabetes and nephropathy are established (Schäfer et al., 2004).
In order to further elucidate the mechanism behind these nephroprotective effects, we investigated the effects of AVE7688 on functional and morphological end points in a setting of established diabetic nephropathy in the presence and absence of the bradykinin B2 receptor antagonist, icatibant. Our data indicate that the beneficial effects of vasopeptidase inhibition in type II diabetic nephropathy are partially, but not completely, mediated by the bradykinin B2 receptor.
Methods
Animals
The animal experiments were performed in accordance with the Aventis Laboratory Animal Science and Welfare (LASW) guidelines and the German law for the protection of animals. Male ZDF rats (Gmi ZDF fa/fa) were purchased from Charles River, Sulzfeld, Germany. The animals were housed individually in standard cages; they received tap water ad libitum and standard chow containing 0.2% sodium and 19% crude protein (Standard diet #1320, Altromin, Lage, Germany).
Groups
At age 21 weeks, after baseline measurements in a random subset of the ZDF rats, the animals were randomly assigned to one of four groups receiving either no specific treatment (n=10, Plac), the bradykinin B2 receptor antagonist icatibant (n=11, Plac+Ica), the vasopeptidase inhibitor AVE7688 (n=12, AVE), or AVE7688 plus icatibant (n=10, AVE+Ica). AVE7688 was administered orally, pressed in chow at a concentration of 450 mg kg−1. Taking into account an average daily food intake of approximately 40 g day−1 per rat, the resulting daily dose of 45 mg kg−1 day−1 was similar to the one which prevented diabetic nephropathy in a previous study (Schäfer et al., 2003). Icatibant was continuously infused via osmotic minipumps at a calculated rate of 500 μg kg−1 day−1, a dose which had previously been demonstrated to yield complete blockade of the bradykinin B2 receptor in rats (Wirth et al., 1991). The minipumps were implanted subcutaneously under isoflurane anaesthesia, as previously described (Gohlke et al., 1994), and exchanged every 4 weeks.
Metabolism and renal function
Metabolic characterization was performed by collecting urine over 24 h in metabolic cages and by drawing blood samples from the retro-orbital plexus under light anaesthesia (3–3.5 vol. % isoflurane in 34 : 66 v v−1 N2O/O2) the following day. At baseline, (age 21 weeks), these measurements were carried out in a random subset of all animals (n=10). After 1 day, the different treatments were started and the measurements repeated in all animals at age 31 weeks. At age 39 weeks, blood samples were again collected from the retro-orbital plexus, but only a spot urine sample was taken to determine urinary albumin – creatinine ratio as a measure of renal function.
Haemodynamics
At age 39 weeks, heart rate and systolic blood pressure were measured noninvasively using the tail-cuff method (TSE GmbH, BP system V2.2, Bad Homburg, Germany) in all animals. The animals were awake and had been accustomed to the apparatus a few days before the measurements.
Post-mortem studies
At age 47 weeks, the rats were killed by quickly excising the heart and kidneys under deep anaesthesia (ketamin 100 mg kg−1 and xylazine 2 mg kg−1 i.m.). In the two groups which received AVE7688, additional blood samples were drawn for determination of M108048, the major metabolite of AVE7688. After removal, the kidneys were weighed and fixed in 10% buffered formalin. Kidney weights were normalized to body weight. Following standard haematoxylin–eosin and periodic acid Schiff (PAS) staining, the incidence and extent of glomeruloscerosis and tubular atrophy were assessed on a semiquantitative scale. Briefly, glomerular damage was scored as either normal appearance (zero points); focal-segmental mesangial thickening in a few isolated glomeruli (one point); mesangial thickening in several glomeruli (two points); diffuse or nodular mesangial thickening, synechia with Bowman's capsule in many (but less than 50%) of glomeruli, or scattered glomerular sclerosis (three points); same criteria as before but present in more than 50% of glomeruli (four points); or diffuse glomerulosclerosis and synechia with Bowman's capsule (five points). Tubulo-interstitial damage was defined as either normal appearance (zero points); luminal narrowing of a few tubules (one point); luminal narrowing and thickening of the epithelial basement membrane (two points); luminal narrowing and/or thickening of basement membrane and mild small-cell infiltration (three points); more frequent occurrence of the above criteria (four points); or diffuse tubular atrophy and small-cell infiltration (five points). The histopathological evaluation was carried out twice by one investigator (H.-L. Schmidts) in a blinded fashion. The mean value of the two evaluations was taken as the semiquantitative score for each animal.
