Abstract
GEA 3162 (1,2,3,4,-oxatriazolium, 5-amino-3-(3,4-dichlorophenyl)-chloride), has powerful effects on neutrophil function and apoptosis, but the underlying mechanisms are unclear, particularly with respect to the possible roles of nitric oxide (NO) and/or peroxynitrite (ONOO−).
Our hypothesis was that GEA 3162 is a generator of ONOO− and that its biological effects on neutrophil apoptosis differ from those of a conventional NO donor. The effects of GEA 3162 were compared to those of the established ONOO− donor, 3-morpholinosydnonimine (SIN-1), and the NO donor, diethylamine diazeniumdiolate (DEA/NO) in neutrophils from healthy volunteers. Electrochemical detection and electron paramagnetic resonance were used to define the NO-related species generated from these agents.
GEA 3162 and SIN-1 influence neutrophil apoptosis differently from DEA/NO. All three compounds induced morphological neutrophil apoptosis. However, both GEA 3162 and SIN-1 paradoxically inhibited internucleosomal DNA fragmentation, whereas DEA/NO induced fragmentation compared to control.
In contrast to DEA/NO, generation of free NO was not detectable in solutions of GEA 3162 or SIN-1 (100 μM). However, Cu/Zn superoxide dismutase (SOD; 50–750 U ml−1) unmasked NO generated from these compounds in a concentration-dependent manner. GEA 3162 and SIN-1 oxidised the O2−- and ONOO−-sensitive dye, dihydrorhodamine 123 (DHR 123; 1 μM), suggesting that ONOO− released from these compounds is responsible for oxidation of DHR 123.
We conclude that GEA 3162 is an ONOO− donor with pro-apoptotic properties that more closely resemble SIN-1 than the NO donor, DEA/NO. Moreover, unlike NO, ONOO− induces apoptosis in neutrophils via a mechanism that does not require DNA fragmentation.
Keywords: Nitric oxide, peroxynitrite, GEA 3162, neutrophil, apoptosis
Introduction
Nitric oxide (NO) is a free radical that was originally identified as an endogenous endothelium-derived vasodilator (Furchgott & Zawadzki, 1980; Palmer et al., 1987), but is now recognised to have a role in a large number of other physiological and pathophysiological processes, including haemostasis, neurotransmission and inflammation (Quinn et al., 1995). The role of NO in inflammation is particularly complex, with macrophages in particular capable of generating high levels of NO through promotion of transcription factor activity and consequent expression of the unregulated, inducible isoform of NO synthase (iNOS) in response to inflammatory stimuli (Hecker et al., 1996). The primary role of iNOS-derived NO is accepted to be that of a powerful antipathogenic agent, but it is also clear that NO has a complex impact on apoptosis (Dimmeler & Zeiher, 1997; Kim et al., 1999; Taylor et al., 2003), a physiological form of cell death which eliminates effete or unhealthy cells. The issue is further complicated by the rapid reaction of NO with superoxide anion (O2−) to form the powerful oxidising agent, peroxynitrite (ONOO−; Kelm et al., 1997).
Despite NO and ONOO− sharing several biological properties (Ronson et al., 1999; Low et al., 2002), including an ability to induce apoptosis (Taylor et al., 2003), key differences have been noted in their biological effects and mechanisms of action. For example, although both NO and ONOO− are capable of blocking mitochondrial respiration, they do so through inhibition of different complexes in the respiratory chain and their effects are differentially susceptible to reversal by thiols and carbohydrates (Lizasoain et al., 1996). These differences highlight the importance of the accurate identification of the nature of the species generated by the so-called ‘NO donors'; ONOO− generated as a result of concomitant release of NO and O2− may exert effects that are similar but mechanistically distinct from pure NO in biological systems. To date, however, little is known about potential differential effects of NO and ONOO− on inflammatory cell apoptosis.
GEA 3162 is an oxatriazole-5-imine derivative with vasodilator (Nurminen & Vapaatalo, 1996) and pro-apoptotic (Ward et al., 2000; Taylor et al., 2001) properties. However, its identity as a ‘pure' NO donor is controversial (Kankaanranta et al., 1996; Holm et al., 1998; Schmidt et al., 2001). In this study, we set out to establish the identity of the NO-related species generated from GEA 3162, and to compare its biological properties to an established NO donor (diethylamine diazeniumdiolate; DEA/NO) and an ONOO− donor (SIN-1) with respect to neutrophil apoptosis. All the three compounds have previously been shown to accelerate the rate of programmed cell death in neutrophils in vitro (Blaylock et al., 1998; Ward et al., 2000; Taylor et al., 2001); however, the precise mechanisms have not been elucidated.
