Abstract
We investigated the effects of serum albumin on inducible nitric oxide synthase (iNOS) expression in RAW 267.4 macrophages. Crude fraction-V type albumin as well as bovine serum albumin filtrated for endotoxin induced concentration-dependent iNOS expression in macrophages. Accordingly, NO production (estimated by supernatant nitrite) was markedly (up to 10-fold) increased in the presence of albumin.
Albumin-induced expression of iNOS protein was inhibited by cycloheximide and NO production was abolished after incubation of the cells with an iNOS inhibitor, NG-monomethyl-L-arginine (LNMMA).
An inhibitor of the NF-κB pathway, pyrrolidine dithiocarbamate (PDTC), as well as inhibitors of JAK2/STAT and ERK, AG490 and U0126, respectively, significantly reduced albumin-induced iNOS expression and NO production, while an inhibitor of the p38 pathway, SB203580, did not significantly affect NO production induced by albumin.
Both types of serum albumin were contaminated with traces of endotoxin. The endotoxin levels were found not to be sufficient for the observed induction of nitrite production in RAW 267.4 cells. In addition, the albumin-stimulated induction of iNOS was not reduced by preincubation of albumin-containing media with polymyxin B, a LPS inhibitor.
Polymerised albumin fractions were detected in the commercially available albumin tested in this study. A monomeric albumin-rich fraction, separated by ultrafiltration, showed a potent inducing effect on iNOS expression and NO production, while a polymer-rich fraction showed a smaller effect.
Advanced glycation endproducts (AGE) of albumin were not formed by interaction with glucose in incubation medium, as AGE was not increased even after long-time (4 weeks) incubation in albumin-containing media [3.2–4.4 μg ml−1 (basal) vs 4.8–5.6 μg ml−1 (in glucose-containing media)]. However, the duration of albumin exposure to glucose influenced the basal stimulatory properties of albumin.
Our results suggest that serum albumin fractions, as gained by cold alcoholic extraction, may include determinants that stimulate or further enhance stimulation of RAW 267.4 cells and are different from endotoxin, polymeric albumin and AGE.
Keywords: Albumin, nitric oxide synthase, macrophages, inflammation
Introduction
Nitric oxide (NO) is a cellular product released by macrophages after inflammatory stimulation and subsequent expression of the inducible form of nitric oxide synthase (iNOS). A well-known stimulatory pathway in macrophages is initiated by binding of a bacterial wall component (lipopolysaccharide, LPS) to the CD14-toll-like receptor that triggers a complex cascade of kinases and finally leads to gene activation and subsequent enhanced expression of proteins such as iNOS (Paul et al., 1995).
A further reproducible exogenic stimulus for macrophage activation has been found in post-translationally modified forms of serum albumin. Two different forms of modified albumin were shown to act as inflammatory stimuli in various cell types: advanced glycation end products (AGE) are formed by nonenzymatic Maillard reaction of albumin with glucose or fructose after early formation of Amadori products and Schiff's base (Baynes et al., 1989). Elevated concentrations of glycation products of albumin have been demonstrated to be involved in vasculopathy (Schmidt et al., 1994) and inflammation (Morohoshi et al., 1995) as well as poor wound healing (Goova et al., 2001) in diabetic patients, in which this form of albumin can be detected at increased concentrations (Brownlee, 1995). The receptor for AGE (RAGE), which is abundant in macrophages (Vlassara et al., 1985; Thornalley, 1998) and many other cell types (Katsuoka et al., 1997; Yonekura et al., 2003), was identified as a multiligand receptor of the immunoglobulin family MHC II class (Schmidt et al., 1996; 2000; Schmidt & Stern, 2001). The second modified form of albumin that represents a potential inflammatory stimulus in some cell types was found in the polymer fraction of albumin. Shacter et al. (1993) investigated the effect of bovine serum albumin on peritoneal macrophages and found the degree of albumin polymerisation responsible for interleukin (IL-6) and prostaglandin (PGE-2) secretion. The receptor of polymerised albumin was found in macrophages and hepatocytes (Thung & Gerber, 1981) and its role for endocytosis of albumin (Yoshioka et al., 1994) as well as hepatitis-B virus docking (Dash et al., 1991) was reported, however, a link of this receptor to inflammatory pathways has not been reported yet. Moreover, the physiological significance of polymerised albumin and the pathways involved in inflammatory responses due to albumin are still elusive.
In this study, we investigated the ability of commercially available bovine serum albumin to induce iNOS expression and subsequent NO production in the RAW 267.4 macrophage cell line. In addition, the role of endotoxin contamination, polymerisation as well as glycation of albumin in this context was also examined.
Methods
Cells
The RAW 267.4 cell line was cultured in DMEM (4500 mg l−1 glucose) containing 5% FCS at constant CO2 (5%). Streptomycin (100 μg ml−1), penicillin (100 U ml−1) and amphotericin B (0.25 μg ml−1) were added to the medium. We confirmed that the presence of antibiotics at the given concentrations as well as FCS (up to 10%) did not affect NO production induced by albumin in RAW 267.4 cells.
For nitrite measurement, culture medium was changed to phenol red-free DMEM without FCS. Cells were seeded at equal density on 24-well plates with 0.5 ml medium each. The nitrite measurement and protein harvest for Western blotting were performed using cells at 100% confluence, which was determined to be equal to a cellular density of about 106 cells per well.
