Abstract
This study compares the role of endothelial factors in α-adrenoceptor contractile responses in mesenteric resistance (MRA) and superior (SMA) mesenteric arteries from ouabain-treated (8.0 μg day−1, 5 weeks) and untreated rats. The role of the renin–angiotensin system was also evaluated.
Ouabain treatment increased systolic blood pressure. In addition, ouabain reduced the phenylephrine response in SMA but did not alter noradrenaline responses in MRA.
Endothelium removal or the nitric oxide synthase (NOS) inhibitor (L-NAME, 100 μM) increased the responses to α-adrenergic agonists in both vessels. After ouabain treatment, both endothelial modulation and the L-NAME effect were increased in SMA, while only the L-NAME effect was increased in MRA. Endothelial NOS expression remained unaltered after ouabain treatment.
Indomethacin (10 μM) similarly reduced the noradrenaline contraction in MRA from both groups; in contrast, in SMA, indomethacin only reduced phenylephrine-induced contractions in segments from untreated rats. Co-incubation of L-NAME and indomethacin leftward shifted the concentration–response curves for noradrenaline more in MRA from ouabain-treated rats; tetraethylammonium (2 mM) shifted the noradrenaline curves further leftward only in MRA from untreated rats.
Losartan treatment prevents the development of hypertension but not all vascular changes observed after ouabain treatment.
In conclusion, a rise in endothelial NO and impaired prostanoid participation might explain the reduction in phenylephrine-induced contraction in SMA after ouabain treatment. An increase in the modulatory effect of endothelial NO and impairment of endothelium-dependent hyperpolarizing factor effect might explain why the ouabain treatment had no effect on noradrenaline responses in MRA.
Keywords: Ouabain, hypertension, endothelium, nitric oxide, endothelium-derived hyperpolarizing factor
Introduction
The role of an endogenous ouabain-like compound in the development and/or maintenance of hypertension has been suggested by findings that show that its plasma levels are elevated in some forms of hypertension. In addition, exogenous ouabain, as well as its structurally related analogs, induces hypertension when chronically administered to normotensive rats (Hamlyn et al., 1982; 1996; Huang et al., 1994; Manunta et al., 1994; 2001; Rossoni et al., 2002a, 2002b; Di Filippo et al., 2003). This hypertension seems to depend, at least in part, on central mechanisms associated with increased sympathetic tone, subsequent to the activation of cerebral renin–angiotensin (Huang & Leenen, 1999; Zhang & Leenen, 2001) and endothelin (Di Filippo et al., 2003) systems.
Additional peripheral mechanisms might occur in the hypertension induced by chronic treatment with ouabain. Previously, we have demonstrated that this hypertensive model is accompanied by a reduction in vascular reactivity to phenylephrine and by increased activity and expression of Na+, K+-ATPase in rat aortae (Rossoni et al., 2002a, 2002b). These alterations are associated with increased endothelium-derived nitric oxide (NO) production and increased expression of the endothelial (eNOS) and neuronal isoforms of NO synthase (Rossoni et al., 2002b; Xavier et al., 2004). Other authors have also shown in aortic rings from ouabain-treated rats a reduction in the contraction induced by phenylephrine in the absence of a development of hypertension (Cargnelli et al., 2000). However, in isolated renal arterial preparations, treatment with ouabain was accompanied by increased K+- and phenylephrine-induced vasoconstriction (Kimura et al., 2000). Collectively, these observations suggest that different vascular beds are differently affected by ouabain treatment.
All the studies mentioned above used conductance arteries. However, at present, there are no published studies on the effects of chronic treatment with ouabain on resistance arteries, despite the fact that resistance arteries have a much greater role in the regulation of the total peripheral vascular resistance and blood pressure than do conductance vessels. The purpose of this study was to compare the effect of hypertension induced by ouabain treatment on the endothelial modulation of vascular responses induced by α-adrenoceptor activation in resistance and conductance arteries. For this purpose, the main and the fourth branch of superior mesenteric arteries were used. We also studied whether antihypertensive treatment with an AT1-angiotensin receptor antagonist (losartan), which has been effectively demonstrated to block the hypertensinogenic effect of ouabain (Zhang & Leenen, 2001), would prevent the possible vascular alterations induced by chronic ouabain treatment.
Methods
Male Wistar rats, 6-week-old, were obtained from colonies maintained at the Animal Quarters of the Facultad de Medicina of the Universidad Autónoma de Madrid. All experiments comply with the current Spanish and European laws (RD 223/88 MAPA and 609/86).
Rats were divided into four treatment groups: untreated, ouabain-treated (≈8.0 μg day−1), losartan-treated (15 mg kg−1 day−1, v.o.) and ouabain plus losartan-treated. Controlled time-release pellets (Innovative Research of America) containing ouabain (0.5 mg) or vehicle (placebo) were subcutaneously implanted, as described previously (Huang et al., 1994). Losartan, administered from the time the pellets were implanted, was dissolved in tap water at a final concentration, calculated as a function of body weight and the volume of water consumed. Systolic blood pressure (SBP) was measured by the tail-cuff method before the beginning of treatment and every week during treatment until week 5.
Vascular reactivity measurements
After 5-weeks' treatment, rats were decapitated and mesenteric arteries removed. Segments of superior mesenteric arteries 4 mm in length were set up in gassed (95% O2 and 5% CO2) Krebs–Henseleit solution (KHS), at 37°C, under a tension of 0.5 g. Isometric tension was recorded using an isometric force displacement transducer (Grass FT03C, MA, U.S.A.) connected to an acquisition system (MacLab/41 ADInstruments Pty Ltd, Castle Hill, Australia). Segments of fourth-order mesenteric resistance arteries, 2 mm in length, were mounted in a small-vessel dual-chamber myograph for measurement of isometric tension according to the method described by Mulvany & Halpern (1977).
