Abstract
The aim of this study was to analyse the effects of eliprodil, a noncardiac drug with neuroprotective properties, on the cardiac repolarisation under in vitro circumstances, under normal conditions and after the attenuation of the ‘repolarisation reserve' by blocking the inward rectifier potassium current (IK1) current with BaCl2.
In canine right ventricular papillary muscle by applying the conventional microelectrode technique, under normal conditions, eliprodil (1 μM) produced a moderate reverse rate-dependent prolongation of the action potential duration (7.4±1.5, 8.9±2.1 and 9.9±1.8% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=9).
This effect was augmented in preparations where IK1 was previously blocked by BaCl2 (10 μM). BaCl2 alone lengthened APD in a reverse frequency-dependent manner (7.0±1.3, 14.2±1.6 and 28.1±2.1% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8). When eliprodil (1 μM) was administered to these preparations, the drug induced a marked further lengthening relative to the APD values measured after the administration of BaCl2 (12.5±1.0, 17.6±1.5 and 20.5±0.9% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8).
In the normal Langendorff-perfused rabbit heart, eliprodil (1 μM) produced a significant QTc prolongation at 1 Hz stimulation frequency (12.7±1.8%, n=9). After the attenuation of the ‘repolarisation reserve' by the IK1 blocker BaCl2 (10 μM), the eliprodil-evoked QTc prolongation was greatly enhanced (28.5±7.9%, n=6). In two out of six Langendorff preparations, this QTc lengthening degenerated into torsade de pointes ventricular tachycardia.
Eliprodil significantly decreased the amplitude of rapid component of the delayed rectifier potassium current (IKr), but slow component (IKs), transient outward current (Ito) and IK1 were not considerably affected by the drug when measured in dog ventricular myocytes by applying the whole-cell configuration of the patch-clamp technique.
The results indicate that eliprodil, under normal conditions, moderately lengthens cardiac repolarisation by inhibition of IKr. However, after the attenuation of the normal ‘repolarisation reserve', this drug can induce marked QT interval prolongation, which may result in proarrhythmic action.
Keywords: Potassium channels, action potential duration, repolarisation reserve, proarrhythmia, eliprodil, BaCl2
Introduction
Prolongation of the effective refractory period by lengthening of the action potential duration (APD) is a common mechanism in the mode of action of certain dysrhythmic drugs (Singh & Vaughan Williams, 1970; Singh, 1988), which was termed by Vaughan Williams as Class III antiarrhythmic effect (Vaughan Williams, 1970). Although lengthening repolarisation can terminate both ventricular tachycardia (Anderson et al., 2002) and atrial fibrillation (Singh et al., 1999), it can, in certain situations, also evoke torsade de pointes (TdP) ventricular arrhythmias, which may degenerate into ventricular fibrillation, causing sudden death. The proarrhythmic potential of Class III antiarrhythmic drugs greatly limit their usefulness in therapy. This was clearly demonstrated in the SWORD study (Waldo et al., 1996) in which D-sotalol, a selective blocker of the rapid delayed rectifier potassium current (IKr), unexpectedly increased the incidence of sudden death-related mortality in postinfarction patients.
Under normal conditions, block of one type of outward potassium channel is not likely to cause excessive and potentially dangerous APD lengthening, since the other types of potassium channels provide sufficient repolarisation strength, which was termed by Roden (1998) as ‘repolarisation reserve'. However, in situations where the density of one or more types of potassium channel is decreased by inheritance (Roden et al., 1996) or remodelling (Tomaselli & Marban, 1999), that is, the repolarisation reserve is impaired, even relatively weak inhibition of another potassium channel may lead to excessive APD prolongation, which can result in increased risk of proarrhythmia.
Recently, we have demonstrated that pharmacological attenuation of the repolarisation reserve evoked greatly increased APD lengthening by blocking the rapid component (IKr), the slow component (IKs) of the delayed rectifier (IK) and the inward rectifier (IK1) potassium currents, and occasionally induced early afterdepolarisations (Biliczki et al., 2002).
In the past few years, it became evident that several noncardiac drugs can also moderately prolong repolarisation (Pinney et al., 1995; De Ponti et al., 2000; Gintant et al., 2001) by inhibition of one or more potassium currents (Antzelevitch et al., 1996; Ducic et al., 1997; Rampe & Murawsky, 1997; Drici & Barhanin, 2000). Eliprodil, a newly developed NMDA (N-methyl-D-aspartate) receptor antagonist neuroprotective agent (Reyes et al., 1998), has been at times observed to prolong the QT interval in patients (Garreau et al., 1992), which may involve the risk of development of proarrhythmic complications. Despite the fact that neuroprotective drugs for acute stroke have appeared to be effective in animals, they have all failed in clinical trials (Gladstone et al., 2002; Ikonomidou & Turski, 2002). However, these new selective NMDA antagonists of the NR2B receptor subtype appear promising tools for acute and chronic pain, and they are currently under clinical investigation (Chizh et al., 2001).