Experimental procedures
Isolation of neutrophils
Neutrophils were isolated from the blood of healthy volunteers as described previously (Ward et al., 1999a). Briefly, whole, citrated blood was centrifuged (200 × g, 20 min) and platelet-rich plasma aspirated. Leukocytes were separated from erythrocytes by dextran sedimentation, then further divided into mononuclear cell and granulocyte populations by centrifugation through a discontinuous Percoll gradient (720 × g, 20 min). Granulocytes were harvested from the 79 : 68% interface of the gradient, and only neutrophil preparations of ⩾95% purity were used. Neutrophil preparations were tested for cellular homogeneity by preparation of a cytospin slide (100 μl of cell suspension, 300 r.p.m., 3 min), which was then stained and examined by oil-immersion light microscopy, with at least 500 cells counted. The percentage of contaminating leukocytes (eosinophils, monocytes and lymphocytes) was then calculated and the cell preparation was discarded if levels of contamination reached or exceeded 5% of the total cell population.
Apoptosis studies
Neutrophils (4.5 × 106 cells ml−1) were suspended in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Paisley, U.K.) containing penicillin (100 U ml−1) and streptomycin (100 μg ml−1), and supplemented with 10% (v v−1) autologous serum. They were cultured in flat-bottomed 96-well Falcon polypropylene plates (37°C, 5% CO2) for 1–20 h in the presence of either phosphate-buffered saline (PBS) at pH 7.4 (controls) or NO donors (100 μM–3 mM). The concentrations of NO donors used in these studies were selected on the basis of previously published data (Taylor et al., 2001), which have been shown to modulate neutrophil apoptosis.
Following incubation, 100 μl of recovered cells were cytocentrifuged in duplicate, fixed in methanol and stained using Diff-Quik™ physiological stain, then observed by light microscopy (× 100 objective) to determine the proportion of darkly stained cells with condensed nuclei. At least 500 cells per slide were counted, with the observer blinded to the experimental conditions.
A further 100 μl of recovered cells were centrifuged (200 × g, 3 min), then fixed and permeabilised in 70% ethanol (4°C, 10 min). The cells were washed (× 3) in PBS without Ca2+/Mg2+ before addition of 60 μl RNase A (0.5 mg ml−1) and 60 μl propidium iodide (PI; 0.1 mg ml−1), then assessed by flow cytometry using an EPICS XL2 (Coulter Electronics, Luton, U.K.), to measure DNA fragmentation.
Dihydrorhodamine 123 studies
Dihydrorhodamine 123 (DHR 123) is a fluorescent dye which is activated by various reactive oxygen species, including ONOO−, O2−, hydrogen peroxide (H2O2) and HOCl, but not by NO (Crow, 1997). This compound can therefore be used to discriminate between agents that release NO only and those that generate NO and O2− simultaneously.
PBS (100 μl), the neutrophil-activating agent, phorbol 12-myristate 13-acetate (PMA; final concentration 10 nM), SIN-1 (1 mM), GEA 3162 (100 μM) or DEA/NO (1 mM) was added to 900 μl PBS in 2 ml Eppendorf tubes. DHR 123 was added to each tube to a final concentration of 1 μM. Tubes were incubated for 60 min (37°C, 5% CO2). A volume of 450 μl was then transferred to a 0.5 ml cuvette, excited at 480 nm and the fluorescence emitted at 500 nm was read using a spectrofluorimeter (Perkin Elmer, U.K.).
Electrochemical detection of NO
Free NO from GEA 3162 or SIN-1 (both 100 μM) and DEA/NO (5 μM) was measured in Iscove's MDM cell culture medium using an NO electrode (Iso-NO II, World Precision Instruments) calibrated daily with DEA/NO (0.1–1.6 μM) in pH 4 buffer. Superoxide dismutase (SOD; 50–750 U ml−1) was added cumulatively in an effort to unmask NO generated from these agents (Lizasoain et al., 1996), and the concentration of NO was measured at plateau approx. 5 min following SOD addition.
