Abstract
The aim was to test the hypothesis that nitric oxide (NO) donor drugs can inhibit the 5-hydroxytryptamine (5-HT) transporter, SERT.
The NO donors, MAHMA/NO (a NONOate; (Z)-1-[N-methyl-N-[6-(N-methylammoniohexyl)-amino]]diazen-1-ium-1,2-diolate), SIN-1 (a sydnonimine; 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride), FK409 (an oxime; (±)-(4-ethyl-2E-(hydroxyimino)-5-nitro-3E-hexenamide)) and peroxynitrite, but not Angeli's salt (source of nitroxyl anion) or sodium nitrite, caused concentration-dependent inhibition of the specific uptake of [3H]-5-HT in COS-7 cells expressing human SERT.
Superoxide dismutase (150 U ml−1) plus catalase (1200 U ml−1), used to remove superoxide and hence prevent peroxynitrite formation, prevented the inhibitory effect of SIN-1 (which generates superoxide) but not of MAHMA/NO or FK409.
The inhibitory effects of the NO donors were not affected by the free radical scavenger, hydroxocobalamin (1 mM) or the guanylate cyclase inhibitor, ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; 3 μM).
L-Cysteine (1 mM; source of excess thiol residues) abolished or markedly reduced the inhibitory effects of MAHMA/NO, SIN-1, FK409 and peroxynitrite.
It is concluded that inhibition of SERT by the NO donors cannot be attributed exclusively to NO free radical nor to nitroxyl anion. It does not involve guanosine-3′,5′-cyclic monophosphate, but may involve nitrosation of cysteine residues on the SERT protein. Peroxynitrite mediates the effect of SIN-1, but not the other drugs.
Data in mice with hypoxic pulmonary hypertension suggest that SERT inhibitors may attenuate pulmonary vascular remodelling. Thus, NO donors may be useful in pulmonary hypertension, not only as vasodilators, but also because they inhibit SERT, provided they display this effect in vivo at appropriate doses.
Keywords: 5-Hydroxytryptamine, 5-HT transporter (SERT), dopamine transporter (DAT), nitric oxide donors, NONOates, SIN-1, FK409, peroxynitrite, transfected COS-7 cells
Introduction
Nitric oxide (NO) donors are known primarily for their vasodilator properties and are used in a variety of cardiovascular diseases such as angina pectoris, heart failure and hypertension. They may also have a role as pulmonary vasodilators in pulmonary hypertension (Tilton et al., 2001; Keefer, 2003). In this condition, they could provide an alternative to NO gas, which is successfully used in neonatal pulmonary hypertension (Golombek, 2000), but is impractical for long-term use in adults (Hoeper et al., 2002). In addition to their vasodilator properties, NO donors have a variety of other pharmacological actions. One of these is the ability to inhibit the dopamine transporter (DAT) in both neuronal and non-neuronal cells (Lonart & Johnson, 1994; Pogun et al., 1994; Cook et al., 1996; Cao & Reith, 2002; Park et al., 2002). The 5-hydroxytryptamine (5-HT) transporter (SERT) is closely related to DAT and this raises the possibility that NO donors may be able to inhibit SERT as well. This could be of particular interest since it has been suggested that inhibitors of SERT may be of value in the treatment of patients with pulmonary hypertension (Marcos et al., 2003).
Both SERT and DAT belong to the monoamine transporter subfamily of the Na+- and Cl−-dependent neurotransmitter transporter family, and share 44% amino-acid identity (Ramamoorthy et al., 1993). The few studies in which the effects of NO donors on SERT have been investigated have provided conflicting results. Some studies have reported increases in 5-HT uptake by the S-nitrosothiol, S-nitrosoacetylpenicillamine, mediated via a guanosine-3′,5′-cyclic monophosphate (c-GMP)-dependent mechanism (Miller & Hoffman, 1994; Kilic et al., 2003) and others have reported decreases in 5-HT uptake by sodium nitroprusside and S-nitrosoacetylpenicillamine (Pogun et al., 1994; Asano et al., 1997) via a c-GMP-independent mechanism (Asano et al., 1997). However, more importantly, these previous studies have mainly examined S-nitrosothiols and sodium nitroprusside, which are not representative of NO donors in general (Feelisch & Stamler, 1996).
The objective of the present study was to test the hypothesis that NO donors inhibit SERT activity and, if they do, to investigate the mechanisms by which inhibition occurs. The NO donors selected belong to different chemical classes, but have in common the ability to generate NO ‘spontaneously' at physiological pH. Thus, they differ from the drugs used in the previous studies (see above), since nitroprusside requires tissue activation to generate NO and S-nitrosothiols can act without the release of free NO (Feelisch & Stamler, 1996). SERT activity was evaluated in this study by determining the specific uptake of [3H]-5-HT in COS-7 cells transiently transfected with the cDNA of human SERT (hSERT). In some parts of the study, the effects of the NO donors on DAT activity (specific uptake of [3H]-dopamine into COS–7 cells transiently transfected with the cDNA of hDAT) have also been determined for comparison with the results for SERT.
A preliminary account of some of these data was communicated to the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (Bryan-Lluka et al., 2003).