Therefore, the aim of the present study was to analyse the effect of eliprodil on the cardiac repolarisation under in vitro circumstances, under normal conditions and after the attenuation of the ‘repolarisation reserve' by blocking the IK1 current with BaCl2.
Methods
All experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (USA NIH publication no. 85-23, revised 1985). The protocols were approved by the Review Board of the Committee on Animal Research of the University of Szeged (54/1999 Oej).
Conventional microelectrode technique
Adult mongrel dogs (8–14 kg) of either sex were used. Following anaesthesia (sodium pentobarbital, 30 mg kg−1 administered intravenously (i.v.)), the heart of each animal was rapidly removed through right lateral thoracotomy. The hearts were immediately rinsed in oxygenated modified Locke's solution containing (in mM): NaCl 120, KCl 4, CaCl2 1.0, MgCl2 1, NaHCO3 22 and glucose, 11. The pH of this solution was 7.35–7.40 when saturated with 95% O2 and 5% CO2 at 37°C. The tip of the papillary muscles obtained from the right ventricle were individually mounted in a tissue chamber (volume ≈50 ml). Each ventricular preparation was initially stimulated (HSE stimulator type 215/II, Hugo Sachs Elektronik, March-Hugstetten, Germany) at a basic cycle length of 1000 ms (frequency=1 Hz), using rectangular constant current pulses 2 ms in duration. These stimuli were isolated from ground and delivered through a bipolar platinum electrode in contact with the preparation. At least 1 h was allowed for each preparation to equilibrate after mounting before experimental measurements were initiated. Temperature of the superfusate was kept constant at 37°C. Transmembrane potentials were recorded using conventional microelectrode technique. Microelectrodes filled with 3 M KCl and having tip resistances of 5–20 MΩ were connected to the input of a high impedance electrometer (HSE microelectrode amplifier, type 309), which was connected to the ground. The first derivative of transmembrane potentials was electronically obtained by an HSE differentiator (type 309). The voltage outputs from all amplifiers were displayed on a dual beam memory oscilloscope (Tektronix 2230 100 MHz digital storage oscilloscope, Beaverton, OR, U.S.A.).
The maximum diastolic potential, action potential amplitude and APD at 50 and 90% of repolarisation (APD50 and APD90) were automatically measured using a software developed in our laboratory (Hugo Sachs Elektronik, Action Potential Evaluation System (HSE-APES) running on a 386-microprocessor-based, IBM-compatible computer containing an ADA 3300 analogue-to-digital data-aquisition board (Real Time Devices Inc., State Collage, PA, U.S.A.)), with a maximum sampling frequency of 40 KHz. In each experiment, baseline action potential characteristics were first determined during continuous pacing at 1 Hz, and then while pacing cycle length was sequentially varied between 300 and 5000 ms. In all, 25 action potentials were evoked at each cycle length and the cycle length was then changed so that ‘quasi' steady-state frequency response relations could be rapidly generated. After control measurements, the preparations were superfused for 40 min with saline containing the compound under study, and then the electrophysiological measurements were resumed. The effects of eliprodil (Gedeon Richter Ltd, Budapest, Hungary) and BaCl2 were studied at 1 and 10 μM concentrations, respectively. Attempts were made to maintain the same impalement throughout each experiment. If, however, an impalement became dislodged, adjustment was attempted, and if the action potential characteristics of the re-established impalement deviated by less than 5% from the previous measurement, the experiment continued.
Electrocardiogram (ECG) measurements in Langendorff-perfused rabbit hearts
New Zealand rabbits weighing 1.5–2.0 kg of either sex were used. Each animal was killed by cervical dislocation after an i.v. injection of 400 IU kg−1 heparin. The chest was opened, the heart quickly removed and immediately immersed in oxygenated modified Locke's solution. The hearts were mounted on a Langendorff column and perfused with oxygenated Locke's solution warmed to 37°C. After flushing blood from the coronary vasculature for 3–5 min, the heart was immersed in a tissue chamber filled with perfusion solution maintained at 37°C while continuing perfusion. Volume-conducted ECGs were obtained as described previously (Zabel et al., 1995). Briefly, four silver–silver chloride electrodes were positioned in a simulated Einthoven configuration with the reference and ‘foot' electrodes situated beneath the heart and the ‘arm' electrodes fixed to the upper walls of the tissue chamber to record the six bipolar ECG leads I through augmented unipolar foot ECG lead. All leads were acquired by an ECG signal processing system (Haemosys, Experimetria Ltd, Budapest, Hungary) utilising a P4-microprocessor-based, IBM-compatible personal computer. After analogue-to-digital conversion, the data were stored on hard disk and analysed off-line. After an 1 h equilibration period, baseline ECGs were obtained and a 20 min perfusion period was initiated with eliprodil either alone or after a 20 min BaCl2 pretreatment.