Electron paramagnetic detection of O2− and ONOO−
Spin-trapping experiments were performed in Iscove's MDM cell culture medium containing 100 μM SIN-1, GEA 3162 or DEA/NO in the presence or absence of SOD (500 U ml−1), and the spin trap Tempone-H (1 mM) (Dikalov et al., 1997) prepared in water containing EDTA (10 mM). Reaction mixtures were incubated at 37°C throughout the experiments and the intensity of the electron paramagnetic resonance (EPR) signal corresponding to the formation of 4-oxo-Tempo (triplet centred at 3364 G) was measured (arbitrary units) at timed intervals for each of the donor drugs (Magnettech® miniscope MS100 with the following parameter settings: field sweep 51.2 G, microwave frequency 9.5 GHz, microwave power 20 mW, modulation amplitude 1500 mG). In control experiments without any NO-generating compounds present, there was a slow increase in EPR signal corresponding to the auto-oxidation of Tempone-H to 4-Oxo-Tempo; these signals have been subtracted from the data shown.
Statistical analysis
Data were assessed for statistical significance using two-way analysis of variance (ANOVA) with a Student–Neuman–Keuls post-test. Probability values of P<0.05 were considered statistically significant.
Results
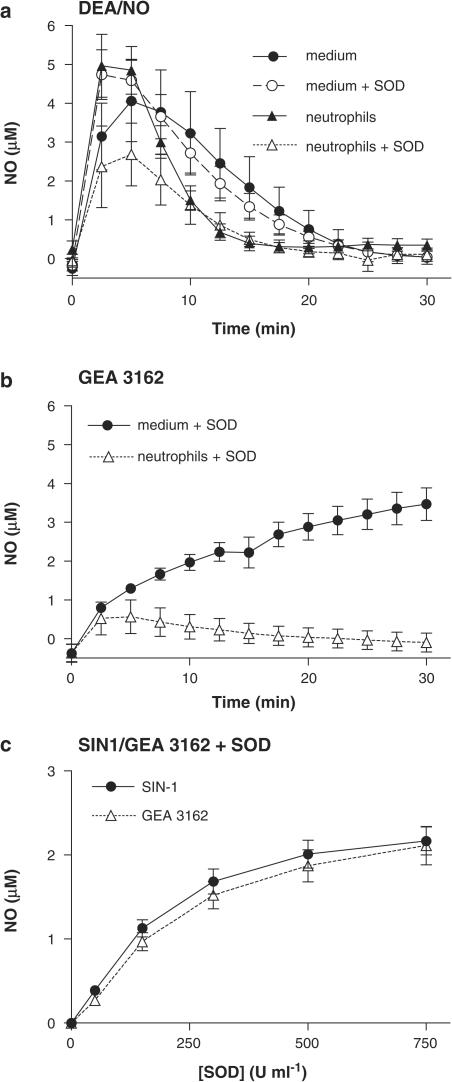
Electrochemical detection of DEA/NO-derived NO revealed that this compound liberated free NO in a spontaneous and predictable manner. A bolus addition of DEA/NO (5 μM) to culture medium caused a rapid transient rise in NO concentration, reaching a maximum of ∼5 μM, before subsiding over the subsequent 30 min (Figure 1a). The signal was not altered by incubation with SOD (150 U ml−1), but the rate of decay of the NO signal was markedly enhanced in the presence of neutrophils (5 × 106 ml−1; Figure 1a). SOD (150 U ml−1) failed to increase the signal seen with neutrophils; instead, it caused a significant and surprising inhibition of NO generation.
Figure 1.
Measurement of NO release from DEA/NO, SIN-1 and GEA 3162 in the absence and presence of neutrophils and/or SOD. Mean NO electrode recordings for (a) DEA/NO (5 μM) and (b) GEA-3162 (100 μM) in Iscove's medium, in the presence and absence of human neutrophils (4.5 × 106 cells ml−1). NO release from GEA 3162 was not detectable in the absence of SOD (150 U ml−1). Concentrations of NO were calculated using a calibration curve generated using DEA/NO (100 nM1.6 μM) in pH 4 buffer. Results are mean±s.e.m. (n=6). (c) Generation of NO by SIN-1 and GEA 3162 (both 100 μM) in the presence of SOD (50–750 U ml−1). Data represent NO concentrations at plateau approx. 5 min following addition of SOD. Results are mean±s.e.m. (n=6).
In contrast to DEA/NO, NO was not detectable from GEA 3162 (100 μM) in medium. However, NO generation from this agent was detected in the presence of SOD (150 U ml−1), with maximal NO concentrations reaching ∼3 μM after 30 min incubations (Figure 1b). In the presence of neutrophils (5 × 106 ml−1) and SOD (150 U ml−1), NO generation was markedly attenuated for incubation periods of >10 min. NO generation bore a nonlinear relationship to SOD concentration for both GEA 3162 and SIN-1 (Figure 1c), and there was no significant difference between the two agents under these conditions (P>0.05).