Male Wistar rats were killed by desanguination, and alveolar macrophages were collected by bronchoalveolar lavage under sterile conditions as described previously (Chandler et al., 1986; Chandler and Fulmer, 1987). Briefly, the trachea of each rat was cannulated with polypropylene tubing attached to an 18-gauge needle. The lung of each rat was lavaged in situ with a total of 50 ml of cold (4°C) PBS (Ca2+- and Mg2+-free phosphate-buffered saline) with gentle massage of the thoracic cavity. The lavage fluid was centrifuged at 400 × g for 10 min at 4°C. Cell pellets of each rat were washed twice with cold PBS (Ca2+ and Mg2+-free) and were resuspended in 4 ml of Dulbecco's modified Eagle's medium (DMEM) with 4 mM glutamate and the antibiotics. Cell numbers and viability were then quantified by trypan blue exclusion using a haemocytometer. Isolated fresh rat alveolar macrophages (2.0 × 106 ml−1) were incubated in 24-well plates. After preincubation for 2 h, the medium was changed to DMEM with LPS (100 nM), albumin (10 mg ml−1) or a vehicle, and the cells were further incubated for 24 h. The study protocols regarding treatment of animals were in accordance with the ‘Guidelines for Experiments Using Laboratory Animals in Yamagata University School of Medicine'.
Albumin
Types of commercial albumin (all from Sigma, St Louis, MO, U.S.A.) used in this study included low endotoxin (<0.1 ng mg−1) and globulin-free bovine serum albumin, fraction-V bovine serum albumin and human serum albumin. Albumin-containing media, including streptomycin (100 μg ml−1), penicillin (100 U ml−1) and amphotericin B (0.25 μg ml−1), were filtrated separately through a 0.2 μm syringe-filter and stored at 4°C.
Polymyxin B
Polymyxin B sulphate (Sigma) was diluted in PBS to gain a 100 μg ml−1 stock solution and this solution was added to albumin- or LPS-containing media at a final concentration of 1 μg ml−1, vortexed and incubated for 30 min before incubation of cells. LDH content of the supernatant was found not to be elevated at the concentration of polymyxin B (1 μg ml−1) used in this study.
Estimation of nitrite concentration
NO generation was estimated by measuring the amount of nitrite in the supernatant of each well after incubation with an albumin-free or an albumin-containing medium for 24 h. Each aliquot (100 μl) of the incubation medium was mixed with 50 μl of a modified Griess reagent [2% sulphanilamide/0.2% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% phosphoric acid]. The mixture was incubated at room temperature for 10 min and the absorbance at 540 nm was then measured using a spectrophotometer (550 Microplate Reader, Bio-Rad, Hercules, CA, U.S.A.). Standard curves were determined using solutions with known concentrations of NaNO2.
Measurement of lactate dehydrogenase (LDH) activity
LDH was measured using a commercially available kit (Liquitech LDH, Boehringer Mannheim). Briefly, 10 μl of the sample was added to 350 μl of a solution containing 0.6 mM pyruvate and 50 mM phosphate buffer (pH 7.5), and incubated at 37°C for 5 min. Next, 70 μl of 0.18 mM NADH solution was added and incubated for further 5 min. Then, absorbance at 340 nm was measured using a spectrophotometer.
Inhibitors
De novo protein synthesis in RAW 267.4 cells was inhibited by cycloheximide (Sigma). NO production by iNOS was blocked by NG-monomethyl-L-arginine (Wako, Osaka, Japan). Cells were incubated with tyrphostin AG490 (Calbiochem, La Jolla, CA, U.S.A.) for inhibition of JAK2/STAT. U0126 (Sigma) was used for blocking of the ERK1/2 pathway. To inhibit p38, SB203580 (Sigma) was used and pyrrolidine dithiocarbamate (PDTC, Sigma) for inhibition of NF-κB. AG490, SB203580 and U0126 were dissolved with DMSO to make stock solutions of 100, 10 and 100 mM, respectively, and all solutions were diluted with PBS just before use in order to make a final concentration of 30 μM each. PDTC was dissolved with PBS to make a stock solution of 1 mM, and diluted with PBS just before use in order to make a final concentration of 30 μM in each well. LNMMA and cycloheximide were dissolved with PBS to obtain concentrations of 100 and 1 mM, respectively, and diluted with PBS just before use in order to make final concentrations of 100 and 1 μM, respectively, in each well. The final concentration of DMSO, as a solvent for the inhibitors, was less than 0.01%.
Preparation of glutaraldehyde-modified albumin
PBS (10 ml) containing 1 g of bovine serum albumin (low-endotoxin type) was incubated with a glutaraldehyde solution (final 0.01% glutaraldehyde w/v) for 24 h at 37°C. The glutaraldehyde-containing albumin solution was further dialysed using a cellulose tube against 2 × 1000 ml PBS for 72 h. Aliquots of the solution were administrated to the culture medium for experiments.
Protein ultrafiltration
Polymeric and monomeric fractions of albumin were separated by ultrafiltration using centrifugal filter devices (Millipore). Albumin dissolved in PBS at a concentration of 1 g ml−1 was centrifuged twice using a 100-kDa nominal-molecular-weight (NMW) filter device at 1000 × g for 1 h. The polymeric fraction was gained by reversing the filter device and centrifugation at 1000 × g for 30 min. The monomeric fraction was further concentrated using a 50-kDa NMW filter device at 1000 × g for 30 min. Protein content of final filtrates was estimated by the Bradford method and distribution of monomeric and polymeric fractions was checked by native polyacrylamide gel electrophoresis.
Native polyacrylamide gel electrophoresis
To analyse the relative content of polymerised albumin in different albumin preparations, we performed native gel electrophoresis using a 7% acrylamide slab gel at 20 mA for 80 min in Tris-buffer (pH 8.3) containing 0.192 M glycine. Samples of 100 ng of each kind of albumin were used for electrophoresis. Protein bands were detected by the standard Coomassie-blue technique.
