Abstract
Sphingosine 1-phosphate (S1P), a bioactive lipid, signals through cell surface receptors to induce vasoconstriction and activate endothelial nitric oxide synthase (eNOS), suggesting a role for S1P in vascular tone modulation.
Using a model of aging in female rats, we investigated the vasoactivity of S1P and the roles of eNOS and estrogen replacement in modulation of that vasoactivity.
Mesenteric arteries from aged female rats were significantly more sensitive to S1P-induced vasoconstriction than arteries from young female rats, and reached greater maximum constriction (58.2±2.98 vs 34.8±4.44%; P<0.005). Modulation of this vasoconstriction by pretreating vessels with the NOS inhibitor L-NAME occurred only in young vessels.
Ovariectomy reduced the maximum S1P-induced vasoconstriction observed in intact aged rats. Estrogen replacement did not appear to have an independent beneficial effect. However, estrogen replacement did restore nitric oxide modulation of S1P-induced vasoconstriction.
Expression of the S1P1 receptor, through which eNOS can be activated, was reduced in vessels from aged rats. S1P1 receptor expression was restored in vessels from the estrogen-replaced group.
S1P is a novel mediator of vascular tone through induction of both vasoconstriction and vasodilation. Reduced S1P1 receptor expression on aging vessels may explain reduced eNOS activity, which results in greater sensitivity to S1P-induced vasoconstriction. Estrogen replacement in aging female rats restores both S1P1 receptor expression and NOS activity, suggesting an important role for estrogen in this novel pathway of vascular tone modulation.
Keywords: Aging, eNOS, endothelium, estrogen, female, mesenteric arteries, ovariectomy, sphingosine 1-phosphate, S1P receptors, vascular tone
Introduction
Aging is a complex physiological process associated with endothelial dysfunction and platelet activation. The vascular endothelium undergoes morphological and functional changes that may lead to altered vascular tone and cardiovascular problems such as atherosclerosis and hypertension (Cooper et al., 1994). A balance between agents mediating vasoconstriction and vasodilation leads to the development of vascular tone. Increased vascular tone and thus hypertension occur when the balance is altered by increased vasoconstriction or decreased vasodilation. The latter occurs in part through altered endothelial nitric oxide synthase (eNOS) activity, leading to reduced availability of a potent vasodilator, nitric oxide (Amrani et al., 1996; Barton et al., 1997; Cernadas et al., 1998; Chou et al., 1998; Matz et al., 2000). The mechanisms by which these endothelial changes occur in aging are largely unknown.
Sphingosine 1-phosphate (S1P) is a bioactive lipid released from activated platelets (Yatomi et al., 1995). In addition to a specific intracellular signaling role (Spiegel et al., 1998), S1P also acts through cell surface receptors to alter many biological activities including proliferation, migration, differentiation and morphogenesis (Levade et al., 2001). S1P may also be a novel regulator of vascular tone (Siess et al., 2000; Bolz et al., 2003; Dantas et al., 2003) by inducing both arterial vasoconstriction (Bischoff et al., 2000b, 2001a, 2001b; Sugiyama et al., 2000; Tosaka et al., 2001; Salomone et al., 2003) and vasodilation by activation of eNOS and release of nitric oxide (Igarashi & Michel, 2000; Dantas et al., 2003). A reduction in S1P-mediated eNOS activation could be one mechanism by which endothelial dysfunction occurs in aging.
Extracellular S1P mediates its biological effects by binding to specific cell surface receptors originally named Endothelial Differentiation Gene (EDG) for discovery of the EDG-1 transcript in differentiated endothelial cells (Hla & Maciag, 1990). S1P binds to five distinct receptors (S1P1/EDG-1, S1P2/EDG-5, S1P3/EDG-3, S1P4/EDG-6 and S1P5/EDG-8), some of which are differentially expressed in vascular endothelial (S1P1 and S1P3) and smooth muscle cells (S1P1, S1P2 and S1P3). Each receptor interacts with a different combination of heterotrimeric G-proteins (Gi, Gq and/or G12/13) to activate intracellular signaling pathways that produce distinct cellular responses (reviewed in Kluk & Hla, 2002). Constriction of smooth muscle cells occurs primarily through S1P2 (Ohmori et al., 2003) and S1P3 (Salomone et al., 2003) by phospholipase C (PLC)/Ca2+ (Gi, Gq) and the rho/rho kinase pathways (G12/13). Strong S1P-induced eNOS activation in endothelial cells occurs primarily through S1P1 (Gi) by PI3-kinase/Akt-mediated phosphorylation (Igarashi et al., 2001) and potentially through S1P3 (Gi, Gq) by PLC-mediated Ca2+/calmodulin. Decreased expression of S1P receptors on vascular endothelium could therefore result in reduced S1P-mediated activation of eNOS, suggesting a mechanism for endothelial dysfunction in aging.
The risk of cardiovascular disease is significantly lower in cycling pre-menopausal women than men or post-menopausal women. This cardiovascular protection has been attributed to the beneficial effect of estrogen on vascular function, in part through eNOS activation in the endothelium (reviewed in Chambliss & Shaul, 2002). Estrogen modulates eNOS in two ways: genomically through putative estrogen response elements in the eNOS promoter (Venema et al., 1994) and nongenomically through estrogen receptors recently identified in caveolae to be functionally coupled to eNOS (Chambliss et al., 2000). Interestingly, the S1P1 receptor, through which eNOS can also be activated, translocates to the caveolae upon ligation by S1P (Igarashi & Michel, 2000). It is intriguing to consider the potential interactions between estrogen, S1P and S1P1 receptor expression and activation of eNOS in the endothelium.
Using an aging female rat model (Armstrong et al., 2002), we investigated the vasoactivity of S1P and the role of estrogen replacement in modulation of that vasoactivity. We hypothesized that mesenteric arteries from aged compared to young female rats are more sensitive to S1P-induced vasoconstriction as a result of impaired eNOS modulation, mediated in part through reduced endothelial S1P1 receptor expression. We further postulated that increasing the level of estrogen in aged female rats will restore eNOS modulation and reduce S1P sensitivity in aged vessels through increased endothelial S1P1 receptor expression.