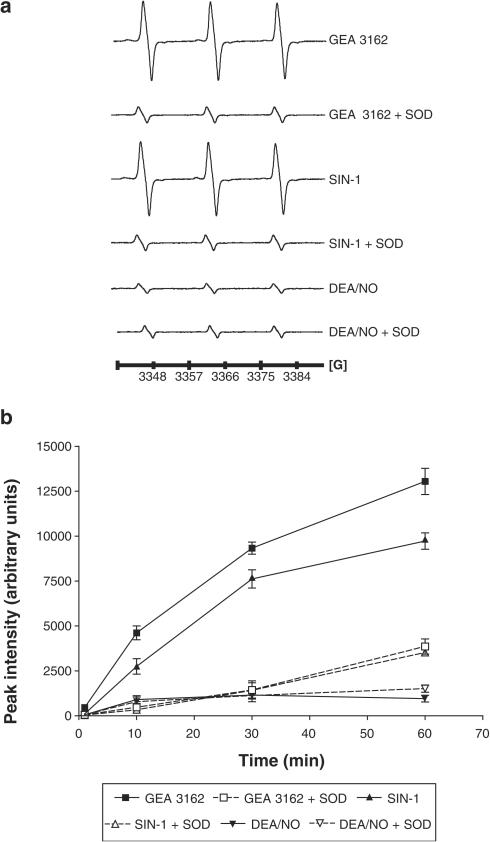
Results from EPR spectroscopy identified a signal corresponding to 4-oxo-Tempo formation from the reduced form of the spin-trap, Tempone-H, after incubation with GEA 3162 or SIN-1 (Figure 2a). The intensity of the signal obtained with both GEA-3162 and SIN-1 was dependent on the incubation time and was severely attenuated in the presence of SOD (500 U ml−1). DEA/NO failed to generate a significant signal under the same conditions (Figure 2b).
Figure 2.
Generation of O2−/ONOO− by donor drugs. (a) Representative EPR spectra showing the signal generated through oxidation of Tempone-H (1 mM) by O2−/ONOO− after incubation (30 min, 37°C) with SIN-1, GEA 3162 and DEA/NO (all 100 μM) in the presence or absence of SOD (500 U ml−1). Control spectra of Iscove's medium and 1 mM Tempone-H have been subtracted from each trace. (b) Relative intensities (arbitrary units) for EPR signals generated over time in the presence of SIN-1, GEA-3162 or DEA/NO (100 μM) in the presence or absence of SOD (500 U ml−1). Results are mean±s.e.m. (n=6).
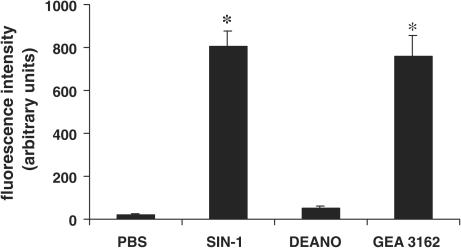
Assessment of the oxidation of DHR 123 to fluorescent rhodamine 123 using a spectrofluorimeter showed that both SIN-1 (1 mM) and GEA 3162 (100 μM) produced high levels of fluorescence (803±74 and 757±100 U, respectively; P<0.05; n=3) compared to control (PBS alone, 23±5 U; Figure 3). In contrast, DEA/NO failed to significantly oxidise DHR 123 (53±9 U; n=3). None of the compounds alone (in the absence of DHR 123) produced a fluorescent signal that exceeded 1.0 arbitrary units (data not shown), thus excluding autofluorescence as a possible explanation for the differential fluorescence observed.
Figure 3.
Spectrofluorimetric assessment of DHR 123 oxidation. DHR 123 (1 μM) was added to solutions of PBS (control), SIN-1 (1 mM), DEA/NO (1 mM) or GEA 3162 (100 μM) and incubated for 60 min at 37°C. The extent of DHR 123 oxidation to rhodamine 123 was assessed using a spectrofluorimeter at an emission wavelength of 500 nm. Results are mean±s.e.m. (n=3). Asterisks represent significant difference (P<0.05) from control fluorescence.