Methods
Cell culture and transfection
COS-7 cells (SV40-transformed African green monkey kidney cells; American Type Culture Collection, Bethesda, MD, U.S.A.) were selected for the study because they do not endogenously express SERT or DAT. The cells were grown at 37°C in a 5% CO2, humidified atmosphere on standard plastic cultureware in Dulbecco's modified Eagle's medium (Invitrogen Australia Pty Ltd, Melbourne, Australia), supplemented with 10% foetal calf serum (Invitrogen) and 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Invitrogen). The cells were subcultured onto 12-well culture plates and transiently transfected with hSERT or hDAT cDNA using Lipofectamine (Invitrogen) as previously described (Paczkowski et al., 1999) for experiments 48 h later.
Uptake assays
Culture medium was removed from the COS-7 cells, and they were then washed twice with 1 ml Krebs/HEPES buffer containing 0.1% bovine serum albumin (Catalogue No. A-7906, Sigma-Aldrich, Sydney, NSW, Australia) at 37°C. The composition of the Krebs/HEPES buffer was 125 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.3 mM CaCl2, 25 mM HEPES, 5.55 mM glucose, 40 μM Na2EDTA, 5 μM U-0521 (to inhibit catechol-O-methyltransferase; only in experiments with [3H]-dopamine) and 1 μM pargyline (to inhibit monoamine oxidase), pH 7.4. The cells were preincubated for 15 min (unless indicated otherwise) at 37°C with 1 ml Krebs/HEPES buffer containing 0.1% bovine serum albumin in the absence or presence of the SERT inhibitor paroxetine (1 μM; 1000 × greater than the Ki of paroxetine for inhibition of SERT (Owens et al., 1997)) for hSERT-expressing cells or the DAT inhibitor GBR 12909 (1 μM; 50 × greater than the Ki of GBR 12909 for inhibition of DAT (Eshleman et al., 1999)) for hDAT-expressing cells (to determine total and non-specific uptake, respectively). NO donors or nucleophiles (12.5–800 μM) or vehicle were present for the last 10 min of preincubation. In some experiments, 1 mM hydroxocobalamin, 150 U ml−1 superoxide dismutase (SOD) plus 1200 U ml−1 catalase (CAT), 1 mM L-cysteine or vehicle was present for the last 13 min of the 15 min preincubation period. In other experiments, 3 μM ODQ or vehicle was present for an extended 20 min preincubation period. After the preincubation period, [3H]-5-HT (10 nM) or [3H]-dopamine (10 nM) was added for an incubation period of exactly 2 min. The cells were then immediately washed three times with Krebs/HEPES buffer at 0°C and lysed by addition of 750 μl of 0.1% Triton X-100 in 10 mM Tris–HCl (pH 7.5). The [3H] content of the cell lysates was determined in a 2500 TR Packard Liquid Scintillation Analyzer (Packard, Melbourne, Australia) after addition of Starscint scintillation medium (Packard) to the samples. The protein content of the cell lysates was measured according to the Lowry method (Lowry et al., 1951) using bovine serum albumin as standard. The presence of the NO donors, nucleophiles or other drugs did not affect the protein content of the wells at the end of the experiments, indicating that they were not toxic to the cells over these short exposure times.
Data analysis
The results of liquid scintillation counting and Lowry protein determinations from duplicate wells for each treatment condition on each 12-well plate were used to calculate [3H]-5-HT or [3H]-dopamine uptake (in fmol (mg protein)−1) into the COS-7 cells during the constant 2 min incubation time. Specific [3H]-5-HT or [3H]-dopamine uptake was then calculated for each 12-well plate as the difference between uptake measured in the absence and presence of paroxetine or GBR 12909, respectively, on the same 12-well plate. In the absence of NO donors, specific uptake was 93.3% of the total uptake of [3H]-5-HT and 92.6% of that of [3H]-dopamine in the cells that were transfected with the cDNA of hSERT and hDAT, respectively. There was no specific uptake of the amines in COS-7 cells that were transfected with the vector plasmid without hSERT or hDAT, that is no endogenous expression of SERT or DAT in the COS-7 cells. Specific [3H]-5-HT or [3H]-dopamine uptake was expressed either as % inhibition of the specific uptake in vehicle-treated controls in the absence of NO donors on the same 12-well plate or as a % of specific uptake in vehicle-treated controls on the same 12-well plate.
Drugs and solutions
The drugs used in the study were: Angeli's Salt (disodium diazen-1-ium-1,2,2-triolate; Cayman Chemical Company, Ann Arbor, MI, U.S.A.), catalase (CAT; Sigma-Aldrich), L-cysteine (Sigma-Aldrich), FK409 ((±)-(4-ethyl-2E-(hydroxyimino)-5-nitro-3E-hexenamide; Cayman)), GBR 12909 (1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride; Research Biochemicals Inc., Natick, MA, U.S.A.), hydroxocobalamin (Sigma-Aldrich), MAHMA/NO ((Z)-1-[N-methyl-N-[6-(N-methylammoniohexyl)-amino]]diazen-1-ium-1,2-diolate; Cayman), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; Cayman), PAPA/NO ((Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate; Cayman), pargyline hydrochloride (Sigma-Aldrich), paroxetine hydrochloride (GlaxoSmithKline, Uxbridge, Middlesex, U.K.), SIN–1 chloride (5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride, linsidomine; Cayman), sodium nitrite (Sigma-Aldrich), sodium peroxynitrite (Cayman), superoxide dismutase (SOD; Sigma-Aldrich), U-0521 (3′,4′-dihydroxy-2-methylpropiophenone; Pharmacia and Upjohn, Kalamazoo, MI, U.S.A.). The radiolabelled compounds used in the study were 5-[1,2-3H(N)]-HT creatinine sulphate ([3H]-5-HT; NET-498, specific activity: 1 Bq fmol−1) and [ring-2,5,6-3H]-dopamine (NET-673, specific activity: 2 Bq fmol−1) from Perkin-Elmer Life Sciences Inc., Boston, MA, U.S.A. All other chemicals were AR grade, molecular biology grade or cell culture tested as appropriate, and were obtained from standard suppliers. All drugs were stored as recommended by the manufacturer.