ECG recordings were monitored continuously and compared to baseline measurements at the end of this period. QT intervals were always measured on lead II from QRS onset to the end of the T wave; biphasic T waves were measured to the time of final baseline return. These QT measurements and simultaneously recorded RR intervals were used to derive heart rate corrected QT intervals using Carlsson's formula IQTc=QT−0.175 (RR−300)) (Carlsson et al., 1993). ECG parameters were averaged from measures of three consecutive complexes and a single observer performed all analyses.
Whole-cell configuration of the patch-clamp technique
Ventricular myocytes were enzymatically dissociated from hearts of mongrel dogs of either sex weighing 10–20 kg following anaesthesia (sodium pentobarbital, 30 mg kg−1 i.v.) as described earlier in detail (Varró et al., 2000).
One drop of cell suspension was placed within a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and individual myocytes were allowed to settle and adhere to the chamber bottom for at least 5 min before superfusion was initiated. Only rod-shaped cells with clear crossstriations were used. HEPES-buffered Tyrode's solution served as the normal superfusate. This solution contained (mM): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, glucose 5.5 and HEPES 5.0 at pH of 7.4.
Patch-clamp micropipettes were fabricated from borosilicate glass capillaries (Clark, Reading, U.K.) using a P-97 Flaming/Brown micropipette puller (Sutter Co, Novato, CA, U.S.A.). These electrodes had resistances between 1.5 and 2.5 MΩ when filled with pipette solution containing (in mM): K-aspartate 100, KCl 45, ATP 3, MgCl2 1, EGTA 10 and HEPES 5. The pH of this solution was adjusted to 7.2 by KOH. Cell capacitance was measured by applying a 10 mV hyperpolarising pulse from −10 mV. The holding potential was −90 mV. Cell capacitance was measured by integration of the capacitive transient divided by the amplitude of the voltage step (10 mV). Measuring K+ currents, nisoldipine (1 μM) (gift from Bayer AG, Leverkusen, Germany) was added to the external solution to eliminate L-type Ca2+ current (ICa). The rapid IKr and slow IKs components of the delayed rectifier potassium current were separated by using the selective IKr blocker E-4031 (1 μM, Institute for Drug Research, Budapest, Hungary) or the IKs blocker L-735,821 (100 nM, a gift from Merck-Sharpe & Dohme, West-Point, PA, U.S.A.). Membrane currents were recorded with Axopatch-1D and 200B patch-clamp amplifiers (Axon Instruments, Union City, CA, U.S.A.) using the whole-cell configuration of the patch-clamp technique. After establishing a high (1–10 GΩ) resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of 1.5 V electrical pulses for 1–5 ms. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocols). Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitised using a 333 kHz analog-to-digital converter (Digidata 1200, Axon Instruments) under software control (pClamp 6.0 and 7.0 Axon Instruments). Analyses were performed using pClamp 6.0 software (Axon) after low-pass filtering at 1 kHz. All patch-clamp data were collected at 37°C.
Statistical analysis
Results were compared using Student's t-tests for paired and unpaired data. Differences were considered significant when P<0.05. Data are expressed as mean±s.e.m.
Results
Effect of eliprodil on ventricular APD in isolated canine ventricular papillary muscle under normal conditions
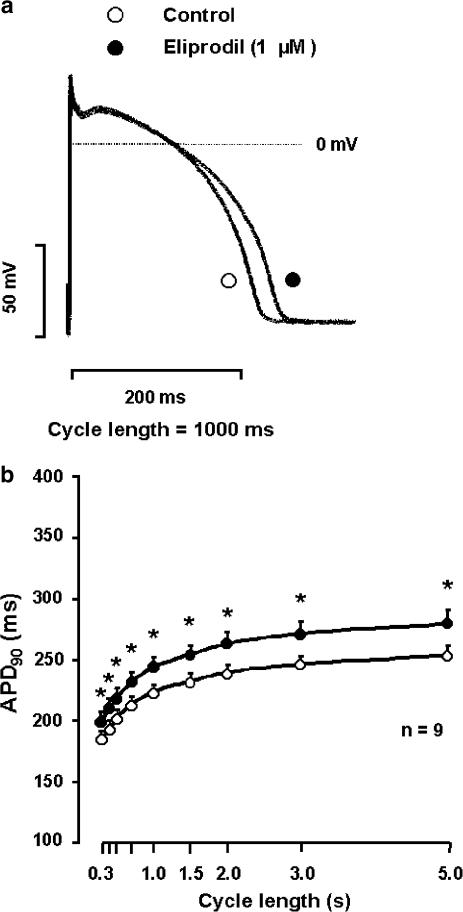
The effect of eliprodil on the action potential in canine ventricular papillary muscle at 1 Hz stimulation frequency is shown in Figure 1a. Eliprodil (1 μM) lengthened APD90 moderately (<10%) from 235.3±5.9 to 257.3±9.0 ms (n=9, P<0.05) without causing significant change in the resting membrane potential, the action potential amplitude and the maximum upstroke velocity (dVdtmax−1). To study the rate-dependent effect of eliprodil on APD and dVdtmax−1, the preparations were stimulated at cycle lengths ranging from 300 to 5000 ms. Under these circumstances, eliprodil did not change the dVdtmax−1. However, as shown in Figure 1b, under normal conditions, the drug produced a moderate reverse rate-dependent APD prolongation (7.4±1.5, 8.9±2.1 and 9.9±1.8% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=9).
