Abstract
The study was designed to test the hypothesis that aspirin may stimulate nitric oxide (NO) release from vascular endothelium, a pivotal factor for maintenance of vascular homeostasis.
Clinical evidence suggests that low-dose aspirin may improve vascular endothelial function. Since other cyclooxygenase (COX) inhibitors showed no beneficial vascular effects, aspirin may exhibit a vasculoprotective, COX-independent mechanism.
Luminal NO release was monitored in real time on dissected porcine coronary arteries (PCA) by an amperometric, NO-selective sensor. Additionally, endothelial NO synthase (eNOS) activity was measured in EA.hy 926 cell homogenates by an L-[3H]citrulline/L-[3H]arginine conversion assay. Superoxide scavenging capacity was assessed by lucigenin-enhanced luminescence.
Aspirin induced an immediate concentration-dependent NO release from PCA with an EC50 of 50 nM and potentiated the NO stimulation by the receptor-dependent agonist substance P. These effects were independent of an increase in intracellular calcium and could be mimicked by stimulation with acetylating aspirin derivatives. The aspirin metabolite salicylic acid or the reversible cyclooxygenase inhibitor indomethacin failed to modulate NO release. Incubation of soluble eNOS for 15 min with 100 μM aspirin or acetylating aspirin analogues increased the L-[3H]citrulline yield by 40–80%, while salicylic acid had no effect. Aspirin and salicylic acid showed a similar, but only modest, magnitude and velocity of superoxide scavenging.
Our findings demonstrate that therapeutically relevant concentrations of aspirin elicit NO release from vascular endothelium. This effect appears to be due to a direct acetylation of the eNOS protein, but is independent of COX inhibition or inhibition of superoxide-mediated NO degradation.
Keywords: Aspirin, nitric oxide, endothelium
Introduction
Low-dose aspirin (75–325 mg day−1) has a well-established efficacy in acute treatment and secondary prevention of coronary and cerebrovascular events (Awtry & Loscalzo, 2000). This effect has been largely attributed to its antithrombotic activity, resulting from the irreversible inhibition (acetylation) of the cyclooxygenase-1 (COX-1) in platelets, thereby preventing synthesis of the platelet-activating prostanoid thromboxane A2 (Vane, 1971; Awtry & Loscalzo, 2000). However, it is still intensively debated whether aspirin in therapeutically relevant concentrations might exert additional cardioprotective effects. Evidence from explorative clinical trials suggests that treatment with low-dose aspirin improves endothelium-dependent arterial relaxation (Husain et al., 1998; Noon et al., 1998; Monobe et al., 2001), which is regarded pivotal for the maintenance of vascular homoeostasis (Loscalzo, 2001). It has been proposed that the underlying mechanism may be an inhibition of the release of vasocontrictive prostanoids from the endothelium, such as thromboxane A2 and prostaglandin F2α. However, since aspirin also inhibits the formation of endothelium-derived vasodilator prostanoids, such as prostacyclin, an alternative, COX-independent mechanism may be involved.
Nitric oxide (NO) is the main vasodilating factor released from vascular endothelium and plays a crucial role for maintenance of vascular homoeostasis and preventing atherothrombotic events. Therefore, in this study, we tested the hypothesis that aspirin may stimulate the release of NO from vascular endothelium.
Methods
Chemicals
o-Acetylsalicylhydroxamic acid (ASHA) and o-(acetoxyphenyl)hept-2-ynyl sulfide (APHS) were obtained from Cayman Chemical, Ann Arbor, MI, U.S.A. N-(3-aminoethyl)benzyl)-acetamide hydrochloride (1400W) was from Alexis, Grunberg, Germany. L-[2,3,4,5-3H]Arginine monohydrochloride was from Amersham, Freiburg, Germany. Superoxide dismutase (SOD) (copper, zinc isoform from bovine erythrocytes, 4200 U mg−1) and all other chemicals were from Sigma, Taufkirchen, Germany.
Real-time measurement of NO
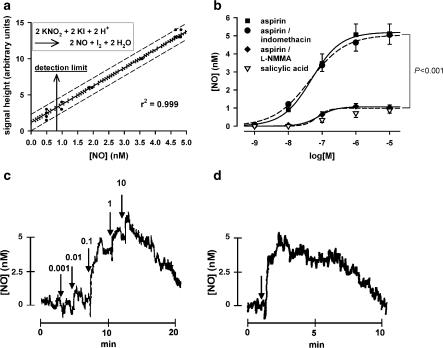
Luminal NO release of porcine coronary conduit arteries (PCA) was monitored ex vivo in real time by an NO-selective microsensor (ISO-NO-Meter, WPI, Sarasota, FL, U.S.A.), as described (Taubert et al., 2002). The amperometric sensor was positioned under microscopic control at a distance of 200–300 μm above the endothelial surface. To avoid measurement artifacts, the absence of vehicle-induced NO signals and the maintenance of constant pH (7.40) and temperature (25°C) were controlled. The detection limit was calculated to be 0.8 nM NO at 99% confidence (Figure 1a).