Stock solutions of GBR 12909 (100 μM), U-0521 (1 mM) and paroxetine and pargyline (10 mM) were prepared in water and stored at −20°C. Stock solutions of hydroxocobalamin (100 mM), L-cysteine (100 mM), SOD (15,000 U ml−1) and CAT (120,000 U ml−1) were prepared in water on the day of the experiment. Stock solutions (80 mM) of the following compounds were prepared on the day of the experiment in the indicated solvents, diluted in the same solvent to 100-fold greater than the final concentrations required and stored on ice until just before they were required: MAHMA/NO, PAPA/NO and Angeli's salt in 10 mM NaOH, FK409 and ODQ in dimethylsulphoxide (not stored on ice), SIN-1 in 100 μM HCl and sodium nitrite in water. Sodium peroxynitrite was purchased as a 19 mM solution in 300 mM NaOH (concentration determined spectrophotometrically at a wavelength of 302 nm just prior to the experiments; extinction coefficient 1670 M−1 cm−1). The maximal final concentration that could be examined to avoid pH changes of the preincubation and incubation solutions was 190 μM. Aliquotted samples of sodium peroxynitrite were stored at −80°C and thawed once only on ice just prior to dilution in 300 mM NaOH. The dilutions were kept on ice until they were used within 20 min of preparation.
Solutions of the nucleophiles, MAHMA and PAPA were prepared prior to the experiments by exposing MAHMA/NO and PAPA/NO, respectively, to conditions under which they readily break down into NO and the relevant nucleophile. MAHMA/NO and PAPA/NO were prepared as 160 mM solutions in 1 M HCl and left for 48 h at room temperature in an open vial, followed by 2 h at 37°C to allow decomposition to the nucleophiles (established by spectrophotometric analysis; loss of absorbance characteristics of MAHMA/NO and PAPA/NO at a wavelength of 250 nm), and the solutions were then neutralised and diluted to 80 mM with 1 M NaOH.
Final dilutions of all drugs (10 μl in 1 ml) to the required concentrations were made in the wells in Krebs/HEPES buffer containing 0.1% bovine serum albumin at the required time in the experiment.
Statistical analysis
The results are shown as arithmetic means±s.e.m. for the indicated number of independent experiments. The controls showed that the specific [3H]-5-HT and [3H]-dopamine uptake was unaffected by the presence of the various solvents used (see above). The significance of differences between values for specific uptake of [3H]-5-HT or [3H]-dopamine in the absence and presence of the various concentrations of the drugs and between drug treatments was analysed by one-factor analysis of variance (ANOVA) and Tukey–Kramer multiple comparison test or Student's t-test as appropriate (see Results; Prism 4, GraphPad Software, San Diego, CA, U.S.A.). Values for percentage inhibition of specific uptake of [3H]-5-HT or [3H]-dopamine by the NO donors were compared with zero by the Student's t-test (Instat 3, GraphPad).
Results
Effects of NO donors on uptake of [3H]-5-HT by SERT and of [3H]-dopamine by DAT
The NONOate MAHMA/NO (12.5–800 μM) caused a concentration-dependent inhibition of specific [3H]-5-HT uptake into COS-7 cells expressing hSERT and more marked inhibition of [3H]-dopamine uptake into COS-7 cells expressing hDAT (Figure 1a). MAHMA, the corresponding nucleophile of MAHMA/NO, had no effect on [3H]-dopamine uptake at the same concentrations and caused a small inhibitory effect on [3H]-5-HT uptake only at the highest concentration used (800 μM) (data not shown). Another NONOate with a longer half-life, PAPA/NO, also caused a concentration-dependent inhibition of specific uptake of [3H]-5-HT and [3H]-dopamine, but its effects were less marked than those of MAHMA/NO, and the inhibition of SERT (but not DAT) could be almost entirely accounted for by the effect of the nucleophile PAPA. Hence, PAPA/NO was not examined further in this study.
Figure 1.
Inhibition of specific [3H]-5-HT or [3H]-dopamine uptake by the NO donors (a) MAHMA/NO, (b) FK409 and (c) SIN-1 in COS-7 cells expressing hSERT (closed symbols) or hDAT (in (a) only; open symbols), respectively. Mean concentration–effect curves from four to five experiments are shown. The data were expressed as % inhibition of specific uptake in the absence of NO donor for each individual 12-well plate, and are shown as mean values with s.e.m. The mean and s.e.m. of the control-specific [3H]-5-HT and [3H]-dopamine uptake values (no NO donor) in these experiments were 572±43 (n=13) and 643±26 fmol (mg protein)−1 (n=4), respectively. One-factor ANOVA of the data in each graph showed that the effects of the NO donors were concentration-dependent (P<0.001). Asterisks indicate that specific uptake was significantly less than the control values in the absence of NO donor (i.e. there was significant inhibition): *0.05>P>0.01, **0.01>P>0.001, ***P<0.001 (Student's t-test).
