Abstract
Cannabidiol (CBD), a nonpsychoactive marijuana constituent, was recently shown as an oral antihyperalgesic compound in a rat model of acute inflammation. We examined whether the CBD antihyperalgesic effect could be mediated by cannabinoid receptor type 1 (CB1) or cannabinoid receptor type 2 (CB2) and/or by transient receptor potential vanilloid type 1 (TRPV1). Rats received CBD (10 mg kg−1) and the selective antagonists: SR141716 (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) for CB1, SR144528 (N-[(1S)-endo-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide) for CB2 and capsazepine (CPZ) for TRPV1 receptors. The intraplantar injection of carrageenan in rats induced a time-dependent thermal hyperalgesia, which peaked at 3 h and decreased at the following times. CBD, administered 2 h after carrageenan, abolished the hyperalgesia to the thermal stimulus evaluated by plantar test. Neither SR141716 (0.5 mg kg−1) nor SR144528 (3 and 10 mg kg−1) modified the CBD-induced antihyperalgesia; CPZ partially at the lowest dose (2 mg kg−1) and fully at the highest dose (10 mg kg−1) reversed this effect. These results demonstrate that TRPV1 receptor could be a molecular target of the CBD antihyperalgesic action.
Keywords: Cannabinoid, cannabidiol, TRPV1, hyperalgesia, carrageenan
Introduction
Cannabidiol (CBD) is a major component of Cannabis sativa that lacks the psychotropic effect of delta-9-tetrahydrocannabinol (THC). This compound has potential broad therapeutic applications as anxiolytic, neuroprotective and anticonvulsive agent (see Mechoulam et al., 2002 for a review). Moreover, CBD possesses antiinflammatory activity in a model of rheumatoid arthritis (Malfait et al., 2000) and of carrageenan-induced acute inflammation (Costa et al., 2004). Few and conflicting are the reports about the antinociceptive activity of CBD in nonpathological conditions, whereas we have recently demonstrated that in acute inflammation the compound shows potent antihyperalgesic effect (Costa et al., 2004). In spite of its wide spectrum of pharmacological activities, little is known about the molecular mechanism of CBD action. CBD has very low affinity for either cannabinoid receptor subtypes cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2) (Pertwee, 1997). In addition, CBD has been reported to inhibit both the fatty acid amide hydrolase (FAAH)-mediated degradation of the endogenous cannabinoid ligand, anandamide (Watanabe et al., 1996) and the RBL-2H3 cell anandamide transporter activity (Rakhshan et al., 2000). These findings raised the possibility that some of the above-quoted pharmacological actions of CBD might be due to inhibition of anandamide degradation, with subsequent enhancement of endogenous level of this mediator. Anandamide has been identified as an endogenous cannabinoid CB1 receptor agonist and as ligand for cannabinoid CB2 receptor albeit with low affinity. Another important site at which anandamide acts is the transient receptor potential vanilloid type 1 (TRPV1) (Zygmunt et al., 1999), which acts as a molecular integrator of noxious physical and thermal stimuli and is important for thermal nociception and inflammatory hyperalgesia (Caterina et al., 2000; Davis et al., 2000). Finally, CBD was found to be a full, although weak, agonist of human TRPV1 (Bisogno et al., 2001). Thus, CBD could activate cannabinoid receptors indirectly through anandamide and vanilloid receptors directly and/or indirectly via this endogenous fatty acid amide. Given the demonstrated capacity of CBD to inhibit thermal hyperalgesia associated with carrageenan paw edema previously reported by us (Costa et al., 2004), the present study was addressed to clarify the mechanism underlying this effect. Considering that CBD could act through anandamide or TRPV1 receptors, we examined whether CBD antihyperalgesic effect could be reversed by selective antagonists of central or peripheral cannabinoid receptors (SR141716 or SR144528, respectively) and/or by an antagonist of TRPV1 receptors, capsazepine (CPZ).
Methods
Animals and treatment
Male Wistar rats (100–120 g, Harlan, Italy) were used. Animals were housed in a room with controlled temperature (22±1°C), humidity (60±10%) and light (12 h per day) for at least 1 week before being used. Food and water were available ad libitum. Experiments performed were in accordance with Italian State regulations, governing the care and treatment of laboratory animals (permission no. 94/2000A) and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (Zimmermann, 1983).