Western blots
Protein expression of iNOS was determined by Western blot analysis. Cells grown in one well were harvested, sonicated shortly in a sample buffer [65 mM Tris/HCl (pH 6.8), 10% glycerol, 2.5% β-mercaptoethanol and 2% sodium dodecylsulphate with 0.01% bromphenol blue] and heated at 100°C for 10 min. As revealed by control experiments, total amount of cellular proteins per sample remained fairly constant under given conditions and can be assumed to be the same in a single experiment. Aliquots (3–10 μl) were subjected to SDS–PAGE on 7.5% polyacrylamide slab gels and blotted onto polyvinylidene difluoride membrane. The blots were blocked for 1 h in Tris-buffered saline (TBS: 150 mM NaCl, 20 mM Tris, pH 7.5) containing 5% nonfat milk and incubated overnight with anti-(mouse iNOS) IgG (diluted 1 : 1000; Transduction Laboratory, Lexington, KY, U.S.A.) at 4°C. After washing with TBS, the membrane was incubated with goat anti-mouse alkaline phosphatase-conjugated antibody (diluted 1 : 1000) for 1 h. After removal of antibodies by washing, proteins were detected by enhanced chemiluminescence method using an immunoblot assay kit (Immun-Blot Assay Kit, BioRad).
In all electrophoresis experiments, a protein standard ladder (Kaleidoscope prestained standard, Bio-Rad) was used for estimation of protein molecular size.
Densitometry of Western blots
Western blots were digitalised using a high-resolution image scanner and densitometrically evaluated by a gel-analysis software.
Estimation of endotoxin content in albumin-containing media
Free-endotoxin content in albumin-containing media was measured by chromogenic endotoxin test (Endospec-SP), a limulus amoebocyte lysate assay (LAL) optimised for endotoxin specificity, as described previously by Obayashi et al. (1986).
Estimation of AGE in glucose-incubated media
AGE content in albumin-containing media was measured using a common ELISA technique as described previously (Ono et al., 1998). Briefly, a microplate was coated with 105-fold-diluted AGE-BSA by overnight incubation. After washing with PBS containing 0.05% Tween-20 (PBST), standards and samples were added to each well. Anti-AGE antibody (dilution 1 : 10,000) was added to each well and incubated overnight. The secondary antibody was added after washing with PBST and detected by incubation with 3, 3′, 5, 5′-tetramethylbenzidine solution for 40 min. The optical density was measured at 450 nm with a microplate ELISA reader after addition of 2 N H2SO4 (50 μl) and subsequent mixing.
Statistical analysis
Observed mean values were tested for significant difference from the control by Student's t-test or one-way ANOVA in combination with Dunnett's multiple comparison post hoc test. Significant difference was considered for values of P<0.05.
Results
Bovine serum albumin-induced iNOS expression in RAW 267.4 cells
Incubation of RAW 264.7 cells for 24 h with commercial bovine serum albumin markedly induced iNOS expression in a concentration-dependent manner. The degree of protein expression was dependent on the kind of albumin preparations used. Low endotoxin-containing albumin strongly induced iNOS expression in macrophages, while fraction-V-type albumin induced less but still significant iNOS expression at the same protein concentration (Figure 1a and b). The maximal absolute levels of albumin-induced nitrite production in RAW 267.4 cells by both albumin preparations were comparable with that induced by purified endotoxin at a concentration of 100 ng ml−1 (Figure 1c).
Figure 1.
Albumin induces iNOS expression in RAW 267.4 cells. Nitrite production in RAW 267.4 cells after 24-h incubation with fraction-V type albumin (a), low-endotoxin albumin (b) and purified lipopolysaccharide (c) at given concentrations. n=5–8. Asterisks (a–c) indicate significant difference from the control without albumin or endotoxin incubation. (d) Western blots of iNOS expression after 24-h incubation with fraction-V type albumin and low-endotoxin albumin at given concentrations. (e) Human serum albumin (HAS, 10 mg ml−1) induces nitrite production in RAW 267.4 cells after 24-h incubation without inhibitors as well as in the presence of 1 μM cycloheximide (CH) or 100 μM NG-monomethyl-L-arginine (LN). n=3. (f) Nitrite production in freshly ex vivo prepared rat alveolar macrophages after 24-h incubation using media without albumin and inhibitors (ctrl), with media containing purified LPS (100 ng ml−1), low-endotoxin (LE) or fraction-V (FV) type of albumin (10 mg ml−1) and in the absence or presence of 1 μM cycloheximide or 100 μM NG-monomethyl-L-arginine. Asterisks (c, f) indicate significant difference from the level after stimulation with albumin or LPS in the absence of inhibitors.
In accordance with these findings, incubation of RAW 267.4 cells for 24 h with media containing fraction-V or low-endotoxin type of albumin upregulated iNOS expression in a concentration-dependent manner (Figure 1d).
Measurement of LDH in the supernatant of albumin-exposed cells revealed that iNOS upregulation by albumin was not linked to increased cytotoxicity, as the LDH levels were not significantly affected by albumin incubation (without albumin, 128.7±12.2 U l−1; with albumin, 153.0±15.5 U l−1).
As confirmed by preliminary experiments, iNOS induction by albumin in RAW cells was not influenced by the presence of antibiotics in the media (not shown).
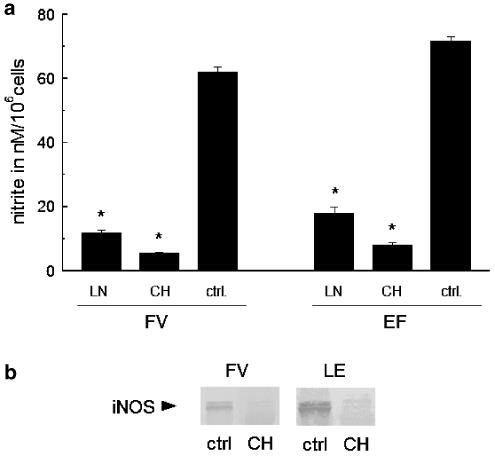
Human serum albumin (10 mg ml−1) also induced NO production in RAW 267.4 cells to a similar degree to that induced by bovine serum albumin (Figure 1e). To determine whether the observed effects were specific to RAW 267.4 cells, we conducted experiments using alveolar macrophages (freshly prepared from rats). NO production by bovine serum albumin was also observed in alveolar macrophages freshly isolated from rats (Figure 1f).