Two different NO donors, FK409 (Figure 1b) and SIN-1 (Figure 1c), also caused a concentration-dependent inhibition of [3H]-5-HT uptake in cells expressing hSERT. These drugs were not examined in cells expressing hDAT.
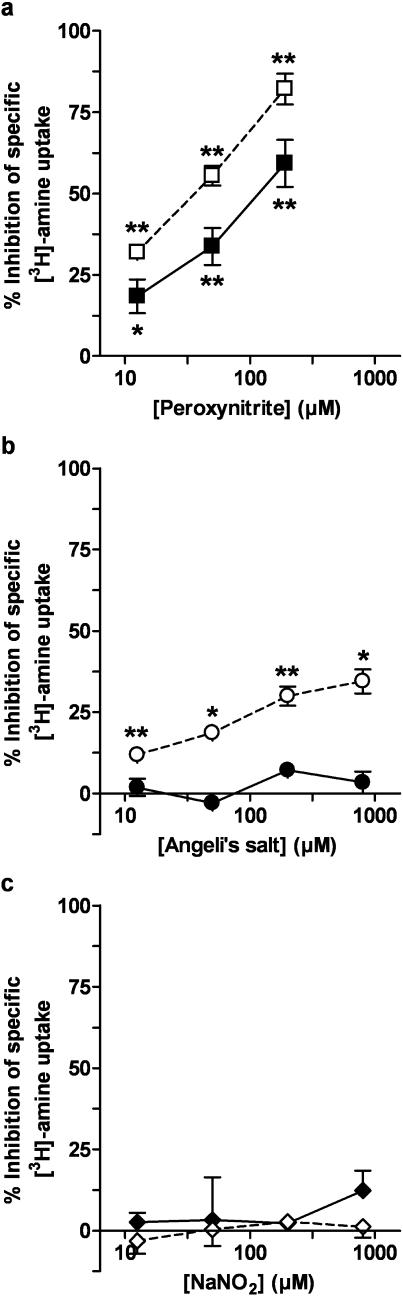
Peroxynitrite (Figure 2a) caused concentration-dependent inhibition of the specific [3H]-5-HT uptake into COS-7 cells expressing hSERT and more marked inhibition of the specific [3H]-dopamine uptake into COS-7 cells expressing hDAT (Figure 2a). The nitroxyl ion donor, Angeli's salt, had no effects on specific [3H]-5-HT uptake into cells expressing hSERT, but caused moderate inhibitory effects on specific [3H]-dopamine uptake in cells expressing hDAT (Figure 2b). Sodium nitrite had no effects on specific [3H]-5-HT or [3H]-dopamine uptake (Figure 2c).
Figure 2.
Inhibition of specific [3H]-5-HT or [3H]-dopamine uptake by (a) peroxynitrite, (b) Angeli's salt and (c) sodium nitrite in COS-7 cells expressing hSERT (closed symbols) or hDAT (open symbols), respectively. Mean concentration–effect curves from three to four experiments are shown. The data were expressed as % inhibition of specific uptake in the absence of drug for each individual 12-well plate and are shown as mean values with s.e.m. The mean and s.e.m. of the control-specific [3H]-5-HT uptake and [3H]-dopamine uptake values (no drug) in these experiments were 640±37 (n=11) and 677±42 fmol (mg protein)−1 (n=9), respectively. One-factor ANOVA of the data in each graph showed that the effects of peroxynitrite for hSERT (0.01>P>0.001) and hDAT (P<0.001) and Angeli's salt for hDAT only (0.01>P>0.001) were concentration-dependent. Asterisks indicate that specific uptake was significantly less than the control values in the absence of drug (i.e. there was significant inhibition): *0.05>P>0.01, **0.01>P>0.001 (Student's t-test).
Effects of superoxide dismutase (SOD) plus catalase (CAT)
SOD (150 U ml−1) plus CAT (1200 U ml−1), which remove superoxide thereby preventing the formation of peroxynitrite, had no effects on the specific uptake of [3H]-5-HT (Figure 3). The inhibitory effects of MAHMA/NO and FK409 were still apparent in the presence of SOD and CAT, but the inhibitory effect of SIN-1 was abolished (Figure 3).
Figure 3.
Effects of superoxide dismutase (SOD; 150 U ml−1) plus catalase (CAT; 1200 U ml−1) on the inhibitory effects of NO donors (800 μM) on specific uptake of [3H]-5-HT in COS-7 cells expressing hSERT. Values for specific [3H]-5-HT uptake were expressed as a percentage of control-specific uptake in the absence of drugs (solid bars) for each individual 12-well plate, and are shown as mean values with s.e.m. (n=3). The mean and s.e.m. of the control-specific [3H]-5-HT uptake values (no NO donor or SOD and CAT) in these experiments were 745±41 fmol (mg protein)−1 (n=9). One-factor repeated-measures ANOVA of the data for each NO donor showed that the drugs had significant effects on specific [3H]-5-HT uptake (P<0.01). Asterisks indicate that the value in the presence of NO donor was significantly less than the corresponding value in the absence of NO donor: **0.01>P>0.001, ***P<0.001 (Tukey–Kramer multiple comparison test).
Effects of hydroxocobalamin
The NO free radical scavenger, hydroxocobalamin (1 mM), reduced the uptake of [3H]-5-HT by about 50% (Figure 4). However, the inhibitory effects of MAHMA/NO, FK409 and SIN-1 on specific [3H]-5-HT uptake were still apparent in the presence of hydroxocobalamin and no less pronounced than in the absence of hydroxocobalamin (Figure 4).