Figure 1.
Effect of eliprodil on the APD (APD90) in canine right ventricular papillary muscle. In (a), he effect of 1 μM eliprodil on the action potential at 1 s stimulation cycle length is shown, while (b) illustrates the frequency-dependent effect of 1 μM eliprodil on the APD90 (mean±s.e.m., *P<0.05 vs control).
Effect of eliprodil on ventricular APD in isolated canine ventricular papillary muscle after IK1 inhibition
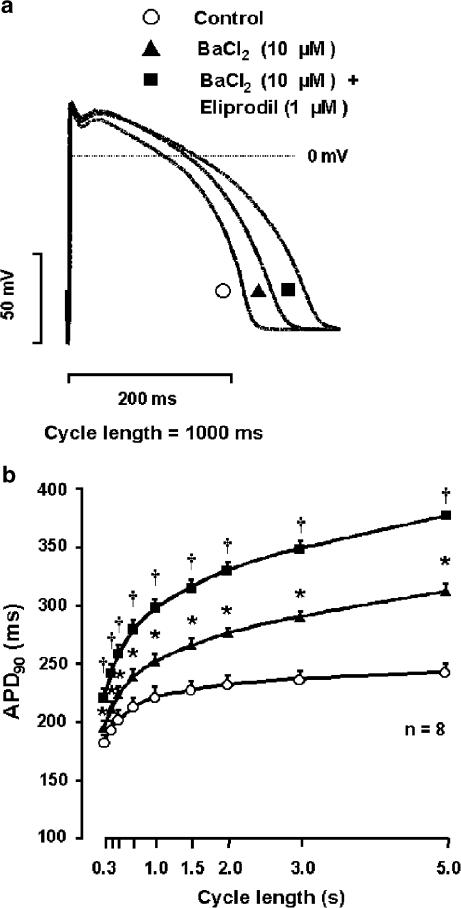
In canine right ventricular papillary muscles, partial block of IK1 by 10 μM BaCl2 (Liu et al., 2001) lengthened APD in a reverse frequency-dependent manner (7.0±1.3, 14.2±1.6 and 28.1±2.1% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8) (Figure 2a and b). In the presence of BaCl2, 1 μM eliprodil was added to these preparations. The drug induced a marked further lengthening relative to the APD values measured after the administration of BaCl2 (12.5±1.0, 17.6±1.5 and 20.5±0.9% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=8) (Figure 2a and b), that is, the APD lengthening effect of eliprodil was significantly augmented in preparations where the ‘repolarisation reserve' was attenuated by previous application and presence of BaCl2.
Figure 2.
Effect of eliprodil on the APD (APD90) in the presence of IK1 block by BaCl2 (10 μM) in canine right ventricular papillary muscle. In (a), representative action potential traces are shown under control conditions (open circle) in the presence of 10 μM BaCl2 alone (triangle) and after application of 1 μM eliprodil in the presence of IK1 block (square) at 1 s stimulation cycle length, while (b) illustrates the APD90 as a function of the stimulation frequency under control conditions (open circles), in the presence of 10 μM BaCl2 alone (triangles) and after application of 1 μM eliprodil in the presence of IK1 block (squares) (mean±s.e.m., *P<0.05 vs control, †P<0.05 vs BaCl2).