Albumin-induced NO production due to upregulated iNOS expression
To confirm the linkage of NO production with albumin-induced iNOS expression, we checked for dependence of nitrite levels on protein synthesis and iNOS activity, using inhibitors (Figure 2). The production of NO as well as iNOS protein expression was abolished by a de novo protein-synthesis inhibitor, cycloheximide (1 μM), and a NOS inhibitor, LNMMA (100 μM). Both inhibitors were highly effective when RAW 267.4 cells were stimulated with low-endotoxin and fraction-V types of albumin, as shown in Figure 2. Thus, the induction of NO production by albumin is due to intracellular upregulation of iNOS.
Figure 2.
Effects of NG-monomethyl-L-arginine (LN) and cycloheximide (CH) on albumin-induced iNOS expression and NO production in macrophages. (a) Inhibition of NO production by 100 μM LN and 1 μM CH in macrophages incubated for 24 h with fraction-V (FV) and low-endotoxin (LE) types of albumin (each 5 mg ml−1). (b) Inhibition of iNOS expression in macrophages by cycloheximide (CH) as described in A, revealed by Western blotting. Asterisks indicate significant difference from the control (ctrl, albumin incubation without inhibitors).
Albumin-activated pathways of intracellular kinases in RAW 267.4 cells
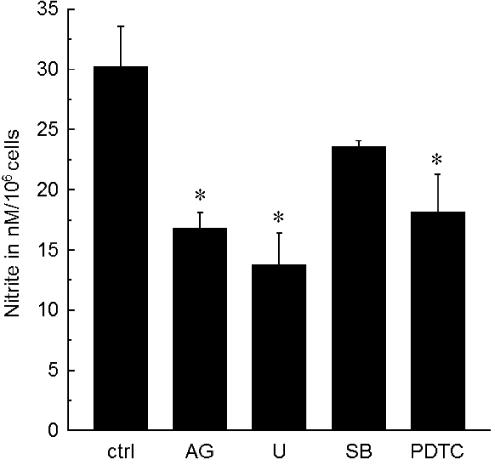
RAW 267.4 cells exposed to albumin (fraction-V type, 2 mg ml−1) were coincubated with different inhibitors that are known to interfere with kinase pathways involved in LPS-induced immune response in macrophages in order to test for the involvement of known general activation pathways in the iNOS-inducing action of albumin (Figure 3). An inhibitor of p38 phosphorylation, SB203850 (30 μM), did not significantly reduce the NO production after incubation with albumin (fraction V). A JAK2/STAT pathway inhibitor, AG490 (30 μM), and an ERK pathway inhibitor, U0126 (30 μM), as well as a NF-κB inhibitor, PDTC (30 μM), significantly reduced nitrite levels in the macrophage culture supernatant after incubation with albumin.
Figure 3.
Effects of inhibition of different enzymatic phosphorylation pathways on albumin-induced NO production in RAW 267.4 cells. Cells were incubated with albumin (fraction-V type, 2 mg ml−1) for 24 h in the absence or presence of tyrphostin AG490 (AG), U0126 (U), SB203580 (SB) and pyrrolidine dithiocarbamate (PDTC), each at 30 μM. Asterisks indicate significant difference from the control (ctrl) value in the absence of inhibitors (P<0.05). n=4–6.
Endotoxin content of the albumin fractions
Commercially available albumin fractions used in this study were contaminated with small amounts of endotoxin. Surprisingly, the albumin fraction, designated as ‘low-endotoxin albumin', contained more LPS (20.72±2.00 pg mg−1 albumin, n=4) than crude fraction-V type albumin (2.9±0.7 pg mg−1 albumin, n=4). However, the estimated contamination with free LPS in both fractions, even in media including 10 mg ml−1 albumin, remained clearly below the threshold-concentration of purified LPS (1 ng ml−1) required for significant macrophage stimulation (Figure 1c).
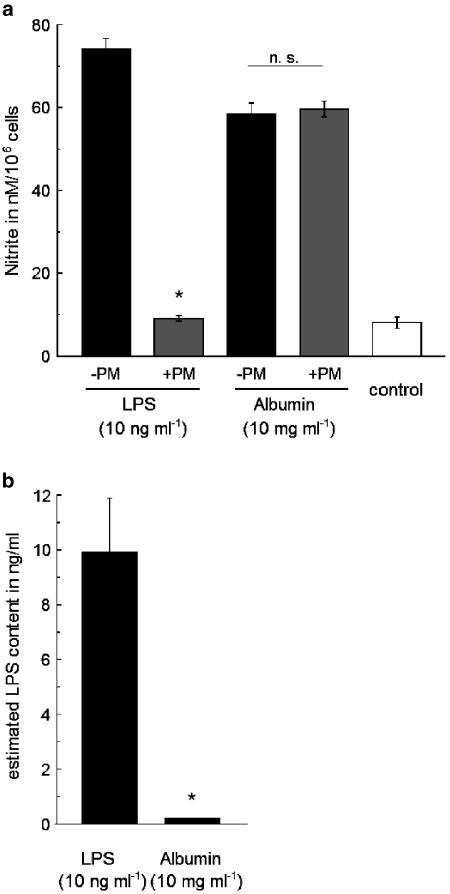
To exclude possible effects of the contaminating traces of endotoxin, we preincubated albumin-containing media with LPS-binding polymyxin B. Although concentrations above 5 μg ml−1 polymyxin B resulted in cytotoxic effects on RAW 267.4 cells, a concentration of 1 μg ml−1 was sufficient to abolish the induction of nitrite production in RAW 267.4 cells by purified LPS (10 ng ml−1). The incubation of albumin-containing media with the same polymyxin B at 1 μg ml−1 did not reduce the nitrite production induced by low-endotoxin type of albumin, while the overall nitrite production was clearly smaller in albumin-incubated control cells than that induced by purified LPS (Figure 4a and b). Thus, the observed stimulatory effects of the albumin fractions on macrophages are independent of contamination with free endotoxin.