Experimental protocols
After a 45 min equilibration period, mesenteric resistance and superior mesenteric arteries were exposed to 120 and 75 mM KCl, respectively, to check their functional integrity. Then, concentration–response curves to acetylcholine (ACh, 0.01 nM–30 μM) were performed in mesenteric resistance and superior mesenteric arteries previously contracted with noradrenaline or phenylephrine, respectively, at a concentration that produced approximately 50% of the contraction induced by KCl in each case.
After a washout period of 60 min, concentration–response curves to phenylephrine (1 nM–10 μM) were constructed in superior mesenteric arteries. In resistance arteries, noradrenaline (10 nM–30 μM) was used to study the vasoconstriction induced by α-adrenoceptor activation because noradrenaline produced responses that were more stable than those produced by phenylephrine; the tonic phase of the contractions induced by phenylephrine was not sustained and this precluded the generation of cumulative concentration–response curves. In some experiments, the role of the endothelium on the contractile responses was studied by removing this vascular component. In other experiments, the effects of the NOS inhibitor N-nitro-L-arginine methyl ester (L-NAME, 100 μM) and the cyclooxygenase (COX) inhibitor indomethacin (10 μM) on the noradrenaline- or phenylephrine-induced response were investigated. These drugs were added 30 min before the concentration–response curve.
In another set of experiments, the role of endothelium-derived hyperpolarizing factor (EDHF) on noradrenaline-induced contraction was assessed in mesenteric resistance arteries from the experimental groups studied. For this, three consecutive concentration–response curves to noradrenaline were performed at intervals of 1 h; L-NAME (100 μM) plus indomethacin (10 μM) and L-NAME plus indomethacin plus tetraethylammonium (TEA, 2 mM) were added 30 min before the second and the third concentration–response curves, respectively. No differences between the three consecutive concentration–response curves to noradrenaline were observed in control experiments (results not shown).
Western blot analysis of eNOS protein expression
Proteins from homogenized mesenteric arteries (30 μg per lane) were separated by 7.5% sodium lauryl sulphate-polyacrylamide gel and then transferred to polyvinyl difluoride membranes overnight. Next, the membranes were incubated for 1 h with mouse monoclonal antibody for eNOS (1 : 2500, Transduction Laboratories, Lexington, U.K.) and, after washing, incubated with antimouse IgG antibody conjugated to horseradish peroxidase (1 : 2000, Transduction Laboratories). The immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus, Amersham International plc, Little Chalfont, U.K.) and subjected to autoradiography (Hyperfilm ECL, Amersham International plc). Signals on the immunoblot were quantified using a computer program (NIH Image V1.56). The same membranes were used to determine α-actin expression using a mouse monoclonal antibody (1 : 3,000,000, Boehringer Mannheim, Mannheim, Germany). Homogenates from human endothelial cells were used for eNOS-positive control.
Solutions, drugs and statistical analysis
The composition of KHS was as follows (mM): NaCl 115, CaCl2 2.5, KCl 4.6, KH2PO4 1.2, MgSO4·7H2O 1.2, NaHCO3 25, glucose 11.1 and Na2EDTA 0.03. Losartan was a gift of Merck Sharp & Dohme (Spain). Drugs used were phenylephrine hydrochloride, noradrenaline hydrochloride, ACh chloride, L-NAME dihydrochloride, indomethacin and TEA (Sigma, St Louis, MO, U.S.A.). Stock solutions (10 mM) of drugs were made in a distilled water, except for noradrenaline, which was dissolved in a NaCl (0.9%)-ascorbic acid (0.01% w v−1) solution, and indomethacin, which was dissolved in 1.5 mM NaHCO3. These solutions were kept at −20°C and appropriate dilutions were made on the day of the experiment.
Contractile responses are expressed as a percentage of the maximum response produced by KCl. The maximum effect (Emax) and the concentration of agonist that produced half of Emax (log EC50) were calculated using nonlinear regression analysis (GraphPad Prism Software, San Diego, CA, U.S.A.). The sensitivity of the agonists is expressed as pD2 (−log EC50). Some results were expressed as ‘differences' of area under the concentration–response curves (dAUC) to phenylephrine or noradrenaline in control and experimental situations. AUC were calculated from the individual concentration–response curves plot (GraphPad Prism Software, San Diego, CA, U.S.A.) and the differences were expressed as a percentage of the difference to AUC of the corresponding control situation. Results of eNOS expression are expressed as the ratio between optical density for NOS and for α-actin.
All values are expressed as means±s.e.m. of the number of animals used in each experiment. Results were analyzed using Student's t-test, by completely randomized two-way ANOVA for comparison between groups, or by repeated-measure two-way ANOVA to compare treatments within the same group. When ANOVA showed a significant treatment effect, Bonferroni's post hoc test was used to compare individual means. Differences were considered statistically significant at P<0.05.
Results
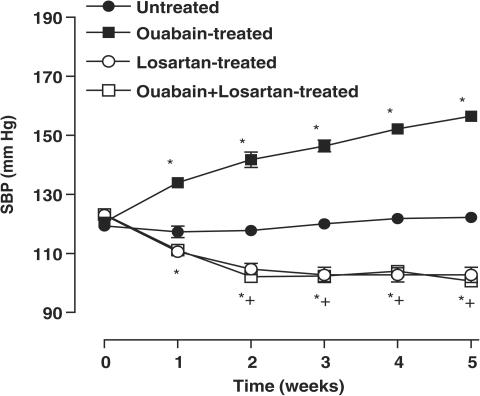
Ouabain treatment induced a progressive increase in SBP from the first week (P<0.05). In both ouabain-treated and untreated rats, treatment with losartan reduced the SBP to a similar level (Figure 1). The body weight was similar in all experimental groups studied (untreated: 324±12; ouabain-treated: 335±15; losartan-treated: 343±29; ouabain+losartan-treated: 326±28 g, P>0.05).
Figure 1.