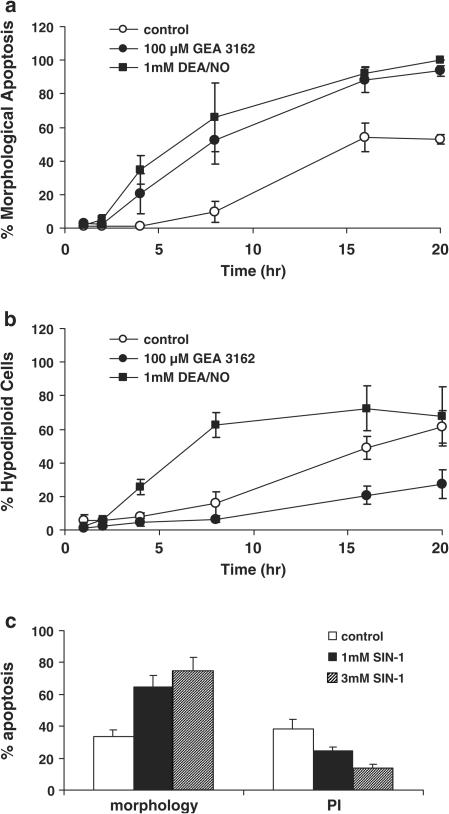
High concentrations of DEA/NO (1 mM) and GEA 3162 (100 μM) accelerated morphological changes characteristic of neutrophil apoptosis over a 20-h time course compared to untreated controls (Figure 4a). Approximately 50–60% of control cells underwent spontaneous apoptosis during the culture period, whereas those treated with either compound were virtually all apoptotic. However, when internucleosomal DNA fragmentation was assessed by flow cytometric measurement of PI intercalation, key differences were observed between the two compounds. Whereas DEA/NO increased DNA fragmentation compared to control, GEA 3162 produced an unexpected inhibition of this process (Figure 4b). The effects of 1–3 mM SIN-1 on morphology and DNA fragmentation at 20 h were then studied in order to determine how ONOO− influences these apoptotic events in neutrophils. This compound had effects similar to GEA 3162 but opposing effects to DEA/NO, in that a concentration-dependent inhibition of DNA fragmentation occurred alongside an induction of apoptotic morphology (Figure 4c).
Figure 4.
Differential effects of compounds on independent parameters of neutrophil apoptosis. Human neutrophils were incubated at 37°C for 1–20 h with PBS (control), DEA/NO (1 mM) or GEA 3162 (100 μM). Apoptosis was then assessed by (a) morphological changes using oil-immersion light microscopy and (b) PI intercalation to show hypodiploid DNA content characteristic of apoptotic cells. Results are mean±s.e.m. (n=3–6). (c) Neutrophils were incubated for 20 h with SIN-1 (1–3 mM) and apoptosis was assessed by morphology and PI intercalation. Results are mean±s.e.m. (n=5).
Discussion
Our results show that the oxatriazole-5-imine-derived compound, GEA 3162, generated an oxidising species that induced apoptosis in human neutrophils via a mechanism that was independent of DNA fragmentation. Similar results were obtained with the known ONOO− generator, SIN-1, but the NO donor, DEA/NO, failed to generate an oxidising species, and apoptosis induction in response to this agent was associated with increased DNA fragmentation. Further experiments to determine the NO-related species generated from these agents established that GEA 3162 and SIN-1 concomitantly generate NO with O2−, resulting in rapid formation of ONOO−. The NO component was unmasked by removal of O2− by high concentrations of the enzyme, SOD. These results were in common with those of the recognised ONOO− generator, SIN-1 (Hogg et al., 1992), but were at odds with the NO donor drug, DEA/NO (Maragos et al., 1991), which was found to generate NO in the absence of O2− scavenger systems, and did not oxidise Tempone-H to generate an EPR signal.
Previous studies have suggested that GEA 3162 does not liberate O2− alongside NO; therefore it was suggested to be a ‘pure' NO donor (Holm et al., 1998). However, in contrast, recent preliminary data have indicated that GEA 3162 resembles SIN-1, in that both compounds simultaneously generate NO and O2− and are therefore both ONOO− donors (Schmidt et al., 2001). Given that SIN-1 is accepted to be an ONOO− donor (Hogg et al., 1992), while DEA/NO generates pure NO (Maragos et al., 1991), our results suggest that ONOO− is a requirement for the inhibition of DNA fragmentation and implies that GEA 3162 is also an ONOO−-generating agent. We therefore carried out a number of experiments to determine the nature of the NO-related species liberated from GEA 3162.