Laboratory measurements
Glucose (in whole blood) and creatinine (in serum and urine) were quantified with standard kits (Roche Diagnostics) using a Hitachi 912 E analyzer. Urinary albumin was quantified using a fluorescence dye-binding assay (Mikroflural, Progen Biotechnik GmbH, Heidelberg, Germany).
The activity of ACE was measured in the plasma using a spectrophotometric assay. Hydrolysis of the tripeptide N-[3-(2-furyl)acryloyl]-L-phenylanalyl-glycyl-glycin (FAPPG) into furylacryloyl – phenylalanine and glycin – glycin is catalysed by ACE. The activity of the enzyme was determined by measuring the decrease in absorption at λ=340 nm (Hitachi type 912 automatic analyzer) in comparison to known standards.
For determination of M108048, the plasma samples were treated with 3 N hydrochloric acid (1 : 10), cooled at 0°C and, after mixing, frozen in a dry ice/ethanol bath and stored at −20°C. Time between blood collection and freezing did not exceed 45 min. Samples were analysed by LC-MS/MS after pretreatment with dithiothreitol for cleavage of disulphide bonds.
Materials
AVE7688 and icatibant were manufactured by Aventis Pharma. The chemical name of AVE7688 is 7-[[(2S)-2-(acetylthio)-1-oxo-3-methylpropyl]amino]-1,2,3,4,6,7,8,12b-octahydro-6-oxo-(4S,7S,12bR)-pyrido[2,1-a][2]benzapin-4-carboxy-acid, its molecular weight is 432.5. The chemical name of icatibant is D-arginyl-L-arginyl-L-prolyl-[(4R)-4-hydroxyprolyl]-glycyl-L-[3-(2-thienyl)analyl]-L-seryl-D-(1,2,3,4-tetrahydroisoquinnoline-3-yl-carbonyl)-L-[(3aS,7aS)-octahydroindol-2-yl-carbonyl]-L-arginine, its molecular weight is 1304.6.
All other reagents were purchased from commercial suppliers in analytical grade quality.
Statistics
Differences were tested for statistical significance between the placebo group and Plac+Ica, AVE and AVE+Ica groups, respectively, and between the AVE and AVE+Ica groups. An unpaired two-sided t-test was used. As corrections for multiple testing (four tests) were carried out by the Bonferroni method, P<0.0125 was considered significant. Data are presented as mean±s.e.m.
Results
Fundamental biometric and laboratory data over time are given in Table 1. In the placebo group, body weight tended to decrease over time, despite an increase in food intake. Concomitant treatment with icatibant increased body weight in the placebo and AVE groups. This effect was not due to an increased food intake in these groups. The marked elevation of blood glucose at age 21 weeks indicates that the animals were overtly diabetic from the beginning of the study. No treatment significantly influenced blood glucose. Plasma ACE activity was drastically reduced by AVE7688, to a similar extent in the absence and presence of icatibant.
Table 1.