Acute inflammation was induced by intraplantar injection of 0.1 ml carrageenan (1% (w v−1)) (Sigma-Aldrich, Milano, Italy) in saline into the right paw. CBD was administered orally at a dose of 10 mg kg−1 (5 ml kg−1) 2 h after the induction of acute inflammation; this dose was chosen since we reported that it was effective in reversing fully the hyperalgesic effect produced by carrageenan injection (Costa et al., 2004). To study the involvement of cannabinoid and/or vanilloid receptor in the CBD antihyperalgesic effect, different treatments were tested: SR141716 (0.5 mg kg−1) or its vehicle (1 ml kg−1), both i.p., was coadministered with CBD or its vehicle; SR144528 (3 or 10 mg kg−1) or its vehicle (5 ml kg−1), both p.o., was administered 1 h before CBD or its vehicle; CPZ (2 or 10 mg kg−1) or its vehicle (5 ml kg−1), both i.p., was administered simultaneously with CBD or its vehicle. Control groups received an intraplantar injection of saline and the vehicles of drugs.
Carrageenan-induced thermal hyperalgesia
Latency for the withdrawal of both hindpaws from a thermal stimulus was measured by use of a radiant heat method (Hargreaves et al., 1988). The Plantar Test (Ugo Basile, Varese, Italy), which provides an infrared emission of 190 mW(cm2)−1 S−1, corresponding to a temperature of about 46°C, was used. After baseline withdrawal latencies (S) had been recorded, pain threshold was estimated 3, 5, 6 and 7 h after carrageenan and expressed as difference of latency vs baseline.
Data analysis
All results are presented as mean±s.e.m. of the differences between the withdrawal latency evaluated before the injection in rat paw and that measured 3 h after. The data were compared by one-way analysis of variance (ANOVA) followed by Tukey's test. A P-value <0.05 was considered significant.
Drugs
CBD, dissolved in methanol, was kindly supplied by GW Pharma Salisbury (U.K.). The methanol was evaporated under reduced pressure and the CBD residue was emulsified in a 1 : 1 : 18 mixture of ethanol : cremophor : saline. SR141716 and SR144528 were kindly supplied by Sanofi-Synthelabo Recherche (Montpellier, France) and were dissolved in a 1 : 2 : 7 mixture of Tween-80 : dimethylsulphoxide : distilled water. The employed doses of SR141716 and SR144528 were chosen since they antagonise cannabinoid-induced effects in rodents (Rinaldi-Carmona et al., 1998; Carta et al., 1999). CPZ was purchased from Sigma-Aldrich (Milano, Italy) and dissolved in a 1 : 1 : 8 mixture of ethanol : Tween-80 : saline. The doses of CPZ used were previously shown to antagonise the effects induced by the selective agonist of TRPV1 receptors, capsaicin, in rats (Di Marzo et al., 2001).
Results
As previously shown by us (Costa et al., 2004), intraplantar injection of carrageenan in rats led to a time-dependent thermal hyperalgesia, which peaked at 3 h and decreased during the subsequent evaluation times (5, 6 and 7 h), although it was still significant. At the peak time, CBD 10 mg kg−1, administered 2 h after carrageenan injection, abolished the hyperalgesia to the thermal stimulus.
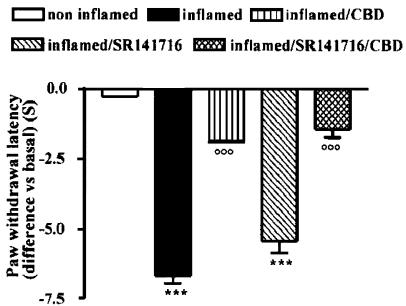
Cannabinoid CB1 receptor selective antagonist SR141716 did not reverse the CBD antihyperalgesic effect
SR141716 (0.5 mg kg−1, i.p.) per se did not modify the nociceptive responses of the contralateral hindpaw (data not shown) and did not affect the carrageenan-induced hyperalgesia either 3 h (Figure 1) or 5, 6 and 7 h after carrageenan (data not shown). When coadministered with CBD, SR141716 did not significantly reverse the CBD-induced antihyperalgesia (Figure 1) (P<0.0001, by one-way ANOVA). SR141716 did not modify the CBD effect during the subsequent evaluation times (data not shown).
Figure 1.
Effect of SR141716 (0.5 mg kg−1, i.p.) administration on CBD-induced antihyperalgesia 3 h after carrageenan injection into the rat hindpaw. Each point represents the mean±s.e.m. of five rats. ***P<0.001 vs non inflamed; °°°P<0.001 vs inflamed.