Figure 1.
(a) Calibration curve of the amperometric NO sensor with 95% confidence interval (dotted lines) and 95% prediction interval (dashed lines) (n=60). Inset: Stoichiometric NO formation from nitrite by reduction with iodide. (b) NO formation from endothelium-preserved PCA induced by cumulative increasing concentrations of aspirin or salicylic acid, and effects of aspirin after 15 min preincubation with 100 μM L-NMMA or 10 μM indomethacin (n=5–8). (c) Original recording of the time course of NO release from PCA caused by cumulative increasing concentrations of aspirin (arrows indicate time points of addition, added concentrations in μM are given above). (d) Original recording of the time course of NO release from PCA by a single concentration of 10 μM aspirin.
Cell culture
Immortalized human umbilical vein endothelial cells (EA.hy 926) were cultured as described (Edgell et al., 1983).
L-Citrulline assay
EA.hy 926 cells were homogenized in 25 mM Tris–HCl (pH 7.4), 1 mM EDTA, and 1 mM EGTA. Cytoplasmatic fractions containing soluble eNOS and particular fractions containing membrane-bound eNOS were separated by centrifugation at 15,000 × g for 15 min at 4°C. Activity of soluble eNOS was determined in the supernatants by monitoring the conversion of L-arginine to L-citrulline using a commercially available NO synthase assay kit (Calbiochem). In all, 10 μg of protein per sample (determined in the Bradford assay) was coincubated with 1 μCi L-[3H]arginine and various concentrations of test compound in 40 μl reaction volume supplemented with 0.75 mM CaCl2, 0.1 μM calmodulin, 1.25 mM NADPH, 3.75 μM tetrahydrobiopterin, 1.25 μM FAD, and 1.25 μM FAD for 15 min at 25°C. The reaction was stopped by addition of 5 mM EDTA (pH 5.5). The mixture was passed through a cation-exchange resin, and L-[3H]citrulline concentration was determined in the eluate by liquid scintillation.
For some experiments, cytosolic fractions were further purified by ultracentrifugation at 150,000 × g for 60 min at 4°C.
Measurement of superoxide scavenging
Superoxide scavenging was measured by lucigenin-enhanced chemiluminescence. EA.hy 926 cells were grown to confluence on glass coverslips, and incubated for 1 h with test compounds at 37°C. The medium was then replaced with HEPES/Krebs buffer (pH 7.4, 25°C) containing 2.5 μM lucigenin, and luminescence intensity was recorded for 90 s using the FB-12 Berthold Sirius Single Probe Luminometer.
Second-order reaction rate constants of the interaction with superoxide were obtained by a nonenzymatic lucigenin-enhanced luminescence assay, as described (Taubert et al., 2003). Briefly, luminescence intensity was monitored for 60 s in 600 μl of a mixture of KO2 crown ether (166.67 μM) as superoxide donor, 100 μM EDTA, and 250 μM lucigenin in HEPES/Krebs buffer (pH 7.4, 25°C) in the presence (IX) and absence (I0) of the test compound X. The intensity ratio IX/I0 was plotted against the concentration ratio [X]/[lucigenin], and the rate constant kX was derived from the slope kX/klucigenin of the linear regression (klucigenin=1.054 × 106 mol−1 l s−1).
Data analysis
Emax and EC50 values of NO measurements are derived from the sigmoid regression curves of cumulative log concentration/response data and expressed as arithmetic means±95% confidence limits (±95% CI). Other values are expressed as means±s.e.m. Differences between means were assessed by two-tailed t-test or ANOVA with Tukey post hoc test for multiple comparisons. P<0.05 was considered statistically significant.