Biometric and laboratory parameters
| Age (weeks) | Placebo | Placebo+Ica | AVE | AVE+Ica |
|---|---|---|---|---|
| Body weight (g) | ||||
| 21 (baseline) | 400±9 | |||
| 31 | 379±6 | 438±18* | 388±10 | 423±13 |
| 39 | 362±8 | 414±16* | 384±9 | 406±13* |
| Food intake (g day−1) | ||||
| 21 (baseline) | 35±2 | |||
| 31 | 51±2 | 44±3 | 39±2 | 43±2 |
| 39 | ND | ND | ND | ND |
| Blood glucose (mmol l−1) | ||||
| 21 (baseline) | 31.5±1.1 | |||
| 31 | 32.7±0.8 | 28.7±1.2 | 29.9±1.1 | 31.4±0.9 |
| 39 | 34.7±1.2 | 32.9±1.2 | 33.3±0.8 | 33.1±1.3 |
| Plasma ACE activity (U l−1) | ||||
| 21 (baseline) | ND | |||
| 31 | 112±2 | 101±4 | 10±3* | 6±0.3* |
| 39 | 116±4 | 101±3 | 10±1* | 10±1* |
P<0.0125 vs Placebo; ND, not determined.
Systolic blood pressure was 164±6, 155±5, 121±11 and 119±6 mmHg in the Plac, Plac+Ica, AVE and AVE+Ica groups, respectively. Both groups receiving AVE7688 had lower blood pressures than Plac (P<0.0125), but there was no difference between AVE and AVE+Ica. Heart rate was 319±7, 313±7, 324±15, 316±12 beats min−1 in the Plac, Plac+Ica, AVE and AVE+Ica groups, respectively. There were no differences between groups.
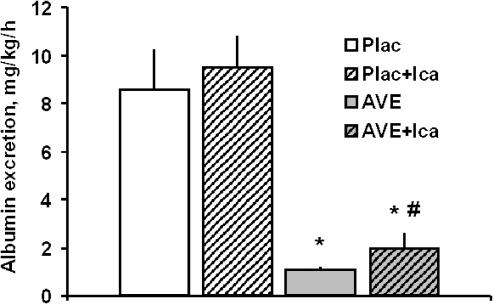
Renal functional parameters are given in Table 2. In the placebo group, creatinine clearance increased from baseline to age 31 weeks. This increase was ameliorated by AVE7688. However, compared to baseline values at age 21 weeks, creatinine clearance was increased at 31 weeks, despite chronic treatment with the AVE7688. Urinary albumin excretion increased markedly from age 21 weeks to 31 weeks in the Plac and Plac+Ica groups. At week 31, AVE7688 had drastically reduced albuminuria compared to placebo, whether expressed as albumin excretion rate (Figure 1) or albumin–creatinine ratio (Table 2). Moreover, the data on albumin–creatinine ratio illustrate that the nephroprotective effect by AVE7688 is maintained during prolonged treatment, up to age week 39 (Table 1). In the AVE+Ica group, albumin excretion rate at week 31 was significantly higher (approximately twofold) compared to AVE alone, but still markedly reduced compared to placebo (Figure 1). Similarly, the urinary albumin–creatinine ratio tended to be higher in the AVE+Ica than in the AVE alone group at weeks 31 and 39, but this effect was not statistically significant (Table 2).
Table 2.
Parameters of renal function
| Age (weeks) | Placebo | Placebo+Ica | AVE | AVE+Ica |
|---|---|---|---|---|
| Creatinine clearance (ml kg−1 min−1) | ||||
| 21 (baseline) | 6.8±0.4 | |||
| 31 | 10.0±0.3 | 9.2±0.3 | 8.4±0.3* | 8.7±0.4* |
| 39 | ND | ND | ND | ND |
| Urinary albumin–creatinine ratio (mg mmol−1) | ||||
| 21 (baseline) | 1331±230 | |||
| 31 | 957±102 | 1193±176 | 135±12* | 211±60* |
| 39 | 1257±206 | 1732±225 | 101±17* | 172±41* |
P<0.0125 vs Placebo; ND, not determined.
Figure 1.