Methods
Reagents
S1P (Biomol; Plymouth Meeting, PA, U.S.A.) was dissolved in HPLC-grade methanol (Sigma; Oakville, ON, Canada), aliquoted and stored at −20°C. Immediately prior to use, the methanol was evaporated under nitrogen and S1P redissolved in 0.01 N NaOH.
Animal model
Female Sprague–Dawley rats (n=37) purchased from Charles River were used at 3–4 months (Young; n=12) or aged for 11–12 months (n=25) in the University of Alberta animal facilities. Some aged rats underwent ovariectomies (n=16) to control for estrogen level variability and received either an estrogen pellet (17β-estradiol, 1.5 mg per pellet, 60-day release, Innovative Research of America; Sarasota, FL, U.S.A.; Estrogen, n=8) or a placebo pellet (Innovative Research of America; Placebo, Placebo, n=8) subcutaneously. The estrogen dose used was based on our previous studies (Davidge & Zhang, 1998). The effectiveness of estrogen replacement in the ovariectomized female rats was confirmed by a significant difference in uterine to body weight ratios (ovariectomy (Ovx)+estrogen: 2.80±0.33 mg g−1, n=8; Ovx+placebo: 1.22±0.15 mg g−1; n=8; P<0.001). At 1 month after Ovx and pellet insertion, rats were anesthesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg kg−1 body wt.) and killed by exsanguination. These animal protocols were examined and approved by the University of Alberta Animal Welfare Committee and found to follow the guidelines set out by the Canada Council on Animal Care.
Vessel preparation and arteriograph mounting
Resistance-sized mesenteric arteries located 5–10 cm distal to the pylorus were dissected free from adipose and connective tissue in ice-cold N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid)-buffered physiological saline solution (HEPES-PSS (pH 7.5): 142 mM NaCl; 4.7 mM KCl; 1.17 mM MgSO4; 1.18 mM KH2PO4; 1.56 mM CaCl2; 10 mM HEPES; 5.5 mM glucose). The proximal end of each artery was mounted and tied to a glass cannula, which was attached to a pressure transducer and connected to a servo-controlled peristaltic pump to alter intraluminal pressures in a dual-chamber arteriograph (Living Systems Instrumentation, Burlington, VT, U.S.A.). Residual blood was then gently flushed from the vessel lumen with HEPES-PSS and the distal end of the vessel was mounted to a second cannula, which was occluded to prevent flow. After mounting, the temperature of the 2.5-ml HEPES-PSS baths was increased and maintained at 37°C. Arterial lumen diameters were measured using a digital filar eyepiece (Lasico, CA, U.S.A.) on a compound microscope as previously described (Halpern et al., 1984).
Experimental protocol for vascular function
All vessels were equilibrated for 1 h at 37°C at an intraluminal pressure of 60 mmHg with bath changes every 10 min. To provide a carrier for S1P, the vessel bath contained 0.1% fatty acid free bovine serum albumin (BSA; Sigma) in HEPES-PSS. For each animal, two vessels of similar diameter were mounted: one vessel was preincubated with 100 μM of NG-nitro-L-arginine methyl ester (L-NAME; Calbiochem, La Jolla, CA, U.S.A.) for 15 min and then both vessels underwent concentration–response curves to S1P. Each concentration was incubated for 5 min before measurement of the lumen diameter. There were no significant differences in lumen diameters at 60 mmHg with and without drug or between treatment groups (data not shown). Percent constriction was calculated as D1−D2 divided by D1 × 100, where D1 is the arterial lumen diameter prior to drug addition and D2 is the arterial lumen diameter after drug addition. To assess passive unpressurized lumen diameter, vessels were incubated for 10 min in EGTA-Ca free-PSS (142 mM NaCl; 4.7 mM KCl; 1.17 mM MgSO4; 1.18 mM KH2PO4; 10 mM HEPES; 2 mM EGTA) and papaverine (Sigma; 0.1 mM).
Immunofluorescence
Sections of rat mesentery were embedded in Optimal Cutting Temperature (OCT) Compound (Tissue-Tek), snap-frozen in liquid nitrogen, and stored at −80°C. The frozen sections were cut on a cryostat in consecutive 8–10 μm sections, mounted on glass slides and fixed in acetone for 10 min. Slides were stored at −80°C prior to use.
Slides were thawed at room temperature for 1 h prior to use, then fixed in ice-cold acetone for 10 min at −20°C and allowed to air dry. Each section was enclosed in a circle using a PAP pen, followed by three 5-min washes in phosphate-buffered saline (PBS). The sections were blocked using 100 μl of 2% BSA in PBS (blocking buffer) for 1 h at room temperature. Following removal of the blocking buffer, 50 μl of the primary antibody diluted in blocking buffer was incubated overnight at 4°C. The primary antibodies used were specific for the S1P1 receptor (EDG-1, goat polyclonal, 1 : 50; Santa Cruz Biotechnology, CA, U.S.A.), von Willebrand factor (rabbit polyclonal, 1 : 200; Sigma) and α actin (mouse monoclonal, 1 : 200; Boehringer Mannheim, Germany). After three 5-min washes in PBS, the appropriate Alexa Fluor-488 tagged secondary antibody (donkey anti-goat, goat anti-rabbit or goat anti-mouse, each at 1 : 200; Molecular Probes, Oregon, U.S.A.) was incubated for 30 min. Control sections were incubated with 2% BSA, followed by the secondary antibody. After extensive washing (three 10-min washes in PBS), slides were mounted with 60 μl of Vectashield H-1200 mounting solution with DAPI (2 : 1 solution; Vector Laboratories Inc) to visualize nuclei. Slides were stored in the dark at 4°C. Stained sections were examined immediately under an Olympus IX81 fluorescent microscope (Carson Scientific Imaging Group; Ontario, Canada) using Slidebook 2D, 3D Timelapse Imaging Software (Intelligent Imaging Innovations Inc.; CO, U.S.A.).