Results
NO production by porcine coronary arteries
Aspirin produced a concentration-dependent increase of endothelial NO release with an EC50 of 48.8 nM aspirin (95% CI: 35.6–66.8 nM) and an extrapolated maximal effect Emax of 5.2 nM NO (95% CI: 4.6–5.8 nM) (n=8). Near-maximal (>90%) effects of aspirin were already observed at 1 μM (Figure 1b and c). After stimulation with single concentrations of 10 μM of aspirin, the peak NO release (Emax=5.1 nM (95% CI: 4.0–6.1 nM)) was reached within 2 min and thereafter returned to baseline within 5–10 min (n=6, Figure 1d). The aspirin-induced NO release was almost completely abolished after inhibition of the NO-synthase with NG-monomethyl-L-arginine (L-NMMA) (100 μM, n=6) (Figure 1b), excluding the possibility that amperometric signals were from oxidation of reactants other than NO. In contrast, preincubation with 1400W (5 μM), a selective inhibitor of the inducible NOS isoform (iNOS), did not reduce aspirin-mediated NO release (Emax=97.8±5.0% of 1400W-free control, P=0.69) (n=4), indicating that the transient NO stimulation by aspirin is solely due to induction of the endothelial NOS isoform (eNOS) but not iNOS. PCA segments lacking NO-reactive endothelium (defined by a substance P-induced NO release of less than 1 nM) failed to show a NO response to aspirin (n=6, data not shown), which further excludes that aspirin interferes with NO production in smooth muscle cells. The NO elevation appears to be selective for aspirin, as salicylate produced no marked NO responses (n=6, Figure 1b). Moreover, since the potent nonselective cyclooxygenase inhibitor indomethacin (10 μM, n=5) did not cause endothelial NO release (data not shown), and pretreatment of arteries with indomethacin (10 μM) had no impact on aspirin effects (n=5, Figure 1b), we concluded that the aspirin-induced NO rise was not mediated by inhibition of prostanoid synthesis. This is further supported by the finding that preincubation with ibuprofen (50 μM), which antagonizes COX inhibition by aspirin by preventing access to the active site of the enzyme, had no effect on NO release induced by 10 μM aspirin (Emax=107.9±11.0% of ibuprofen-free control, P=0.51) (n=6).
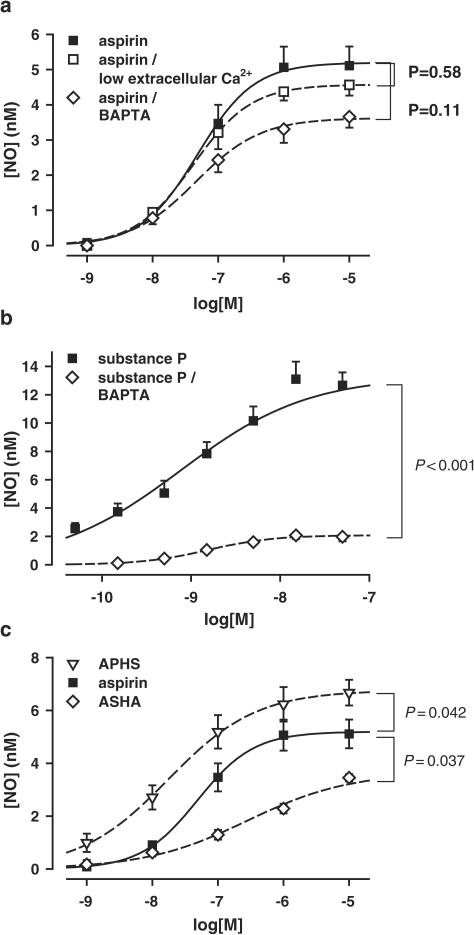
The aspirin-induced NO release was not affected by the absence of the transmembraneous calcium gradient ([Ca2+]e=50 nM) (n=5, Figure 2a), indicating that the NO stimulation is independent of an external calcium entry. Reducing the free intracellular calcium concentration by pretreatment of the vessels with the cell-permeable Ca2+-chelating agent 1,2-bis-O-aminophenoxyethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM, 10 μM) also exerted only minor inhibitory effects on the aspirin-induced release of NO (n=5, Figure 2a), while the substance P-mediated NO release was diminished to 15.6±6.9% of the BAPTA-free control (n=5, Figure 2b).
Figure 2.
(a) NO formation from PCA induced by aspirin in normal calcium buffer, in nominally calcium-free buffer ([Ca2+]e=50 nM), and after 15 min preincubation with intracellular calcium chelating agent BAPTA-AM (10 μM) (n=5). (b) NO formation from PCA induced by substance P in normal HEPES/Krebs buffer (n=10), and after 15 min preincubation with BAPTA-AM (10 μM) (n=5). (c) NO formation from PCA induced by aspirin and the acetylating aspirin derivatives APHS or ASHA (n=5).
Involvement of Ca2+-independent eNOS activation via phosphatidylinositol-3′-OH-kinase (PI3K) or cAMP-dependent protein kinase A (PKA) signaling cascades was ruled out by preincubation of PCA for 15 min with the PI3K blocker wortmannin (1 μM) or the PKA inhibitor H-89 (20 μM), which failed to reduce aspirin-induced NO release from PCA: Emax (aspirin/wortmannin)=93.7±12.6% of wortmannin-free control, P=0.46 (n=4) and Emax (aspirin/H-89)=103.4±14.1% of H-89-free control, P=0.57 (n=4).