Effect of eliprodil on QTc interval in isolated Langendorff-perfused rabbit hearts in the absence and presence of IK1 block
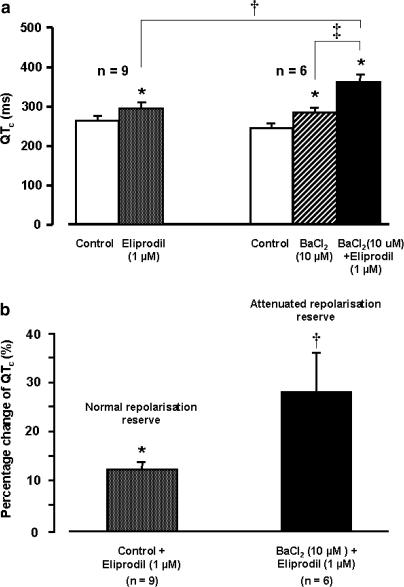
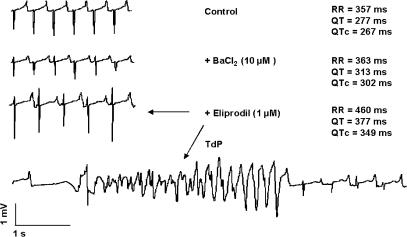
In the normal Langendorff-perfused rabbit heart, eliprodil (1 μM) produced a significant QTc prolongation (12.7±1.8%, n=9) (Figure 3a and b). After the attenuation of the ‘repolarisation reserve' by the IK1 blocker BaCl2 (10 μM), this eliprodil-evoked QTc prolongation was greatly enhanced (28.5±7.9%, n=6) (Figure 3a and b). In two out of six Langendorff preparations, the QTc lengthening degenerated into TdP ventricular tachycardia (Figure 4).
Figure 3.
(a) Effect of 1 μM eliprodil on QTc interval of the volume-conducted ECG recorded in isolated Langendorff-perfused rabbit heart in the absence and presence of 10 μM BaCl2 (mean±s.e.m., *P<0.05 eliprodil or BaCl2 vs control in normal Locke's solution, †P<0.05 eliprodil in solution containing 10 μM BaCl2 vs eliprodil in normal Locke's solution, ‡P<0.05 BaCl2 vs BaCl2 and eliprodil in the organ bath). (b) The percentage change of the eliprodil-evoked QTc lengthening in normal and in attenuated repolarisation reserve preparations. The changes in the attenuated repolarisation reserve preparations was calculated by using values as baseline after application of 10 μM BaCl2.
Figure 4.
Proarrhythmic effect of 1 μM eliprodil after the administration of 10 μM BaCl2 on volume-conducted ECG recorded in isolated Langendorff-perfused rabbit heart.
Effect of eliprodil on the transmembrane potassium currents in canine ventricular myocytes
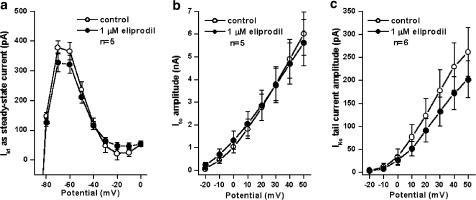
IK1 and transient outward current (Ito) was measured by applying 400 ms long depolarising voltage pulses in the voltage range of −120 mV to +60 mV with 3 s pulse intervals from the holding potential of −90 mV. IK1 was measured as the steady-state current at the end of the test pulse in the voltage range between −80 to 0 mV. Ito was measured as the difference of the peak outward current at the beginning of the pulse and the steady-state current at the end of the pulse. As Figure 5 shows, eliprodil (1 μM) does not considerably influence IK1 or Ito in canine ventricular myocytes (IK1 current values at −60 mV: 365.7±29.2 pA as control and 321.5±30.5 pA in the presence of 1 μM eliprodil, n=5; Ito current values at 50 mV: 6017.2±963.0 pA as control and 5617.5±1025.0 pA in the presence of 1 μM eliprodil, n=5).
Figure 5.
Lack of effect of 1 μM eliprodil in canine ventricular myocytes on the inward rectifier potassium current (IK1) measured as the steady-state current at the end of the test pulse in the voltage range between −80 to 0 mV (a), on the transient outward current (Ito) (b) and on the slow component of the delayed rectifier potassium current (IKs) (c). Panels show current–voltage relationships under control conditions and in the presence of 1 μM eliprodil (mean±s.e.m.).
IKs was measured by applying 5 s long depolarising voltage pulses from a holding potential of −40 mV. Test pulses were applied every 10 s with 10 mV increments in the voltage range from −30 to +50 mV. On clamping the cells back to the holding potential of −40 mV, an outward tail current was measured that was attributed to IKs. In these measurements, 1 μM E-4031 was used to block completely IKr. As Figure 5 indicates, 1 μM eliprodil does not significantly affect IKs in canine ventricular myocytes (IKs tail current was 261.6±53.0 pA under control conditions and 202.4±40.0 pA after application of 1 μM eliprodil, at 50 mV of potential of activation, n=6).