Figure 4.
Effects of hydroxocobalamin (1 mM) on the inhibitory effects of NO donors (800 μM) on specific uptake of [3H]-5-HT in COS-7 cells expressing hSERT. Values of specific [3H]-5-HT uptake were expressed as a percentage of control-specific uptake in the absence of drugs (solid bars) for each individual 12-well plate and are shown as mean values with s.e.m. (n=3). The mean and s.e.m. of the control-specific [3H]-5-HT uptake values (no NO donor or hydroxocobalamin) in these experiments were 646±39 fmol (mg protein)−1 (n=9). One-factor repeated-measures ANOVA of the data for each NO donor showed that the drugs had significant effects on specific [3H]-5-HT uptake (P<0.001). Asterisks indicate that the value in the presence of NO donor was significantly less than the corresponding value in the absence of NO donor: ***P<0.001; hashes indicate that, in the absence of NO donor, the value in the presence of hydroxocobalamin was significantly less than the corresponding control value (no drugs): ###P<0.001 (Tukey–Kramer multiple comparison test).
Effects of ODQ
The inhibitor of soluble guanylate cyclase, ODQ (3 μM), had no effects on the specific uptake of [3H]-5-HT (Figure 5). The inhibitory effects of MAHMA/NO, FK409 and SIN-1 on specific [3H]-5-HT uptake were unaffected by ODQ (Figure 5).
Figure 5.
Effects of ODQ (3 μM) on the inhibitory effects of NO donors (800 μM) on specific uptake of [3H]-5-HT in COS-7 cells expressing hSERT. Values for specific [3H]-5-HT uptake were expressed as a percentage of control-specific uptake in the absence of drugs (solid bars) for each individual 12-well plate and are shown as mean with s.e.m. (n=3). The mean and s.e.m. of the control-specific [3H]-5-HT uptake values (no NO donor or ODQ) in these experiments were 775±25 fmol (mg protein)−1 (n=9). One-factor repeated-measures ANOVA of the data for each NO donor showed that the drugs had significant effects on specific [3H]-5-HT uptake (P<0.001). Asterisks indicate that the value in the presence of NO donor was significantly less than the corresponding value in the absence of NO donor: **0.01>P>0.001, ***P<0.001.
Effects of L-cysteine
L-Cysteine (1 mM), a source of excess thiol residues, had no effect on the specific uptake of [3H]-5-HT (Figure 6). L-Cysteine abolished or markedly reduced the inhibitory effects of MAHMA/NO, FK409, SIN-1 and peroxynitrite on specific [3H]-5-HT uptake (Figure 6).
Figure 6.
Effects of L-cysteine (1 mM) on the inhibitory effects of NO donors (800 μM MAHMA/NO, FK409 and SIN-1; 190 μM peroxynitrite) on specific uptake of [3H]-5-HT in COS-7 cells expressing hSERT. Values for specific [3H]-5-HT uptake were expressed as a percentage of control-specific uptake in the absence of drugs (solid bars) for each individual 12-well plate and are shown as mean values with s.e.m. (n=3). The mean and s.e.m. of the control-specific [3H]-5-HT uptake values (no NO donor or L-cysteine) in these experiments were 707±33 fmol (mg protein)−1 (n=12). One-factor repeated-measures ANOVA of the data for each NO donor showed that the drugs had significant effects on specific [3H]-5-HT uptake (P<0.01). Asterisks indicate that the value in the presence of NO donor was significantly less than the corresponding value in the absence of NO donor: **0.01>P>0.001, ***P<0.001 (Tukey–Kramer multiple comparison test).
Discussion
The results of this study in COS-7 cells expressing hSERT substantiate the hypothesis that NO donor drugs are able to inhibit the uptake of 5-HT by its specific transporter. The drugs for which this has been demonstrated (MAHMA/NO, SIN-1 and FK409) have diverse chemical structures, but all of them can generate NO ‘spontaneously' at physiological pH. PAPA/NO (another NONOate) also inhibited 5-HT uptake, but its effect was complicated by the pronounced inhibitory effect of the nucleophile, PAPA; this did not apply to MAHMA, the nucleophile for MAHMA/NO. Since the common feature of these diverse compounds is their ability to produce NO, it is most likely that NO, in one of its redox states, is responsible for their inhibitory effect on SERT. Nitrite is another potential by-product of the decomposition of the NO donors, but it cannot account for the effects of the compounds because sodium nitrite did not inhibit either SERT or DAT.
This study was initially prompted by reports that NO donors can inhibit the uptake of dopamine by DAT in a variety of cells (see Introduction). Therefore, for comparison, some of the experiments on SERT were repeated using uptake of dopamine (instead of 5-HT) into COS-7 cells expressing DAT (instead of SERT). MAHMA/NO was found to inhibit dopamine uptake, reflecting results with PAPA/NO in a previous study (Cao & Reith, 2002). However, the effects of MAHMA/NO were less pronounced on SERT than on DAT. A number of other differences between SERT and DAT were also observed. For example, the effects of peroxynitrite (like those of MAHMA/NO) were more pronounced on DAT than on SERT, and Angeli's salt inhibited DAT but not SERT. Furthermore, the nucleophile PAPA inhibited SERT but not DAT. These discrepancies could point to differences in the sites or mechanisms of action of the drugs on the two transporters.