To test whether an acetylation may be responsible for the NO stimulation, we employed two aspirin analogs with different structures and different acetylating potencies: the highly active thioether APHS, in which the acetyl is attached to the phenol, and the less active ASHA, in which the acetyl is attached to a hydroxamate group. Both compounds also stimulated a transient NO release from PCA (n=5, Figure 2c) that was blocked in the presence of L-NMMA (100 μM, n=5, data not shown).
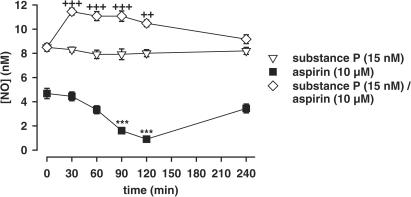
Repeated exposure of PCA to 10 μM aspirin every 30 min was associated with a progressive reduction of the NO response that was reactivated after 2 h of incubation in aspirin-free buffer. This fall in aspirin-induced NO activation was not the result of an eNOS substrate or cofactor impoverishment, since repeated stimulation with the receptor-dependent eNOS activator substance P failed to show a decrease in NO activation. A single preincubation of PCA with 10 μM aspirin for 15 min enhanced the NO response to subsequent repeated stimulation with substance P over a period of at least 2 h (n=4, Figure 3).
Figure 3.
NO formation from PCA induced by repeated stimulation with 10 μM aspirin, 15 nM substance P, and 15 nM substance P after a single 15-min preincubation with 10 μM aspirin (n=4). After each stimulation, the aspirin- or substance P-containing incubation buffer was changed with test substance-free buffer. ***P<0.001 indicates a significant difference in NO response to aspirin compared to the initial aspirin-induced NO release (t=0). ++P<0.01, +++P<0.001 indicate significant differences in NO response to substance P after aspirin pretreatment compared to the respective value without aspirin pretreatment.
NO production by EA.hy 926 cell homogenates
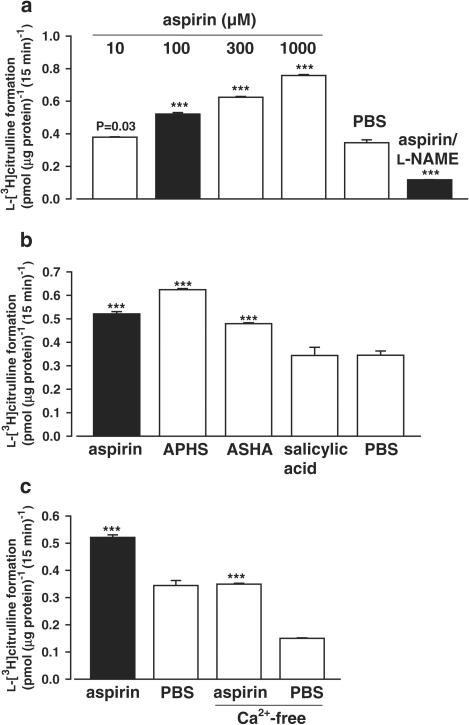
The amperometric NO determinations on intact PCA were supported by monitoring eNOS activity in soluble homogenates of EA.hy 926 cells via L-arginine/L-citrulline conversion. After 15 min of incubation with aspirin, L-[3H]citrulline yield increased in a concentration-dependent fashion; for example, with 100 μM aspirin to 151.2±2.7% of the aspirin-free phosphate-buffered saline (PBS) control (n=4, Figure 4a). This effect was abolished by coincubation with 1 mM of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (n=4, Figure 4a).
Figure 4.
(a) L-[3H]citrulline formation by EA.hy 926 cell homogenates in the L-arginine/L-citrulline conversion assay after 15 min incubation with 10, 100, 300, or 1000 μM aspirin and 100 μM aspirin concomitant with 1 mM L-NAME compared to the PBS control (n=4) (***P<0.001). (b) Incubation with 100 μM salicylic acid, or 100 μM of the acetylating compounds APHS or ASHA compared to 100 μM aspirin (n=4) (***P<0.001). (c) Incubation with 100 μM aspirin in normal calcium buffer and in nominally calcium-free buffer ([Ca2+]e=50 nM) compared to the respective PBS controls (n=4) (***P<0.001).
Incubation with 100 μM salicylic acid for 15 min did not increase eNOS activity (n=4), whereas 100 μM of the acetylating agents APHS or ASHA caused an eNOS stimulation to 181.1±1.4 and 139.0±1.1% of the PBS control (n=4) (Figure 4b).