Effect of AVE7688 and icatibant on albuminuria at age 31 weeks (after 10 weeks chronic treatment). Icatibant alone has no effect on albuminuria but antagonizes part of the antialbuminuric effect of AVE7688. Plac, Placebo group; Ica, icatibant. *P<0.0125 vs Plac; #P<0.0125 vs AVE.
The plasma levels of M108048 were 23±3 and 18±3 μg l−1 in the AVE and AVE+Ica groups, respectively. These concentrations were not different from each other. They exceed the in vitro IC50s for ACE (0.05 nmol l−1) and neutral endopeptidase (5.0 nmol l−1) (Schäfer et al., 2003) by approximately one order of magnitude.
The kidney weights were reduced by AVE7688 (0.45±0.02 g (100 g−1)) compared to Plac (0.53±0.02 g (100 g−1)), P<0.0125. Icatibant did not influence this effect (Plac+Ica: 0.47±0.02 g (100 g−1), P=NS vs Plac; AVE+Ica: 0.44±0.02 g (100 g−1), P=NS vs AVE).
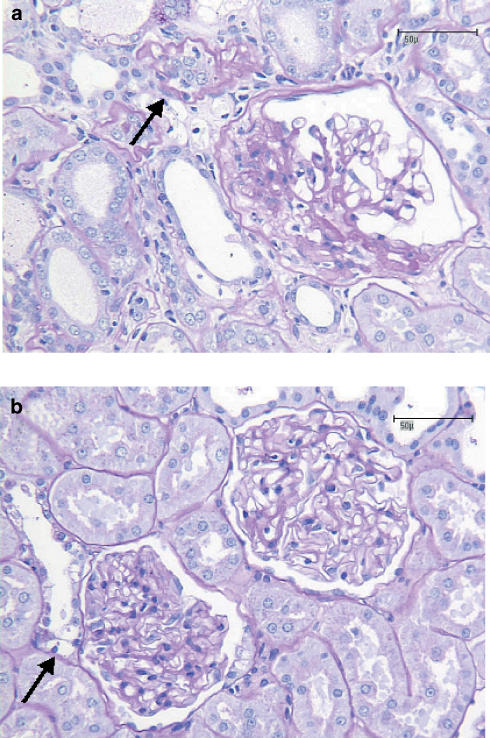
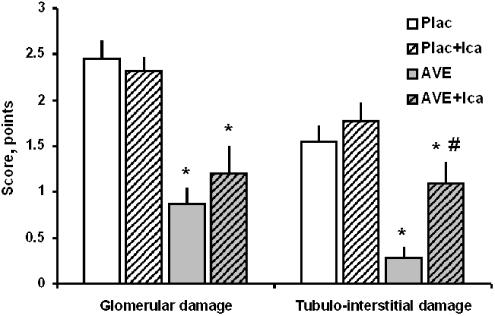
Representative microscopic pictures of renal specimens from a placebo and an AVE7688-treated rat are shown in Figure 2. The maximum number of points in the semiquantitative glomerular or tubulo-interstitial damage score that was reached by any single animal was 3; higher degrees of damage were not observed in the present study. The summary of the histopathologic scoring of the kidneys is represented in Figure 3. Icatibant alone had no effect on glomerular or tubulo-interstitial damage. Compared to Placebo, AVE7688 reduced glomerular and tubulo-interstitial damage markedly. In the presence of icatibant, the morphological benefit of AVE7688 was reduced. This effect was statistically significant with respect to tubulo-interstitial damage, but did not reach statistical significance (P=0.29) with respect to glomerulosclerosis.
Figure 2.
Histomorphological features of nephropathy in ZDF rats. In the placebo group (panel a), segmental glomerular fibrosis was frequently observed together with adjacent synechia and rarefaction of the remnant glomerulus. A neighbouring tubulus (arrow) is atrophic and shows thickening of basal membrane. Diabetic nephropathy was ameliorated in the AVE7688 group, as illustrated by two normal glomeruli in panel b. An adjacent tubulus shows a few enlarged cells with a clear cytoplasm (Armanni–Epstein cells, arrow), indicating a history of diabetes and glucosuria. Bars indicate 50 μm.