SBP for Wistar rats, which received vehicle (untreated N=17), ouabain (N=23), losartan (N=7) or ouabain plus losartan (N=8). Results are expressed as means±s.e.m. for the number of animals used. *P<0.05 vs untreated rats; +P<0.05 vs untreated/ouabain-treated rats.
KCl (120 mM in mesenteric resistance arteries and 75 mM in superior mesenteric arteries) evoked contractions in vessels from ouabain-treated rats that were similar to those obtained in vessels from untreated rats for both the mesenteric resistance (untreated: 9.5±0.8 vs ouabain-treated: 9.8±0.8 mN, P>0.05) and superior mesenteric (untreated: 12.2±1.0 vs ouabain-treated: 13.3±0.6 mN, P>0.05) arteries. Treatment with losartan did not change KCl response (results not shown) in either type of arteries from ouabain-treated and untreated rats. The endothelium-dependent relaxation to ACh (0.01 nM–30 μM) also remained unmodified in all study groups (results not shown).
Contractile responses mediated by α-receptor activation in ouabain-treated and untreated rats
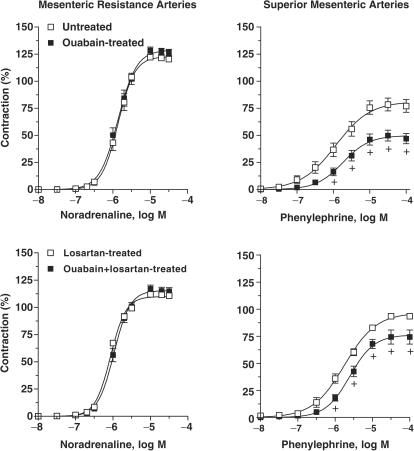
Contractile responses to noradrenaline were similar in mesenteric resistance arteries from ouabain-treated and untreated rats (Figure 2 and Table 1) and remained unmodified after losartan treatment (Figure 2 and Table 2). However, in superior mesenteric arteries, the contractile responses produced by phenylephrine were smaller in ouabain-treated than in untreated rats (Figure 2 and Table 1) and remained smaller in segments from rats that received ouabain plus losartan (Figure 2 and Table 2).
Figure 2.
Contractile responses to α-adrenoceptor activation in superior mesenteric arteries and mesenteric resistance arteries from untreated (N=7, 9) and ouabain-treated (N=7, 9) or losartan-treated (N=7, 7) and ouabain plus losartan-treated (N=8, 8) rats. Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): +P<0.05.
Table 1.
Effect of endothelium denudation (E−), L-NAME and indomethacin treatment on Emax and pD2 to noradrenaline in MRA and to phenylephrine in SMA from rats subcutaneously receiving vehicle (untreated) and ouabain (Oua) for 5 weeks
| MRA | SMA | |||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| Control | ||||
| Untreated | 121±2 | 5.93±0.04 | 80±6 | 6.00±0.09 |
| Oua-treated | 128±3 | 5.98±0.03 | 52±5+ | 5.67±0.10 |
| E− | ||||
| Untreated | 122±3 | 6.58±0.08* | 103±5* | 6.87±0.09* |
| Oua-treated | 129±7 | 6.42±0.08* | 100±4* | 6.66±0.09* |
| L-NAME | ||||
| Untreated | 127±5 | 6.17±0.05* | 135±4* | 6.38±0.12* |
| Oua-treated | 145±5*+ | 6.38±0.05*+ | 128±8* | 6.21±0.11* |
| Indomethacin | ||||
| Untreated | 113±4 | 5.67±0.06 | 51±9* | 5.54±0.03* |
| Oua-treated | 125±6 | 5.63±0.03 | 49±7 | 5.68±0.07 |
Values represent means±s.e.m. t-test:
P<0.05 vs Control;P<0.05, ouabain-treated vs untreated. N=6–9.
P<0.05, ouabain-treated vs untreated. N=6–9.
Table 2.
Effect of endothelium denudation (E−), L-NAME and indomethacin treatment on Emax and pD2 to noradrenaline in MRA and to phenylephrine in SMA from rats treated with losartan (Los) or ouabain+losartan (Oua+Los) for 5 weeks
| MRA | SMA | |||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| Control | ||||
| Los-treated | 114±1 | 6.07±0.02 | 95±2 | 5.78±0.09 |
| Oua+Los-treated | 114±2 | 6.00±0.02 | 76±6+ | 5.65±0.12 |
| E− | ||||
| Los-treated | 115±2 | 6.60±0.03* | 122±4* | 6.15±0.11* |
| Oua+Los-treated | 117±3 | 6.56±0.10* | 117±3* | 6.19±0.07* |
| L-NAME | ||||
| Los-treated | 120±2 | 6.34±0.05* | 126±4* | 6.14±0.06* |
| Oua+Los-treated | 131±5*+ | 6.32±0.05* | 120±4* | 6.35±0.08* |
| Indomethacin | ||||
| Los-treated | 110±3 | 5.90±0.05* | 49±9* | 5.26±0.12* |
| Oua+Los-treated | 119±3 | 5.84±0.06* | 70±8 | 5.73±0.13 |
Values represent means±s.e.m. t-test:
P<0.05 vs Control;
P<0.05, Oua+Los-treated vs Los-treated. N=7–8.