Specific eNOS activation (determined as L-[3H]citrulline yield in the presence of aspirin minus the yield in the absence of aspirin: 0.18±0.04 pmol (μg protein)−1 (15 min)−1) did not change when the CaCl2 was omitted from the incubation buffer: 0.20±0.01 pmol (μg protein)−1 (15 min)−1, suggesting that full eNOS activation by aspirin already occurs at resting calcium levels (n=4, Figure 4c). When the incubation period was extended to 30 min, the L-[3H]citrulline yield in the presence of 100 μM aspirin increased to 0.80±0.02 pmol (μg protein)−1 (30 min)−1 and the absence of aspirin to 0.61±0.02 pmol (μg protein)−1 (30 min)−1, whereas the specific yield remained constant: 0.19±0.03 pmol (μg protein)−1 (30 min)−1 (n=4, data not shown), indicating that direct aspirin-induced eNOS activation is limited to the first 15 min of incubation.
Further purification of the cytosolic protein fraction by ultracentrifugation had no impact on eNOS stimulation by aspirin: L-[3H]citrulline yield after 15 min of incubation with 100 μM aspirin was 148.6±0.7% of the PBS control (P=0.44, n=4). This suggests that the particular cell fraction containing the eNOS localized to plasma or Golgi membrane had been already removed by the initial centrifugation procedure.
Superoxide scavenging
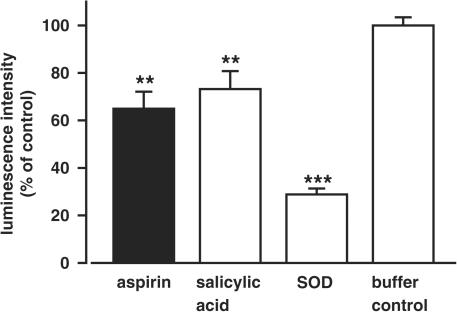
Treatment of EA.hy 926 cells with 1 μM aspirin or salicylic acid for 1 h reduced basal superoxide production to 64.9±7.2% (n=8) and 73.2±7.5% (n=7), respectively, of the test compound-free control (=100%) (Figure 5); the difference between aspirin and salicylic acid was not significant (P=0.27). Incubation of the cells with 1 μM SOD for 1 h, serving as a positive control, reduced the basal superoxide level to 28.8±2.5% (n=6, Figure 5). However, stimulation of PCA with increasing concentrations of SOD (up to 10 μM) did not elevate NO availability (n=5, data not shown), suggesting that in the current experimental setting superoxide scavenging is not implicated in delaying NO destruction.
Figure 5.
Reduction of basal superoxide production in EA.hy 926 cells after treatment with 1 μM aspirin, salicylic acid or SOD for 1 h. Values represent the lucigenin-enhanced chemiluminescence intensity relative to the test substance-free control with HEPES/Krebs buffer (=100%) (n=6–8, **P<0.01, ***P<0.001).
The second-order rate constants of aspirin and salicylic acid for the reaction with superoxide were also in the same range: (5.0±0.6) × 104 and (7.3±0.8) × 104 mol−1 l s−1, respectively (P=0.47).
Discussion and conclusions
This is the first report demonstrating that aspirin mediates a direct stimulation of NO release from native vascular endothelium as well as an increase of eNOS activity in endothelial cell homogenates.
Principally, the bioavailability of NO can be increased either by preventing its degradation or by enhanced synthesis.
Biological half-life of NO in vascular tissue is limited (3–5 s) by the rapid inactivation with superoxide to form peroxynitrite. Although our findings indicate that aspirin scavenges superoxide at a therapeutically relevant concentration, an antioxidant mechanism appears not to be involved in the aspirin-mediated increase of endothelial NO release, since the equally effective superoxide scavenger salicylic acid did not increase NO levels. This may be explained by the slow reaction rate of aspirin and salicylic acid with superoxide compared to the nearly diffusion-controlled interaction between NO and superoxide (k=1.6 × 1010 mol−1 l s−1) (Nauser & Koppenol, 2002). Thus, to effectively compete with NO for superoxide aspirin has to exceed the apparent NO concentration by more than 105-fold, that is toxic millimolar aspirin concentrations would be required to delay NO degradation. Moreover, failure of the more effective and rapid superoxide scavenger SOD (k=1.4 × 109 mol−1 l s−1; Taubert et al., 2003) to enhance NO availability from PCA indicates that under physiological conditions (i.e. in the absence of excessive oxidative stress) the vascular superoxide levels are below NO concentrations, so that even a near-complete depression of superoxide formation would not markedly enhance NO release.