Figure 3.
Histopathological effects of chronic treatment with AVE7688 and icatibant. Icatibant alone has no effect on morphological kidney damage, but antagonizes part of the tubulo-interstitial benefit of AVE7688. Plac, Placebo group; Ica, icatibant. *P<0.0125 vs Plac; #P<0.0125 vs AVE.
Discussion
The present study demonstrates that the vasopeptidase inhibitor AVE7688 ameliorates albuminuria and morphological kidney damage when type II diabetes and nephropathy are already fully established. The extent of the nephroprotective effect is impressive, given a reduction of albuminuria by more than 80% in the present study, which is independent of glucose control. Data from recent studies indicate that vasopeptidase inhibition is superior over pure ACE inhibition in the prevention and treatment of nephropathies of various origin (Tikkanen et al., 1998; Taal et al., 2001; Davis et al., 2003; Schäfer et al., 2003), but the exact reason for this extra benefit has not yet been identified.
The vasoactive peptide bradykinin has recently been implicated to play a major role in diabetic nephropathy, based on its modulatory effect on intrarenal haemodynamics and its antiproliferative effects. In addition, bradykinin is known to be an important component of cardiovascular actions of ACE inhibitors (Linz et al., 1995). Moreover, an upregulation of the bradykinin B2 receptor has been described in streptozotocin-treated, diabetic rats (Tschöpe et al., 1999). Bradykinin B2 receptor blockade has been demonstrated to antagonize the benefit of ACE inhibitors, for example, in myocardial hypertrophy (Linz & Schölkens, 1992; Rouleau et al., 2001), endothelial dysfunction (Berkenboom et al., 1997), myocardial ischaemia (Martorana et al., 1990) and uraemia (Amann et al., 2000). As bradykinin is degraded by ACE and neutral endopeptidase, the vasopeptidase inhibitors prevent the degradation of bradykinin more effectively than pure ACE inhibition, leading to higher tissue and plasma concentrations (Blais Jr et al., 2001).
Interestingly, no investigations have been reported so far on the relative role of bradykinin in the cardiovascular actions of vasopeptidase inhibition. We hypothesized that part of the nephroprotection afforded by vasopeptidase inhibition is mediated by activation of the bradykinin B2 receptor. The present study demonstrates that this is indeed the case. While icatibant alone does not influence diabetic nephropathy, albuminuria and morphological kidney damage are approximately 70–100% increased in the simultaneous presence of the B2 antagonist and AVE7688 compared to AVE7688 alone. Icatibant had no influence on systemic blood pressure, heart rate or creatinine clearance. These findings make it unlikely that the bradykinin B2 receptor-dependent nephroprotection seen in the present study is mediated by systemic or local renal haemodynamic effects.
Interestingly, icatibant partially antagonized the AVE7688-mediated morphological improvement in the tubulo-interstitium, but not significantly the glomeruli. This finding is in good agreement with previous data in a model of renal fibrosis. Schanstra et al. (2002) demonstrated that genetic ablation (B2(−/−) mice) or pharmacological blockade of the bradykinin B2 receptor increased renal interstitial fibrosis following unilateral ureteral obstruction in mice, whereas transgenic rats expressing increased endogenous bradykinin showed reduced interstitial fibrosis after ureteral obstruction. However, despite the evidence for a role of the bradykinin B2 receptor in tubulo-interstitial damage, the nephroprotective pharmacological actions of ACE inhibition are not exclusively dependent on activation of the bradykinin B2 receptor, as has been demonstrated recently (Schanstra et al., 2003). The present study shows that a major part of protection from diabetic nephropathy by chronic vasopeptidase inhibition is bradykinin B2 receptor independent, given the marked reduction of albuminuria even in the presence of icatibant.