The NO donors studied can each produce NO in one or more of its different redox states, viz. NO-free radical (NO•), nitroxyl (NO−) or nitrosonium (NO+) (Feelisch & Stamler, 1996). The data obtained in this study showed that nitroxyl appears not to be responsible for the inhibition of SERT because Angeli's salt, which generates nitroxyl, was without effect on [3H]-5-HT uptake. It is unlikely that the effects of the NO donors on SERT are due exclusively to free radical NO since their effects were not prevented by the free radical scavenger, hydroxocobalamin. This scavenger has been shown to inhibit responses to solutions of NO gas (NO free radical) as well as NO donors in other biological systems (Rand & Li, 1993; La et al., 1996; Wanstall et al., 2001). It was found that hydroxocobalamin itself inhibited SERT, but sufficient uptake remained to demonstrate that the inhibition of SERT by the NO donors was no less pronounced in the presence of hydroxocobalamin than in its absence. This finding is in contrast to the findings with DAT reported by Cao & Reith (2002). These authors showed that the inhibitory effects of SIN-1 and DEA/NO on DAT were prevented by hydroxocobalamin, leading them to conclude that NO was responsible for the effects they observed. In the present study, we showed that, in contrast to the report of Cao & Reith (2002), hydroxocobalamin itself abolished uptake of [3H]-dopamine by DAT (data not shown) and hence we were unable to confirm whether it abolished the inhibitory effects of the NO donors on [3H]-dopamine uptake.
Many of the pharmacological effects of NO donors are mediated by activation of soluble guanylate cyclase, production of c-GMP and subsequent activation of G-kinase. However, this pathway appears not to be involved in the inhibitory effects on SERT seen in the present study because the actions of the various NO donors were not prevented by the guanylate cyclase inhibitor, ODQ. The lack of involvement of c-GMP is in agreement with the findings of Asano et al. (1997) in rat brain synaptosomes. Interestingly, in contrast, the augmentation of 5-HT uptake by S-nitrosoacetylpenicillamine reported by Kilic et al. (2003) was inhibited by ODQ.
Since neither nitroxyl nor NO-free radical appeared to account for the effects of the NO donors on SERT, and since c-GMP is not involved, the possibility of direct nitrosation of SERT via NO+ was considered. Nitrosation of the thiol site of cysteine residues on the noradrenaline transporter (NET) has been proposed to account for the inhibitory effects of NO donors on NET (Kaye et al., 1997). The evidence to support this view included the finding that the inhibitory action of an NO donor on NET was attenuated by L-cysteine, which was used to provide excess thiol groups to act as a ‘sink' (Kaye et al., 1997). In our present study, L-cysteine abolished or markedly attenuated the inhibitory effects of each of the NO donors on SERT. This observation is compatible with the conclusion that the inhibition of SERT by the NO donors occurs by nitrosation of cysteine residues of the transporter protein, a process that by definition involves NO+. It should be noted that L-cysteine (via a different mechanism) can also inhibit effects of NO donors when nitroxyl ions contribute to the response (Wanstall et al., 2001), but this cannot explain the present findings because the experiments with Angeli's salt indicated that nitroxyl was not involved (see above).
Each of the NO donors examined for their effects on SERT can produce NO+, with the exception of SIN-1 (Feelisch & Stamler, 1996). However, SIN-1 differs from the remaining NO donors studied in that it generates not only NO but also superoxide (Feelisch & Stamler, 1996). NO and superoxide interact to produce peroxynitrite, which can then produce NO+ (even though SIN-1 does not generate NO+ directly). Peroxynitrite was shown to effectively inhibit SERT. The effects of SIN-1, but not the other NO donors studied, were blocked by SOD and CAT, which remove superoxide, thereby preventing the formation of peroxynitrite. Furthermore, the inhibitory effect of peroxynitrite on SERT, like that of SIN-1, was blocked by L-cysteine. Taken together, these data suggest that the effect of SIN-1 on SERT is probably mediated by peroxynitrite. Peroxynitrite was also found to markedly inhibit DAT in the present study. Park et al. (2002) also reported inhibition of DAT by peroxynitrite (although at higher concentrations than in the present study), but, in contrast, a lack of effect of peroxynitrite on DAT activity was reported by Cao & Reith (2002). We currently have no explanation for these discrepancies between the effects of peroxynitrite on DAT activity in the different studies, but peroxynitrite is very unstable and great care has to be taken to establish that breakdown of the compound has not occurred during storage, and to minimise the possibility of its breakdown during the experimental protocol.
Our findings that NO donors inhibit SERT are in keeping with those of two other studies in a different experimental system, rat brain synaptosomes, where inhibition of 5-HT uptake was reported for sodium nitroprusside, two S-nitrosothiols and SIN-1 (Pogun et al., 1994; Asano et al., 1997). They do, however, conflict with the results of a study in rat basophilic leukaemia RBL2H3 cells in which the S-nitrosothiol S-nitrosoacetylpenicillamine was shown to increase 5-HT uptake by about 45% (Miller & Hoffman, 1994), and with a recent study that reported an increase in 5-HT uptake by S-nitrosoacetylpenicillamine in hSERT-expressing COS-7 cells (Kilic et al., 2003). However, in the latter study, the authors comment that they sometimes observed inhibition instead of augmentation of 5-HT uptake at concentrations of S-nitrosoacetylpenicillamine greater than 100 μM (Kilic et al., 2003).