Western blot analysis
Arteries were dissected from the entire mesenteric arcade and sonicated in homogenization buffer (2% SDS, 100 mmol l−1 dithiothreitol and 60 mmol l−1 Tris, pH 6.8). at 4°C. Protein content was analyzed using the Bradford protein assay. Each sample containing 10 μg of protein was diluted in 4 × SDS gel loading buffer (100 mmol l−1 Tris–HCl, pH 6.8, 200 mmol l−1 dithiothreitol, 4% SDS, 0.2% bromophenol blue and 20% glycerol). The samples were heated at 100°C for 5 min, run on a 9% polyacrylamide gel and electrotransferred to nitrocellulose at 4°C. Membranes were then blocked in 5% nonfat dry milk in PBS for 3 h, washed with PBS–Tween (0.1%) and incubated with the S1P1 receptor antibody (1 : 1000 rabbit polyclonal; Biomol, PA, U.S.A.) or the α actin antibody (1 : 1000 goat polyclonal, Santa Cruz) for 3 h at room temperature. After washing with PBS-Tween (0.1%), the secondary goat anti-rabbit or donkey anti-goat antibody (1 : 4000; Santa Cruz) was added to the membrane for 1 h at room temperature. Following washing, enzyme-linked chemiluminescence detection was carried out according to the manufacturer's instructions (Amersham). Imaging was conducted using a Fluor-S MultiImager (BioRad).
Statistics
Entire curves were compared using a two-way ANOVA and the Bonferroni post hoc test. Maximum constriction to S1P (mean±s.e.m.) was compared among groups using a one-way ANOVA with the Tukey post hoc test or one-way ANOVA on ranks using Dunn's method of multiple comparisons where appropriate. Two groups were compared where appropriate using a Student's t-test. Statistical significance was accepted at P<0.05.
Results
S1P-induced vasoconstriction in mesenteric arteries from young and intact aged female rats
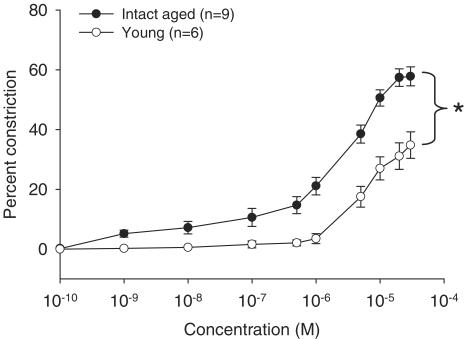
Resistance-sized arteries dissected from the mesenteric arcade of intact aged or young female rats were mounted and pressurized on a pressure myograph system. Using increasing S1P concentrations (0.1 nM–30 μM), we compared S1P-induced vasoconstriction in arteries matched for passive unpressurized diameter (intact aged: 201±12.7 μm (n=9) vs young: 217±5.92 μm (n=6)). Arteries obtained from intact aged females were significantly more sensitive to S1P-induced vasoconstriction than arteries from young females (Figure 1). As well, the maximum constriction observed was significantly higher in vessels from aged compared to young females (Figure 1).
Figure 1.
Vasoconstriction to S1P in mesenteric arteries from intact aged vs young female rats. Mesenteric arteries from intact aged or young female rats were assessed for constriction in response to increasing concentrations of S1P. Data from intact aged (n=9) and young (n=6) animals are summarized and expressed as the mean±s.e.m. percent decrease in lumen diameter at each S1P concentration compared to arteries in the absence of S1P. Passive unpressurized vessel diameters (μm) in each group were similar (intact aged: 201±12.7 and young: 217±5.92). A significant difference between the curves as measured by ANOVA is denoted by an asterisk (P<0.05).
NOS modulation of S1P-induced vasoconstriction in mesenteric arteries from intact aged and young female rats
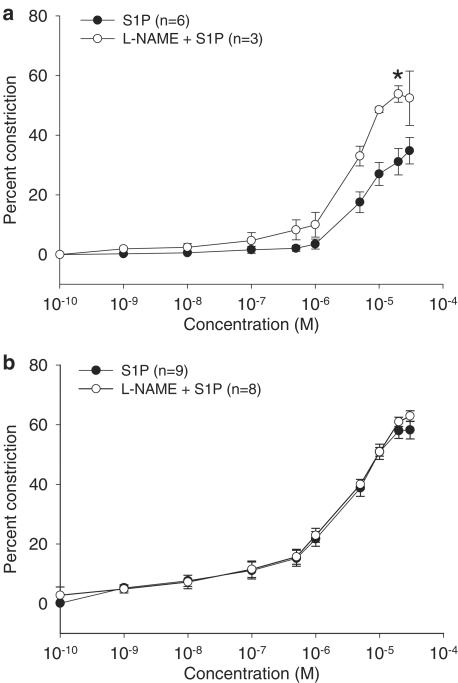
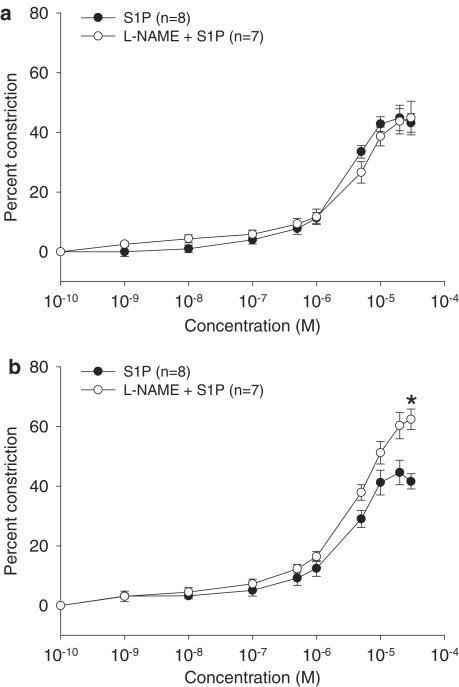
Arterial vasoconstriction can be modified by the vasodilator nitric oxide, the availability of which may be reduced in aging (Amrani et al., 1996; Barton et al., 1997; Cernadas et al., 1998; Chou et al., 1998; Matz et al., 2000) through a number of mechanisms including reduced eNOS activity. Since S1P can increase eNOS activity (Dantas et al., 2003; Igarashi et al., 2001), we investigated whether S1P-induced vasoconstriction in vessels from intact aged and young rats was modulated by nitric oxide by pretreating vessels with L-NAME, a NOS inhibitor. Vasoconstriction in arteries from young (Figure 2a) but not intact (Figure 2b) aged female rats was enhanced by pretreatment with L-NAME, suggesting that modulation of S1P-induced vasoconstriction by NOS activity occurs in vessels from young female rats, but is lost in vessels from intact aged female rats.