Possible alternative explanations for the marked nephroprotection during treatment with AVE7688 include activation of the bradykinin B1 receptor, or stimulation of the particulate guanylyl cyclase via the natriuretic peptides. The bradykinin B1 receptor has been shown to be upregulated in the kidney in streptozotocin-induced treated diabetic rats (Mage et al., 2002) and during chronic ACE inhibitor treatment (Marin-Castano et al., 2002). In the present study, the presence of both diabetes and ACE inhibition may have potentiated the expression and, hence, the potential benefit of activation of the bradykinin B1 receptor via increased tissue concentrations of bradykinin. Unfortunately, at the present time there is no information available regarding renal bradykinin B1 or B2 receptor expression in type II diabetes and during vasopeptidase inhibitor treatment.
Various studies in animals and humans have demonstrated an increase in plasma or urinary natriuretic peptides, or cGMP after administration of the prototype vasopeptidase inhibitor, omapatrilat (Bäcklund et al., 2001; Chen et al., 2001; Azizi et al., 2002). Therefore, in addition to its bradykinin-dependent actions, it seems very plausible to assume that natriuretic peptides mediate at least part of the pharmacological effects of AVE7688, based on the dual mode of action of the vasopeptidase inhibitors.
In summary, chronic vasopeptidase inhibition with AVE7688 reduces albuminuria and morphological kidney damage in rats with established diabetes and nephropathy. Activation of the bradykinin B2 receptor mediates part of this nephroprotective effect. If the data from the present study and from different animal models can be reproduced in man, vasopeptidase inhibition may represent an encouraging novel therapeutic principle for intervention in diabetic nephropathy.
Acknowledgments
We thank Peter Hainz and Gerald Fischer for excellent technical support. The present study is part of the ‘Cardiovascular and Renal Endpoints in Diabetes (CARED)' preclinical study program at Aventis Pharma.
Abbreviations
- ACE
angiotensin-converting enzyme
- AVE
AVE7688
- Ica
icatibant
- Plac
placebo
- ZDF
Zucker diabetic fatty
References
- AMANN K., GASSMANN P., BUZELLO M., ORTH S.R., TORNIG J., GROSS M.L., MAGENER A., MALL G., RITZ E. Effects of ACE inhibition and bradykinin antagonism on cardiovascular changes in uremic rats. Kidney Int. 2000;58:153–161. doi: 10.1046/j.1523-1755.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- AZIZI M., LAMARRE-CLICHÉ M., LABATIDE-ALANORE A., BISSERY A., GUYENE T.T., MENARD J. Physiologic consequences of vasopeptidase inhibition in humans: effect of sodium intake. J. Am. Soc. Nephrol. 2002;13:2454–2463. doi: 10.1097/01.asn.0000030142.80452.11. [DOI] [PubMed] [Google Scholar]
- BÄCKLUND T., PALOJOKI E., GRONHOLM T., ERIKSSON A., VUOLTEENAHO O., LAINE M., TIKKANEN I. Dual inhibition of angiotensin converting enzyme and neutral endopeptidase by omapatrilat in rat in vivo. Pharmacol. Res. 2001;44:411–418. doi: 10.1006/phrs.2001.0875. [DOI] [PubMed] [Google Scholar]