Figure 4.
Effects of albumin and lipopolysaccharide-induced nitrite production in RAW 267.4 cells after incubation with media containing polymyxin B. (a) Nitrite production of RAW 267.4 cells after 24-h incubation with media containing either 10 ng ml−1 purified lipopolysaccharide (LPS) or 10 mg ml−1 albumin (low-endotoxin type) in the presence and absence of polymyxin B (PM). Both media were incubated with polymyxin B (1 μg ml−1) for 30 min before cell exposure. n=5. (b) Content of free endotoxin as estimated by limulus amoebocyte lysate assay in the medium containing 10 ng ml−1 purified endotoxin or 10 mg ml−1 albumin (low-endotoxin type) n=4. Asterisks indicate significant difference from the level in the absence of polymyxin B (a) or the level in the medium containing LPS (b).
Polymeric albumin
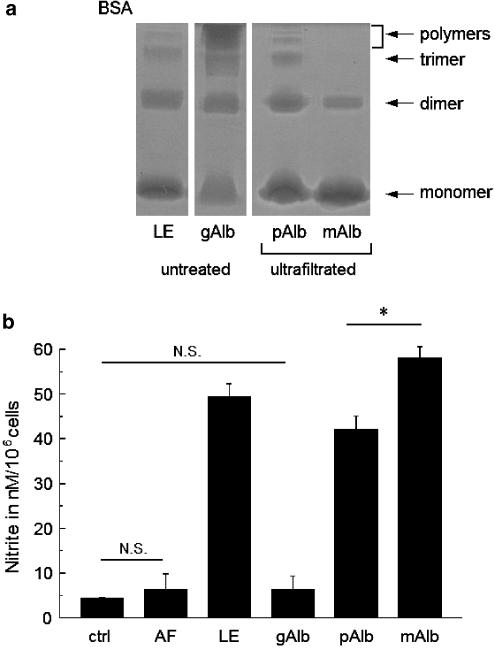
Monomer and polymer fractions of bovine serum albumin were separated and concentrated by ultrafiltration. We obtained a high-molecular-weight filtrate, which contained less monomeric protein and polymeric forms at higher concentration, as well as a low-molecular-weight filtrate including monomers and a smaller portion of dimers but no polymers (Figure 5a).
Figure 5.
Role of polymerisation for albumin-induced NO production in RAW 267.4 cells. (a) Results of native gel electrophoresis of albumin solutions containing 5 mg ml−1 albumin. The contents of polymers, trimer, dimer and monomer in untreated purified albumin (low-endotoxin type, LE), glutaraldehyde-treated albumin (gAlb) and a polymer-containing fraction (pAlb) as well as in a mainly monomeric ultrafiltrated albumin (mAlb) fraction are shown. (b) NO production in RAW 267.4 cells after 24-h incubation with the following media: a control solution (ctrl), an essentially albumin-free low-molecular-weight filtrate (AF), a medium containing 5 mg ml−1 untreated low-endotoxin albumin (LE), glutaraldehyde-treated albumin (gAlb) and an ultrafiltrate containing 5 mg ml−1 polymeric fraction (pAlb) as well as an ultrafiltrate containing mainly monomeric albumin (mAlb, 5 mg ml−1) as shown in (a). An asterisk indicates significant difference from the control value in the absence of albumin fractions (AF, LE, gAlb) or significant difference between the effects of monomeric and polymeric fractions (mAlb, pAlb). N.S., no significant difference. n=4–6.
When RAW 267.4 cells were incubated with these filtration products at the same protein concentrations, the low-molecular-weight filtrate induced higher levels of iNOS expression than the polymeric fraction. Accordingly, NO production was higher in the cells incubated with the low-molecular-weight filtrate (mAlb, Figure 5b) than those incubated with untreated albumin or the polymeric filtrate (pAlb). Thus, polymerisation is not the responsible determinant of the basal stimulatory properties of bovine serum albumin.
The virtually albumin-free filtrate (AF) that contained the remaining lower-molecular-weight components (smaller than 50 kDa) after ultrafiltration was nominally protein-free (not shown) and did not stimulate NO production of macrophages (Figure 5b).
To further analyse the role of albumin polymerisation in macrophage activation, we treated low-endotoxin albumin with a low concentration of glutaraldehyde to gain a strongly polymerised-albumin fraction. Treatment with glutaraldehyde increased the polymer content strongly as shown in Figure 5a. The glutaraldehyde-treated sample showed a clear shift from monomeric and dimeric forms to highly polymeric forms (> pentamer). After removal of glutaraldehyde by dialysis, the stimulatory potential of glutaraldehyde-treated albumin was compared with untreated albumin samples. Albumin treated with glutaraldehyde (gAlb) only slightly enhanced NO production, while untreated albumin (LE) induced considerable NO production (Figure 5b).
To rule out inhibitory effects by traces of glutaraldehyde remaining in the preparations, we administrated the albumin-free but glutaraldehyde-treated medium after dialysis to RAW 267.4 cells and then stimulated with LPS. LPS (10 nM)-induced nitrite production was only slightly reduced after incubation with the dialysate [nitrite/106 cells: 51±6.2 nM in glutaraldehyde-treated medium versus 56.7±0.7 nM in the control medium, (n=4)], indicating that the main cellular pathway for iNOS activation by LPS remained intact after incubation with the dialysate.