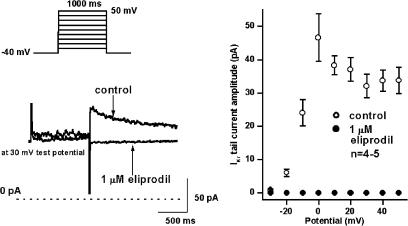
IKr was determined by applying 1 s long depolarising voltage pulses in the voltage range of −30 and +50 mV with 20 s pulse intervals from the holding potential of −40 mV. The amplitude of the tail current after the end of the test pulse was considered as IKr. In these experiments, 100 nM L-735,821 was used to eliminate IKs. As Figure 6 indicates, 1 μM eliprodil abolished IKr tail current completely.
Figure 6.
Effect of 1 μM eliprodil on the rapid component of the delayed rectifier potassium current (IKr) in canine ventricular myocytes. (Left panel) Original current traces under control conditions and after application of 1 μM eliprodil. (Right panel) The current–voltage relationship of IKr under control conditions and in the presence of 1 μM eliprodil (mean±s.e.m.). The applied voltage protocol is shown on the top on the left.
Discussion
The most important finding of this study is that eliprodil, which blocks IKr current without considerably interfering with IK1, IKs and Ito, caused moderate APD and QTc lengthening when it was applied alone, but when the ‘repolarisation reserve' was attenuated by BaCl2, it evoked augmented prolongation of repolarisation, occasionally resulting in TdP ventricular tachycardia.
In our experiments, we applied 10 μM BaCl2 to partially but selectively inhibit IK1 (Liu et al., 2001). At this concentration, BaCl2 does not affect IKr, IKs or Ito but depresses IK1 by about 60% (Biliczki et al., 2002). Eliprodil, which has an estimated effective plasma concentration (Garrigou-Gadenne et al., 1995; Malavasi et al., 1996) around the micromolar range and of whose effect on cardiac potassium channels has not been characterised so far, did not influence potassium currents other than IKr in our experimental conditions. Therefore, the present results extend our previous observation that simultaneous blockade of different potassium channels decreases the ‘repolarisation reserve' and enhances APD prolongation (Biliczki et al., 2002).
Vos et al. (1995) developed an experimental TdP arrhythmia model in the dog, in which they found that after complete atrioventricular block downregulation of potassium currents increased the ability of certain Class III antiarrhythmic drugs to produce excessive QTc lengthening and TdP arrhythmia, which is in good agreement with our present results. It is also known that various potassium channels are downregulated during heart failure (Beuckelmann et al., 1993; Näbauer et al., 1993; Näbauer & Kääb, 1998), resulting in longer APD (Kääb et al., 1996) and increased risk of proarrhythmia. Furthermore, in some forms of inherited long QT syndrome, mutation in the channel protein genes (Roden et al., 1996; Priori et al., 2001) does not necessarily lead to marked or even manifest QTc lengthening (Priori et al., 1998; Swan et al., 1998), but individuals with these alterations are probably more susceptible to drugs that affect repolarisation. These observations argue the role of the ‘repolarisation reserve', that is, that different potassium channels compensate each other to secure the repolarisation process (Roden, 1998; Biliczki et al., 2002).
The present experiments may have important therapeutical and practical implications. Some noncardiac drugs exhibit weak inhibition of one or more potassium, most frequently the IKr (HERG/MiRP) channel. Since this effect does not markedly influence repolarisation in normal situation, their effect on QT is often masked. Therefore, the potential proarrhythmic danger can be easily underestimated in individuals who have decreased ‘repolarisation reserve' in spite of their baseline QTc falls within the normal range. Accordingly, eliprodil or any drug, which is known to inhibit potassium current and exert only moderate or not even consistent repolarisation lengthening, should be administered under repeated or continuous ECG control, and if QTc prolongation longer than expected is noticed, the therapy with such a drug should be discontinued. Also, the concept of attenuated ‘repolarisation reserve' should be considered during safety pharmacology studies, since the rabbit and guinea-pig possessing fast heart rate or even the dog, all of which probably have relatively strong repolarisation reserve, can not be expected to respond with significant QT lengthening when drugs partially block only one type of cardiac potassium channels. Instead of studying drug effects on the cardiac repolarisation and proarrhythmic risk in the normal heart, it would certainly be more useful to develop and apply screening tests where repolarisation reserve is attenuated.
Conclusion
We conclude that the neuroprotective drug eliprodil under normal conditions only moderately lengthens cardiac repolarisation by inhibition of IKr, but this drug can induce marked QT interval/APD prolongation under circumstances where the repolarisation reserve is attenuated, which may greatly enhance proarrhythmic risk.