- BERKENBOOM G., LANGER I., CARPENTIER Y., GROSFILS K., FONTAINE J. Ramipril prevents endothelial dysfunction induced by oxidized low-density lipoproteins: a bradykinin-dependent mechanism. Hypertension. 1997;30:371–376. doi: 10.1161/01.hyp.30.3.371. [DOI] [PubMed] [Google Scholar]
- BLAIS C., JR, LAPOINTE N., ROULEAU J.L., CLEMENT R., GERVAIS N., GEADAH D., ADAM A. Effects of the vasopeptidase inhibitor omapatrilat on cardiac endogenous kinins in rats with acute myocardial infarction. Peptides. 2001;22:953–962. doi: 10.1016/s0196-9781(01)00401-6. [DOI] [PubMed] [Google Scholar]
- BRENNER B.M., COOPER M.E., ZEEUW D., KEANE W.F., MITCH W.E., PARVING H.H., REMUZZI G., SNAPINN S.M., ZHANG Z., SHAHINFAR S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- CHEN H.H., LAINCHBURY J.G., MATSUDA Y., HARTY G.J., BURNETT J.C., JR Endogenous natriuretic peptides participate in renal and humoral actions of acute vasopeptidase inhibition in experimental mild heart failure. Hypertension. 2001;38:187–191. doi: 10.1161/01.hyp.38.2.187. [DOI] [PubMed] [Google Scholar]
- DAVIS B.J., JOHNSTON C.I., BURRELL L.M., BURNS W.C., KUBOTA E., CAO Z., COOPER M.E., ALLEN T.J. Renoprotective effects of vasopeptidase inhibition in an experimental model of diabetic nephropathy. Diabetologia. 2003;46:961–971. doi: 10.1007/s00125-003-1121-9. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., LINZ W., SCHÖLKENS B.A., KUWER I., BARTENBACH S., SCHNELL A., UNGER T. Angiotensin-converting enzyme inhibition improves cardiac function. Role of bradykinin. Hypertension. 1994;23:411–418. doi: 10.1161/01.hyp.23.4.411. [DOI] [PubMed] [Google Scholar]
- HEART OUTCOMES PREVENTION EVALUATION STUDY INVESTIGATORS Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- ISMAIL N., BECKER B., STRZELCZYK P., RITZ E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55:1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- LEWIS E.J., HUNSICKER L.G., BAIN R.P., ROHDE R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- LEWIS E.J., HUNSICKER L.G., CLARKE W.R., BERL T., POHL M.A., LEWIS J.B., RITZ E., ATKINS R.C., ROHDE R., RAZ I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- LINZ W., SCHÖLKENS B.A. A specific B2-bradykinin receptor antagonist HOE 140 abolishes the antihypertrophic effect of ramipril. Br. J. Pharmacol. 1992;105:771–772. doi: 10.1111/j.1476-5381.1992.tb09054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZ W., WIEMER G., GOHLKE P., UNGER T., SCHÖLKENS B.A. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol. Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- MAGE M., PECHER C., NEAU E., CELLIER E., DOS REISS M.L., SCHANSTRA J.P., COUTURE R., BASCANDS J.L., GIROLAMI J.P. Induction of B1 receptors in streptozotocin diabetic rats: possible involvement in the control of hyperglycemia-induced glomerular Erk 1 and 2 phosphorylation. Can. J. Physiol. Pharmacol. 2002;80:328–333. doi: 10.1139/y02-024. [DOI] [PubMed] [Google Scholar]
- MARIN-CASTANO M.E., SCHANSTRA J.P., NEAU E., PRADDAUDE F., PECHER C., ADER J.L., GIROLAMI J.P., BASCANDS J.L. Induction of functional bradykinin b(1)-receptors in normotensive rats and mice under chronic angiotensin-converting enzyme inhibitor treatment. Circulation. 2002;105:627–632. doi: 10.1161/hc0502.102965. [DOI] [PubMed] [Google Scholar]