Figure 2.
Effect of L-NAME on S1P-induced vasoconstriction in intact aged and young female rats. S1P-induced vasoconstriction described in Figure 1 was also examined in the presence of L-NAME in mesenteric arteries from intact aged rats (S1P: 201±12.7 μm, n=9; L-NAME+S1P: 202±16.1 μm, n=8) and size-matched vessels from young female rats (S1P: 217±5.92 μm, n=6; L-NAME+S1P: 202±15.3 μm, n=3). Data are presented as the mean±s.e.m. percent decrease in lumen diameter compared to arteries in the absence of drugs. The L-NAME curves are presented with their corresponding curves from Figure 1 for: (a) young and (b) intact aged female rats. A significant difference in maximum constriction is denoted by an asterisk (P<0.05).
S1P-induced vasoconstriction vs vessel size
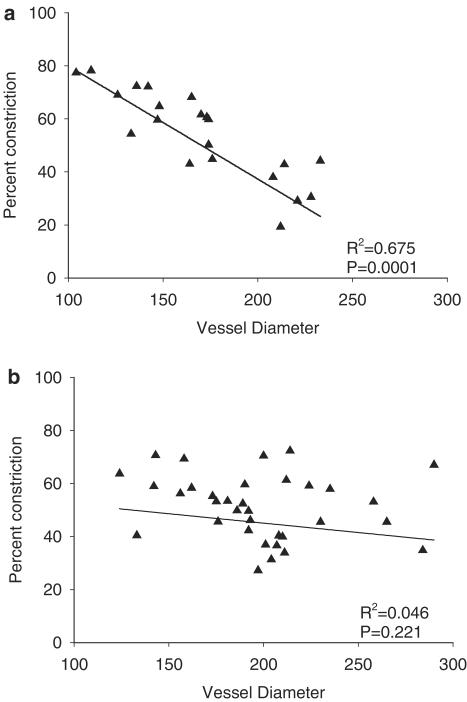
Interestingly, if maximum constriction to S1P is plotted against passive unpressurized lumen diameter in all vessels tested, a negative correlation was found in mesenteric arteries from young (Figure 3a) but not aged rats (Figure 3b). Thus, the smaller the vessel the greater the maximum constriction to S1P. We also found that although S1P-induced vasoconstriction in larger vessels (202±15.3 μm) from young female rats could be modulated by NOS activity (Figure 2a), there was no such modulation in smaller vessels (133±11.0 μm; data not shown).
Figure 3.
The relationship of S1P-induced vasoconstriction to arterial lumen diameter. The maximum percent constriction to S1P was plotted against the passive unpressurized arterial lumen diameter (μm) in vessels from (a) young rats or (b) aged rats regardless of treatment. Linear regression lines were calculated using SigmaPlot software to obtain R2 and P-values.
Role of estrogen in S1P-induced vasoconstriction
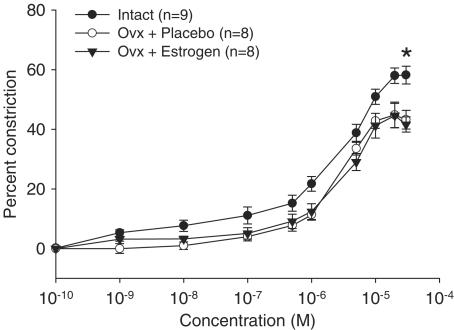
Since estrogen is reduced in aging females and plays a role in eNOS activation (Chambliss & Shaul, 2002), we investigated whether estrogen affects S1P-induced vasoconstriction. Aged female rats were ovariectomized (Ovx) to remove residual estrogen and a pellet containing either 17β-estradiol or a placebo was inserted subcutaneously. At 1 month after treatment, the rats were killed and the mesentery dissected. The response of mesenteric arteries to S1P was then compared in all aged groups (Figure 4). While Ovx itself in aged females significantly reduced maximum constriction to S1P when compared to intact aged females, this did not appear to be an estrogen-specific effect (intact aged: 58.2±2.98%; Ovx+placebo: 44.8±4.27%; Ovx+estrogen: 44.6±4.09%; P<0.05).
Figure 4.
Effect of ovary removal and estrogen replacement on S1P-induced vasoconstriction in mesenteric arteries from aged female rats. S1P-induced vasoconstriction was compared in mesenteric arteries from intact aged (intact; n=9) from Figure 1, aged ovariectomized placebo-treated (Ovx+placebo; n=8) or aged ovariectomized estrogen-replaced (Ovx+estrogen; n=8) female rats. Data are summarized and expressed as the mean±s.e.m. percent decrease in lumen diameter at each S1P concentration compared to vessels in the absence of S1P. Passive unpressurized vessel diameters (μm) in each group were similar (intact: 201±12.7, Ovx+placebo: 196±10.3 and Ovx+estrogen: 212±15.2). A significant difference in maximum constriction is denoted by an asterisk (P<0.05).
NOS modulation of S1P-induced vasoconstriction in mesenteric arteries from Ovx female rats
We next determined NOS modulation to S1P-induced vasoconstriction in the Ovx groups. L-NAME did not alter maximum constriction to S1P in vessels from the aged placebo-treated Ovx group (Figure 5a; 44.8±5.62 vs 44.8±4.27%) similar to the results found in the intact aged group (Figure 2a; 62.9±1.82 vs 58.2±2.98%). However, maximum constriction to S1P was significantly increased in L-NAME-treated compared to non-treated vessels from the aged Ovx estrogen-replaced rats (Figure 5b; 62.4±3.43 vs 44.6±4.09%; P<0.05) similar to that found in the young group (Figure 2a). These results suggest that estrogen replacement restored NOS activity in aged female rats.