AGE production by interaction of albumin with glucose
The level of AGE in glucose- and albumin-containing media was found to be low, compared with the values that can be detected in human blood (Ono et al., 1998). The AGE content was estimated to range between 4.8 and 5.6 μg ml−1 (1.2–1.4 mU ml−1) in glucose-treated media, containing 10 mg ml−1 albumin and 4500 mg ml−1 glucose, while glucose-free media contained AGE at concentrations between 3.2 and 4.4 μg ml−1 (0.8–1.1 mU ml−1). Thus, formation of AGE may not be responsible for the strong enhancing effect of glucose-treated albumin on iNOS expression in macrophages in our experiments.
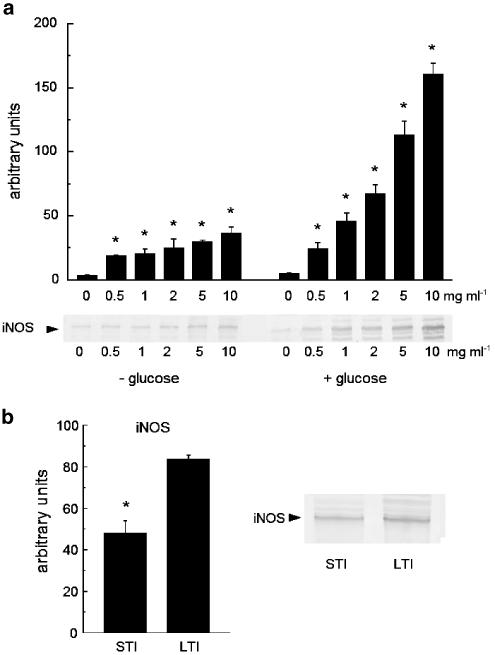
Even in the absence of glucose in the medium, albumin was able to concentration-dependently stimulate iNOS expression in RAW 267.4 cells. Nevertheless, both albumin-induced NO production and iNOS expression in a glucose-free medium were lower than those observed in the presence of a high concentration of glucose (4.5 g l−1) (Figure 6a). A comparison of levels of iNOS expression induced by albumin after exposure to glucose for different times [short duration (24 h) and long duration (3 weeks)] revealed that the duration of incubation with glucose influenced the degree of the stimulatory effect of albumin (Figure 6b). However, the exposure of albumin to glucose for only 24 h was sufficient to induce a significant iNOS expression in macrophages.
Figure 6.
Role of glucose and glycated albumin for albumin-induced iNOS-expression in RAW 267.4 cells. (a) Western blot and its densitometric evaluation (upper panel) of iNOS expression in RAW 267.4 cells after 24-h incubation with the medium containing low-endotoxin albumin at given concentrations and in the presence as well as in the absence of glucose in the medium. (b) Western blot and its densitometric evaluation of iNOS expression in RAW 267.4 cells after 24-h incubation with the medium containing 10 mg ml−1 low-endotoxin-type albumin that was preincubated with 4.5 g l−1 glucose for either 24 h (short time incubation, STI) or 3 weeks (long time incubation, LTI). Asterisks indicate significant difference from the control in the absence of albumin (a) or the level after long time incubation (b). n=4.
Discussion
Albumin is the most prevalent protein in blood and has many important functions including roles in colloid-osmotic homeostasis, binding and transport of smaller molecules and as a storage form of metabolic energy. Aside these well-known functions, albumin was reported to directly influence the immune-system after post-translational modifications like polymerisation or glycation, introducing additional biological implications. We observed a strong induction of iNOS expression and NO production by bovine serum albumin in RAW 267.4 macrophages and investigated possible determinants in the albumin fractions responsible for this stimulatory effects.
As the albumin-induced pathways of enzymatic phosphorylations in RAW 267.4 cells appeared to include NF-kB, JAK/STAT and ERK (a profile that mirrors classical LPS-activated pathways), we investigated the role of contaminating LPS in the albumin fractions. Endotoxin contamination of commercially available albumin fractions may be enhanced during the industrial purification process and does not necessarily require bacterial infection of the animal blood source. However, the albumin fractions used in this study included only very low levels of endotoxin, as determined by a limulus test. The estimated LPS contamination in both albumin types remained clearly below the threshold concentrations required for activation of RAW 267.4 cells by purified LPS from Escherichia coli. To test for the involvement of the estimated traces of endotoxin in the observed activation of macrophages by albumin, we preincubated albumin-containing media with polymyxin B, a compound that binds and functionally removes LPS. Polymyxin B completely failed to influence the albumin-induced nitrite production in RAW 267.4 cells, while it was highly effective to prevent LPS-induced NO production in the cells. These results suggest that endotoxin cannot be responsible for the iNOS-inducing effects of albumin.
LPS is a major pyrogen and a trace amount of LPS is detected even in the blood of healthy humans. However, our present study demonstrated that LPS is not involved in the observed iNOS-inducing action of albumin in macrophages. On the other hand, the amount of the wall particles originated from other kinds of microorganisms, such as Gram-positive bacterias, is expected to be much less in healthy blood compared with LPS. Thus, it appears to be very unlikely that different kinds of particles originating from microorganisms besides Gram-negative bacterias are involved in the observed effects of albumin.
As all albumin preparations used in this study were found to contain polymeric fractions, and polymeric albumin was reported to stimulate macrophages (Shacter et al., 1993), we investigated the role of albumin polymerisation in stimulatory effects of nonglycated albumin. Surprisingly, a fraction gained by ultrafiltration, which mainly consisted of monomers and dimers, induced stronger iNOS expression than a fraction containing a large part of polymeric forms. Furthermore, the stimulatory potential of the albumin preparations was not reflected by their polymer content as revealed by native gel electrophoresis. An albumin preparation, which contained a very large polymeric albumin fraction, as gained by treatment of albumin with glutaraldehyde, induced only low levels of iNOS expression in macrophages. Thus, polymerisation of albumin is not involved in induction of iNOS expression in RAW 267.4 cells by serum albumin.