Effect of endothelium removal on α-adrenergic responses
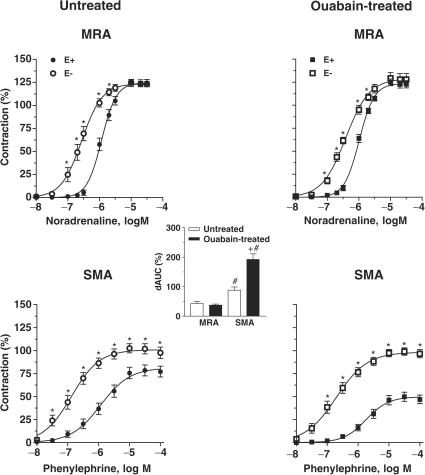
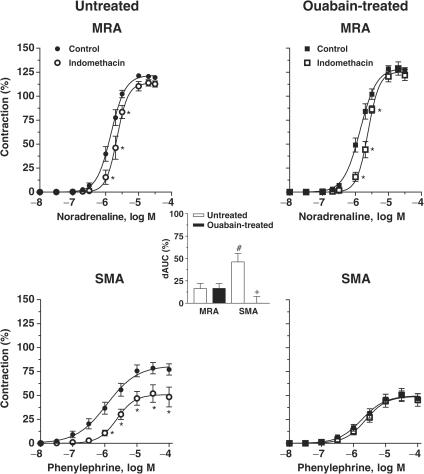
In both ouabain-treated and untreated rats, endothelium removal increased the potency of the agonists in both types of artery, while the maximum response was only increased for phenylephrine in superior mesenteric arteries (Figure 3 and Table 1). The effect of endothelium removal was larger in superior mesenteric than in mesenteric resistance arteries (see dAUC graph in Figure 3).
Figure 3.
Effect of endothelium denudation on the concentration-dependent response curves to noradrenaline and phenylephrine in MRA and SMA, respectively, from ouabain-treated (N=7–9) and untreated (N=7–9) rats. Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): *P<0.05. The inset graph shows the dAUC to noradrenaline or phenylephrine in endothelium-intact (E+) and -denuded (E−) arteries. dAUC values (means±s.e.m.) are expressed as a percentage of the difference of the corresponding AUC for segments with intact endothelium (unpaired t-test, #P<0.05, SMA vs MRA; +P<0.05 ouabain-treated vs untreated rats).
In mesenteric resistance arteries, the potentiation of noradrenaline-induced contraction by endothelium removal was similar in both experimental groups. In contrast, in superior mesenteric arteries, the potentiation of the phenylephrine response was greater in preparations from ouabain-treated than those from untreated rats (see dAUC graph in Figure 3).
Treatment with losartan did not alter the effect induced by endothelium removal on α-adrenergic responses in both arteries from ouabain-treated and untreated rats (Table 2).
Effect of NOS inhibition on α-adrenergic responses
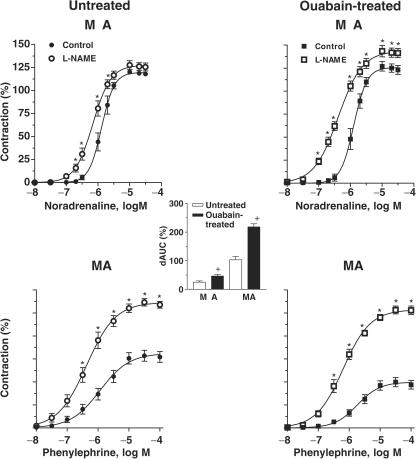
To assess the contribution of endothelium-derived NO to the alpha-adrenergic responses, segments were incubated with the NO synthase inhibitor L-NAME (100 μM). This drug increased the potency of agonists in both arteries from both groups. The maximum response was also increased in all preparations except in mesenteric resistance arteries from untreated rats (Figure 4 and Table 1). The enhancement induced by L-NAME in α-adrenergic responses was greater in superior mesenteric than in mesenteric resistance arteries (see dAUC graph in Figure 4).
Figure 4.
Effect of L-NAME (100 μM) on the concentration-dependent response curves to noradrenaline and to phenylephrine performed in MRA and SMA from ouabain-treated (N=6–9) and untreated (N=7–9) rats. Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): *P<0.05. The inset graph shows the dAUC to noradrenaline or phenylephrine in segments in the absence and in the presence of L-NAME. dAUC values (means±s.e.m.) are expressed as a percentage of the differences of the corresponding AUC for segments in the absence of L-NAME (unpaired t-test, #P<0.05, SMA vs MRA; +P<0.05 ouabain-treated vs untreated rats).
In both mesenteric resistance and superior mesenteric arteries, the potentiation induced by L-NAME was greater in arteries from ouabain-treated rats (see dAUC comparison in Figure 4).
In mesenteric resistance arteries from untreated rats, the analysis of the dAUC shows that the increase in the response to noradrenaline was larger after endothelium removal (46.7±6.5) than after L-NAME incubation (25.2±4.2%, P<0.05); in contrast, in segments from ouabain-treated rats, the potentiation after endothelium removal (37.3±3.7) or L-NAME incubation (46.6±6.1%, P>0.05) was similar. In superior mesenteric arteries, both endothelium removal and L-NAME similarly increased the concentration–response curve for phenylephrine in segments from untreated (dAUC: endothelium removal: 91.8±10.2 vs L-NAME: 103.0±16.8%, P>0.05) and ouabain-treated rats (dAUC: endothelium removal: 192.0±17.6 vs L-NAME: 218.0±7.8%, P>0.05).
Treatment with losartan did not alter the differences observed in L-NAME effects on α-adrenergic responses in both types of artery from ouabain-treated and untreated rats (Table 2).
Effect of COX inhibition on α-adrenergic responses
As shown in Figure 5 and Table 1, indomethacin shifted the concentration–response curves for noradrenaline or phenylephrine rightward in both arteries from untreated rats; this effect was greater in superior mesenteric arteries (see dAUC graph in Figure 5). Ouabain treatment did not alter the effect of indomethacin in mesenteric resistance arteries, but abolished it in superior mesenteric arteries (Figure 5 and Table 1).
Figure 5.
Effect of indomethacin (10 μM) on concentration-dependent response curves to noradrenaline and to phenylephrine performed in MRA and SMA from ouabain-treated (N=6–9) and untreated (N=6–9) rats. Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): *P<0.05. The inset graph shows the dAUC to noradrenaline or phenylephrine in segments in the absence and in the presence of indomethacin. dAUC values (means±s.e.m.) are expressed as a percentage of the difference of the corresponding AUC for segments in the absence of indomethacin (unpaired t-test, #P<0.05, SMA vs MRA; +P<0.05 ouabain-treated vs untreated rats).