Therefore, we propose that aspirin stimulates endothelial NO release by activating its biosynthesis. It is well established that eNOS can be activated by calcium-mobilizing agonists and by diverse stimuli that are independent of an increase in [Ca2+]i such as fluid shear stress. The finding that the specific NO stimulation by aspirin was largely unaffected by removal or chelation of Ca2+ suggests that aspirin induces NO formation without elevating intracellular Ca2+ concentrations. The rapid response of intact vessels and cell homogenates to aspirin and failure of 1400W to abrogate NO formation excludes direct induction of the inducible NOS isoform, which is known to require only low resting [Ca2+]i levels for full enzyme activation. Thus, aspirin appears to specifically activate eNOS by a calcium-independent mechanism. Although it has been proposed that eNOS-derived NO in nanomolar concentrations may upregulate the iNOS expression (Connelly et al., 2003), Western blot analysis of porcine aortic endothelial cell cultures revealed the lack of iNOS protein under basal conditions, but also after 48 h of incubation with an eNOS activator (Berkels et al., 2001). These findings do not support the idea that aspirin may cause a more pronounced NO formation by indirectly triggering iNOS expression in the endothelium. Stimulation of the eNOS by phosphorylation (and coordinated dephosphorylation) of serine and threonine residues via protein kinases, which represents a common calcium-independent signaling pathway of eNOS activation (Dimmeler et al., 1999; Boo et al., 2002), appears not to be involved in the aspirin action. Acetylation of COX could be excluded to contribute; however, another acetylating process appears to underlie NO induction, since the stimulating effects of aspirin on NO release of intact endothelium as well as activation of isolated eNOS could be mimicked by acetylating aspirin analogs. It is intriguing that the magnitude of the NO stimulation (Emax) correlates with the acetylating potency of these compounds (APHS>aspirin>ASHA) (Kalgutkar et al., 1998; Loll et al., 2001). A covalent modification by acetylation also accords with the progressive deactivation of the NO stimulation after repeated exposure to aspirin as well as the prolonged sensitization of the substance P-mediated NO stimulation. The acetylation appears to be essentially irreversible, since regeneration of basal enzyme activity required at least 120 min of incubation, while the half-life of the eNOS protein has been determined as 80 min (Ramet et al., 2003), indicating that enzyme activity is restored by de novo synthesis. Modulation of upstream signaling pathways implicated in eNOS stimulation seems unlikely, since aspirin-mediated eNOS activation was not restricted to intact endothelial cells but could also be demonstrated in the soluble eNOS fraction, which lacks the key signaling mediators of eNOS activation that are targeted to the membrane fractions. The higher concentrations of aspirin required to activate NO production in the soluble eNOS homogenates in comparison with the coronary arteries are consistent with the observation that loss of plasma membrane targeting is associated with a fall in enzyme activity (Govers & Rabelink, 2001). Additionally, differences between native endothelium and cultured endothelial cells have to be considered (e.g. regarding eNOS concentration or eNOS interaction with regulatory proteins like HSP-90); hence, employing the amperometric technique of NO measurement, it was a common observation that the NO release from cells grown on dishes or coverslips was lower and required 10–100-fold higher agonist concentrations to stimulate NO formation compared to experiments performed on PCA (unpublished observations). Although consistent, our results present only indirect evidence for eNOS acetylation by aspirin. However, previous studies have already shown that various types of proteins were acetylated, preferentially at the ɛ-amino group of lysine residues, when they were incubated with aspirin (Bridges et al., 1975; Burch & Blazer-Yost, 1981; Green & Jung, 1981; Hun-Opfer & Mata-Segreda, 1986). At which position of the eNOS molecule or associated regulatory proteins (scaffolds, chaperons, kinases) a functionally relevant acetylation occurs and what are the molecular consequences of this acetylation (e.g. a reduced Ca2+ dependency for the enzyme activation by calmodulin) have to be assessed in subsequent studies.
Impaired NO-dependent endothelial function was recently found to be a strong predictor of cardiovascular events (Halcox et al., 2002; Targonski et al., 2003). Therefore, vascular NO is regarded as a new therapeutic target in treatment and prevention of cardiovascular diseases (Loscalzo, 2001). However, it was suggested that endothelial dysfunction in several vascular diseases is accompanied by eNOS uncoupling, leading to production of reactive oxygen species rather than NO (Landmesser et al., 2003). This pathological situation may be aggravated by stimulatory effects of aspirin on eNOS activity. However, our data provide no evidence that aspirin may cause an increase of basal eNOS activity or an induction of eNOS or iNOS expression, excluding potentially detrimental effects of a persistent NOS activation. Accordingly, clinical studies found an improvement of NO-mediated vasodilation in patients with coronary atherosclerosis (Husain et al., 1998; Noon et al., 1998) or hypertension (Monobe et al., 2001) by low-dose aspirin administration, indicating that aspirin may restore the eNOS response to physiologic stimuli.