Acknowledgments
This work was supported by grants from the Hungarian National Research Fundation (OTKA T-032558, T-035018 and T-037520), Hungarian Ministry of Health (ETT 188/2003 and 03144/2001), National Research and Development Programmes (NKFP 1A/0011/2002), from the Hungarian Academy of Sciences and by János Bolyai Research Scholarship (for LV).
Abbreviations
- APD
action potential duration
- dVdtmax−1
maximum upstroke velocity
- ICa
L-type calcium current
- IK1
inward rectifier potassium current
- IKr
rapid component of the delayed rectifier potassium current
- IKs
slow component of the delayed rectifier potassium current
- Ito
transient outward current
- TdP
torsade de pointes ventricular tachycardia
References
- ANDERSON M.E., AL-KHATIB S.M., RODEN D.M., CALIFF R.M., DUKE CLINICAL RESEARCH INSTITUTE/AMERICAN HEART JOURNAL EXPERT MEETING ON REPOLARIZATION CHANGES Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am. Heart J. 2002;144:769–781. doi: 10.1067/mhj.2002.125804. [DOI] [PubMed] [Google Scholar]
- ANTZELEVITCH C., SUN Z.Q., ZHANG Z.Q., YAN G.X. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J. Am. Coll. Cardiol. 1996;28:1836–1848. doi: 10.1016/S0735-1097(96)00377-4. [DOI] [PubMed] [Google Scholar]
- BEUCKELMANN D.J., NÄBAUER M., ERDMANN E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- BILICZKI P., VIRÁG L., IOST N., PAPP J.G., VARRÓ A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON L., ABRAHAMSSON C., ANDERSSON B., DUKER G., SCHILLER-LINHARDT G. Proarrhythmic effects of the class III agent almokalant: importance of infusion rate, QT dispersion, and early afterdepolarisations. Cardiovasc. Res. 1993;27:2186–2193. doi: 10.1093/cvr/27.12.2186. [DOI] [PubMed] [Google Scholar]
- CHIZH B.A., HEADLEY P.M., TZSCHENTKE T.M. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol. Sci. 2001;22:636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., POLUZZI E., MONTANARO N. QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur. J. Clin. Pharmacol. 2000;56:1–18. doi: 10.1007/s002280050714. [DOI] [PubMed] [Google Scholar]
- DRICI M.D., BARHANIN J. Cardiac K+ channels and drug-acquired long QT syndrome. Therapie. 2000;55:185–193. [PubMed] [Google Scholar]
- DUCIC I., KO C.M., SHUBA Y., MORAD M. Comparative effects of loratadine and terfenadine on cardiac K+ channels. J. Cardiovasc. Pharmacol. 1997;30:42–54. doi: 10.1097/00005344-199707000-00007. [DOI] [PubMed] [Google Scholar]
- GARREAU M., GIROUX C., L'HERITIER C., COUPEZ M., EICH F., MORSELLI P.L. Pilot studies on the effect of SL 82.0715 in psychotic syndromes. Clin. Neuropharmacol. 1992;15 Suppl 1:699A–700A. doi: 10.1097/00002826-199201001-00361. [DOI] [PubMed] [Google Scholar]
- GARRIGOU-GADENNE D., THENOT J.P., MORSELLI P L. Influence of the rate of intravenous administration of eliprodil (SL 82.0715), a new anti-ischaemic agent, on its distribution in rat plasma and tissues. J. Pharmacokinet. Biopharm. 1995;23:147–161. doi: 10.1007/BF02354269. [DOI] [PubMed] [Google Scholar]
- GINTANT G.A., LIMBERIS J.T., McDERMOTT J.S., WEGNER C.D., COX B.F. The canine Purkinje fiber: an in vitro model system for acquired long QT syndrome and drug-induced arrhythmogenesis. J. Cardiovasc. Pharmacol. 2001;37:607–618. doi: 10.1097/00005344-200105000-00012. [DOI] [PubMed] [Google Scholar]
- GLADSTONE D.J., BLACK S.E., HAKIM A.M. Toward wisdom from failure. Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C., TURSKI L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury. Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- KÄÄB S., NUSS H.B., CHIAMVIMONVART N., O'ROURKE B., KASS D.A., MARBAN E., TOMASELLI G.F. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ. Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- LIU G.X., DERST C., SCHLICHTHÖRL G., HEINEN S., SEEBOHM G., BRÜGGEMANN A., KUMMER W., VEH R.W., DAUT J., PREISIG-MÜLLER R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J. Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAVASI B., RIPAMONTI M., ROUCHOUSE A., ASCALONE V. Stereoselective determination of unchanged and glucuroconjugated eliprodil, a new anti-ischaemic drug, in human plasma and urine by precolumn derivatization and column-switching high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A. 1996;729:323–333. doi: 10.1016/0021-9673(95)00893-4. [DOI] [PubMed] [Google Scholar]