It is interesting that there is now evidence that NO donors can inhibit all members of the monoamine neurotransmitter transporter family, that is, SERT, DAT and NET. However, there are some discrepancies between the results of the various studies (see above), so it is not yet clear whether the mechanisms involved for the three transporters are the same or not. Nitrosation of cysteine residues has been claimed to be the mechanism of the inhibitory effects of peroxynitrite on DAT, via inactivation of cysteine residue 342 in intracellular loop 3 (Park et al., 2002), and of S-nitrosoacetylpenicillamine on NET, via inactivation of cysteine 351 in transmembrane domain 7 (Kaye et al., 2000). It remains to be determined which particular amino-acid residues are implicated in the effects of NO donors on SERT. Cysteine residue 342 of DAT is also present in SERT and NET and cysteine residue 351 of NET is present in SERT but not in DAT. In view of the differences between SERT and DAT with respect to the effects of NO donors, noted above, it is tempting to speculate that cysteine residue 351 may be involved.
The finding that NO donors can inhibit SERT reveals another potentially valuable pharmacological property of this group of drugs. Admittedly, the concentrations of NO donors used in this study are somewhat higher than those which cause, for example, vasorelaxation. However, the concentrations are comparable to those used in other laboratories in studies on DAT and NET, and it must be remembered that concentrations of drugs that are effective in cell culture experiments are not necessarily predictive of those required in intact tissues. It will be interesting to determine whether these in vitro effects of NO donors on SERT translate into comparable effects in vivo and, if so, at what dosage levels they occur.
If inhibitory effects of NO donors on SERT can be demonstrated in vivo, and at doses that correspond to plasma levels of NO byproducts associated with clinical haemodynamic effectiveness (e.g. equal to or less than 100 μM; Preiser et al., 1998), then this could be a clinically useful property of the drugs. Inhibition of SERT may be of particular value in the treatment of pulmonary hypertension. There is circumstantial evidence that 5-HT may contribute to pulmonary vascular remodelling in pulmonary hypertension, and that uptake of 5-HT by SERT into pulmonary vascular smooth muscle cells may be a prerequisite for this effect (Lee et al., 1991; Eddahibi et al., 1999). Furthermore, there is increased expression of SERT in pulmonary arteries from patients with pulmonary hypertension (Eddahibi et al., 2001), and in mice with hypoxic pulmonary hypertension inhibitors of SERT have been shown to attenuate pulmonary vascular remodelling (Marcos et al., 2003). NO donors could have a therapeutic future as vasodilators in pulmonary hypertension, especially if given by the inhalational route to achieve pulmonary selectivity. The results of this study indicate that inhibition of SERT may possibly be an additional benefit.
Acknowledgments
The financial support of the National Health and Medical Research Council of Australia is gratefully acknowledged. We would like to thank Professor R. Blakely (Center for Molecular Neuroscience, Vanderbilt University Medical Center, Nashville, TN, U.S.A.) and Professor M. Caron (Department of Cell Biology, Duke University Medical Center, Durham, NC, U.S.A.) for their generous provision of hSERT and hDAT cDNAs, respectively, and Pharmacia and Upjohn (Kalamazoo, MI, U.S.A.) and GlaxoSmithKline (Uxbridge, Middlesex, U.K.) for the donations of U-0521 and paroxetine hydrochloride, respectively.
Abbreviations
- ANOVA
analysis of variance
- CAT
catalase
- DA
dopamine (in figures only)
- DAT
dopamine transporter
- FK409
(±)-4-ethyl-E-(hydroxyimino)-5-nitro-3E-hexenamide
- GBR 12909
1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride
- c-GMP, guanosine-3′
5′-cyclic monophosphate
- h
human (with DAT or SERT)
- HCob
hydroxocobalamin (in figures only)
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethane sulphonic acid
- 5-HT
5-hydroxytryptamine (serotonin)
- MAHMA/NO, (Z)-1-[N-methyl-N-[6-(N-methylammoniohexyl)-amino]]diazen-1-ium-1
2-diolate
- Na2EDTA
disodium ethylenediaminetetraacetate
- NET
noradrenaline transporter
- NO
nitric oxide
- NONOates
diazeniumdiolates
- PAPA/NO, (Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino]diazen-1-ium-1
2-diolate
- SERT
5-HT (serotonin) transporter
- SIN-1
5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride (linsidomine)
- SOD
superoxide dismutase
- U-0521
3′, 4′-dihydroxy-2-methylpropiophenone
References
- ASANO S., MATSUDA T., NAKASU Y., MAEDA S., NOGI H., BABA A. Inhibition by nitric oxide of the uptake of [3H]serotonin in rat brain synaptosomes. Jpn. J. Pharmacol. 1997;75:123–128. doi: 10.1254/jjp.75.123. [DOI] [PubMed] [Google Scholar]
- BRYAN-LLUKA L.J., PAPACOSTAS M.H., WANSTALL J.C. Inhibition of the 5-HT transporter (SERT) by nitric oxide (NO) donors. Proc. Australas. Soc. Clin. Exp. Pharmacol. Toxicol. 2003;10:43. [Google Scholar]