Glycated-albumin products are well recognised as inflammatory stimuli, especially in diabetic patients. In spite of the relatively high glucose concentration, our experimental conditions were not optimal for generation of advanced glycation endproducts (AGE), which have been described as a macrophage-stimulating glycation product (Koj et al., 1994; Morohoshi et al., 1995), because of comparable low temperature (4°C) and low albumin concentrations during incubation. As expected, the concentration of AGE detected in glucose-treated media was found to be very low, even when compared with the levels reported to occur in human blood. The mean AGE levels were reported to be 13.2 μg ml−1 (3.3 mU ml−1) in the blood of healthy humans and rose to 28.8 μg ml−1 (7.2 mU ml−1) in diabetic patients (Ono et al., 1998). Pentosidine, a far advanced AGE that is not detected by the assay, is unlikely to be produced under the conditions in our experiments. Nevertheless, exposure time of albumin to high glucose clearly influenced the stimulatory properties of albumin, indicating involvement of some form of glycation in the observed effects.
A possible involvement of albumin-associated globulins (opsonisation) can also be ruled out, as globulins were removed from the low-endotoxin albumin fraction. Moreover, the lack of stimulatory effects in albumin-ultrafiltration products smaller than 50 kDa and the lower stimulatory effect in polymeric fractions indicate that the stimulatory substance is closely associated with albumin itself.
Furthermore, macrophages may be stimulated by contact with nonglobulin proteins of a different species (cattle, humans). However, bovine serum albumin also induced iNOS expression and nitrite production in rat aortic smooth muscle cells (data not shown). Thus, even cell types that lack the sophisticated immunological apparatus of macrophages, such as vascular smooth muscle cells, can be stimulated by purified serum albumin, indicating a more common mechanism of protein–cell interaction for the iNOS-inducing action of serum albumin.
Our findings suggest that an unidentified determinant in the serum albumin fractions may elicit or strongly enhance iNOS production in macrophages. Currently, we cannot exclude involvement of a contaminating moiety, and further investigations are necessary to clarify if albumin itself may obtain such strong stimulatory properties. This question may be of importance for methodological considerations as well as physiological effects of albumin.
Serum inherent albumin contained in FCS failed to induce iNOS expression in RAW 267.4 cells even at a high concentration (10%) of FCS (∼4 mg ml−1 albumin) in media (data not shown). Possible reasons for this may be the foetal origin of albumin in FCS or differences in thermic treatment of commercial FCS and albumin. In addition, we cannot completely rule out the possibility, that a stimulating moiety is introduced during the albumin purification process. However, the observed effects may at least be important for experimental methods, cell culture and pharmacological usage of purified albumin.
At this point of our investigation, we can only speculate about the physiological implications of the observed effects of albumin. In renal tubointerstitial inflammatory disease, albumin overload due to proteinuria has been suggested to stimulate tissue inflammatory pathomechanisms through activation of NF-κB (Drumm et al., 2001). The present study demonstrated that albumin induces iNOS expression in macrophages which is dependent on NF-κB activation (Xie et al., 1994). Thus, albuminuria possibly stimulates macrophages in renal tubules (Rodriguez-Iturbe et al., 2001) and aggravates tubointerstitial inflammation. Macrophages at high density, which may be sensitive to albumin, can be found in atherosclerotic plaques (Okimoto et al., 2002), and iNOS has recently been reported to be involved in acceleration of atherosclerotic progress (Behr-Roussel et al., 2000; Detmers et al., 2000). On the other hand, inducible NO is known to be involved in bacteriocidal action of macrophages (Nathan & Hibbs, 1991; Anthony et al., 1992). Low albuminemia is a complication in a variety of diseases, such as malnutrition, liver cirrhosis and nephrotic syndrome, and induces susceptibility to infection. Thus, it can be speculated that impaired immunity in such diseases could be in part related to a decrease in inducible NO production due to low albuminaemia. Further studies, including in vivo experiments and clinical investigations are needed to test the above hypothesis.
In conclusion, our results show that albumin purified from serum induces iNOS expression in macrophages independently of polymerisation, endotoxin contamination and AGE, while this stimulation is influenced by interaction of albumin with glucose.
Acknowledgments
This work was supported by the Yamagata University 21st Century COE program from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan.