- MARTORANA P.A., KETTENBACH B., BREIPOHL G., LINZ W., SCHÖLKENS B.A. Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur. J. Pharmacol. 1990;182:395–396. doi: 10.1016/0014-2999(90)90301-l. [DOI] [PubMed] [Google Scholar]
- MOLINARO G., ROULEAU J.L., ADAM A. Vasopeptidase inhibitors: a new class of dual zinc metallopeptidase inhibitors for cardiorenal therapeutics. Curr. Opin. Pharmacol. 2002;2:131–141. doi: 10.1016/s1471-4892(02)00138-8. [DOI] [PubMed] [Google Scholar]
- ROULEAU J.L., KAPUKU G., PELLETIER S., GOSSELIN H., ADAM A., GAGNON C., LAMBERT C., MELOCHE S. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation. 2001;104:939–944. doi: 10.1161/hc3401.093149. [DOI] [PubMed] [Google Scholar]
- SCHÄFER S., LINZ W., BUBE A., GERL M., HUBER J., KÜRZEL G.U., BLEICH M., SCHMIDTS H.L., BUSCH A.E., RÜTTEN H. Vasopeptidase inhibition prevents nephropathy in Zucker diabetic fatty rats. Cardiovasc. Res. 2003;60:447–454. doi: 10.1016/s0008-6363(03)00544-3. [DOI] [PubMed] [Google Scholar]
- SCHÄFER S., LINZ W., VOLLERT H., BIEMER-DAUB G., RÜTTEN H., BLEICH M., BUSCH A.E. The vasopeptidase inhibitor AVE7688 ameliorates Type 2 diabetic nephropathy. Diabetologia. 2004;47:98–103. doi: 10.1007/s00125-003-1264-8. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., DUCHENE J., DESMOND L., NEAU E., CALISE D., ESTAQUE S., GIROLAMI J.P., BASCANDS J.L. The protective effect of angiotensin converting enzyme inhibition in experimental renal fibrosis in mice is not mediated by bradykinin B2 receptor activation. Thromb. Haemost. 2003;89:735–740. [PubMed] [Google Scholar]
- SCHANSTRA J.P., NEAU E., DROGOZ P., AREVALO GOMEZ M.A., LOPEZ NOVOA J.M., CALISE D., PECHER C., BADER M., GIROLAMI J.P., BASCANDS J.L. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J. Clin. Invest. 2002;110:371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAAL M.W., NENOV V.D., WONG W., SATYAL S.R., SAKHAROVA O., CHOI J.H., TROY J.L., BRENNER B.M. Vasopeptidase inhibition affords greater renoprotection than angiotensin-converting enzyme inhibition alone. J. Am. Soc. Nephrol. 2001;12:2051–2059. doi: 10.1681/ASN.V12102051. [DOI] [PubMed] [Google Scholar]
- TIKKANEN T., TIKKANEN I., ROCKELL M.D., ALLEN T.J., JOHNSTON C.I., COOPER M.E., BURRELL L.M. Dual inhibition of neutral endopeptidase and angiotensin-converting enzyme in rats with hypertension and diabetes mellitus. Hypertension. 1998;32:778–785. doi: 10.1161/01.hyp.32.4.778. [DOI] [PubMed] [Google Scholar]
- TSCHÖPE C., REINECKE A., SEIDL U., YU M., GAVRILUK V., RIESTER U., GOHLKE P., GRAF K., BADER M., HILGENFELDT U., PESQUERO J.B., RITZ E., UNGER T. Functional, biochemical, and molecular investigations of renal kallikrein–kinin system in diabetic rats. Am. J. Physiol. 1999;277:H2333–H2340. doi: 10.1152/ajpheart.1999.277.6.H2333. [DOI] [PubMed] [Google Scholar]
- WIRTH K., HOCK F.J., ALBUS U., LINZ W., ALPERMANN H.G., ANAGNOSTOPOULOS H., HENK S., BREIPOHL G., KONIG W., KNOLLE J. Hoe 140: a new potent and long acting bradykinin-antagonist: in vivo studies. Br. J. Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRTH K.J., LINZ W., WIEMER G., SCHÖLKENS B.A. Kinins and cardioprotection. Pharmacol. Res. 1997;35:527–530. doi: 10.1006/phrs.1997.0181. [DOI] [PubMed] [Google Scholar]