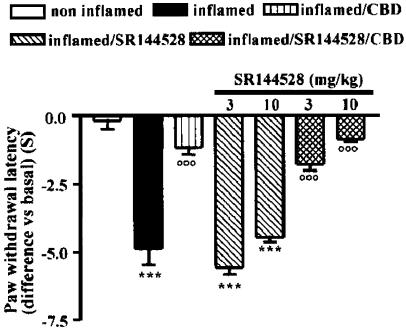
Cannabinoid CB2 receptor specific antagonist SR144528 did not reverse the CBD antihyperalgesic effect
At all evaluation times, no differences in nociceptive thresholds were found in both hindpaws of animals treated orally with SR144528 alone (3 and 10 mg kg−1) or with vehicle. Neither dose of CB2 antagonist administered 1 h before CBD inhibited the antihyperalgesic effect of CBD: 3 h after carrageenan the paw withdrawal latency of SR144528 plus CBD-treated animals did not differ from that of animals receiving CBD alone (Figure 2) (P<0.0001, by one-way ANOVA). At all the following evaluation times, SR144528 did not modify the effects of CBD (data not shown).
Figure 2.
Effect of SR144528 (3 and 10 mg kg−1, p.o.) administration on CBD-induced antihyperalgesia 3 h after carrageenan injection into the rat hindpaw. Each point represents the mean±s.e.m. of five rats. ***P<0.001 vs non inflamed; °°°P<0.001 vs inflamed.
TRPV1 receptor selective antagonist CPZ reversed the CBD antihyperalgesic effect
CPZ (2 and 10 mg kg−1) on its own did not modify the withdrawal latency of both contralateral (data not shown) and ipsilateral hindpaws (Figure 3). At 3 h after carrageenan, the lowest dose of CPZ partially reversed the antihyperalgesic action induced by CBD; in fact, CBD, coadministered with 2 mg kg−1 CPZ, inhibited the carrageenan-induced hyperalgesia by about only 40%. The highest dose of the TRPV1 receptor antagonist fully reversed the CBD-induced antihyperalgesic effect (Figure 3) (P<0.0001, by one-way ANOVA). At 5, 6 and 7 h following carrageenan injection, CPZ per se did not alter thermal hyperalgesia and when given together with CBD preserved its antagonistic effect (data not shown).
Figure 3.
Effect of CPZ (2 and 10 mg kg−1, i.p.) administration on CBD-induced antihyperalgesia 3 h after carrageenan injection into the rat hindpaw. Each point represents the mean±s.e.m. of five rats. ***P<0.001,**P<0.01 vs non inflamed; °°°P<0.001,°P<0.05 vs inflamed; ###P<0.001, #P<0.05 vs inflamed/CBD.
Discussion
Here, we report for the first time that the antihyperalgesic effect of CBD is mediated by TRPV1 receptors and does not involve the cannabinoid receptor subtypes CB1 and CB2. These findings highlight TRPV1 as a molecular target for CBD in vivo. So far only one study, in vitro, has demonstrated a pharmacological effect of CBD on TRPV1 (Bisogno et al., 2001). In this study, we demonstrate that the TRPV1-specific antagonist, CPZ, is able to antagonise the ability of CBD to abolish the hyperalgesia in the model of carrageenan-induced inflammation. TRPV1 receptor is a nonselective cation channel that, when activated, allows the influx of monovalent and divalent cations, predominantly Ca2+. This receptor is a critical mediator of the thermal hyperalgesia that occurs in the setting of tissue injury, namely that elicited by carrageenan, mustard oil or complete Freund's adjuvant (Caterina et al., 2000; Davis et al., 2000). These observations indicate that the contribution of TRPV1 to thermal sensing is greatly upregulated by inflammatory mediators, a finding in good agreement with the facilitatory action of mild acidification and bradykinin on TRPV1 activation in recombinant and native systems. There is strong evidence that not only the sensitivity but also the density of TRPV1 is enhanced in dorsal root ganglia neurons during inflammatory conditions (Amaya et al., 2003) and within nerve fibres at the site of inflammation (Carlton & Coggeshall, 2001). The TRPV1 agonist capsaicin, an irritant vanilloid derived from chilli peppers, excites sensory neurons directly by acting on TRPV1 receptors present in sensory nerve terminals; this first initiates the generation of action potentials perceived as burning pain and, second, it evokes a refractory state traditionally referred to as desensitisation in which the previously excited neurons no longer respond to painful stimuli. This latter phenomenon is believed to underlie the analgesia caused by capsaicin and other TRPV1 agonists. As CBD binds to TRPV1 receptors (Bisogno et al., 2001), we can hypothesise that CBD, like capsaicin, leads to desensitisation of TRPV1 receptors, with subsequent ‘paradoxical analgesic effects'.