Figure 5.
Effect of L-NAME on S1P-induced vasoconstriction in aged ovariectomized placebo and estrogen-replaced female rats. S1P-induced vasoconstriction described in Figure 4 in the two Ovx groups was also measured in the presence of L-NAME. Data are presented as the mean±s.e.m. percent decrease in lumen diameter compared to vessels in the absence of drugs. The L-NAME curves are presented with their corresponding curves from Figure 4: (a) Ovx+placebo and (b) Ovx+estrogen. Passive unpressurized lumen diameters (μm) in L-NAME-treated vessels (Ovx+placebo: 196±4.36 and Ovx+estrogen: 214±16.0) did not differ from vessels treated with S1P alone (see Figure 4). A significant difference in maximum constriction is denoted by asterisk (P<0.05).
S1P1 receptor expression in female rat mesentery
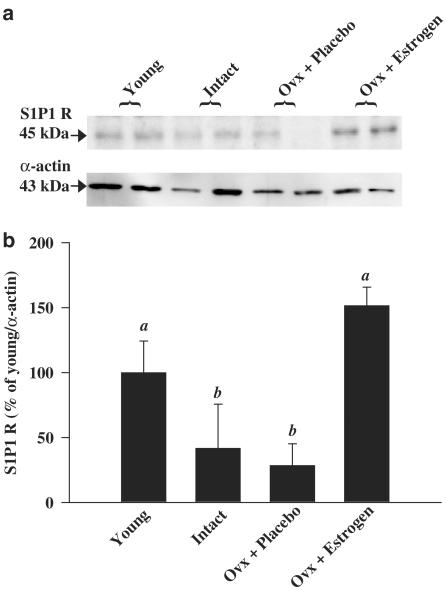
S1P induces eNOS activation by signals transmitted through the S1P1 receptor (Igarashi et al., 2001). Changes in eNOS activation could therefore be mediated by altered S1P1 receptor expression. Arteries from the entire mesenteric arcade were dissected, washed and processed for Western blot analysis. S1P1 receptor protein expression in mesenteric arteries was reduced in aged compared to young rats and restored to young levels in Ovx estrogen-replaced but not placebo-treated rats (Figure 6).
Figure 6.
S1P1 receptor expression in all animal groups by Western blot analysis. (a) Representative gel for S1P1 receptor expression. (b) Summary of S1P1 receptor expression in each experimental group (n=4) expressed as percent change from the young group. Bar graphs represent mean±s.e.m. Bars with different letters are significantly different at P<0.05.
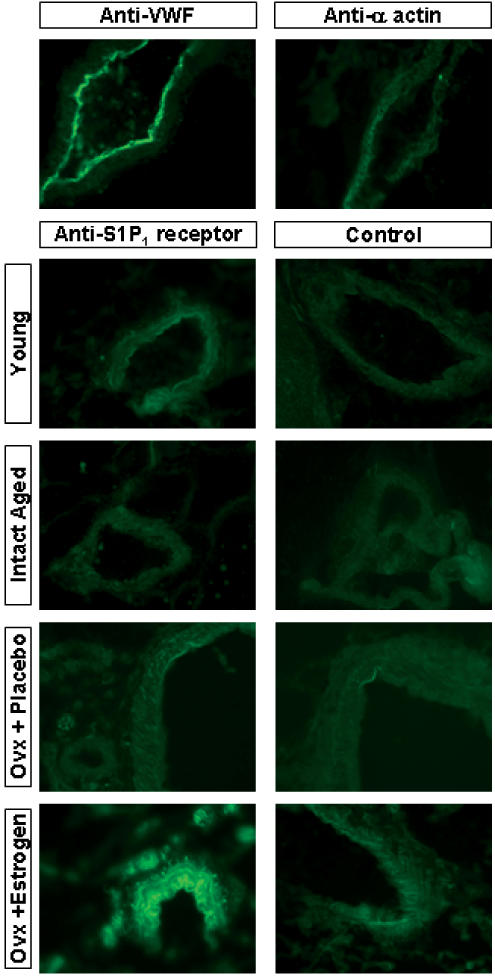
Western analysis included both endothelial and smooth muscle cells and both cell types express the S1P1 receptor. To determine if the receptor changes observed by Western analysis were specific for endothelial or smooth muscle cells, we compared S1P1 receptor expression on vessels in frozen sections of mesentery from all four animal groups by immunofluorescence (Figure 7). To confirm the presence of intact endothelium and to demonstrate smooth muscle-specific staining, mesenteric sections were stained for von Willebrand factor and α-actin, respectively. The S1P1 receptor is localized to both the vascular endothelium and smooth muscle cells of vessels from young female rats. The expression of S1P1 receptor on both cell types is reduced in vessels from intact aged female rats. S1P1 receptor expression is restored on both endothelial and smooth muscle cells in the Ovx estrogen-replaced group.
Figure 7.
S1P1 receptor expression in all animal groups by immunofluorescence. Thin sections of mesentery (8–10 μm) were assessed by immunofluorescence for expression of von Willebrand factor (VWF), α actin, S1P1 receptor or a no primary antibody control. Tissues were obtained from young, intact aged. Ovx + Placebo aged or Ovx + Estrogen aged female rats.
Discussion
The focus of this study was to compare the vascular reactivity of the bioactive lipid, S1P, in mesenteric arteries obtained from young and aged female rats. Arteries from the mesenteric bed were chosen because they contribute to the overall peripheral resistance (Christensen & Mulvany, 1993). Our data demonstrate greater sensitivity to S1P-induced vasoconstriction in vessels from aged compared to young female rats. This response to S1P was modulated by NOS activity in vessels from young but not intact aged females. We also show that while Ovx reduces the maximum constriction observed in vessels from aged female rats, only Ovx rats treated with estrogen appear to have restored NOS modulation of S1P-induced vasoconstriction. Furthermore, the expression of S1P1 receptors, through which eNOS can be activated, is reduced in the endothelium of aged compared to young female vessels and appears to be restored by estrogen treatment of Ovx aged rats.