- CAO B.-J., REITH M.E.A. Nitric oxide inhibits uptake of dopamine and N-methyl-4-phenylpyridinium (MPP+) but not release of MPP+ in rat C6 glioma cells expressing human dopamine transporter. Br. J. Pharmacol. 2002;137:1155–1162. doi: 10.1038/sj.bjp.0704974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK J.A., WINK D.A., BLOUNT V., KRISHNA M.C., HANBAUER I. Role of antioxidants in the nitric oxide-elicited inhibition of dopamine uptake in cultured mesencephalic neurons. Insights into potential mechanisms of nitric oxide-mediated neurotoxicity. Neurochem. Int. 1996;28:609–617. doi: 10.1016/0197-0186(95)00125-5. [DOI] [PubMed] [Google Scholar]
- EDDAHIBI S., FABRE V., BONI C., MARTRES M.P., RAFFESTIN B., HAMON M., ADNOT S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ. Res. 1999;84:329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- EDDAHIBI S., HUMBERT M., FADEL E., RAFFESTIN B., DARMON M., CAPRON F., SIMONNEAU G., DARTEVELLE P., HAMON M., ADNOT S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J. Clin. Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHLEMAN A.J., CARMOLLI M., CUMBAY M., MARTENS C.R., NEVE K.A., JANOWSKY A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J. Pharmacol. Exp. Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- FEELISCH M., STAMLER J.S.Donors of nitrogen oxides Methods in Nitric Oxide Research 1996Chichester, U.K.: John Wiley & Sons Ltd; 71–115.ed. Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- GOLOMBEK S.G. The use of inhaled nitric oxide in newborn medicine. Heart Dis. 2000;2:342–347. [PubMed] [Google Scholar]
- HOEPER M.M., GALIÈ N., SIMONNEAU G., RUBIN L.J. New treatments for pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2002;165:1209–1216. doi: 10.1164/rccm.200110-028PP. [DOI] [PubMed] [Google Scholar]
- KAYE D.M., WIVIOTT S.D., KOBZIK L., KELLY R.A., SMITH T.W. S-nitrosothiols inhibit neuronal norepinepherine transport. Am. J. Physiol. 1997;272:H875–H883. doi: 10.1152/ajpheart.1997.272.2.H875. [DOI] [PubMed] [Google Scholar]
- KAYE D.M., GRUSKIN S., SMITH A.I., ESLER M.D. Nitric oxide mediated modulation of norepinephrine transport: identification of a potential target for S-nitrosylation. Br. J. Pharmacol. 2000;130:1060–1064. doi: 10.1038/sj.bjp.0703416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEEFER L.K. Progress toward clinial application of the nitric oxide-releasing diazeniumdiolates. Annu. Rev. Pharmacol. Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- KILIC F., MURPHY D.L., RUDNICK G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol. Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- LA M., LI C.G., RAND M.J. Comparison of the effects of hydroxocobalamin and oxyhaemoglobin on responses to NO, EDRF and the nitrergic transmitter. Br. J. Pharmacol. 1996;117:805–810. doi: 10.1111/j.1476-5381.1996.tb15264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S.L., WANG W.W., MOORE B.J., FANBURG B.L. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ. Res. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- LONART G., JOHNSON K.M. Inhibitory effects of nitric oxide on the uptake of [3H] dopamine and [3H] glutamate by striatal synaptosomes. J. Neurochem. 1994;63:2108–2117. doi: 10.1046/j.1471-4159.1994.63062108.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O., ROSEBROUGH N., FARR A., RANDALL R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARCOS E., ADNOT S., PHAM M.H., NOSJEAN A., RAFFESTIN B., HAMON M., EDDAHIBI S. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2003;168:487–493. doi: 10.1164/rccm.200210-1212OC. [DOI] [PubMed] [Google Scholar]
- MILLER K.J., HOFFMAN B.J. Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J. Biol. Chem. 1994;269:27351–27356. [PubMed] [Google Scholar]
- OWENS M.J., MORGAN W.N., PLOTT S.J., NEMEROFF C.B. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J. Pharmacol. Exp. Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- PACZKOWSKI F.A., BRYAN-LLUKA L.J., PÖRZGEN P., BRÜSS M., BÖNISCH H. Comparison of the pharmacological properties of cloned rat, human and bovine norepinephrine transporters. J. Pharmacol. Exp. Ther. 1999;290:761–767. [PubMed] [Google Scholar]
- PARK S.U., FERRER J.V., JAVITCH J.A., KUHN D.M. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J. Neurosci. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGUN S., BAUMANN M.H., KUHAR M.J. Nitric oxide inhibits [3H]dopamine uptake. Brain Res. 1994;641:83–91. doi: 10.1016/0006-8993(94)91818-x. [DOI] [PubMed] [Google Scholar]
- PREISER J.-C., DE BACKER D., DEBELLE F., VRAY B., VINCENT J.-L. The metabolic fate of long-term inhaled nitric oxide. J. Crit. Care. 1998;13:97–103. doi: 10.1016/s0883-9441(98)90012-0. [DOI] [PubMed] [Google Scholar]
- RAMAMOORTHY S., BAUMAN A.L., MOORE K.R., HAN H., YANG-FENG T., CHANG A.S., GANAPATHY V., BLAKELY R.D. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Differential effects of hydroxocobalamin on relaxations induced by nitrosothiols in rat aorta and anococcygeus muscle. Eur. J. Pharmacol. 1993;241:249–254. doi: 10.1016/0014-2999(93)90210-9. [DOI] [PubMed] [Google Scholar]
- TILTON R.G., BROCK T.A., DIXON R.A. Therapeutic potential of endothelin receptor antagonists and nitric oxide donors in pulmonary hypertension. Expert Opin. Investig. Drugs. 2001;10:1291–1308. doi: 10.1517/13543784.10.7.1291. [DOI] [PubMed] [Google Scholar]
- WANSTALL J.C., JEFFERY T.K., GAMBINO A., LOVREN F., TRIGGLE C.R. Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br. J. Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]