Moreover, our present findings indicate that the cannabinoid system is not involved in the antihyperalgesic effect of CBD. Anandamide possesses well-established analgesic and antihyperalgesic properties via cannabinoid receptors (Calignano et al., 1998), and it has been reported that CBD inhibits the intracellular uptake of anandamide (Rakhshan et al., 2000) and its subsequent hydrolysis (Watanabe et al., 1996), leading to enhanced extracellular levels of this endogenous fatty acid amide. However, the possibility that CBD inhibits the carrageenan-induced hyperalgesia through anandamide acting on CB receptors seems unlikely in the present study, since the administration of the selective CB1 and CB2 receptor antagonists did not reverse the antihyperalgesia evoked by CBD.
In conclusion, the present study suggests that the antihyperalgesic action of the natural cannabinoid CBD is mediated by TRPV1. In pathological conditions, such as neuropathy and rheumatoid arthritis, in which TRPV1 receptor sensitivity and expression are increased (Amaya et al., 2003; Rashid et al., 2003), the nontoxic and nonpsychoactive compound CBD, may represent an useful pharmacological alternative in the treatment of the disease-associated chronic pain.
Acknowledgments
We are grateful to GW Pharma for kindly supplying cannabidiol and to Sanofi-Synthelabo for the gift of SR141716 and SR144528.
Abbreviations
- CBD
cannabidiol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CPZ
capsazepine
- FAAH
fatty acid amide hydrolase
- SR141716
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR144528
N-[(1S)-endo-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide
- THC
delta-9-tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid type 1
References
- AMAYA F., OH-HASHI K., NARUSE Y., IIJIMA N., UEDA M., SHIMOSATO G., TOMINAGA M., TANAKA Y., TANAKA M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., HANUS L., DE PETROCELLIS L., TCHILIBON S., PONDE D.E., BRANDI I., SCHIANO MORIELLO A., DAVIS J.V., MECHOULAM R., DI MARZO V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CARLTON S.M., COGGESHALL R.E. Peripheral capsaicin receptors increase in the inflamed hindpaw: a possible mechanism for peripheral sensitisation. Neurosci. Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- CARTA G., GESSA G.L., NAVA F. Dopamine D2 receptor antagonists prevent Δ9-tetrahydrocannabinol-induced antinociception in rats. Eur. J. Pharmacol. 1999;384:153–156. doi: 10.1016/s0014-2999(99)00696-2. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- COSTA B., COLLEONI M., CONTI S., PAROLARO D., FRANKE C., TROVATO A.E., GIAGNONI G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:294–299. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., LASTRES-BECKER I., BISOGNO T., DE PETROCELLIS L., MILONE A., DAVIS J.B., FERNANDEZ-RUIZ J.J. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur. J. Pharmacol. 2001;420:123–131. doi: 10.1016/s0014-2999(01)01012-3. [DOI] [PubMed] [Google Scholar]
- HARGREAVES K.M., DUBNER R., BROWN S., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- MALFAIT A.M., GALLILY R., SUMARIWALLA P.F., MALIK A.S., ANDREAKOS E., MECHOULAM R., FELDMANN M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHOULAM R., PARKER L.A., GALLILY R. Cannabidiol: an overview of some pharmacological aspects. J. Clin. Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- RAKHSHAN F., DAY T.A., BLAKELY R.D., BARKER E.L. Carrier-mediated uptake of the endogenous cannabinoid anandamide in RBL-2H3 cells. J. Pharmacol. Exp. Ther. 2000;124:315–322. [PubMed] [Google Scholar]
- RASHID M.H., INOUE M., BAKOSHI S., UEDA H. Increased expression of vanilloid receptor 1 on myelinated primary afferent neurons contributes to the antihyperalgesic effect of capsaicin cream in diabetic neuropathic pain in mice. J. Pharmacol. Exp. Ther. 2003;306:709–717. doi: 10.1124/jpet.103.050948. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J-M, CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J-C, Le FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- WATANABE K., KAYANO Y., MATSUNAGA T., YAMAMOTO I., YOSHIMURA H. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol. Pharm. Bull. 1996;19:1109–1111. doi: 10.1248/bpb.19.1109. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]