An NO-specific electrode detected NO release from DEA/NO in culture medium, as has been shown previously (Crane et al., 2002). Release of NO from this compound (5 μM) occurred spontaneously at physiological temperature and pH. The signal was not significantly affected by SOD alone, suggesting that there was no concurrent release of O2− under these conditions. In the presence of neutrophils, there was a mild acceleration of the decay of NO signal from DEA/NO, suggesting a scavenging effect of the cells. However, NO detection in the presence of neutrophils and SOD was not enhanced; indeed, it was surprisingly blunted, perhaps indicating a scavenging effect of the enzyme under these conditions. On the other hand, SIN-1 and GEA 3162 failed to produce measurable free NO under the same conditions, despite the relatively high concentrations of these drugs used (100 μM). The limit for NO detection of the electrode used in these studies is ∼50 nM. Rapid scavenging of NO through reaction with O2− generated simultaneously might account for the lack of detectable NO under these conditions. It is well established that this is the case for SIN-1, which is recognised to be an ONOO− donor rather than a pure NO donor (Hogg et al., 1992), but it was a matter of debate whether NO release from GEA 3162 also involves the concomitant liberation of O2−. In order to test this, the buffer into which the electrode was immersed was preincubated with varying concentrations of Cu/Zn SOD, which rapidly converts O2− to H2O2, in an effort to ‘unmask' NO by removal of O2−. If SIN-1 and GEA 3162 both liberate a combination of NO and O2−, then free NO might be detectable in the presence of SOD, which would prevent at least some of the NO being oxidised to ONOO− (Lizasoain et al., 1996). With reference to the rate constants, the relative rates of reaction can be estimated as follows (for a concentration of 1 μM NO and assuming the amount of NO generated is matched by O2−):
From these reaction rates, it is reasonable to predict that some NO might be unmasked in the presence of SOD, but that the amount observed would be a relatively small percentage of the total amount generated, on account of the fact that the rate of reaction of NO with O2− is three times faster than that catalysed by SOD. In the event, NO was detected from both SIN-1 and GEA 3162; indeed, the profile of NO release from the two compounds in the presence of SOD was virtually identical, and correlated well with previously published data for SIN-1 (Lizasoain et al., 1996). The role of oxygen-derived free radicals in quenching of NO was confirmed by EPR experiments using the O2− and ONOO−-specific spin-trap, Tempone-H. A signal corresponding to 4-oxo-Tempo increased in intensity during incubations with both SIN-1 and GEA-3162, but was not seen with DEA/NO. The 4-oxo-Tempo signal was quenched by SOD, indicating that the species responsible for oxidation of Tempone-H was O2−, or a downstream product of O2− (e.g. ONOO−). Taken together, these results indicate that GEA-3162 is indeed an ONOO− generator, with characteristics that are very similar to SIN-1 and quite distinct from the NO donor, DEA/NO.
DHR 123 is oxidised to the fluorochrome, rhodamine 123, by a number of oxidative and nitrosative species, including ONOO−, O2−, H2O2 and HOCl, and is widely used in flow cytometric assessment of respiratory burst in neutrophils (Smith & Weidemann, 1993; Ruchaud-Sparagano et al., 1997). However, it has been demonstrated that NO does not have the capacity to oxidise this molecule (Crow, 1997). This makes DHR 123 an ideal tool for discriminating between agents that release NO and those that liberate ONOO−. Addition of DHR 123 to solutions of SIN-1 (1 mM) and GEA 3162 (100 μM) led to the generation of fluorescent rhodamine 123, whereas with PBS and DEA/NO (1 mM), no fluorescence could be detected. This suggests that both SIN-1 and GEA 3162 generate species distinct from NO, such as ONOO−, which does react with DHR 123.
Functionally, SIN-1 and GEA 3162 share properties that differ from DEA/NO. Apoptosis studies showed that all the three compounds accelerated morphological neutrophil apoptosis at high concentrations, as has previously been demonstrated by this group (Ward et al., 2000; Taylor et al., 2001). Only extremely high concentrations of DEA/NO were able to induce neutrophil apoptosis (Taylor et al., 2001), which are likely to be suprapathophysiological, thereby suggesting that even high concentrations of NO generated from iNOS during excessive inflammation are unable to promote neutrophil apoptosis by themselves. Therefore, endogenously formed NO, whatever its source, is unlikely to induce apoptosis in this cell type if it is not converted to ONOO−.