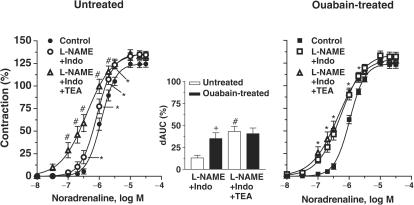
In mesenteric resistance arteries from both groups, incubation with L-NAME (100 μM) plus indomethacin (10 μM) shifted the concentration–response curves for noradrenaline leftward (Figure 6 and Table 3). However, this effect was larger in segments from ouabain-treated rats, as shown by the values for dAUC (Figure 6). The addition of TEA (2 mM), together with L-NAME and indomethacin, produced a further leftward shift of the curve for noradrenaline in segments from untreated rats, but not in those from ouabain-treated rats (Figure 6).
Figure 6.
Effect of preincubation with L-NAME (100 μM) plus indomethacin (Indo, 10 μM) and with L-NAME plus Indo plus TEA (2 mM) on concentration–response curves to noradrenaline in mesenteric resistance arteries from untreated (N=8) and ouabain-treated (N=8) rats. Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): *P<0.05, L-NAME+Indo vs Control; #P<0.05, L-NAME+Indo+TEA vs L-NAME+Indo. The inset graph shows the dAUC to noradrenaline in segments in the absence and in the presence of L-NAME+Indo and L-NAME+Indo+TEA. dAUC values (means±s.e.m.) are expressed as a percentage of the difference of the corresponding AUC for segments in the absence of drugs (unpaired t-test, #P<0.05, L-NAME+Indo+TEA vs L-NAME+Indo; +P<0.05 ouabain-treated vs untreated rats).
Table 3.
Effect of TEA treatment on pD2 and Emax values to noradrenaline in endothelium intact mesenteric resistance arteries, previously incubated with L-NAME plus indomethacin (Indo), from rats treated with vehicle (untreated), ouabain, losartan or ouabain plus losartan for 5 weeks
| Control | L-NAME+Indo | L-NAME+Indo+TEA | ||||
|---|---|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | Emax | pD2 | |
| Untreated | 124±3 | 5.93±0.04 | 132±4 | 6.11±0.06* | 135±4 | 6.43±0.09*+ |
| Ouabain-treated | 125±4 | 5.98±0.03 | 134±5 | 6.30±0.04* | 129±5 | 6.39±0.08* |
| Losartan-treated | 109±4 | 6.04±0.05 | 114±3 | 6.31±0.04* | 122±3 | 6.57±0.09*+ |
| Ouabain+Losartan-treated | 113±3 | 6.00±0.03 | 131±4 | 6.26±0.07* | 130±4 | 6.29±0.09* |
Values represent means±s.e.m. t-test:
P<0.05 vs Control;
P<0.05 vs L-NAME+Indo. N=7–8.
Treatment with losartan modified neither the effect of indomethacin on α-adrenergic responses in mesenteric resistance and superior mesenteric arteries (Table 2) nor the effect of indomethacin plus L-NAME plus TEA in mesenteric resistance arteries from either group (Table 3).
Western blot analysis of eNOS expression
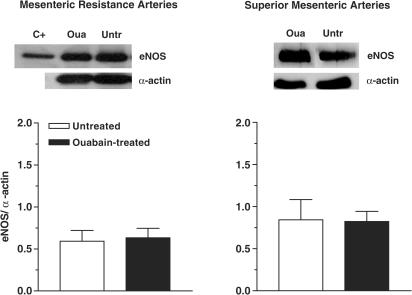
Figure 7 shows the eNOS isoform expression detected by the Western blot technique in mesenteric resistance and superior mesenteric arteries from ouabain-treated and untreated rats. eNOS protein expression was not affected by chronic ouabain treatment in either type of artery.
Figure 7.
Upper panel: Representative blot for eNOS protein expression in mesenteric resistance and superior mesenteric arteries from ouabain-treated (Oua) and untreated rats (Untr). The first lane shows the positive control (C+) for eNOS. Lower panel: Densitometric analysis of the Western blot for eNOS protein expression. Results (means±s.e.m.) are expressed as the ratio between the signal for the eNOS protein and the signal for α-actin. The number of arterial samples used was 7–8.
Discussion
It is now established that chronic treatment with ouabain, centrally or peripherally administered, induces hypertension in rats (Huang et al., 1994; Manunta et al., 1994; 2001; Rossoni et al., 2002a, 2002b; Di Filippo et al., 2003). This hypertension has been associated with an increased sympathetic tone that may be due to activation of the central regulatory systems involving renin–angiotensin (Huang & Leenen, 1999; Zhang & Leenen, 2001) and endothelin (Di Filippo et al., 2003). Regional differences in vascular reactivity following chronic treatment with ouabain have been reported. Reduction in the response to phenylephrine in aortae and superior mesenteric arteries (Cargnelli et al., 2000; Rossoni et al., 2002a, 2002b) and no change in caudal arteries (Rossoni et al., 2002a, 2002b) have been found in this model; however, in renal arteries, contractions produced by phenylephrine and K+ were increased (Kimura et al., 2000). In addition, Di Filippo et al. (2003) reported increased renal vascular resistance and decreased renal blood flow, suggesting an important role of the renal vascular bed in the development and/or maintenance of the hypertension in rats chronically treated with ouabain. The reason for these regional differences is not known, but different roles of these vascular beds in the development and/or maintenance of ouabain-induced hypertension would explain them. The above-mentioned results let us to speculate that resistance and conductance vessels might respond differently to chronic ouabain treatment. The present study was designed to compare the effect of hypertension induced by chronic treatment with ouabain on the endothelial modulation of the responses mediated by α-adrenoceptor activation in the main, capacitance, and the fourth-order, resistance, branches of the mesenteric artery and the effect of an antihypertensive treatment with an AT1-angiotensin receptor antagonist, losartan, on these responses.