Abbreviations
- DMEM
Dulbecco's modified Eagle medium
- DMSO
dimethylsulphoxide
- ERK1/2
extracellular-regulated kinase 1/2
- FCS
foetal calf serum
- JAK2
Janus kinase 2
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- STAT
signal transducer and activator of transcription
References
- ANTHONY L.S., MORRISSEY P.J., NANO F.E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-γ-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J. Immunol. 1992;148:1829–1834. [PubMed] [Google Scholar]
- BAYNES J.W., WATKINS N.G., FISHER C.I., HULL C.J., PATRICK J.S., AHMED M.U., DUNN J.A., THORPE S.R. The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 1989;304:43–67. [PubMed] [Google Scholar]
- BEHR-ROUSSEL D., RUPIN A., SIMONET S., BONHOMME E., COUMAILLEAU S., CORDI A., SERKIZ B., FABIANI J.N., VERBEUREN T.J. Effect of chronic treatment with the inducible nitric oxide synthase inhibitor N-iminoethyl-L-lysine or with L-arginine on progression of coronary and aortic atherosclerosis in hypercholesterolemic rabbits. Circulation. 2000;102:1033–1038. doi: 10.1161/01.cir.102.9.1033. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- CHANDLER D.B., FULLER W.C., JACKSON R.M., FULMER J.D. Fractionation of rat alveolar macrophages by isopycnic centrifugation: morphological, cytochemical, biochemical, and functional properties. J. Leukocyte Biol. 1986;39:371–383. doi: 10.1002/jlb.39.4.371. [DOI] [PubMed] [Google Scholar]
- CHANDLER D.B., FULMER J.D. Prostaglandin synthesis and release by subpopulations of rat alveolar macrophages. J. Immunol. 1987;139:893–898. [PubMed] [Google Scholar]
- DASH S., RAO K.V., JOSHI B., NAYAK N.C., PANDA S.K. Significance of natural polymerized albumin and its receptor in hepatitis B infection of hepatocytes. Hepatology. 1991;13:134–142. [PubMed] [Google Scholar]
- DETMERS P.A., HERNANDEZ M., MUDGETT J., HASSING H., BURTON C., MUNDT S., CHUN S., FLETCHER D., CARD D.J., LINSOCK J., WEIKEL R., BERGSTROM J.D., SHEVELL D.E., HERMANOWSKI-VOSATKA A., SPARROW C.P., CHAO Y.S., RADER D.J., WRIGHT S.D., PURE E. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- DRUMM K., GASSNER B., SILBERNAGL S., GEKLE M. Albumin in the mg l−1-range activates NF-κB in renal proximal tubule-derived cell lines via tyrosine kinases and protein kinase C. Eur. J. Med. Res. 2001;6:247–258. [PubMed] [Google Scholar]
- GOOVA M.T., LI J., KISLINGER T., QU W., LU Y., BUCCIARELLI L.G., NOWYGROD S., WOLF B.M., CALISTE X., YAN S.F., STERN D.M., SCHMIDT A.M. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATSUOKA F., KAWAKAMI Y., ARAI T., IMUTA H., FUJIWARA M., KANMA H., YAMASHITA K. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem. Biophys. Res. Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- KOJ A., GUZDEK A., POTEMPA J., KORZUS E., TRAVIS J. Origin of circulating acute phase cytokines: modified proteins may trigger IL-6 production by macrophages. Preliminary report. J. Physiol. Pharmacol. 1994;45:69–80. [PubMed] [Google Scholar]
- MOROHOSHI M., FUJISAWA K., UCHIMURA I., NUMANO F. The effect of glucose and advanced glycosylation end products on IL-6 production by human monocytes. Ann. N.Y. Acad. Sci. 1995;748:562–570. doi: 10.1111/j.1749-6632.1994.tb17362.x. [DOI] [PubMed] [Google Scholar]
- NATHAN C.F., HIBBS J.B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- OBAYASHI T., TAMURA H., TANAKA S., OHKI M., TAKAHASHI S., KAWAI T. Endotoxin-inactivating activity in normal and pathological human blood samples. Infect. Immun. 1986;53:294–297. doi: 10.1128/iai.53.2.294-297.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKIMOTO T., IMAZU M., HAYASHI Y., FUJIWARA H., UEDA H., KOHNO N. Atherosclerotic plaque characterization by quantitative analysis using intravascular ultrasound: correlation with histological and immunohistochemical findings. Circ. J. 2002;66:173–177. doi: 10.1253/circj.66.173. [DOI] [PubMed] [Google Scholar]
- ONO Y., AOKI S., OHNISHI K., YASUDA T., KAWANO K., TSUKADA Y. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res. Clin. Pract. 1998;41:131–137. doi: 10.1016/s0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- PAUL A., PENDEREIGH R.H., PLEVIN R. Protein kinase C and tyrosine kinase pathways regulate lipopolysaccharide-induced nitric oxide synthase activity in RAW 264.7 murine macrophages. Br. J. Pharmacol. 1995;114:482–488. doi: 10.1111/j.1476-5381.1995.tb13252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-ITURBE B., PONS H., HERRERA-ACOSTA J., JOHNSON R.J. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–1640. doi: 10.1046/j.1523-1755.2001.0590051626.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A.M., HORI O., BRETT J., YAN S.D., WAUTIER J.L., STERN D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A.M., HORI O., CAO R., YAN S.D., BRETT J., WAUTIER J.L., OGAWA S., KUWABARA K., MATSUMOTO M., STERN D. RAGE: a novel cellular receptor for advanced glycation end products. Diabetes. 1996;45 Suppl 3:S77–S80. doi: 10.2337/diab.45.3.s77. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A.M., HOFMANN M., TAGUCHI A., YAN S.D., STERN D.M. RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin. Thromb. Hemost. 2000;26:485–493. doi: 10.1055/s-2000-13204. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A.M., STERN D.M. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front. Biosci. 2001;6:D1151–1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- SHACTER E., ARZADON G.K., WILLIAMS J.A. Stimulation of interleukin-6 and prostaglandin E2 secretion from peritoneal macrophages by polymers of albumin. Blood. 1993;82:2853–2864. [PubMed] [Google Scholar]
- THORNALLEY P.J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell. Mol. Biol. (Noisy-le-grand) 1998;44:1013–1023. [PubMed] [Google Scholar]
- THUNG S.N., GERBER M.A. Presence of receptors for polymerized albumin in HbsAg-containing hepatocytes and hepatoma cell line. Hepatology. 1981;1:132–136. doi: 10.1002/hep.1840010208. [DOI] [PubMed] [Google Scholar]
- VLASSARA H., BROWNLEE M., CERAMI A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc. Natl. Acad. Sci. 1985;82:5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE Q.W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- YONEKURA H., YAMAMOTO Y., SAKURAI S., PETROVA R.G., ABEDIN M.J., LI H., YASUI K., TAKEUCHI M., MAKITA Z., TAKASAWA S., OKAMOTO H., WATANABE T., YAMAMOTO H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIOKA T., YAMAMOTO K., KOBASHI H., TOMITA M., TSUJI T. Receptor-mediated endocytosis of chemically modified albumins by sinusoidal endothelial cells and Kupffer cells in rat and human liver. Liver. 1994;14:129–137. doi: 10.1111/j.1600-0676.1994.tb00061.x. [DOI] [PubMed] [Google Scholar]