On the other hand, therapeutic relevance of the aspirin-induced short-term increase in NO availability and the relative low peak NO elevation may be questionable. Aspirin in concentrations up to 1 mM did not relax rings of PCA in organ bath experiments (data not shown), which accords with our previous findings demonstrating that vasodilation requires a higher maximal NO release than the 5 nM elicited by aspirin (Taubert et al., 2002). Nevertheless, the missing relaxation does not exclude a potential cardioprotection, since NO has been shown to exert additional beneficial effects, such as antiapoptotic effects on endothelial cells, negative inotropic effects on cardiomyocytes, inhibitory effects on platelets, and anti-inflammatory actions (Heydrick, 2000). The rapid decline in NO release is a characteristic feature of eNOS stimulation with receptor-dependent agonists, like substance P or bradykinin, but it is also seen after applying direct eNOS activators, like L-arginine. The endothelial NO formation is tightly controlled by negative feedback mechanisms (Buga et al., 1993) that may also be involved in the rapid termination of the aspirin-induced NO signal. However, rather than this transient direct effect, the more prolonged potentiating effect of aspirin on receptor-mediated endothelial NO generation by vascular autacoids (like substance P or bradykinin) or by shear stress may constitute the relevant mechanism underlying the improvement of endothelial function under aspirin therapy.
So far, several COX-independent mechanisms have been proposed to contribute to the cardiovascular benefit of aspirin (Awtry & Loscalzo, 2000). However, most of these effects have been demonstrated only with high concentrations (⩾1 mM) in vitro, questioning their clinical relevance. In contrast, our findings indicate that remarkably small concentrations of aspirin, even below the IC50 of 2–20 μM for inhibiting mammalian COX-1 (Amann & Peskar, 2002), stimulate endothelial NO release. Despite the short half-life of aspirin (approx. 20 min in systemic circulation), which is deacetylated to NO-inactive salicylic acid, potentially NO-active plasma concentrations (above the EC50 of 50 nM for NO stimulation) are maintained for about 3 h after a single oral dose of 80 mg aspirin, yielding peak plasma concentrations in the range of 5 μM (Benedek et al., 1995). Therefore, lower aspirin doses than those currently employed for cardioprotection may be sufficient to cause physiologically relevant NO elevations without inhibiting COX activity, which is responsible for adverse bleeding effects limiting the overall benefit of aspirin, particularly in primary prevention (Hayden et al., 2002). However, to definitely assess the functional relevance of our findings clinical trials have to be conducted.
Summarizing, aspirin-stimulated endogenous NO release may preserve endothelial function, thereby reducing cardiovascular risk in a prostanoid-independent fashion.
Abbreviations
- APHS
o-(acetoxyphenyl)hept-2-ynyl sulfide
- ASHA
o-acetylsalicylhydroxamic acid
- BAPTA-AM
1,2-bis-O-aminophenoxyethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester
- COX-1/2
cyclooxygenase-1/2
- EA.hy 926
immortalized human umbilical vein endothelial cells
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- NO
nitric oxide
- PCA
porcine coronary conduit arteries
- PI3K
phoshatidylinositol-3′-OH-kinase
- SOD
superoxide dismutase
References
- AMANN R., PESKAR B.A. Anti-inflammatory effects of aspirin and sodium salicylate. Eur. J. Pharmacol. 2002;447:1–9. doi: 10.1016/s0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]
- AWTRY E.H., LOSCALZO J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- BENEDEK I.H., JOSHI A.S., PIENIASZEK H.J., KING S.Y., KORNHAUSER D.M. Variability in the pharmacokinetics and pharmacodynamics of low dose aspirin in healthy male volunteers. J. Clin. Pharmacol. 1995;35:1181–1186. doi: 10.1002/j.1552-4604.1995.tb04044.x. [DOI] [PubMed] [Google Scholar]
- BERKELS R., EGINK G., MARSEN T.A., BARTELS H., ROESEN R., KLAUS W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension. 2001;37:240–245. doi: 10.1161/01.hyp.37.2.240. [DOI] [PubMed] [Google Scholar]