Extracellular S1P interacts with specific cell surface receptors to effect numerous cellular responses (Spiegel et al., 2002), including vasoconstriction in canine basilar arteries (Tosaka et al., 2001), canine coronary arteries (Sugiyama et al., 2000), rat cerebral arteries (Coussin et al., 2002) and rat renal and mesenteric arteries (Bischoff et al., 2000a, 2000b). Vascular reactivity to S1P varies depending on the vascular bed, with cerebral arteries more sensitive than the aorta (Coussin et al., 2002) and renal vasculature more sensitive than the mesentery (Bischoff et al., 2000b). At lower doses, S1P also induces transient vasodilation in mesenteric arteries preconstricted with norepinephrine (Dantas et al., 2003). We show for the first time that vascular reactivity to S1P in the mesentery is also dependent on the age of the animal from which the vessel is taken and also dependent on vessel size in young animals.
The concentration of S1P at which vasoconstriction in mesenteric arteries from young female rats was first detected is at levels slightly higher than reported in normal plasma (0.2–0.5 μM; Yatomi et al., 2001). This suggests that vascular constriction will occur only in circumstances where circulating or localized levels of S1P are elevated. These results correlate well with those found by Dantas et al. (2003) in which S1P induces vasodilation with an EC50 of 10 nM, well within the physiological range. Thus, in normal young rats, the overall response at normal circulating S1P concentrations is likely vasodilation. In situations where local or systemic S1P concentrations are increased, perhaps as a result of platelet activation, the overall response may be vasoconstriction. These opposing functions, which are dependent on S1P concentration, support a role for this lipid in modulation of vascular tone.
In aging female mesenteric arteries, however, S1P-induced vasoconstriction begins at a 100-fold lower concentration than in vessels from young rats, which is within the normal range found in plasma. In addition, aging is also associated with increased platelet activation (Todd et al., 1994; Gleerup & Winther, 1995), which in turn could lead to elevated S1P levels where we observe significantly enhanced vasoconstriction compared to that found in vessels from young rats. S1P could therefore potentially be a mediator of vasoconstriction in aging.
The enhanced sensitivity to S1P-induced vasoconstriction in vessels from aged rats could be explained by increased vasoconstrictor release, reduced vasodilator production or through altered S1P receptor expression on endothelial and/or smooth muscle cells. With respect to increased vasoconstrictor release, we have previously shown an increase in prostaglandin H synthase (PGHS)-mediated vasoconstriction in aging female rats (Davidge & Zhang, 1998) that can be reduced by estrogen replacement (Armstrong et al., 2002). Although S1P induces PGHS-2 production (Davaille et al., 2000; Kim et al., 2003; Pettus et al., 2003), evidence supporting a role for this lipid in increased release of vasoconstrictors through the PGHS pathway is currently unknown.
Activation of eNOS by S1P in vascular endothelial cells (Igarashi et al., 2001; Dantas et al., 2003) and the emerging importance of nitric oxide in S1P-induced angiogenesis (Rikitake et al., 2002) and protection from apoptosis (Kwon et al., 2001) suggest a role for this potent vasodilator in S1P modulation of vascular tone. We demonstrate that S1P-induced vasoconstriction in mesenteric arteries from young rats is modulated by NOS activity and that this modulation is absent in vessels from aged rats. Interestingly, we found that the size of mesenteric vessels from young but not aged rats is negatively correlated with the maximum S1P-induced vasoconstriction response. Additionally, modulation of the response by nitric oxide only occurred in large and not small vessels from young rats. These results may be explained by the observation that the smaller the vessel, the lower the expression of eNOS (Laughlin et al., 2003) and the less dependent it is on nitric oxide-mediated vasodilation (Campbell & Harder, 2001).
The loss of nitric oxide-induced modulation of S1P-induced vasoconstriction in aged vessels may be independent of the ability of S1P to activate eNOS through the S1P1 receptor. However, the findings of reduced endothelial S1P1 receptor expression along with reduced NOS modulation of S1P-induced vasoconstriction in the aged compared to young arteries suggests that S1P-induced eNOS activation is essential to balance the vasoconstriction properties of at least S1P, and that S1P operates as a novel agent in maintenance of vascular tone. An imbalance in these activities could result in hypertension. Interestingly, vasoconstriction induced by another sphingolipid, sphingosylphosphorylcholine (SPPC), was unaffected by inhibition of NOS activity in mesenteric vessels from young rats (Bischoff et al., 2000a), suggesting a specific role for S1P-induced activation of eNOS in modulation of its own vasoconstriction.
Estrogen replacement therapy in menopausal women may reduce endothelial dysfunction observed in aging (Hayashi et al., 1995; Wellman et al., 1996; Meyer et al., 1997; Zhang & Davidge, 1999; Armstrong et al., 2002). We therefore investigated the effects of estrogen replacement on S1P-induced vasoconstriction in the aging female rat. We controlled for residual estrogen levels in aged animals by performing Ovx followed by implantation of pellets containing either 17β-estradiol or a placebo. We found that Ovx itself significantly reduced the maximum constriction to S1P observed in intact aged females. The agent(s) in the aging ovary or produced through the hypothalamic pituitary axis, which is dependent on feedback from the ovary, that is responsible for enhanced sensitivity to S1P-induced vasoconstriction is currently unknown. Interestingly, while estrogen replacement does not appear to affect S1P vasoactivity independently of Ovx, estrogen treatment does restore the loss of nitric oxide modulation observed in vessels from aged female rats, suggesting the importance of estrogen to the function of eNOS in the context of S1P-mediated vasoconstriction.