Only DEA/NO produced the expected rise in the level of internucleosomal DNA fragmentation that mirrored the morphological changes observed. In the presence of both SIN-1 and GEA 3162, this process was inhibited compared to control, indicating an uncoupling of DNA fragmentation from other apoptotic events with these two compounds, and provides further evidence that the species generated by GEA 3162 more closely resembles that from SIN-1 than from DEA/NO. Although this study did not investigate the potential underlying mechanism, this phenomenon could potentially be due to the inhibitory modification of tyrosine (nitration) or reduced cysteine (S-nitrosation) residues (Kuo & Kocis, 2002) in critical proteins of the DNA fragmentation pathway such as caspase 3 (Kim et al., 1997) or DFF40 (Widlak, 2000), thus affecting the activity of these proteins and therefore the DNA fragmentation process.
Overall, we have demonstrated that GEA 3162 is an ONOO− donor that induces neutrophil apoptosis via a mechanism that is not dependent on DNA fragmentation. Chemically and biologically, therefore, GEA 3162 resembles SIN-1 and is clearly distinct from DEA/NO. The induction of neutrophil apoptosis may be of therapeutic benefit in a number of inflammatory conditions in which resolution of inflammation is impaired (such as arthritis, pancreatitis, pneumonia and asthma), which causes neutrophils to persist in the tissue and then potentially subsequently die by necrosis (Ward et al., 1999b; Taylor et al., 2003). ONOO− is an important molecule in inflammation as iNOS-derived NO and O2− are both produced by inflammatory cells and the resulting ONOO− may contribute to the pathophysiology of a number of chronic inflammatory conditions (Szabo, 1996).
Acknowledgments
E.L.T and C.A.S. are funded by the Wellcome Trust, as part of a 4-year Ph.D. programme in Cardiovascular Biology within the Cardiovascular Research Initiative at the University of Edinburgh, and FPDR was funded by a Wellcome Trust vacation scholarship (VS/02/EDI/3). The EPR miniscope was supported by a BHF core facility grant (CUI/99010).
Abbreviations
- DEA/NO
diethylamine diazeniumdiolate
- DHR 123
dihydrorhodamine 123
- EPR
electron paramagnetic resonance
- GEA 3162
1,2,3,4-oxatriazolium, 5-amino-3-(3,4-dichlorophenyl)-chloride
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- IMDM
Iscove's modified Dulbecco's medium
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2−
superoxide anion
- ONOO−
peroxynitrite
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PMA
phorbol 12-myristate 13-acetate
- SIN-1
3-morpholinosydnonimine
- SOD
superoxide dismutase
References
- BLAYLOCK M.G., CUTHBERTSON B.H., GALLEY H.F., FERGUSON N.R., WEBSTER N.R. The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Radic. Biol. Med. 1998;25:748–752. doi: 10.1016/s0891-5849(98)00108-7. [DOI] [PubMed] [Google Scholar]
- CRANE M.S., OLLOSSON R., MOORE K.P., ROSSI A.G., MEGSON I.L. Novel role for low molecular weight plasma thiols in nitric oxide-mediated control of platelet function. J. Biol. Chem. 2002;277:46858–46863. doi: 10.1074/jbc.M208608200. [DOI] [PubMed] [Google Scholar]
- CROW J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- DIKALOV S., SKATCHKOV M., BASSENGE E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem. Biophys. Res. Commun. 1997;231:701–704. doi: 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., ZEIHER A.M. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide: Biol. Chem. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- HECKER M., PREISS C., KLEMM P., BUSSE R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophages: role of nuclear factor kappa B and interferon regulatory factor 1. Br. J. Pharmacol. 1996;118:2178–2184. doi: 10.1111/j.1476-5381.1996.tb15660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGG N., DARLEY-USMAR V.M., WILSON M.T., MONCADA S.Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide Biochem. J. 1992281419–424.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLM P., KANKAANRANTA H., METSA-KETELA T., MOILANEN E. Radical releasing properties of nitric oxide donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur. J. Pharmacol. 1998;346:97–102. doi: 10.1016/s0014-2999(98)00009-0. [DOI] [PubMed] [Google Scholar]