- NÄBAUER M., KÄÄB S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- NÄBAUER M., BEUCKELMANN D.J., ERDMANN E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993;73:386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- PINNEY S.P., KOLLER B.S., FRANZ M.R., WOOSLEY R.L. Terfenadine increases the QT interval in isolated guinea pig heart. J. Cardiovasc. Pharmacol. 1995;25:30–40. doi: 10.1097/00005344-199501000-00006. [DOI] [PubMed] [Google Scholar]
- PRIORI S.G., BLOISE R., CROTTI L. The long QT syndrome. Europace. 2001;3:16–27. doi: 10.1053/eupc.2000.0141. [DOI] [PubMed] [Google Scholar]
- PRIORI S.G., NAPOLITANO C., BLOISE R., SCHWARTZ P.J. Low penetrance in the long QT syndrome: the importance of molecular diagnosis. Eur. Heart J. 1998;19 Abstract suppl.:424. [Google Scholar]
- RAMPE D., MURAWSKY M.K. Blockade of the human cardiac K+ channel Kv1.5 by the antibiotic erythromycin. Naunyn-Schmiedebergs Arch. Pharmacol. 1997;355:743–750. doi: 10.1007/pl00005008. [DOI] [PubMed] [Google Scholar]
- REYES M., REYES A., OPITZ T., KAPIN M.A., STATON P.K. Eliprodil, a non-competetive, NR2B-selective NMDA antagonist, protects pyramidal neurons in hippocampal slices from hypoxic/ischaemic damage. Brain Res. 1998;782:212–218. doi: 10.1016/s0006-8993(97)01280-8. [DOI] [PubMed] [Google Scholar]
- RODEN D.M. Taking the ‘idio' out of ‘idiosyncratic': predicting torsades de pointes. PACE. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- RODEN D.M., LAZZARA R., ROSEN M., SCHWARTZ P.J., TOWBIN J., VINCENT G.M., for the SADS Foundation Task Force on LQTS Multiple mechanisms in the long-QT syndrome current knowledge, gaps, and future directions. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- SINGH B.N.Comparative mechanisms of action of antiarrhythmic agents: significance of lengthening repolarization Control of Cardiac Arrhythmias by Lengthening Repolarization 1988New York: Futura Publishing Co; 53–127.ed. Singh, B.N. pp [Google Scholar]
- SINGH B.N., MODY F.V., LOPEZ B., SARMA J.S. Antiarrhythmic agents for atrial fibrillation: focus on prolonging atrial repolarization. Am. J. Cardiol. 1999;84:161R–173R. doi: 10.1016/s0002-9149(99)00718-3. [DOI] [PubMed] [Google Scholar]
- SINGH B.N., VAUGHAN WILLIAMS E.M. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. Br. J. Pharmacol. 1970;39:675–687. doi: 10.1111/j.1476-5381.1970.tb09893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAN H., SAARINEN K., KONTULA K., TOIVONEN L., VIITASALO M. Evaluation of QT interval duration and dispersion and proposed clinical criteria in diagnosis of long QT syndrome in patients with a genetically uniform type of LQT1. J. Am. Coll. Cardiol. 1998;32:486–491. doi: 10.1016/s0735-1097(98)00248-4. [DOI] [PubMed] [Google Scholar]
- TOMASELLI G.F., MARBAN E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc. Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., BALÁTI B., IOST N., TAKÁCS J., VIRÁG L., LATHROP D.A., LENGYEL C., TÁLOSI L., PAPP J.G.Y. The role of the delayed component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN WILLIAMS E.M.Classification of antiarrhythmic drugs Symposium on Cardiac Arrhythmias. Sedertalje 1970Sweden: AB Astra; 449–472.eds. Sandoe, E., Flensted-Jensen, E., Olesen, K.H. pp [Google Scholar]
- VOS M.A., VERDUYN S.C., GORGELS A.P.M., LIPCSEI G.C., WELLENS H.J.J. Reproducible induction of early afterdepolarizations and torsade de pointes arrhythmias by D-sotalol and pacing in dogs with chronic atrioventricular block. Circulation. 1995;91:846–872. doi: 10.1161/01.cir.91.3.864. [DOI] [PubMed] [Google Scholar]
- WALDO A.L., CAMM A.J., DERUYTER H., FRIEDMAN P.L., MACNEIL D.J., PAULS J.F., PITT B., PRATT C.M., SCHWARTZ P.J., VELTRI E.P. Effect of D-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- ZABEL M., PORTNOY S., FRANZ M.R. Electrocardiographic indexes of dispersion of ventricular repolarization: an isolated heart validation study. J. Am. Coll. Cardiol. 1995;25:746–752. doi: 10.1016/0735-1097(94)00446-W. [DOI] [PubMed] [Google Scholar]