After ouabain treatment, contractile responses mediated by α-adrenoceptor activation were reduced in superior mesenteric artery, as described previously (Rossoni et al., 2002a), and remained unaltered in mesenteric resistance arteries, confirming the regional differences after ouabain treatment. Both endothelium removal and NOS inhibition by L-NAME potentiated the contractile responses mediated by α-adrenoceptors to a greater extent in the superior mesenteric artery than in the resistance mesenteric arteries. These observations suggest that the endothelial modulation of responses mediated by α-adrenoceptors is greater in mesenteric conductance than in resistance arteries.
In superior mesenteric artery, the increases in phenylephrine responses after endothelium removal or L-NAME treatment were similar and they were greater in vessels from ouabain-treated animals. This suggests that NO is the main modulator of phenylephrine responses and that chronic treatment with ouabain increases the release of endothelial NO, which would be responsible for the reduced effects of phenylephrine observed in superior mesenteric artery, in agreement with our previous observation in rat aorta (Rossoni et al., 2002b). In mesenteric resistance arteries from ouabain-treated rats, L-NAME induced a greater increment in the curves to noradrenaline than in the arteries from untreated rats, as observed in superior mesenteric artery. However, removal of the endothelium potentiated the α-adrenergic contractile responses similarly in segments from ouabain-treated and untreated rats. On the other hand, in resistance arteries from untreated rats, the effect of endothelium removal was greater than that of NO synthesis inhibition with L-NAME. Nevertheless, in segments from ouabain-treated rats, both endothelium removal and L-NAME similarly increased the curves to noradrenaline. These observations suggest that in mesenteric resistance vessels ouabain treatment increases endothelial NO modulation of α-adrenergic responses, as also occurred in superior mesenteric arteries, but decreases the participation of a non-NO mediator.
Chronic treatment with ouabain did not alter the expression of eNOS in either the mesenteric resistance or superior mesenteric arteries, discarding the involvement of upregulated eNOS expression in the increased NO production in the arteries used in this study. This is in contrast to our previous results in rat aorta from ouabain-treated animals in which we found that the increased NO modulation is accompanied by increased expression of eNOS (Rossoni et al., 2002b). Nevertheless, an increase in the activity of this isoform cannot be discarded since increased eNOS activity has been associated to hypertension (Nava et al., 1995; Hayakawa & Raij, 1997; Vaziri et al., 1998). However, the relaxation induced by ACh remained unmodified after ouabain treatment. The mechanisms by which ouabain alters the NO release induced by α-adrenoceptor but not by muscarinic receptor activation are not known. Other investigators have demonstrated that, following its acute administration, ouabain increased basal NO release but did not affect bradykinin-stimulated NO release in rat carotid arteries (Xie et al., 1993).
Previous studies reported that ouabain induces prostacyclin release from bovine endothelial cells (Nagakawa et al., 1987) and enhances its production in aortic endothelial cells stimulated by ATP or bradykinin (Boeynaems & Ramboer, 1989). In addition, alterations in the synthesis of prostaglandins in hypertension have also been reported (Vanhoutte, 1996; Taddei et al., 1998). In the current work, indomethacin reduced the potency but not the maximum response to noradrenaline in mesenteric resistance arteries from ouabain-treated and untreated rats. This suggests the participation of vasoconstrictor prostanoids rather than vasodilator prostanoids in the modulation of responses mediated by α-adrenoceptors in these arteries. The effect of indomethacin was similar in both groups, suggesting that ouabain-induced hypertension is not accompanied by changes in the synthesis and release of vasoconstrictor prostanoids in mesenteric resistance arteries. Opposite results have been reported in mesenteric resistance arteries from SHR, where the participation of contractile prostanoids in α-adrenergic responses has been found to be reduced (Briones et al., 2002).
In superior mesenteric artery from untreated animals, indomethacin produced a nonparallel shift to the right, with a reduction in the maximum response in the concentration–response curves for phenylephrine. However, in segments from ouabain-treated rats, indomethacin did not alter phenylephrine responses, suggesting that ouabain treatment causes the loss of a COX-derived positive modulator for responses mediated by α-adrenoceptors. Thus, in addition to the increase in NO release mentioned above, this mechanism might account for the reduced responses to phenylephrine in the superior mesenteric artery following chronic treatment with ouabain.
It is established that EDHF induces hyperpolarization and vasodilatation through activation of K+ channels (Feletou & Vanhoutte, 1996). In addition, nanomolar concentrations of ouabain induce the release of an endothelium-derived relaxing factor that seems to open KCa channels in the rat-tail arterial bed preparation (Rossoni et al., 1999). Therefore, the effects of TEA in mesenteric resistance arteries previously treated with L-NAME plus indomethacin were analyzed. In arteries from both groups, pretreatment with L-NAME plus indomethacin produced a leftward shift in the concentration–response curve for noradrenaline; this effect was greater in segments from ouabain-treated rats, confirming the increased modulation of NO in noradrenaline responses after ouabain treatment. Subsequent administration of TEA shifted the concentration–response curves for NE further leftward in segments from untreated rats, suggesting that EDHF is released together with NO during the smooth muscle contraction produced by NE, as reported previously (Dora et al., 2000). However, TEA had no additional effect in arteries from ouabain-treated rats, suggesting that only NO mediates endothelial modulation in these arteries.