Estrogen receptors, eNOS and S1P1 receptors have all been localized to plasma membrane caveolae (Igarashi & Michel, 2000; Chambliss & Shaul, 2002). Estrogen increases eNOS activity through nongenomic mechanisms via estrogen receptors that are functionally coupled to eNOS and located in caveolae (Chambliss et al., 2000). The S1P1 receptor which has also been shown to activate eNOS has also recently been shown to relocate to the caveolae upon stimulation with S1P (Igarashi & Michel, 2000). S1P1 receptor expression is known to increase in endothelial cells in response to differentiation (Hla & Maciag, 1990), fluid shear stress (Takada et al., 1997) and treatment with tumour necrosis factor α and vascular endothelial growth factor (Osada et al., 2002; Igarashi et al., 2003). It is intriguing to speculate a role for estrogen in maintaining S1P1 receptor expression through which eNOS can be activated. We show that S1P1 receptor expression on the endothelium of vessels isolated from estrogen-replaced Ovx aged female rats is elevated compared to vessels from intact or placebo-treated Ovx aged female rats. This expression appears to reach and even exceed that found on the endothelium of young female rat vessels. We are currently investigating whether estrogen can directly alter S1P receptor expression in endothelial cells. Interestingly, estradiol has also been reported to reduce platelet aggregation (Chen et al., 2001; Haque et al., 2001), which may therefore reduce plasma S1P levels, providing a further mechanism of protection from S1P-induced vasoconstriction.
In summary, we demonstrate that mesenteric vessels from aged rats are more sensitive to vasoconstriction induced by S1P, a bioactive lipid released from activated platelets. This enhanced sensitivity may be both dependent and independent of altered nitric oxide modulation, with the former potentially as a result of decreased S1P1 receptor expression. We show a role for estrogen replacement in restoring both receptor expression and nitric oxide modulation. These results and those of others (Siess et al., 2000; Bolz et al., 2003; Dantas et al., 2003), in conjunction with work demonstrating the vasodilatory properties of this lipid (Igarashi & Michel, 2000; Dantas et al., 2003), confirm that S1P is involved in development and maintenance of vascular tone. Changes to the balance of either S1P-induced vasoconstriction or vasodilation may result in pathologies including hypertension. Further examination of the role of this lipid in vascular function, particularly with respect to receptor expression and intracellular signaling pathways, will lead to targeted therapeutic interventions for hypertension in aging and hypertensive diseases such as pre-eclampsia.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR). D.G. Hemmings is a post-doctoral fellow supported jointly by Heart and Stroke Foundation of Canada and CIHR. S.T. Davidge is a Canada Research Chair in Women's Cardiovascular Health and a Senior Scholar of the Alberta Heritage Foundation for Medical Research. We thank Stephen Armstrong and Amy Ellett for their assistance.
Abbreviations
- EDG
endothelial differentiation gene
- eNOS
endothelial nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- Ovx
ovariectomy
- PGHS
prostaglandin H synthase
- S1P
sphingosine 1-phosphate
- S1P1 receptor
sphingosine 1-phosphate 1 receptor
References
- AMRANI M., GOODWIN A.T., GRAY C.C., YACOUB M.H. Ageing is associated with reduced basal and stimulated release of nitric oxide by the coronary endothelium. Acta Physiol. Scand. 1996;157:79–84. doi: 10.1046/j.1365-201X.1996.451171000.x. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG S.J., ZHANG Y., STEWART K.G., DAVIDGE S.T. Estrogen replacement reduces PGHS-2-dependent vasoconstriction in the aged rat. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H893–H898. doi: 10.1152/ajpheart.00148.2002. [DOI] [PubMed] [Google Scholar]
- BARTON M., COSENTINO F., BRANDES R.P., MOREAU P., SHAW S., LUSCHER T.F. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., FETSCHER C., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000a;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br. J. Pharmacol. 2000b;130:1878–1883. doi: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., FINGER J., MICHEL M.C. Nifedipine inhibits sphinogosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;364:179–182. doi: 10.1007/s002100100446. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetized rats. Br. J. Pharmacol. 2001b;132:1925–1933. doi: 10.1038/sj.bjp.0703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLZ S.S., VOGEL L., SOLLINGER D., DERWAND R., BOER C., PITSON S.M., SPIEGEL S., POHL U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- CAMPBELL W.B., HARDER D.R. Prologue: EDHF – what is it. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2413–H2416. doi: 10.1152/ajpheart.2001.280.6.H2413. [DOI] [PubMed] [Google Scholar]
- CERNADAS M.R., SANCHEZ DE MIGUEL L., GARCIA-DURAN M., GONZALEZ-FERNANDEZ F., MILLAS I., MONTON M., RODRIGO J., RICO L., FERNANDEZ P., DE FRUTOS T., RODRIGUEZ-FEO J.A., GUERRA J., CARAMELO C., CASADO S., LOPEZ F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ. Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- CHAMBLISS K.L., SHAUL P.W. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- CHAMBLISS K.L., YUHANNA I.S., MINEO C., LIU P., GERMAN Z., SHERMAN T.S., MENDELSOHN M.E., ANDERSON R.G., SHAUL P.W. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- CHEN F.P., LEE N., SOONG Y.K., HUANG K.E. Short- and long-term effects of hormone replacement therapy on cardiovascular risk factors in postmenopausal women. Chang Gung Med. J. 2001;24:431–439. [PubMed] [Google Scholar]
- CHOU T.C., YEN M.H., LI C.Y., DING Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN K.L., MULVANY M.J. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J. Vasc. Res. 1993;30:73–79. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- COOPER L.T., COOKE J.P., DZAU V.J. The vasculopathy of aging. J. Gerontol. 1994;49:B191–B196. doi: 10.1093/geronj/49.5.b191. [DOI] [PubMed] [Google Scholar]
- COUSSIN F., SCOTT R.H., WISE A., NIXON G.F. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ. Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- DANTAS A.P., IGARASHI J., MICHEL T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- DAVAILLE J., GALLOIS C., HABIB A., LI L., MALLAT A., TAO J., LEVADE T., LOTERSZTAJN S. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. J. Biol. Chem. 2000;275:34628–34633. doi: 10.1074/jbc.M006393200. [DOI] [PubMed] [Google Scholar]