- BOO Y.C., SORESCU G., BOYD N., SHIOJIMA I., WALSH K., DU J., JO H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- BRIDGES K.R., SCHMIDT G.J., JENSEN M., CERAMI A., BUNN H.F. The acetylation of hemoglobin by aspirin. In vitro and in vivo. J. Clin. Invest. 1975;56:201–207. doi: 10.1172/JCI108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUGA G., GRISCAVAGE J., ROGERS N., IGNARRO L. Negative feedback regulation of endothelial cell function by nitric oxide. Circ. Res. 1993;73:808–812. doi: 10.1161/01.res.73.5.808. [DOI] [PubMed] [Google Scholar]
- BURCH J.W., BLAZER-YOST B. Acetylation of albumin by low doses of aspirin. Thromb. Res. 1981;23:447–452. doi: 10.1016/0049-3848(81)90205-x. [DOI] [PubMed] [Google Scholar]
- CONNELLY L., JACOBS A.T., PALACIOS-CALLENDER M., MONCADA S., HOBBS A.J. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J. Biol. Chem. 2003;278:26480–26487. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., FLEMING I., FISSLTHALER B., HERMANN C., BUSSE R., ZEIHER A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- EDGELL C.J., MCDONALD C.C., GRAHAM J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOVERS R., RABELINK T.J. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. Renal. Physiol. 2001;280:F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- GREEN F.A., JUNG C.Y. Acetylation of erythrocytic membrane peptides by aspirin. Transfusion. 1981;21:55–58. doi: 10.1046/j.1537-2995.1981.21181127484.x. [DOI] [PubMed] [Google Scholar]
- HALCOX J.P.J., SCHENKE W.H., ZALOS G., MINCEMOYER R., PRASAD A., WACLAWIW M.A., NOUR K.R.A., QUYYUMI A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- HAYDEN M., PIGNONE M., PHILLIPS C., MULROW C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- HEYDRICK S.Cellular signal transduction and nitric oxide Contemporary Cardiology: Nitric Oxide and the Cardiovascular System 2000Totowa, NJ: Humana Press Inc; 33–49.ed. Loscalzo, J. & Vita, J.A. pp [Google Scholar]
- HUN-OPFER C., MATA-SEGREDA J.F. Non-enzymatic acetylation of proteins by aspirin as protection against the secondary complications of diabetes mellitus. Acta Physiol. Pharmacol. Latinoam. 1986;36:313–316. [PubMed] [Google Scholar]
- HUSAIN S., ANDREWS N.P., MULCAHY D., PANZA J.A., QUYYUMI A.A. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716–720. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- KALGUTKAR A.S., KOZAK K.R., CREWS B.C., HOCHGESANG G.P., JR, MARNETT L.J. Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators. J. Med. Chem. 1998;41:4800–4818. doi: 10.1021/jm980303s. [DOI] [PubMed] [Google Scholar]
- LANDMESSER U., DIKALOV S., PRICE S.R., MCCANN L., FUKAI T., HOLLAND S.M., MITCH W.E., HARRISON D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOLL P.J., SHARKEY C.T., O'CONNOR S.J., DOOLEY C.M., O'BRIEN E., DEVOCELLE M., NOLAN K.B., SELINSKY B.S., FITZGERALD D.J. O-acetylsalicylhydroxamic acid, a novel acetylating inhibitor of prostaglandin H2 synthase: structural and functional characterization of enzyme–inhibitor interactions. Mol. Pharmacol. 2001;60:1407–1413. doi: 10.1124/mol.60.6.1407. [DOI] [PubMed] [Google Scholar]
- LOSCALZO J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- MONOBE H., YAMANARI H., NAKAMURA K., OHE T. Effects of low-dose aspirin on endothelial function in hypertensive patients. Clin. Cardiol. 2001;24:705–709. doi: 10.1002/clc.4960241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUSER T., KOPPENOL W.H. The rate constant of reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J. Phys. Chem. A. 2002;106:4084–4086. [Google Scholar]
- NOON J.P., WALKER B.R., HAND M.F., WEBB D.J. Impairment of forearm vasodilatation to acetylcholine in hypercholesterolemia is reversed by aspirin. Cardiovasc. Res. 1998;38:480–484. doi: 10.1016/s0008-6363(98)00013-3. [DOI] [PubMed] [Google Scholar]
- RAMET M.E., RAMET M., LU Q., NICKERSON M., SAVOLAINEN M.J., MALZONE A., KARAS R.H. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J. Am. Coll. Cardiol. 2003;41:2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- TARGONSKI P.V., BONETTI P.O., PUMPER G.M., HIGANO S.T., HOLMES D.R., JR, LERMAN A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- TAUBERT D., BERKELS R., KLAUS W., ROESEN R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: essential structural features. J. Cardiovasc. Pharmacol. 2002;40:701–713. doi: 10.1097/00005344-200211000-00008. [DOI] [PubMed] [Google Scholar]
- TAUBERT D., BREITENBACH T., CENSAREK P., LAZAR A., HARLFINGER S., BERKELS R., KLAUS W., ROESEN R. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radic. Biol. Med. 2003;35:1599–1607. doi: 10.1016/j.freeradbiomed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]