It has been described previously that the hypertensinogenic effect of chronic ouabain is associated to increased activity in sympathoexcitatory pathways and decreased activity in sympathoinhibitory pathways, and that both effects can be prevented by concomitant treatment with losartan (Zhang & Leenen, 2001). In the current work, treatment with losartan significantly reduced the arterial blood pressure in both ouabain-treated and untreated rats, abolishing differences between both groups. In spite of this, losartan treatment did not alter the following findings observed after ouabain treatment: (1) the reduced adrenergic responses in superior mesenteric artery and the unaltered response in mesenteric resistance artery; (2) the large NO participation and the impairment of the COX-dependent release of vasoconstrictor prostanoids in response to phenylephrine observed in the superior mesenteric artery; and (3) the increased modulatory effect of NO and the reduced effect of EDHF in the mesenteric resistance artery. These results allow us to suggest that the peripheral vascular alterations observed after chronic ouabain treatment are not a consequence of the elevated arterial blood pressure or the activation of the renin–angiotensin system, but are probably related to the high circulating levels of ouabain. In agreement, Cargnelli et al. (2000) demonstrated that chronic ouabain treatment reduced the vasoconstrictor response induced by phenylephrine in rat aorta, an effect that was independent of elevated blood pressure. On the other hand, other authors have found that chronic treatment with other cardiac glycoside such as digoxin does not raise the blood pressure (Manunta et al., 2000) but normalizes the ouabain-dependent hypertension (Huang et al., 1999; Manunta et al., 2000). In addition, ouabain treatment, but not digoxin treatment, induced changes in renal vascular reactivity (Kimura et al., 2000). These results suggest that the induction of hypertension by ouabain in rats may be independent of Na pump inhibition. Thus, new binding sites for ouabain in adrenocortical cells have been described (Ward et al., 2002), that could be involved in the vascular changes observed in this hypertension model.
The results presented here show that, in superior mesenteric artery, ouabain treatment reduces the vasoconstrictor response to phenylephrine; this may be explained by an increase in the NO pathway and an impairment of the release of contractile prostanoids. In mesenteric resistance arteries, a greater modulatory effect of endothelial NO and an impairment of EDHF participation would help to explain the unaltered noradrenaline-induced contraction after ouabain treatment. Differences in the endothelial modulation of α-adrenergic responses would explain the effect of ouabain treatment in both arteries. In this sense, differences in the participation of endothelial factors in endothelium-dependent relaxation in resistance and conductance arteries have been described (Freitas et al., 2003). The different role of conductance and resistance arteries in the development and/or maintenance of ouabain-induced hypertension would be also implicated. In any case, these results highlight that extrapolations from conductance to resistance vessels may not be appropriate when hypertension is studied. Although losartan prevented the development of ouabain-induced hypertension, the above-noted vascular alterations were still observed. In conclusion, chronic ouabain treatment produces different effects on the vascular reactivity induced by α-adrenoceptor activation in rat conductance and resistance mesenteric arteries, and these effects are independent of the elevated arterial blood pressure or the renin–angiotensin system.
Acknowledgments
We are grateful to Dr M.C. Fernández-Criado for the care of animals, Miss Patricia Alonso for her technical assistance and Professor Louis A. Barker for his suggestions in the preparation of the article. This work was supported by DGICYT (SAF 2003-00633 and BFI 2001-1324) and Banco Santander Central Hispano. Fabiano E. Xavier was supported by CAPES (PDEE-BEX0339/02-4, Brazil) and by FISS (C03/01, Spain), Luciana V. Rossoni and Dalton V. Vassallo were supported by CNPq/Brazil.
Abbreviations
- ACh
acetylcholine
- COX
cyclooxygenase
- dAUC
difference of area under the concentration–response curves
- EDHF
endothelium-derived hyperpolarizing factor
- EDTA
ethylenediamine-tetraacetic acid
- Emax
maximum response
- eNOS
endothelial nitric oxide synthase
- KHS
Krebs–Henseleit solution
- MRA
mesenteric resistance arteries
- L-NAME
N-nitro-L-arginine methyl ester
- NO
nitric oxide
- pD2
negative logarithm of concentrations producing 50% of maximum response
- SBP
systolic blood pressure
- SMA
superior mesenteric arteries
- TEA
tetraethylammonium
References
- BOEYNAEMS J.M., RAMBOER I. Effects of changes in extra- and intracellular K+ on the endothelial production of prostacyclin. Br. J. Pharmacol. 1989;98:966–972. doi: 10.1111/j.1476-5381.1989.tb14627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIONES A., ALONSO M.J., HERNANZ R., TOVAR S., VILA E., SALAICES M. Hypertension alters the participation of contractile prostanoids products and superoxide anion in lipopolysaccharide effects on small mesenteric arteries. Life Sci. 2002;71:1997–2014. doi: 10.1016/s0024-3205(02)01967-7. [DOI] [PubMed] [Google Scholar]
- CARGNELLI G., TREVISI L., DEBETTO P., LUCIANI S., BOVA S. Effect of long-term ouabain treatment on contractile responses of rat aortae. J. Cardiovasc. Pharmacol. 2000;35:538–542. doi: 10.1097/00005344-200004000-00004. [DOI] [PubMed] [Google Scholar]
- DI FILIPPO C., FILIPPELLI A., RINALDI B., PIEGARI E., ESPOSITO F., ROSSI F., D'AMICO M. Chronic peripheral ouabain treatment affects the brain endothelin system of rats. J. Hypertens. 2003;21:747–753. doi: 10.1097/00004872-200304000-00018. [DOI] [PubMed] [Google Scholar]