- DAVIDGE S.T., ZHANG Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ. Res. 1998;83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- GLEERUP G., WINTHER K. The effect of ageing on platelet function and fibrinolytic activity. Angiology. 1995;46:715–718. doi: 10.1177/000331979504600810. [DOI] [PubMed] [Google Scholar]
- HALPERN W., OSOL G., COY G.S. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann. Biomed. Eng. 1984;12:463–479. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- HAQUE S.F., MATSUBAYASHI H., IZUMI S., SUGI T., ARAI T., KONDO A., MAKINO T. Sex difference in platelet aggregation detected by new aggregometry using light scattering. Endocr. J. 2001;48:33–41. doi: 10.1507/endocrj.48.33. [DOI] [PubMed] [Google Scholar]
- HAYASHI T., YAMADA K., ESAKI T., KUZUYA M., SATAKE S., ISHIKAWA T., HIDAKA H., IGUCHI A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- HLA T., MACIAG T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- IGARASHI J., BERNIER S.G., MICHEL T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- IGARASHI J., ERWIN P.A., DANTAS A.P., CHEN H., MICHEL T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGARASHI J., MICHEL T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J. Biol. Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- KIM J.I., JO E.J., LEE H.Y., CHA M.S., MIN J.K., CHOI C.H., LEE Y.M., CHOI Y.A., BAEK S.H., RYU S.H., LEE K.S., KWAK J.Y., BAE Y.S. Sphingosine 1-phosphate in amniotic fluid modulates cyclooxygenase-2 expression in human amnion-derived WISH cells. J. Biol. Chem. 2003;278:31731–31736. doi: 10.1074/jbc.M300625200. [DOI] [PubMed] [Google Scholar]
- KLUK M.J., HLA T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- KWON Y.G., MIN J.K., KIM K.M., LEE D.J., BILLIAR T.R., KIM Y.M. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J. Biol. Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- LAUGHLIN M.H., TURK J.R., SCHRAGE W.G., WOODMAN C.R., PRICE E.M. Influence of coronary artery diameter on eNOS protein content. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1307–H1312. doi: 10.1152/ajpheart.00792.2002. [DOI] [PubMed] [Google Scholar]
- LEVADE T., AUGE N., VELDMAN R.J., CUVILLIER O., NEGRE-SALVAYRE A., SALVAYRE R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ. Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- MATZ R.L., DE SOTOMAYOR M.A., SCHOTT C., STOCLET J.C., ANDRIANTSITOHAINA R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER M.C., CUMMINGS K., OSOL G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am. J. Physiol. 1997;272:H2264–H2270. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- OHMORI T., YATOMI Y., OSADA M., KAZAMA F., TAKAFUTA T., IKEDA H., OZAKI Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P(2) Cardiovasc. Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- OSADA M., YATOMI Y., OHMORI T., HOSOGAYA S., OZAKI Y. Modulation of sphingosine 1-phosphate/EDG signaling by tumor necrosis factor-alpha in vascular endothelial cells. Thromb. Res. 2002;108:169–174. doi: 10.1016/s0049-3848(02)00385-7. [DOI] [PubMed] [Google Scholar]
- PETTUS B.J., BIELAWSKI J., PORCELLI A.M., REAMES D.L., JOHNSON K.R., MORROW J., CHALFANT C.E., OBEID L.M., HANNUN Y.A. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- RIKITAKE Y., HIRATA K., KAWASHIMA S., OZAKI M., TAKAHASHI T., OGAWA W., INOUE N., YOKOYAMA M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- SALOMONE S., YOSHIMURA S., REUTER U., FOLEY M., THOMAS S.S., MOSKOWITZ M.A., WAEBER C. S1P(3) receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur. J. Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- SIESS W., ESSLER M., BRANDL R. Lysophosphatidic acid and sphingosine 1-phosphate: two lipid villains provoking cardiovascular diseases. IUBMB Life. 2000;49:167–171. doi: 10.1080/713803618. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., CUVILLIER O., EDSALL L.C., KOHAMA T., MENZELEEV R., OLAH Z., OLIVERA A., PIRIANOV G., THOMAS D.M., TU Z., VAN BROCKLYN J.R., WANG F. Sphingosine-1-phosphate in cell growth and cell death. Ann. N.Y. Acad. Sci. 1998;845:11–18. doi: 10.1111/j.1749-6632.1998.tb09658.x. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., ENGLISH D., MILSTIEN S. Sphingosine 1-phosphate signaling: providing cells with a sense of direction. Trends Cell Biol. 2002;12:236–242. doi: 10.1016/s0962-8924(02)02277-8. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., YATOMI Y., OZAKI Y., HASHIMOTO K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc. Res. 2000;46:119–125. doi: 10.1016/s0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- TAKADA Y., KATO C., KONDO S., KORENAGA R., ANDO J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- TODD M.K., GOLDFARB A.H., KAUFFMAN R.D., BURLESON C. Combined effects of age and exercise on thromboxane B2 and platelet activation. J. Appl. Physiol. 1994;76:1548–1552. doi: 10.1152/jappl.1994.76.4.1548. [DOI] [PubMed] [Google Scholar]
- TOSAKA M., OKAJIMA F., HASHIBA Y., SAITO N., NAGANO T., WATANABE T., KIMURA T., SASAKI T. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: possible role in pathogenesis of cerebral vasospasm. Stroke. 2001;32:2913–2919. doi: 10.1161/hs1201.099525. [DOI] [PubMed] [Google Scholar]
- VENEMA R.C., NISHIDA K., ALEXANDER R.W., HARRISON D.G., MURPHY T.J. Organization of the bovine gene encoding the endothelial nitric oxide synthase. Biochim. Biophys. Acta. 1994;1218:413–420. doi: 10.1016/0167-4781(94)90195-3. [DOI] [PubMed] [Google Scholar]
- WELLMAN G.C., BRAYDEN J.E., NELSON M.T. A proposed mechanism for the cardioprotective effect of oestrogen in women: enhanced endothelial nitric oxide release decreases coronary artery reactivity. Clin. Exp. Pharmacol. Physiol. 1996;23:260–266. doi: 10.1111/j.1440-1681.1996.tb02608.x. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., OZAKI Y., OHMORI T., IGARASHI Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., RUAN F., HAKOMORI S., IGARASHI Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- ZHANG Y., DAVIDGE S.T. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1999;34:1117–1122. doi: 10.1161/01.hyp.34.5.1117. [DOI] [PubMed] [Google Scholar]