- KANKAANRANTA H., RYDELL E., PETERSSON A.S., HOLM P., MOILANEN E., CORELL T., KARUP G., VUORINEN P., PEDERSEN S.B., WENNMALM A., METSA-KETELA T. Nitric oxide-donating properties of mesoionic 3-aryl substituted oxatriazole-5-imine derivatives. Br. J. Pharmacol. 1996;117:401–406. doi: 10.1111/j.1476-5381.1996.tb15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELM M., DAHMANN R., WINK D., FEELISCH M. The nitric oxide/superoxide assay. Insights into the biological chemistry of the NO/O-2 interaction. J. Biol. Chem. 1997;272:9922–9932. doi: 10.1074/jbc.272.15.9922. [DOI] [PubMed] [Google Scholar]
- KIM Y.M., BOMBECK C.A., BILLIAR T.R. Nitric oxide as a bifunctional regulator of apoptosis. Circ. Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- KIM Y.M., TALANIAN R.V., BILLIAR T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- KUO W.N., KOCIS J.M. Nitration/S-nitrosation of proteins by peroxynitrite-treatment and subsequent modification by glutathione S-transferase and glutathione peroxidase. Mol. Cell. Biochem. 2002;233:57–63. doi: 10.1023/a:1015510207489. [DOI] [PubMed] [Google Scholar]
- LIZASOAIN I., MORO M.A., KNOWLES R.G., DARLEY-USMAR V., MONCADA S.Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose Biochem. J. 1996314877–880.(Part 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW S.Y., SABETKAR M., BRUCKDORFER K.R., NASEEM K.M. The role of protein nitration in the inhibition of platelet activation by peroxynitrite. FEBS Lett. 2002;511:59–64. doi: 10.1016/s0014-5793(01)03279-3. [DOI] [PubMed] [Google Scholar]
- MARAGOS C.M., MORLEY D., WINK D.A., DUNAMS T.M., SAAVEDRA J.E., HOFFMAN A., BOVE A.A., ISAAC L., HRABIE J.A., KEEFER L.K. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- NURMINEN M.L., VAPAATALO H. Effect of intracerebroventricular and intravenous administration of nitric oxide donors on blood pressure and heart rate in anaesthetized rats. Br. J. Pharmacol. 1996;119:1422–1426. doi: 10.1111/j.1476-5381.1996.tb16054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- QUINN A.C., PETROS A.J., VALLANCE P. Nitric oxide: an endogenous gas. Br. J. Anaesth. 1995;74:443–451. doi: 10.1093/bja/74.4.443. [DOI] [PubMed] [Google Scholar]
- RONSON R.S., NAKAMURA M., VINTEN-JOHANSEN J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc. Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- RUCHAUD-SPARAGANO M.H., STOCKS S.C., TURLEY H., DRANSFIELD I. Activation of neutrophil function via CD66: differential effects upon beta 2 integrin mediated adhesion. Br. J. Haematol. 1997;98:612–620. doi: 10.1046/j.1365-2141.1997.2523070.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT K., SCHRAMMEL A., GORREN A.C.F., KOESLING D., MAYER B. Decomposition of the ‘NO donor' GEA 3162 results in the formation of peroxynitrite. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:161S. [Google Scholar]
- SMITH J.A., WEIDEMANN M.J. Further characterization of the neutrophil oxidative burst by flow cytometry. J. Immunol. Methods. 1993;162:261–268. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- SZABO C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia–reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- TAYLOR E.L., MEGSON I.L., HASLETT C., ROSSI A.G. Dissociation of DNA fragmentation from other hallmarks of apoptosis in nitric oxide-treated neutrophils: differences between individual nitric oxide donor drugs. Biochem. Biophys. Res. Commun. 2001;289:1229–1236. doi: 10.1006/bbrc.2001.6122. [DOI] [PubMed] [Google Scholar]
- TAYLOR E.L., MEGSON I.L., HASLETT C., ROSSI A.G. Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ. 2003;10:418–430. doi: 10.1038/sj.cdd.4401152. [DOI] [PubMed] [Google Scholar]
- WARD C., CHILVERS E.R., LAWSON M.F., PRYDE J.G., FUJIHARA S., FARROW S.N., HASLETT C., ROSSI A.G. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 1999a;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- WARD C., DRANSFIELD I., CHILVERS E.R., HASLETT C., ROSSI A.G. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol. Sci. 1999b;20:503–509. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- WARD C., WONG T.H., MURRAY J., RAHMAN I., HASLETT C., CHILVERS E.R., ROSSI A.G. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem. Pharmacol. 2000;59:305–314. doi: 10.1016/s0006-2952(99)00329-9. [DOI] [PubMed] [Google Scholar]
- WIDLAK P. The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim. Pol. 2000;47:1037–1044. [PubMed] [Google Scholar]