- DORA K.A., HINTON J.M., WALKER S.D., GARLAND C.J. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br. J. Pharmacol. 2000;129:381–387. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor. Clin. Exp. Pharmacol. Physiol. 1996;23:1082–1090. doi: 10.1111/j.1440-1681.1996.tb01174.x. [DOI] [PubMed] [Google Scholar]
- FREITAS M.R., SCHOTT C., CORRIU C., SASSARD J., STOCLET J.C., ANDRIANTSITOHAINA R. Heterogeneity of endothelium-dependent vasorelaxation in conductance and resistance arteries from Lyon normotensive and hypertensive rats. J. Hypertens. 2003;21:1505–1512. doi: 10.1097/00004872-200308000-00014. [DOI] [PubMed] [Google Scholar]
- HAMLYN J.M., HAMILTON B.P., MANUNTA P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. J. Hypertens. 1996;14:151–171. doi: 10.1097/00004872-199602000-00002. [DOI] [PubMed] [Google Scholar]
- HAMLYN J.M., RINGEL R., SCHAEFFER J., LEVINSON P.D., HAMILTON B.P., KOWEARSKI A.A., BLAUSTEIN M.P. A circulating inhibitor of (Na+, K+) ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- HAYAKAWA H., RAIJ L.The link among nitric oxide synthase activity, endothelial function, and aortic and ventricular hypertrophy in hypertension Hypertension 199729235–241.(Part 2) [DOI] [PubMed] [Google Scholar]
- HUANG B.S., HUANG X., HARMSEN E., LEENEN F.H.H. Chronic central versus peripheral ouabain, blood pressure, and sympathetic activity in rats. Hypertension. 1994;23:1087–1090. doi: 10.1161/01.hyp.23.6.1087. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., LEENEN F.H.H. Brain renin–angiotensin system and ouabain-induced sympathetic hyperactivity and hypertension in Wistar rats. Hypertension. 1999;34:107–112. doi: 10.1161/01.hyp.34.1.107. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., KUDLAC M., KUMARATHASAN R., LEENEN F.H.H. Digoxin prevents ouabain and high salt intake-induced hypertension rats with sinoaortic denervation. Hypertension. 1999;34:733–738. doi: 10.1161/01.hyp.34.4.733. [DOI] [PubMed] [Google Scholar]
- KIMURA K., MANUNTA P., HAMILTON B.P., HAMLYN J.M. Different effects of in vivo ouabain and digoxin on renal artery function and blood pressure in the rat. Hypertens. Res. 2000;23:S67–S76. doi: 10.1291/hypres.23.supplement_s67. [DOI] [PubMed] [Google Scholar]
- MANUNTA P., HAMILTON B.P., HAMLYN J.M. Structure–activity relationship for the hypertensinogenic activity of ouabain: role of the sugar and lactone ring. Hypertension. 2001;37:471–477. doi: 10.1161/01.hyp.37.2.472. [DOI] [PubMed] [Google Scholar]
- MANUNTA P., HAMILTON B.P., ROGOWSKI A.C., HAMILTON B.P., HAMLYN J.M. Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens. Res. 2000;23:S77–S85. doi: 10.1291/hypres.23.supplement_s77. [DOI] [PubMed] [Google Scholar]
- MANUNTA P., ROGOWSKI A.C., HAMILTON B.P., HAMLYN J.M. Ouabain-induced hypertension in the rat: relationships among plasma and tissue ouabain and blood pressure. J. Hypertens. 1994;12:549–560. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAGAKAWA M., TAKAMATSU H., TOYODA T., SAWADA S., TSUJI H., IJICHI H. Effect of inhibition of Na+–K+-ATPase on the prostacyclin generation of cultured human vascular endothelial cells. Life Sci. 1987;40:351–357. doi: 10.1016/0024-3205(87)90136-6. [DOI] [PubMed] [Google Scholar]
- NAVA E., NOLL G., LÜSCHER T.F. Increased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertension. Circulation. 1995;91:2310–2313. doi: 10.1161/01.cir.91.9.2310. [DOI] [PubMed] [Google Scholar]
- ROSSONI L.V., CUNHA V., FRANÇA A., VASSALLO D.V. The influence of nanomolar ouabain on vascular pressor responses is modulated by the endothelium. J. Cardiovasc. Pharmacol. 1999;34:887–892. doi: 10.1097/00005344-199912000-00017. [DOI] [PubMed] [Google Scholar]
- ROSSONI L.V., SALAICES M., MARÍN J., VASSALLO D.V., ALONSO M.J. Alterations on vascular reactivity to phenylephrine and Na+, K+-ATPase activity and expression in hypertension induced by chronic administration of ouabain. Br. J. Pharmacol. 2002a;135:771–781. doi: 10.1038/sj.bjp.0704501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSONI L.V., SALAICES M., MIGUEL M., BRIONES A.M., BARKER L.A., VASSALLO D.V., ALONSO M.J. Ouabain-induced hypertension is accompanied by increases in endothelial vasodilator factors. Am. J. Physiol. 2002b;283:H2110–H2118. doi: 10.1152/ajpheart.00454.2002. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., GHIADONI L., SALVETTI A. The role of endothelium in human hypertension. Curr. Opin. Nephrol. Hypertens. 1998;7:203–209. doi: 10.1097/00041552-199803000-00010. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelial dysfunction in hypertension. J. Hypertens. 1996;14:S83–S93. [PubMed] [Google Scholar]
- VAZIRI N.D., NI Z., OVEISI F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- WARD S.C., HAMILTON B.P., HAMLYN J.M. Novel receptors for ouabain: studies in adrenocortical cells and membranes. Hypertension. 2002;39:536–542. doi: 10.1161/hy0202.103048. [DOI] [PubMed] [Google Scholar]
- XAVIER F.E., SALAICES M., MARQUEZ-RODAS I., ALONSO M.J., ROSSONI L.V., VASSALLO D.V., BALFAGÓN G. Neurogenic nitric oxide release increases in mesenteric arteries from ouabain hypertensive rats. J. Hypertens. 2004;22:949–957. doi: 10.1097/00004872-200405000-00017. [DOI] [PubMed] [Google Scholar]
- XIE J., WANG Y., SUMMER W.R., GREENBERG S.S. Ouabain enhances basal release of nitric oxide from carotid artery. Am. J. Med. Sci. 1993;305:157–163. doi: 10.1097/00000441-199303000-00005. [DOI] [PubMed] [Google Scholar]
- ZHANG J., LEENEN F.H.H. AT(1) receptor blockers prevent sympathetic hyperactivity and hypertension by chronic ouabain and hypertonic saline. Am. J. Physiol. 2001;280:H1318–H1323. doi: 10.1152/ajpheart.2001.280.3.H1318. [DOI] [PubMed] [Google Scholar]